Abstract
We previously described that mitochondrial ATP-Binding Cassette transporter, ABCme (ABCB10) is induced by GATA-1 in erythroid cells and that its over-expression leads to increased heme production. However, the role of ABCme in vivo and the hematologic phenotype of its deficiency have not been described thus far. Here we report for the first time that ABCme is essential for embryonic blood development in vivo. ABCme deficient mouse was generated on 129SvEvBrd/C57BL6J background and backcrossed to n = 4 on the C57BL6J. The homozygous KO mouse is embryonic lethal. ABCme KO dies at 12.5 days of gestation and displayed aplastic embryonic anemia by 10.5 days of gestation, manifested as improper blood development as well as erythroid progenitor apoptosis (Figure 1). Ex vivo analysis of maturation of isolated yolk sac blood determined that ABCme KO erythroid cells fail to differentiate beyond the stage of basophilic erythroblast and exhibit 55% of apoptosis in CD71 positive cells in comparison with 29.25% in ABCme HET, and 17.5% in WT. Erythroid colony assay indicated 50% reduction in the formation of CFU-E and an 80% reduction in BFU-E in comparison with WT. Disruption of heme synthesis may result in accumulation of heme intermediates that are pro-oxidants. To test the hypothesis that apoptosis is induced by oxidative damage we tested both mitochondrial oxidation and the rescue effects of mitochondrial antioxidants in the ABCme KO. Isolated mitochondria of ABCme KO erythroid cells demonstrated significantly elevated levels (5 fold) of oxidized protein in comparison to both WT and HET cells. This elevated level of superoxide were confirmed and identified to be mitochondrial in nature by FACS analysis of superoxide sensitive dye MitoSox. Using antioxidants we tested the patho-physiological role of the elevated ROS in the hematologic phenotype. Treatment of ABCme KO cells with the SOD2 mimetic TBAP resulted in partial and complete rescue of the apoptotic phenotypes of the KO and the HET respectively. Our findings suggest that ABCme is required for erythroid development in vivo and that its deficiency results in impaired erythroid maturation, hemoglobinzation, and increased mitochondrial oxidative damage.
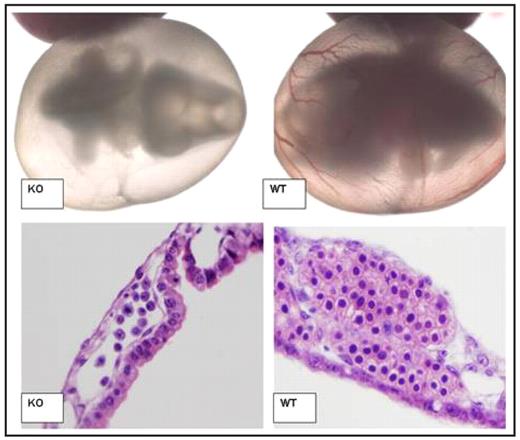
SEQ Figure \* ARABIC 1: Impaired Blood Development of ABCme deficient embryos
Light microscopy of Day 10.5 p.c. Embryo and Yolk Sac (Top). At this stage KO embryos are still alive as denoted by heartbeat as well as normal development of other organ systems. However, lack of red cell development is apparent. H/E staining of day 10.5 p.c. yolk sac blood islands (Bottom). KO blood islands demonstrate reduced number, increased nuclear fragmentation associated with apoptosis, and a lack of developed erythroid progenitors. (WT= Wildtype mice, KO= ABCme −/− mice.)
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal