Abstract
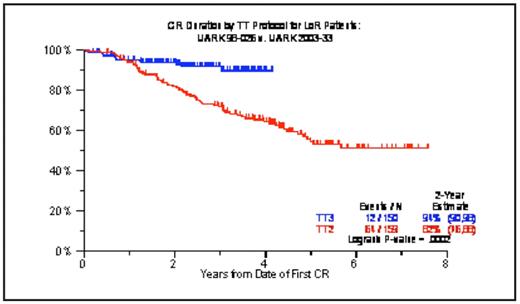
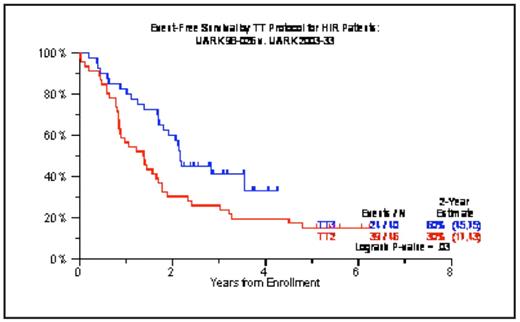
We have previously reported on the remarkable activity of the TT3 program that incorporated both bortezomib (V) and thalidomide (T) into the up-front management of 303 patients. TT3 consisted of 2 cycles each of induction prior to and of dose-reduced consolidation therapy with VTD-PACE (cisplatin, doxorubicin, cyclophosphamide, etoposide) after melphalan 200mg/m2 (M200)-based tandem transplants, followed by maintenance therapy for 3 years with VTD and, in later stages, VRD (substituting T for lenalidomide, R). Characteristics included a median age of 59yr (range, 33–75yr), B2M >=4mg/L in 37%, albumin <3.5g/dL in 26%, ISS stages II and III in 33% and 21%, cytogenetic abnormalities (CA) in 33% and gene expression profiling (GEP)-defined high-risk MM in 15% of the 275 patients with such data. With a median follow-up of 39 months, 4-yr overall survival (OS) and event-free survival (EFS) estimates were 78% and 71%, respectively, including 84% and 77% among the 85% with GEP-defined low-risk MM contrasting with 43% and 33% in the remainder with high-risk MM (both p<0.0001). Near-CR and CR, attained in 86% and 63%, were sustained at 4 years from response onset in 78% and 87%, which pertained to 83% and 90% with low-risk MM but to only 44% and 57% with high-risk MM (all p <0.0001). These results were corroborated in a TT3 extension trial (TT3E) that enrolled 175 additional patients, comprising higher proportions of CA (42%) and GEP-defined high-risk MM (21%). Two-year estimates of OS and EFS are 85% and 85%, with 94% and 92% in low-risk patients versus 61% and 62% in high-risk MM (p=0.0001, p=0.0003); the 2-yr estimate of remaining in CR is 93% including 100% in low-risk and 77% in high-risk MM (p=0.01). Multivariate analysis of features linked to OS in TT3 included GEP-defined high-risk, CA, B2M and LDH elevation, collectively accounting for 41% of outcome variability by R2 statistics; the corresponding R2 values for EFS and n-CR duration were 38% and 39%. Compared to the predecessor trial, TT2, that evaluated the role of T in a randomized trial design in 668 patients, TT3 data were superior for OS (p=0.08), EFS (<0.0001), n-CR duration (p<0.0001) and CR duration (p=0.0002). In the low-risk subgroup, EFS (p=0.0001), n-CR duration (p<0.0001) and CR duration (Figure 1a; p=0.0002) all were superior in TT3 versus TT2; whereas, in the high-risk MM group, outcomes remained poor also with TT3 despite superior EFS (Figure 1b; p=0.03). Based on these data, we have now started a GEP-risk-based algorithm of assigning separate therapies to good-risk (TT4) and poor-risk MM (TT5). As the TT3 results for low-risk are difficult to improve upon, TT4 randomizes patients between standard TT3 and TT3-LITE that employs only 1 cycle each of induction and consolidation (with anticipated further improvement in compliance) and 4-day-fractionated M50×4 to enable the addition of VTD and thus exploit synergistic drug interactions to occur. In order to sustain tolerable effective therapies for at least 3 years and prevent recurrence from previous drug-free or insufficiently effective phases in TT3, TT5 for high-risk MM employs less dose-intense and more dose-dense highly synergistic combination therapy, utilizing M10-VTD-PACE for induction, M80 (in 4 daily fractions of M20) plus VRD-PACE tandem transplants, separated by 2 cycles of M20 (in 4 daily fractions of M5) plus VTD-PACE, and followed by 2 years of monthly alternating R-VD and M-VD.
Disclosures: Barlogie:NCI: Research Funding; Millenium: Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; IMF: Honoraria, Membership on an entity’s Board of Directors or advisory committees; MMRF: Membership on an entity’s Board of Directors or advisory committees; SWOG: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Genzyme: Consultancy, Speakers Bureau; Novartis: Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal