Abstract
There is an accumulation of in vivo (graft-versus-leukemia effect) and in vitro (spontaneous remissions after infections) data providing evidence that CLL might be effectively targeted by T-cell based immunotherapy. Earlier, we characterized the receptor for hyaluronic acid mediated motility (RHAMM) as antigen associated with proliferation and negative prognosis in CLL. We also demonstrated that RHAMM-derived epitope(R3)- primed T cells were able to lyse RHAMM+ target CLL cells. Therefore, we initiated a small phase I/II clinical trial with R3 peptide vaccination for patients with CLL. Six CLL patients in Binet stage 0 of the disease were vaccinated four times at a biweekly interval with HLA-A2 restricted RHAMM-derived epitope R3 (ILSLELMKL, 300μg/dose on day 3) emulsified in incomplete Freund’s adjuvant (IFA) with concomitant administration of GM-CSF (100μg/dose, days 1–5). R3-specific T-cell responses were assessed by tetramer staining and ELISPOT assays. T-cell subsets which play a role in regulation of immune responses including CD3+CD4+CD25hiCD127loFOXP3+ T regs, Th17, CD8+CD137+, CD8+CD103+ and IL-17 producing CD8+ T cells (CD8+IL-17+) were evaluated by flow cytometry.
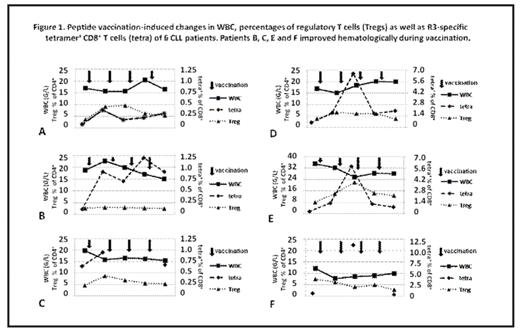
No severe adverse events greater than CTC Io skin toxicity could be observed. Four of six patients showed a reduction of WBC during vaccination. Although these WBC changes did not meet the NCI response criteria, we described these favorable hematological changes achieved in short period of immunotherapy as hematological improvement (defined as at least 20% reduction of WBC during vaccination). The immune responses were found in 5/6 patients as assessed by tetramer-staining (positive response defined as an increase of R3-specific CD8+ T cell frequency by more than 100% after vaccination) and confirmed in 4/5 as assessed by ELISPOT assay. Patients included in this study showed median Tregs frequency of 4.2%, range: 2.5–8%. There was no significant difference of Tregs percentages between patients who improved clinically when compared with non-responders (median 6.1% vs. 3.7%). Vaccination induced Tregs in 4 patients (2 non-responders and 2 responders). Two other patients who improved hematologically did not significantly change frequency of Tregs or even reduced it during vaccination (Figure 1). Median expression of CD103 on CD8+ T cells was 1.84%, range: 0.41–5.63%. In one non-responder, we observed an increase in frequency of CD103+CD8+ T-cells during vaccination from 1.46% to 2.56%. During vaccination, changes in CD8+CD103+ T cell subset did not correlate with the frequency of Tregs, nonetheless we could find an inverse correlation with inflammatory Th17 T cells (r2=−0.5, p<0.05). We could find a correlation between the frequency of Tregs and activated CD8+CD69+ T cells (r2=0.51, p<0.05). Interestingly, CD8+CD137+ cells correlated with CD8+IL-17+ T cells (r2=0.54, p<0.05).
In conclusion, peptide vaccination in CLL patients is safe and feasible to mount immune responses against the tumor antigen RHAMM. Most of patients benefited hematologically from vaccination. Although in some patients we observed an induction of tumor-specific T cells without induction of Tregs there is a rationale to add novel active agents against Tregs in future vaccination trials.
Peptide vaccination induced changes in WBC, percentages of regulatory T cells (Tregs) as well as R3 specific tetramer ‘CD’ T cells (tetra) of A CLL patients. Patients B, C, E and F improved hematologically during vaccination.
Peptide vaccination induced changes in WBC, percentages of regulatory T cells (Tregs) as well as R3 specific tetramer ‘CD’ T cells (tetra) of A CLL patients. Patients B, C, E and F improved hematologically during vaccination.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal