Abstract
The role of proteinases in the mobilization of hematopoietic progenitor cells (HPCs) after granulocyte colony-stimulating factor (G-CSF) remains unclear. Here we report that genetic loss of the plasminogen activator inhibitor Pai-1 or of the plasmin inhibitor α2-antiplasmin increases HPC mobilization in response to G-CSF. Moreover, thrombolytic agents, such as tenecteplase and microplasmin, enhance HPC mobilization in mice and humans. Taken together, these findings identify a novel role for plasmin in augmenting HPC mobilization in response to G-CSF.
Introduction
Autologous or allogeneic granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood (PB) is an important source of hematopoietic progenitor cells (HPCs) for subsequent transplantation in various hematologic diseases, but the therapeutic success to mobilize sufficient numbers of CD34+ cells is limited in a number of patients, in part because of their refractoriness or poor response to G-CSF.1 Therefore, a better understanding of the underlying mechanisms regulating HPC mobilization in response to G-CSF might offer novel therapeutic opportunities for HPC mobilization.
Studies in mice genetically deficient for G-CSF or its receptor (G-CSF-R) have shown that G-CSF does not mobilize HPCs by binding to its receptor on these cells but, instead, by binding to G-CSF-R+ resident cells within the bone marrow (BM) microenvironment and stimulating HPC mobilization via paracrine signals.2 Other studies underscored an important role for BM-derived proteinases, such as neutrophil elastase and matrix metalloprotease-9, which inactivate adhesive signals anchoring HPCs in the BM.3-6 However, loss of these proteinases did not necessarily impair HPC mobilization after G-CSF.7,8
Using gene-deficient mice, we recently documented a role for the proteinase plasmin in HPC mobilization in response to G-CSF (M.T. and P.C., revised manuscript submitted September 2008). Plasmin is generated from its zymogen plasminogen via proteolytic cleavage by 2 plasminogen activators (PA), ie, tissue-type PA (tPA) and urokinase-type PA, whereas inactivation of plasmin occurs by α2-antiplasmin (AP) and its generation is prevented by PAI-1 (which neutralizes PA activity) (reviewed in Collen9 ). Apart from regulating hemostasis, plasmin also cleaves cell-surface receptors, activates matrix metalloproteases, remodels the extracellular matrix, and liberates matrix-bound growth factors, explaining why this proteinase system has such a pleiotropic role in tissue healing, regeneration, and malignancy.9 A recent report showed that administration of recombinant human tPA promotes the hematopoietic recovery of myeloablated mice, but the authors did not investigate its effects on HPC mobilization after G-CSF therapy.10 Here we investigated whether plasmin could enhance HPC mobilization in response to G-CSF.
Methods
Animal studies
All experiments were performed according to the guidelines for care and use of laboratory animals approved by the institutional ethical animal care committee.
Patient study
The protocol of this nonrandomized clinical study was approved by the local medical ethical committee (ML2526) and was performed in accordance with the Declaration of Helsinki. (Full Experimental Methods section available on the Blood website; see the Supplemental Materials link at the top of the online article.)
Results and discussion
Genetic loss of Pai-1 or α2-antiplasmin increases HPC mobilization
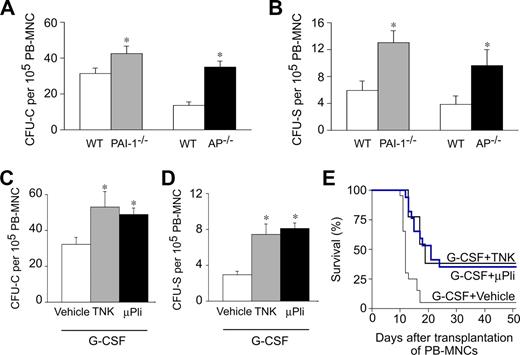
We first analyzed mobilization of HPCs in response to G-CSF in mice lacking Pai-1 (Pai-1−/−) or α2-antiplasmin (Ap−/−), the primary inhibitors of PAs and plasmin, respectively. No genotypic differences in circulating HPCs were detectable in steady-state conditions (not shown). After G-CSF treatment (200 μg/kg per day subcutaneously for 5 days), circulating HPCs were detectable in the PB of the respective wild-type (WT) controls, as analyzed by counting the number of colony-forming units–cells (CFU-C; Figure 1A) and colony-forming units–spleen (CFU-S; Figure 1B) per 105 PB mononuclear cells (PB-MNCs). Notably, Pai-1−/− or Ap−/− mice mobilized more HPCs in the PB (Figure 1A,B). Indeed, compared with their respective WT controls, the number of circulating CFU-C and CFU-S was increased by approximately 1.4-fold and approximately 2.2-fold in Pai-1−/− mice, and by approximately 2.6-fold and approximately 2.5-fold in Ap−/− mice, respectively (Figure 1A,B). Thus, genetic loss of Pai-1 or Ap enhances HPC mobilization after G-CSF.
Plasmin enhances HPC mobilization after G-CSF. (A,B) Compared with WT mice, AP−/− mice had more circulating CFU-C (A) and CFU-S (B) at 5 days after G-CSF. *P < .05 vs WT (n = 10-15). (C,D) Compared with vehicle, treatment with 100 mg/kg tenecteplase (TNK, daily intraperitoneal) or 100 μg/day microplasmin (μPli, osmotic minipump) during 5 days increased the number of circulating CFU-C (C) and CFU-S (D), mobilized in response to G-CSF. Vehicle groups behaved similarly (not shown) and were therefore pooled together. *P < .05 vs WT (n = 10-15). (E) More lethally irradiated syngeneic WT recipients survived when they were transplanted with 1 × 105 PB-MNCs from G-CSF–treated WT mice, receiving TNK or μPli than vehicle (P < .05, Cox regression; n = 13-20).
Plasmin enhances HPC mobilization after G-CSF. (A,B) Compared with WT mice, AP−/− mice had more circulating CFU-C (A) and CFU-S (B) at 5 days after G-CSF. *P < .05 vs WT (n = 10-15). (C,D) Compared with vehicle, treatment with 100 mg/kg tenecteplase (TNK, daily intraperitoneal) or 100 μg/day microplasmin (μPli, osmotic minipump) during 5 days increased the number of circulating CFU-C (C) and CFU-S (D), mobilized in response to G-CSF. Vehicle groups behaved similarly (not shown) and were therefore pooled together. *P < .05 vs WT (n = 10-15). (E) More lethally irradiated syngeneic WT recipients survived when they were transplanted with 1 × 105 PB-MNCs from G-CSF–treated WT mice, receiving TNK or μPli than vehicle (P < .05, Cox regression; n = 13-20).
Thrombolytic agents enhance HPC mobilization in mice
The results cited in “Genetic loss of Pai-1 or α2-antiplasmin increases HPC mobilization” indicate that plasmin augments G-CSF–mediated mobilization. We therefore evaluated whether thrombolytic compounds, which generate or increase plasmin and are currently being used in the clinic (tenecteplase, recombinant human tPA) or are in clinical development (microplasmin, staphylokinase) might be useful to stimulate HPC mobilization. For the mouse study, we used 3 thrombolytics: recombinant human tPA (rtPA), tenecteplase (TNK; a rtPA mutant, which has a prolonged half-life and is used for treatment of acute cardiovascular and cerebrovascular syndromes), and microplasmin (μPli; a plasmin variant lacking the 5 aminoterminal kringle domains, which has an improved safety profile [less bleeding] and is easier to produce as recombinant protein than plasmin11 ). Because mice are well known to be approximately 10-fold less responsive to these thrombolytic agents, especially when given as intraperitoneal bolus injection,12-14 their dose was adapted accordingly. In steady-state conditions, administration of the thrombolytic compounds TNK (daily intraperitoneal bolus injection of 100 mg/kg) or μPli (100 μg/day, continuously over a period of 5 days via osmotic minipumps) failed to induce HPC mobilization in WT mice (not shown), raising the question of whether these agents might only enhance HPC mobilization in conjunction with G-CSF. Indeed, when coadministered with G-CSF (200 μg/kg per day subcutaneously for 5 days), TNK enhanced the mobilization of CFU-C and CFU-S by approximately 1.7-fold and 2.6-fold, respectively, compared with G-CSF alone (Figure 1C,D). Coadministration of μPli and G-CSF also stimulated mobilization of CFU-C and CFU-S by approximately 1.5-fold and approximately 2.8-fold, respectively (Figure 1C,D). These cells were capable of reconstituting hematopoiesis, as transplantation of these cells increased the survival of lethally irradiated WT-recipient mice (Figure 1E).
It is well known that human rtPA has a short half-life in mice (ie, in the order of minutes15 ), resulting from its rapid sequestration and a low efficiency to convert mouse plasminogen to plasmin (> 40-fold lower than for human plasminogen12-14 ); we therefore used a 10-fold higher dose than normally administered to humans.12-14 Nonetheless, daily intraperitoneal bolus injection of human rtPA (100 mg/kg) failed to enhance HPC mobilization in steady-state conditions or after G-CSF administration in mice (not shown). Our data thus differ from recent findings by Heissig et al that a 100-fold lower dose of human rtPA in mice increases HPC mobilization in steady-state conditions and after chemomyeloablation10 ; the precise reason for this discrepancy remains unexplained. Thus, TNK or μPli enhances HPC mobilization after G-CSF.
Thrombolytic agents enhance HPC mobilization in humans
We also evaluated whether thrombolytic agents would be capable of inducing HPC mobilization in humans as well. Our mouse results indicate that thrombolytic agents only enhance HPC mobilization, when these cells are already primed by another mobilization stimulus (such as G-CSF). However, ethical reasons precluded us from testing whether thrombolytic agents enhance HPC mobilization in response to G-CSF in healthy volunteers. We therefore studied the effect of thrombolytic agents in the setting of acute myocardial ischemia, as this is a well-known stimulus for HPC mobilization.16 Therefore, PB samples were collected before and 24 hours after thrombolytic treatment (TNK or staphylokinase) of patients, who were admitted to the Coronary Care Unit with ST-segment elevation myocardial infarction (STEMI). As control group, blood samples were collected from STEMI patients before and 24 hours after percutaneous coronary intervention (PCI) because this procedure does not induce HPC mobilization at this early time point.16
Mobilization of HPCs was determined by quantifying CFU-C using methylcellulose culture assays and the percentage of CD34+ cells using flow cytometry. A pilot group of 11 patients was analyzed: 6 received first-line PCI (PCI group), whereas 5 received thrombolytic therapy (thrombolysis group: 3 treated with TNK, 2 treated with staphylokinase); additional characteristics of the study group are listed in Table S1. At the time of admission (ie, before onset of treatment), both the PCI and thrombolysis groups had comparable numbers of circulating HPCs (CFU-C/106 MNCs: 28% ± 6% vs 19% ± 10%; percentage of CD34+ cells: 1.45 ± 0.22 vs 0.71 ± 0.34; P = not significant). After 24 hours, PCI treatment failed to mobilize HPCs. Indeed, when expressed as a percentage of baseline, post-PCI values were: 90% ± 30% for the CFU-C and 105% ± 13% for the CD34+ cells. This lack of mobilization after PCI is consistent with previous findings that PCI only mobilizes progenitors at later time points (ie, at 3-7 days after treatment).16,17 In contrast, thrombolytic therapy significantly increased HPC mobilization at 24 hours after treatment. Compared with the pretreatment levels, corresponding values after thrombolytic therapy were: 690% ± 230% for the CFU-C (P < .05) and 385% ± 75% for the CD34+ cells (P < .005). Taken together, even though only a small number of patients was analyzed in a nonrandomized study design (and larger cohorts will have to be analyzed in the future), these findings suggest that thrombolytic agents enhance the mobilization of HPCs in STEMI patients at 24 hours after myocardial infarction.
In conclusion, the main finding of this study is that plasmin augments HPC mobilization after G-CSF. Our findings that fibrinolytic agents stimulate HPC mobilization warrant further exploration of their therapeutic potential in patients who mobilize poorly in response to G-CSF. Especially the use of microplasmin might be recommended in perspective of its reduced risk to induce bleeding.11
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Carton, B. Hermans, A. Manderveld, M. Meurrens, H. Moreau, J. Souffreau, S. Terclavers, A. Van Nuffelen, M. Van Russelt, P. Van Wesemael, and B. Vanwetswinkel (Leuven) for assistance, W. Landuyt (Radiobiology, Leuven) for help with irradiation, and Thrombogenics for generously providing microplasmin for our mouse studies.
This work was supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO G.0209.07), the Belgian Science Policy (project no. IAP-P5/02), and an unrestricted Bristol-Myers-Squibb grant (P.C.). S.J. is a clinical investigator of the FWO. M.T. is a research fellow of the Belgian Instituut voor de Aanmoediging van Innovatie door Wetenschap en Technologie (IWT) and FWO.
Authorship
Contribution: M.T. designed and performed experiments, analyzed data, and participated in discussion and manuscript writing; S.J. organized and supervised the collection of patient samples; P.C. designed and analyzed data, participated in discussion and manuscript writing, and provided scientific direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
M.T.'s current address is Leibniz Unit, Centre for Molecular Medicine, Institute of Cardiovascular Regeneration, University of Frankfurt, Frankfurt, Germany.
Correspondence: Peter Carmeliet, Vesalius Research Center, Flanders Institute for Biotechnology (VIB), K.U. Leuven, Campus Gasthuisberg, Herestraat 49, B-3000, Leuven, Belgium; e-mail: peter.carmeliet@med.kuleuven.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal