Abstract
The role of allogeneic HSCT in patients with AML/MDS in CR1 is not well established. Historically, high rates of non-relapse mortality (NRM) associated with HSCT have negatively counterbalanced the reduced relapse rate associated with the graft-versus leukemia effect. We hypothesized that if NRM is reduced, allogeneic HSCT will improve relapse-free survival (RFS) when compared to chemotherapy alone. Here we compared outcomes of patients treated with busulfan 130 mg/m2 and fludarabine 40 mg/m2 for 4 days (our ablative regimen with the lowest NRM rate) and matched controls treated without HSCT.
Methods: Between January, 2001 and December, 2004, 36 patients with AML (n=28) or high-risk MDS (n=8) in CR1 were transplanted. Year 2004 was chosen as the cutoff to allow for significant follow up time. Controls were 110 patients treated in the leukemia department without HSCT between 1980 and 2004, selected to match according to age, cytogenetics, and time in CR1 (in order to compensate for the time in CR1 that preceded HSCT, which was a median of 3.3 months, range 0.36–12.4 months). All patients received induction regimen containing high dose cytarabine. Eligible for HSCT were patients in remission that did not have good prognosis cytogenetics that had a performance status of 0 or 1, adequate organ function, no uncontrolled infection and age 18–65 years. Primary end point was RFS (measured from CR1 date to relapse or death).
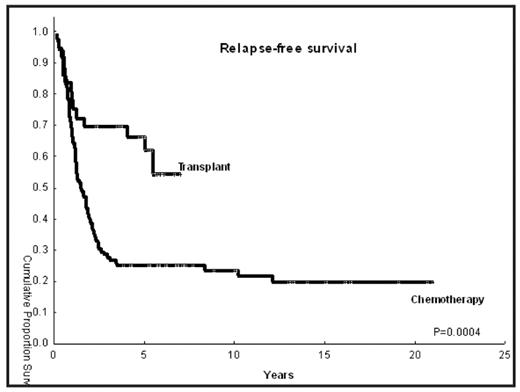
Results: Median age was 42 years in both groups (range 21–62). Proportion of patients with intermediate risk and high-risk cytogenetics in the transplant and chemo groups was as follows: 67% versus 72%, and 33% versus 28%, respectively. The median time from diagnosis to CR1 was 1.5 month (range, 0.6–17.2) and 1.15 month (range, 0.13–5) in the HSCT and chemotherapy groups respectively. Patients in the control group had de novo AML in 92% of the cases (n=102) versus 64% in the HSCT cohort (n=23), while more patients in the transplant group had secondary AML or MDS (36% vs 8%). Donors were HLA matched related in 22 cases (61%), and a matched unrelated donor in 12 patients (39%). Stem cell source was the peripheral blood (n=20; 56%) or bone marrow (n=16, 44%). Median follow-up of surviving patients is 5.2 years for the HSCT (range, 2.4–7) versus 9.1 years (3.9–21) for the chemotherapy group, respectively. Twelve HSCT patients have died (33%), versus 76 (69%) in the chemotherapy group. Relapse occurred in 12/36 patients (33%) in the HSCT group versus 79/110 (72%) in the control group. NRM in the HSCT cohort was zero at 100 days, 7% at one year and 15% at 3 years (1 patient died of chronic GVHD and 1 died of pneumonia). RFS is shown in the picture. RFS of transplant patients remains superior if we only used control patients treated in the same time frame in which the transplants were performed (ie, year 2001 to 2004; p=0.0003)
Conclusion: These results indicate improved survival with HSCT and support the conduct of a randomized comparison of chemotherapy versus allogeneic HSCT using Busulfan Fludarabine preparative regimen for AML in CR1.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal