Abstract
Stem cell factor (SCF) plays critical roles in proliferation, survival, migration, and function of hematopoietic progenitor and mast cells through binding to Kit receptor. Previous studies have implicated the adaptor protein Lnk as an important negative regulator of SCF signaling. However, the molecular mechanism underlying this regulation is unclear. Here, we showed that the Src homology 2 domain (SH2) of Lnk binds directly and preferentially to phosphorylated tyrosine 567 in Kit juxtamembrane domain. Using Lnk−/− bone marrow mast cells (BMMCs) transduced with different Lnk proteins, we demonstrated that Lnk down-regulates SCF-induced proliferation with attenuation of mitogen-activated protein kinase (MAPK) and c-jun N-terminal kinase signaling. Furthermore, we showed that Lnk−/− BMMCs displayed increased SCF-dependent migration compared with wild-type cells, revealing a novel Lnk-mediated inhibitory function. This correlated with enhanced Rac and p38 MAPK activation. Finally, we found that Lnk domains and carboxy-terminal tyrosine contribute differently to inhibition of in vitro expansion of hematopoietic progenitors. Altogether, our results demonstrate that Lnk, through its binding to Kit tyrosine 567, negatively modulates specific SCF-dependent signaling pathways involved in the proliferation and migration of primary hematopoietic cells.
Introduction
Mast cells are multifunctional hematopoietic cells, important for both innate and specific immunity.1 Immature mast cells leave the bone marrow to migrate to target tissues, mostly mucosal and connective tissues, where they undergo terminal differentiation and perform their biologic functions.2
Stem cell factor (SCF) and its receptor Kit are essential for mast cell development in vivo, as shown by the phenotype of mice with null mutations in the Kit (White Spotting or W) or SCF (Steel or Sl) loci. Indeed, these mice lack tissue mast cells and also exhibit defects in hematopoiesis, pigmentation, and reproduction.3 In bone marrow mast cells (BMMCs), activation of Kit, a type III receptor tyrosine kinase (RTK), stimulates cellular responses such as proliferation, survival, differentiation, chemotaxis, cell adhesion, and degranulation.4 Upon SCF binding, the Kit receptor dimerizes, autophosphorylates, and subsequently transphosphorylates specific tyrosines (Y). The resulting phosphotyrosine (pY) residues serve as docking sites for Src homology 2 (SH2) domain–containing proteins, which control intracellular signaling pathways such as all 3 mitogen-activated protein kinases (MAPKs; ERK, p38, and c-jun N-terminal kinase [JNK]), phosphatidylinositol 3-kinase (PI-3K), and Janus kinases (JAK)/signal transducers and activators of transcription (STAT) pathways.4,5 Recruitment of particular targets is mediated by the ability of SH2 domains to recognize specific pY-containing motifs on the activated receptor. Analyses of individual docking sites on Kit have provided valuable information about the activation of pathways emanating from this receptor.6 Juxtamembrane Y567 and Y569 are critical recruitment sites for different regulatory signaling molecules. These include activators such as Src family kinases (Fyn and Lyn), Shp-2 phosphatase and Shc adaptor protein, or negative modulators such as Shp-1 phosphatase, Csk-homology kinase (Chk), suppressors of cytokine signaling (SOCS) proteins, and APS adaptor protein.5,7,8 Mutational and “knock-in” studies on Kit Y567/Y569 have revealed their important role in cell development, proliferation, survival, and migration.6,9-11 However, the biologic significance of these interactions is still poorly understood.
Lnk is a member of the adaptor protein family that includes APS and SH2-B. All 3 members share common protein-protein interaction domains and motifs: a dimerization domain at the amino-terminus, a pleckstrin homology (PH) domain, an SH2 domain, and a conserved tyrosine near the carboxy-terminus.12 Mice deficient for members of this family have demonstrated the positive (SH2-B) and negative (Lnk and APS) role of these adaptors in growth factor, cytokine, and antigen-receptor signaling.13-18 In particular, Lnk nullizygous mice show mainly a profound perturbation in hematopoiesis. These animals exhibit splenomegaly together with fibrosis, expansion of hematopoietic stem cells (HSCs), and myeloid and B lymphoid progenitors.17-19 Recent analysis of Lnk−/−-derived HSC and myeloid (megakaryocytic and erythroid) lineages has shown that Lnk negatively regulates thrombopoietin (TPO) and erythropoietin (EPO) signaling in these cells.20-23
Lnk-deficient mice have also revealed an essential role for Lnk in B-cell lymphopoiesis.17,18 The abnormal proliferation of pro/pre-B and immature B cells is partly due to hypersensitivity to SCF. Indeed, Lnk−/− mice on a KitW/+ background exhibit partial but significant normalization of B-cell overproduction displayed by Lnk-deficient animals, confirming the negative role of Lnk in SCF/Kit signaling in these cells.19 Studies on hematopoietic primary cells and established cell lines have shown that Lnk interacts with Kit receptor and becomes phosphorylated upon SCF binding.19,24,25 Moreover, analysis of Lnk-deficient cells showed an altered activation of the MAPK pathway and in consequence, an increased proliferative response to SCF.18
Despite several reports implicating Lnk in SCF signaling, the mechanism by which Lnk modulates Kit-dependent pathways has not yet been identified. In the present study, we showed that the SH2 domain of Lnk binds directly and preferentially to Y567 in the Kit juxtamembrane domain. To address the functional significance of the Lnk-Kit interaction, we have studied the contribution of the different Lnk domains to Kit-dependent proliferation and migration by reconstitution of Lnk-deficient BMMCs and analyzed the signaling pathways affected. We demonstrate that the SH2 domain of Lnk is essential for inhibiting BMMC proliferation and down-regulation of JNK and MAPK activation. Furthermore, we show that Lnk−/− BMMCs display an increased capacity to migrate in response to SCF compared with wild-type cells. This correlates with enhanced and prolonged activation of the Rac and p38 MAPK signaling pathways. Moreover, our results indicate that the Lnk domains and Y536 residue contribute differently to inhibition of in vitro expansion of hematopoietic progenitors. Taken together, our findings demonstrate that Lnk down-regulates specific Kit-dependent signaling pathways, which are implicated in hematopoietic primary cell proliferation and migration.
Methods
Mice and cell cultures
Wild-type and Lnk-deficient mice18 were housed under pathogen-free conditions at the Institut Cochin Animal facility. The Ba/F3 cells expressing wild-type, Y567F, or Y569F Kit7 were cultured in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, and antibiotics in the presence of 0.1% X63-conditioned medium as a source of interleukin-3 (IL-3). BMMCs from 8- to 12-week-old wild-type and Lnk−/− mice were prepared26 and maintained in OPTIMEM medium (Invitrogen, Carlsbad, CA) with 5% FBS, 2 mM l-glutamine, and antibiotics in the presence of 3% X63-conditioned medium. Mature mast cell population was obtained after 4 weeks' culture and verified by Kit and FcϵRI expression (data not shown). This study received institutional review board approval for the use of mice from the Institute Cochin and the Université Paris Descartes.
Immunoprecipitation and immunoblotting
BMMCs were starved in OPTIMEM plus 1% bovine serum albumin (BSA) for 4 to 6 hours before stimulation with SCF for the indicated times at 37°C. Cells were lysed in ice-cold 0.1% Triton X-100 or 1% Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 10% glycerol, and 1 mM EDTA) containing phosphatase and protease inhibitors for 30 minutes on ice. Insoluble material was removed by centrifugation at 27 000g for 10 minutes at 4°C, and total cell lysates (TCLs) were immediately analyzed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels or immunoprecipitated for 2 hours at 4°C. Immunocomplexes were solubilized in 2 × SDS sample buffer, analyzed on SDS-PAGE gels, and subjected to immunoblotting with the following antibodies: anti-Kit (kindly provided by Dr A. Bernstein, Mount Sinai Hospital, Toronto, ON), anti–p-Kit (Tyr719), anti–p-ERK1/2 (Thr202/Tyr204), anti–p-Akt (Ser473), anti–p-SAPK/JNK (Thr183/Tyr185), anti–p-p38 MAPK (Thr180/Tyr182), anti–p-STAT3 (Tyr705), anti-p38 MAPK, anti-STAT3, anti-Akt rabbit monoclonal (Cell Signaling Technology, Beverly, MA), anti-Erk1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-pTyr mouse monoclonal (clone 4G10), and anti-SAPK/JNK (Millipore, Billerica, MA). Antibody complexes were detected with either horseradish peroxidase (HRP)–conjugated sheep anti–mouse or anti–rabbit immunoglobulin (Cell Signaling Technology) and revealed by enhanced chemiluminescence (ECL; GE Healthcare, Little Chalfont, United Kingdom).
Glutathione-S transferase and peptide pull-down experiments
The SH2 domain (residues 315-476) and amino-terminus plus PH domain (residues 1-330) of Lnk were generated by polymerase chain reaction (PCR), sequenced, and subcloned in pGEX (GE Healthcare) to create in-frame fusion proteins with glutathione-S transferase (GST). The constructs were expressed in BL21 bacteria and purified on glutathione-Sepharose beads (GE Healthcare). Cells were starved and treated with SCF before solubilizing in lysis buffer. Lysates were incubated with 5 μg GST fusion protein for 2 hours at 4°C. For peptide pull-down experiments, 15-mer peptides (INGNNYVYIDPTQLP) comprising phosphorylated or unphosphorylated Y567/Y569 of Kit were coupled to n-hydroxysuccinimide (NHS)-activated sepharose beads (GE Healthcare) and incubated with TCLs for 2 hours at 4°C. The precipitated proteins were separated by SDS-PAGE and subjected to immunoblotting.
Peptide binding assay
13-mer peptides comprising phosphorylated or unphosphorylated tyrosine residues of Kit intracellular domain were synthesized on cellulose membranes as described previously.27 Membranes were blocked in 5% skim milk, incubated with 0.5 μM purified GST fusion protein for 2 hours at 4°C, washed 5 times, and probed with HRP-conjugated mouse monoclonal anti-GST antibody (Santa Cruz Biotechnology) followed by ECL.
BMMC retroviral transduction
Lnk point mutations were generated by QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). Appropriate primers were used to mutate arginine 364 to methionine (R364M), tryptophan 270 to alanine (W270A), and tyrosine 536 to phenylalanine (Y536F). The mutated cDNAs were sequenced and subcloned into the EcoRI and BglII sites of MSCV-IRES-GFP (MIG) retroviral vector (provided by Dr D. Duménil, Institut Cochin, Paris, France). A total of 5 μg of each retroviral construct was cotransfected with equimolar amounts of gag-, pol-, and vsv-g expression plasmids into a 100-mm plate of 293 Epstein Barr nuclear antigen (EBNA)–expressing cells28 using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). The resulting supernatants were collected at 48 hours and filtered (0.2 μm pore), and the viral titer was determined in 3T3 cells. Lnk−/− BMMCs or lineage-negative (Lin−) progenitor cells were incubated twice with retroviral supernatants in media containing 10 μg/mL polybrene (Sigma-Aldrich, St Louis, MO) for 48 hours. After infection, green fluorescent protein (GFP)+ cells were purified by flow cytometer sorting (Elite ESP; Beckman Coulter, Fullerton, CA) and maintained in appropriate media.
Rac activation assay
The GST-PAK1 Cdc42/Rac-interactive binding (CRIB) fusion protein (provided by Dr O. Dorseuil, Institut Cochin, Paris, France) was purified on the day of the assay. BMMCs (107 cells) were stimulated with SCF, lysed in 1% NP-40 lysis buffer, and incubated with GST-PAK1 CRIB beads (∼50 μg) for 40 minutes at 4°C. Bound proteins were washed 3 times with lysis buffer, subjected to SDS-PAGE, and immunoblotted with Rac1 monoclonal antibody (Millipore).
BMMC in vitro proliferation assay
BMMCs in OPTIMEM complete medium were starved of IL-3 for 12 hours. A total of 5 × 104 cells/well were seeded in triplicate into 96-well plates and stimulated with the indicated concentrations of SCF or IL-3 (PromoCell, Heidelberg, Germany) for 24 hours at 37°C. Cells were pulsed for 4 or 12 hours with 0.0185 MBq (0.5 μCi) of [3H]thymidine (PerkinElmer, Waltham, MA). The labeled cells were then harvested and [3H]thymidine incorporation was measured by a TopCount Liquid Scintillation Counter (PerkinElmer).
BMMC migration assay
In vitro migration assays were performed by using transwell migration plates (5-μm pore size; Costar, Corning, NY). A total of 5 × 105 BMMCs in 100 μL chemotaxis medium (OPTIMEM with 1% BSA) were added to the upper well of a transwell chamber. Lower chambers contained 500 μL chemotaxis medium supplemented with different concentrations of SCF. Controls without SCF were maintained in each experiment to account for passive diffusion of cells. The plates were incubated for 4 hours at 37°C. SCF-induced cell migration into the lower chamber was counted by flow cytometry (FACScan; Becton Dickinson, Franklin Lakes, NJ) for 2 minutes at medium flow rate. The results are reported as percentage of input cell migration. Cell flow counting accuracy was verified with fluorescent calibration beads (Trucount; Becton Dickinson).
Lin− progenitor cells purification and methylcellulose colony assays
Single-cell suspensions were prepared from bone marrow (BM) of wild-type or Lnk−/− mice injected 4 days earlier with 5-fluorouracil (5-FU, 150 mg/kg intraperitoneally; Merck Chemicals, Nottingham, United Kingdom). Lineage-committed cells were removed through negative selection. Specifically, BM cells were labeled with a biotinylated cocktail of lineage-specific antibodies (BD Pharmingen, San Diego, CA), followed by Imag streptavidin particles-DM (BD Pharmingen). Lin− progenitor cells were purified using an Imag magnet (BD Pharmingen) as recommended by the manufacturer. Cells were stimulated for 24 hours in Iscove modified Dulbecco medium (IMDM) containing 10% FBS, 2 mM l-glutamine, antibiotics, 50 μM β-mercaptoethanol, 50 ng/mL SCF, Flt-3 ligand and thrombopoietin (TPO), 20 ng/mL IL-6, and 10 ng/mL each IL-3 and IL-7. Cells were then retrovirally infected as described earlier. After 48 hours of infection, the GFP+ cells were purified using flow cytometric sorting and allowed to recover 16 hours. Triplicate samples of 104 cells were plated in methylcellulose media (MethoCult M3630; StemCell Technologies, Vancouver, BC) and pre-B lymphoid progenitor colonies were scored microscopically 7 days later. GFP+ colonies were visualized with a fluorescent microscope (DMI6000, Leica, Wetzlar, Germany). All recombinant cytokines were purchased from PromoCell.
Reverse-transcriptase–PCR
Total RNA from wild-type or Lnk−/− Lin−-transduced cells was prepared using Trizol reagent (Invitrogen) and reverse transcribed using the Superscript II first-strand synthesis system (Invitrogen). PCR was performed using specific primers for Lnk and for the HPRT gene. PCR products were visualized in a 1.5% agarose gel.
Results
The SH2 domain of Lnk associates with the juxtamembrane region of activated Kit receptor
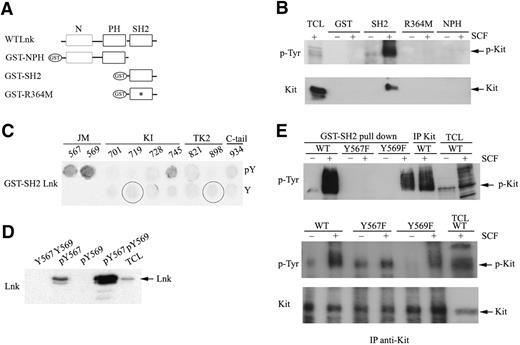
Previous studies have reported that Lnk associates with the Kit receptor through its SH2 domain in response to SCF stimulation.24,25 We therefore examined whether the other Lnk domains could also participate in the interaction with the receptor. For this, we performed GST pull-down experiments on Ba/F3 cells expressing wild-type (WT) Kit. Cells were treated with or without SCF, lysed, and incubated with the Lnk SH2 domain (SH2), the SH2 inactive form (R364M), and the Lnk amino-terminus plus PH domains (NPH) GST fusion proteins (Figure 1A). Only the WT SH2 domain was able to bind to phosphorylated Kit, whereas neither the R364M form, nor the NPH protein could associate with the activated receptor (Figure 1B). These results demonstrate that a functional SH2 domain is sufficient to mediate the interaction between Lnk and Kit and suggest the existence of a binding site(s) for Lnk SH2 domain within the cytoplasmic domain of the receptor.
Lnk SH2 domain associates with the juxtamembrane domain of Kit receptor. (A) Schematic representation of GST-Lnk fusion proteins. *R364M mutation. (B) BaF3 cells expressing WT Kit receptor were unstimulated (−) or stimulated (+) with SCF (100 ng/mL) for 1 minute and incubated with GST alone or different GST-fused Lnk domains. Immunoblotting with anti-pTyr and anti-Kit antibodies allowed detection of bound activated Kit protein. TCL indicates total cell lysate. (C) 13-mer peptides containing either phosphorylated (pY) or nonphosphorylated (Y) tyrosine residues from Kit intracellular domain were synthesized on a peptide spot array. The resulting membrane was incubated with either GST-Lnk SH2 protein (0.5 μM) or GST alone as a control. Positive spots with GST are circled in black. Immunoblotting with anti-GST antibodies identified the interacting peptides. JM indicates juxtamembrane; KI, kinase insert; TK2, distal kinase domain; and C-tail, carboxyl-tail. Numbers indicate tyrosine position in Kit. (D) BMMC lysates were incubated with 2 nmol peptides containing nonphosphorylated (Y567Y569) and either one (pY567 or pY569) or both tyrosines phosphorylated (pY567pY569). Lnk binding was revealed with anti-Lnk antibodies. Lysate is used as a control. (E) Lysates from BaF3 cells expressing either WT or mutant Kit Y567F or Y569F receptors were prepared as in panel B and incubated with GST-Lnk SH2 fusion protein (top panel). Associated Kit was subsequently detected by immunoblotting with anti-pTyr antibodies. Immunoprecipitation (IP) with anti-Kit antibodies showed phosphorylation and expression of Kit proteins (bottom panels).
Lnk SH2 domain associates with the juxtamembrane domain of Kit receptor. (A) Schematic representation of GST-Lnk fusion proteins. *R364M mutation. (B) BaF3 cells expressing WT Kit receptor were unstimulated (−) or stimulated (+) with SCF (100 ng/mL) for 1 minute and incubated with GST alone or different GST-fused Lnk domains. Immunoblotting with anti-pTyr and anti-Kit antibodies allowed detection of bound activated Kit protein. TCL indicates total cell lysate. (C) 13-mer peptides containing either phosphorylated (pY) or nonphosphorylated (Y) tyrosine residues from Kit intracellular domain were synthesized on a peptide spot array. The resulting membrane was incubated with either GST-Lnk SH2 protein (0.5 μM) or GST alone as a control. Positive spots with GST are circled in black. Immunoblotting with anti-GST antibodies identified the interacting peptides. JM indicates juxtamembrane; KI, kinase insert; TK2, distal kinase domain; and C-tail, carboxyl-tail. Numbers indicate tyrosine position in Kit. (D) BMMC lysates were incubated with 2 nmol peptides containing nonphosphorylated (Y567Y569) and either one (pY567 or pY569) or both tyrosines phosphorylated (pY567pY569). Lnk binding was revealed with anti-Lnk antibodies. Lysate is used as a control. (E) Lysates from BaF3 cells expressing either WT or mutant Kit Y567F or Y569F receptors were prepared as in panel B and incubated with GST-Lnk SH2 fusion protein (top panel). Associated Kit was subsequently detected by immunoblotting with anti-pTyr antibodies. Immunoprecipitation (IP) with anti-Kit antibodies showed phosphorylation and expression of Kit proteins (bottom panels).
We next aimed to identify the tyrosine residues in Kit involved in the interaction with Lnk. For this, we screened 9 peptides, phosphorylated and unphosphorylated, comprising tyrosine residues present in the juxtamembrane, kinase insert, distal kinase, and carboxy-tail domains of the Kit receptor. The peptides were immobilized on a membrane and subjected to far-Western blotting using the GST-Lnk SH2 domain as a probe. Peptides containing pY567, pY569, and to a lesser extent pY745 were the major peptides recognized by GST-Lnk SH2 (Figure 1C). Our result demonstrates that Lnk interacts directly with Kit via a major binding site consisting of juxtamembrane Y567/Y569.
To confirm the requirement of Y567/Y569 for Lnk binding, peptide and GST pull-down experiments were done. First, BMMC lysates were incubated with peptides containing either phosphorylated or unphosphorylated Y567/Y569. Lnk binding was only detected when Y567 was phosphorylated (Figure 1D). Next, Ba/F3 cells expressing WT, Y567 (Y567F), or Y569 (Y569F) mutant Kit receptors were used in GST pull-down assays. All Kit proteins were expressed and activated after SCF stimulation (Figure 1E bottom panels). GST-Lnk SH2 or GST control proteins were mixed with cell lysates previously stimulated or not with SCF. GST-Lnk SH2 but not GST strongly associated with WT Kit after SCF stimulation (Figure 1E top panel; Figure 1B). The Y569F Kit receptor also bound the SH2 domain of Lnk, but with slightly lesser affinity than WT Kit. In contrast, the Y567F mutation completely abolished the interaction (Figure 1E top panel). Together, these results indicate that this tyrosine residue is indeed required for Lnk binding.
The SH2 domain of Lnk is required for its phosphorylation in response to SCF
To assess the role of the different domains of Lnk in its regulatory function, we reintroduced WT or Lnk mutated at individual domains into Lnk-deficient BMMCs by retroviral gene transduction. For this, we used the bicistronic MIG vector (Figure 2A). This vector allows selecting Lnk-expressing cells by GFP fluorescence. We then examined the effect of Lnk mutations on their association with the Kit receptor. W270A and Y536F mutants were able to interact with activated Kit, as did WT Lnk. In contrast, R364M mutant failed to interact with the receptor (Figure 2B).
The Lnk SH2 domain is required for Lnk phosphorylation in BMMCs. (A) Schematic representation of WT and Lnk mutant forms cloned into the MIG retroviral vector. Point mutations in the different domains and in the carboxy-terminus tyrosine are indicated with an asterisk (*) or an F. (B) Lnk−/− BMMCs transduced with control vector or Lnk forms were stimulated with SCF for 5 minutes. Cell lysates were immunoprecipitated with anti-Lnk antibodies. Western blot analysis with anti-pKit antibodies allowed identification of associated protein (top panel). Lnk protein expression is shown (bottom panel). White lines indicate repositioned gel lanes. (C) BMMCs expressing Lnk mutant forms were stimulated with SCF for the indicated times. Lnk phosphorylation was analyzed by immunoprecipitation with anti-pTyr, followed by immunoblotting with anti-Lnk antibodies.
The Lnk SH2 domain is required for Lnk phosphorylation in BMMCs. (A) Schematic representation of WT and Lnk mutant forms cloned into the MIG retroviral vector. Point mutations in the different domains and in the carboxy-terminus tyrosine are indicated with an asterisk (*) or an F. (B) Lnk−/− BMMCs transduced with control vector or Lnk forms were stimulated with SCF for 5 minutes. Cell lysates were immunoprecipitated with anti-Lnk antibodies. Western blot analysis with anti-pKit antibodies allowed identification of associated protein (top panel). Lnk protein expression is shown (bottom panel). White lines indicate repositioned gel lanes. (C) BMMCs expressing Lnk mutant forms were stimulated with SCF for the indicated times. Lnk phosphorylation was analyzed by immunoprecipitation with anti-pTyr, followed by immunoblotting with anti-Lnk antibodies.
To assess whether Lnk association with the receptor was necessary for its phosphorylation, we stimulated the BMMC clones with SCF for different periods of time and then immunoprecipitated the phosphorylated proteins with anti-pTyr antibodies. The presence of Lnk was revealed with anti-Lnk antibodies. WT and W270A mutant proteins were phosphorylated after 10 minutes of SCF stimulation (Figure 2C). Interestingly, Y536F mutant was also phosphorylated in response to SCF, suggesting that Lnk is phosphorylated at tyrosine residues other than Y536. In contrast, the R364M mutation dramatically reduced its phosphorylation (Figure 2C). These results demonstrate that association of Lnk with the receptor is necessary for subsequent phosphorylation of the adaptor.
Lnk domains contribute differently to the proliferation of primary hematopoietic cells
Lnk-deficient BMMCs exhibit increased proliferation upon treatment with low concentrations of SCF compared with WT cells.18 To examine the role of the different domains of Lnk in SCF-dependent growth of BMMCs, we measured the [3H] thymidine incorporation rate of Lnk−/− BMMCs expressing WT or Lnk mutant forms. Expression of R364M Lnk showed cell growth similar to vector-expressing cells (Figure 3A). In contrast, WT Lnk protein dramatically inhibited SCF-dependent proliferation, while W270A and Y536F mutants moderately compromised the inhibitory function of Lnk (Figure 3A), suggesting that these 2 mutants play a minor role in this Lnk-dependent function.
A functional SH2 domain of Lnk is required for inhibition of Kit-dependent proliferation and MAPK activation. (A,B) BMMCs expressing different Lnk forms were cultured with the indicated concentrations of SCF (A) or IL-3 (B) for 24 hours, and proliferation was measured as [3H]thymidine incorporation. The values are the mean counts per minute plus or minus SD (error bars) of triplicate determinations and are representative of at least 2 independent experiments. (C) Total BMMC lysates were stimulated with SCF for the indicated times and then subjected to immunoblot analysis with anti–phospho-Erk1/2 (p-Erk) and anti-Erk1/2 antibodies.
A functional SH2 domain of Lnk is required for inhibition of Kit-dependent proliferation and MAPK activation. (A,B) BMMCs expressing different Lnk forms were cultured with the indicated concentrations of SCF (A) or IL-3 (B) for 24 hours, and proliferation was measured as [3H]thymidine incorporation. The values are the mean counts per minute plus or minus SD (error bars) of triplicate determinations and are representative of at least 2 independent experiments. (C) Total BMMC lysates were stimulated with SCF for the indicated times and then subjected to immunoblot analysis with anti–phospho-Erk1/2 (p-Erk) and anti-Erk1/2 antibodies.
We next examined the effect of Lnk mutant forms on IL-3–dependent growth. In contrast to SCF, no difference in proliferation was observed between vector, WT, R364M, or W270 Lnk-expressing BMMCs, confirming that Lnk-mediated cell growth inhibition is specific to Kit signaling (Figure 3B). Interestingly, IL-3–induced proliferation was considerably lower (2- to 3-fold) in Y536F-expressing cells compared with the other Lnk-expressing BMMCs (Figure 3B). Consistent with this, we found that the GFP+ fraction of Y536F-expressing cells was dramatically reduced (40%) after 6 weeks in IL-3 compared with control or Lnk-expressing GFP+ cells (100%) over the same period of time (data not shown). These data suggest that Y536 plays a role in IL-3–dependent proliferation of BMMCs.
We previously demonstrated that increased SCF-induced proliferation of Lnk-deficient BMMCs correlated with an altered kinetic of MAPK activation.18 Therefore, we determined if variations in MAPK activation might account for the differences observed in proliferation. R364M-expressing cells showed MAPK activation similar to control cells with maximal ERK1/2 phosphorylation at 5 minutes and a decrease to 10% of the maximum at 30 minutes (Figure 3C). In contrast, WT Lnk expression drastically inhibited activation of MAPK (90% from control cells) at 5 minutes, resulting in undetectable levels of phosphorylated MAPK at 30 minutes. Interestingly, W270A and Y536F mutants moderately affected the inhibitory function of Lnk (Figure 3C). Total ERK protein levels were equivalent in all cells (Figure 3C bottom panel). Together, these results indicate that the SH2 domain of Lnk is essential for inhibiting SCF-dependent proliferation and MAPK activation, whereas the PH domain and Y536 play a minor role in this function.
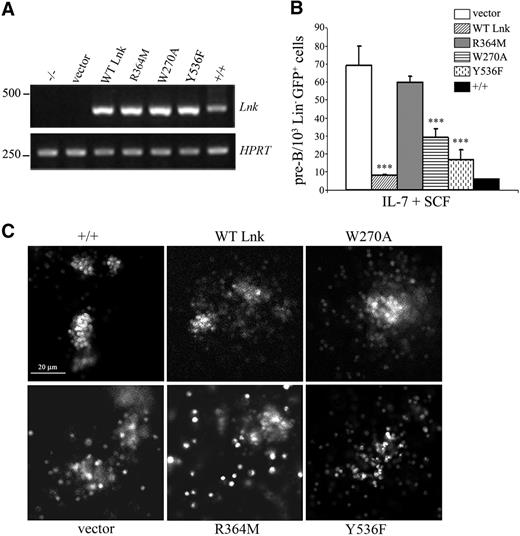
SCF is also important for the survival and proliferation of HSCs and progenitors by synergizing with other growth factors.29 In bone marrow cells, SCF supports the proliferation of B-cell precursors in combination with IL-7.30 Previous studies have shown that Lnk deficiency causes abnormal proliferation of B-cell clonogenic progenitors in the presence of IL-7; this effect was further enhanced with SCF.17,18 To determine the domain(s) of Lnk controlling proliferation of pre–B-cell progenitors, we retrovirally expressed WT or mutant Lnk forms in Lin− progenitor cells derived from Lnk-deficient animals. Lin−-transduced cells were then sorted for GFP+ expression, and equal numbers of cells were plated into methylcellulose with IL-7 and SCF to assess their proliferative potential. The expression of the different Lnk proteins in Lin− cells was confirmed by reverse transcriptase (RT)–PCR (Figure 4A). Expression of WT Lnk restablished normal numbers of pre-B lymphoid colonies similarly to Lin− cells derived from wild-type animals. Moreover, expression of either W270A or Y536F mutant moderately restablished pre-B colony numbers compared with WT cells. In contrast, the R364M mutant displayed comparable colony-forming numbers to vector-transduced Lnk−/− cells (Figure 4B). Consistent with the colony numbers, a difference in the colony size from the different Lin−-transduced clones was observed (Figure 4C). These results indicate that the individual domains and Y536 of Lnk contribute differently to its inhibitory function during expansion of hematopoietic progenitors.
Lnk domains and Y536 contribute differently to the proliferation of hematopoietic progenitors in vitro. (A) Total RNA was extracted from wild-type (+/+) or Lnk−/− Lin−GFP+ progenitor cells transduced with either vector alone, WT or mutant forms of Lnk and subjected to RT-PCR with specific primers for Lnk (top panel) or HPRT (bottom panel) as control. (B) Wild-type or Lnk−/− Lin−GFP+ transduced cells were assessed for their in vitro proliferative capacity in methylcellulose media containing IL-7 and SCF. Data represent the mean plus or minus SD (error bars) of number of colonies per 103 Lin−GFP+ cells from triplicate samples from 3 independent assays using 3 to 5 mice of each genotype. Statistical significance was determined using the Student t test: ***P ≤ .01. (C) Typical appearance of GFP+ pre-B colonies from Lin− transduced progenitor cells in response to IL-7 + SCF after 7 days of incubation in methylcellulose media. GFP+ colonies were acquired with a 40×/0.55 NA dry objective and a charge-coupled device (CCD) camera (MicroMax 13004, Princeton Instruments, Trenton, NJ). Images were processed with MetaMorph software (Molecular Devices, Downingtown, PA). Scale bar equals 20 μm.
Lnk domains and Y536 contribute differently to the proliferation of hematopoietic progenitors in vitro. (A) Total RNA was extracted from wild-type (+/+) or Lnk−/− Lin−GFP+ progenitor cells transduced with either vector alone, WT or mutant forms of Lnk and subjected to RT-PCR with specific primers for Lnk (top panel) or HPRT (bottom panel) as control. (B) Wild-type or Lnk−/− Lin−GFP+ transduced cells were assessed for their in vitro proliferative capacity in methylcellulose media containing IL-7 and SCF. Data represent the mean plus or minus SD (error bars) of number of colonies per 103 Lin−GFP+ cells from triplicate samples from 3 independent assays using 3 to 5 mice of each genotype. Statistical significance was determined using the Student t test: ***P ≤ .01. (C) Typical appearance of GFP+ pre-B colonies from Lin− transduced progenitor cells in response to IL-7 + SCF after 7 days of incubation in methylcellulose media. GFP+ colonies were acquired with a 40×/0.55 NA dry objective and a charge-coupled device (CCD) camera (MicroMax 13004, Princeton Instruments, Trenton, NJ). Images were processed with MetaMorph software (Molecular Devices, Downingtown, PA). Scale bar equals 20 μm.
Lnk-deficient BMMCs exhibit increased SCF-dependent migration and activation of the Rac/JNK and p38 MAPK pathways
Signaling through Kit receptor is important for mast cells chemotaxis.4,31 To address whether Lnk may play a role in SCF-induced migration of mast cells, we performed a transwell migration assay with WT and Lnk−/− BMMCs. Lnk-deficient BMMCs displayed an increase in migration efficiency at low doses of SCF compared with WT cells (Figure 5A). Interestingly, we also observed an increase in the basal migration of Lnk−/− compared with WT BMMCs in the absence of factor (data not shown).
Lnk down-regulates SCF-dependent migration and activation of Rac/JNK and p38 MAPK pathways. (A) Wild-type and Lnk−/− BMMCs were assessed for migration in response to the indicated concentrations of SCF in a transwell assay as described in “BMMC migration assay.” Data represent the mean plus or minus SD (error bars) of triplicate samples from 3 independent experiments. Significance was determined by Student t test: *P ≤ .05. (B) BMMCs from wild-type and Lnk−/− mice were stimulated with SCF (50 ng/mL) for the indicated times. Cell lysates were used in a GST-PAK1 effector pull-down assay. Activated Rac1 (Rac1-GTP; top panel) and total Rac1 (bottom panel) were examined by immunoblot with anti-Rac1 antibodies. Similar results were seen in 3 independent experiments. White lines indicate repositioned gel lanes. (C,D) Total lysates from wild-type and Lnk−/− (top panels) or mutant Lnk-expressing BMMCs (bottom panels) were prepared as in Figure 3C. JNK (C) or p38 MAPK (D) activation was assessed by immunoblotting with anti–phospho-JNK (p-JNK) or anti–phospho-p38 (p-p38) MAPK antibodies, respectively. Total JNK and p38 MAPK levels were analyzed by immunoblotting (bottom panels). Data shown represent one of 3 independent experiments with similar results.
Lnk down-regulates SCF-dependent migration and activation of Rac/JNK and p38 MAPK pathways. (A) Wild-type and Lnk−/− BMMCs were assessed for migration in response to the indicated concentrations of SCF in a transwell assay as described in “BMMC migration assay.” Data represent the mean plus or minus SD (error bars) of triplicate samples from 3 independent experiments. Significance was determined by Student t test: *P ≤ .05. (B) BMMCs from wild-type and Lnk−/− mice were stimulated with SCF (50 ng/mL) for the indicated times. Cell lysates were used in a GST-PAK1 effector pull-down assay. Activated Rac1 (Rac1-GTP; top panel) and total Rac1 (bottom panel) were examined by immunoblot with anti-Rac1 antibodies. Similar results were seen in 3 independent experiments. White lines indicate repositioned gel lanes. (C,D) Total lysates from wild-type and Lnk−/− (top panels) or mutant Lnk-expressing BMMCs (bottom panels) were prepared as in Figure 3C. JNK (C) or p38 MAPK (D) activation was assessed by immunoblotting with anti–phospho-JNK (p-JNK) or anti–phospho-p38 (p-p38) MAPK antibodies, respectively. Total JNK and p38 MAPK levels were analyzed by immunoblotting (bottom panels). Data shown represent one of 3 independent experiments with similar results.
Mast cell migration requires activation of the Rac/JNK pathway through the Kit receptor.6,32,33 We then examined the requirement for Lnk in SCF-dependent Rac activation. WT and Lnk-deficient BMMCs were stimulated with SCF and assayed for Rac activation at different time points by a pull-down assay with GST-PAK1 as a substrate for Rac-GTP. The levels of activated Rac1 were considerably augmented in Lnk−/− BMMCs compared with WT cells independently of SCF stimulation (Figure 5B).
We next analyzed the effect of Lnk on SCF-mediated activation of JNK kinase. Consistent with the increase in Rac activation, SCF-induced phosphorylation of JNK was markedly enhanced and prolonged in the absence of Lnk (Figure 5C top panel). Expression of WT or W270A Lnk significantly inhibited JNK phosphorylation, whereas R364M mutant protein showed similar JNK activation to vector-expressing cells (Figure 5C bottom panel). Immunoblotting with anti-JNK antibodies revealed similar levels of the protein in all samples (Figure 5C).
Several studies have also implicated p38 MAPK in SCF-induced migration of mast cells.10,32,34 We therefore examined the phosphorylation status of p38 MAPK in SCF-stimulated BMMCs. Activation of p38 MAPK was increased and prolonged in Lnk-deficient compared with WT BMMCs (Figure 5D top panel). Similarly to JNK activation, expression of WT or W270A Lnk inhibited p38 MAPK activation, whereas R364M mutants displayed similar p38 activation as vector-expressing BMMCs (Figure 5D bottom panel). All samples showed similar levels of p38 MAPK protein by immunoblotting (Figure 5D).
Together, these results confirm that the effect observed on JNK and p38 activation is due specifically to Lnk deficiency. Moreover, they suggest that Lnk down-modulates mast cell migration through regulation of Rac/JNK and p38 MAPK pathways.
Discussion
Lnk-deficient mice display an abnormal accumulation of HSCs and myeloid and lymphoid progenitors in the different hematopoietic compartments. This phenotype is partly due to enhanced Kit signaling, indicating the importance of Lnk in the regulation of this RTK. In the present study, we have investigated the mechanism by which Lnk regulates Kit-mediated proliferation and chemotaxis in hematopoietic progenitor and primary mast cells. We used Lnk−/−-derived BMMCs and Lin− cells expressing WT or Lnk mutated forms, to analyze the contribution of the different domains of Lnk to Kit-dependent biologic functions. Our results show that, by binding to Kit juxtamembrane domain, Lnk directly down-modulates specific Kit signaling pathways and controls cell proliferation and migration.
Previous studies have shown that Lnk interacts with phosphorylated Kit via its SH2 domain. Here, we further mapped the Lnk SH2 domain binding site to Y567/Y569 in Kit juxtamembrane domain. Although Lnk SH2 domain could also recognize a peptide containing Y745, this interaction does not seem to be relevant in vivo (Figure 1E). APS, one of the members of the Lnk adaptor family, was shown to bind to Y567 and Y934 in Kit.35 Together with our results, this suggests that these adaptors display common and specific binding sequence preferences. Whereas peptide binding assays indicated that Lnk interacts individually with either pY567 or pY569, our peptide and GST pull-down experiments showed that Y567 is the major Lnk binding site in vivo. These results also suggest that Y569 is part of Y567 binding site, since the interaction was diminished but not abolished in the Y569F mutant cells. In this regard, it is possible that binding of Lnk to Kit depends on the kinetics of phosphorylation of these tyrosines after SCF stimulation. Interestingly, it has been shown that the juxtamembrane domain of Kit serves an autoinhibitory function and that its tyrosine phosphorylation abolishes this repressing activity.36 The resulting phosphorylated juxtamembrane tyrosines may then serve as docking sites for SH2-containing molecules. It is then possible that Lnk binding to pY567 inhibits further phosphorylation of Y569 in the same Kit molecule and therefore blocks association of positive signaling proteins to the di-tyrosine motif Y567/Y569.
Association of Lnk with the Kit receptor allows phosphorylation of the adaptor and its proper localization at the signaling complex. These 2 events are necessary for Lnk inhibitory function and are primarily mediated by Lnk SH2 domain. Previous reports showed that the PH mutant (W191A) protein moderately affected Lnk modulation of TPO- or EPO-dependent biologic responses.22,23 In agreement, we found that a different PH mutant, W270A, exerted a similar effect on Kit-dependent signaling. Moreover, we have observed that the Lnk PH domain displays moderate affinity and little specificity to phosphoinositides in vitro (data not shown). It is therefore possible that the Lnk PH domain may down-regulate membrane targeting of Lnk in the absence of docking site for the SH2 domain and increase binding stability to membrane receptors when the SH2 domain is engaged.
The conserved Y536 of Lnk was suggested to be a main site for phosphorylation upon SCF stimulation of the MC9 cell line.19 However, we found that Lnk mutated at this tyrosine still gets phosphorylated upon Kit activation in BMMCs. This result suggests that Lnk is phosphorylated at sites other than Y536. Indeed, a similar result was reported with human Lnk mutated at this residue in Jurkat cells after T-cell activation.37 On the other hand, the biologic relevance of Y536 seems to depend on the signaling pathway analyzed. Y536 is dispensable for the negative function of Lnk in lymphoid development as well as TPO- or SCF-dependent signaling pathways (Figure 3A).22,38 However, it might play a regulatory role in IL-3– and EPO-mediated proliferation (Figure 3B).23
Previous studies identified Y567 in Kit as an important site for activation of the Rac/JNK and ERK pathways controlling mast cell proliferation.6,39 Analysis of Lnk−/− BMMCs demonstrated that Lnk can modulate the kinetics of activation of ERK and JNK MAPKs and that this correlates with attenuation of SCF-induced proliferation. Our results thus reveal a novel pathway regulated by Lnk (via its SH2 domain) in BMMCs and strongly suggest that Lnk controls mast cell proliferation by down-modulating both MAPK and Rac/JNK pathways. This might explain the striking increase in proliferation exhibited by Lnk−/− cells.
Migration of mast cells in vitro and in vivo is coordinated through Rac GTPases. Involvement of Kit Y567/569 in the activation of p38 MAPK and chemotaxis has been shown in primary mast cells and c-Kit–transfected leukemia cell lines.10,32,34 Consistent with these reports, we found that Lnk-deficient BMMCs exhibit an increased response in migration toward SCF, which correlates with increased activation of Rac and p38 MAPK pathways. Thus, our results reveal a novel regulatory role for Lnk in SCF-dependent migration through down-regulation of Kit-dependent signaling pathways involved in this process.
Rac GTPases play an essential role in regulating actin polymerization, essential in hematopoietic and other cells for changing cell shape, migration, and other processes.40,41 Interestingly, several pieces of evidence have implicated the family of Lnk adaptor proteins in the regulation of actin cytoskeleton. SH2-B interacts with Rac and enhances growth hormone–induced actin reorganization and cell migration, while APS may associate with guanine nucleotide exchange factors (GEFs) of the Vav family or with the actin-associated cytoskeleton protein Enigma.42-44 In addition, human Lnk has been shown to interact with the actin-binding protein 280, ABP280 (or filamin A), in Cos7 and Jurkat cells.45 It is then possible that down-modulation of Rac GTPases by Lnk might account for the regulation of actin cytoskeleton that, in turn, results in modifications in cell migration. We are currently investigating whether actin reorganization in BMMCs is also affected by Lnk deficiency.
One important question deals with the identification of Lnk-interacting proteins mediating its inhibitory function on cell proliferation and migration. In this regard, Shp2 has lately been implicated in Kit Y567–induced pathways controlling chemotaxis via its activation by Fyn.32 Moreover, it can also participate in modulating proliferation by interacting with Gab2.46 A previous report has shown that overexpression of Lnk in the MC9 mast cell line inhibits phosphorylation of Gab2 upon SCF stimulation.19 It is then possible that Shp2 and Gab2 serve as mediators of Lnk inhibition accordingly to the biologic response evoked.
Previous studies on lyn−/− and fyn−/− BMMCs confirmed the importance of these Src kinases for SCF-dependent proliferation and chemotaxis. Binding of either Lyn or Fyn to Y567/Y569 in Kit results in regulation of several signaling pathways in mast cells, which are implicated in SCF-dependent proliferation and chemotaxis.47-49 In contrast, Fyn promotes phosphorylation of Shp2 and activation of p38 MAPK and is also essential for chemotaxis.32 The importance of Src pathways was further underscored by a study using several c-Kit mutant receptors to analyze single biochemical pathways elicited by this receptor. The study demonstrated that the Src kinase pathway plays a unique role in the control of multiple c-Kit functions and that this pathway alone is sufficient to restore the activation of Ras, PI-3K, ERK, Rac, JNK, and Akt.6 Interestingly, our results show that association of Lnk with Kit via Y567/Y569 leads to modulation of the same signaling cascades. It is conceivable that Lnk binding to these tyrosine residues blocks subsequent interaction of Src kinases or other activators with the receptor, resulting in inhibition of Kit signaling pathways controlling proliferation and migration.
Of great interest is the fact that these 2 cellular responses are often deregulated in oncogenesis. Gain-of-function mutations of Kit are frequent in gastrointestinal stromal tumors (GISTs) and in mastocytosis.50 Most Kit mutations in GISTs are in-frame deletions in exon 11, which codes for the juxtamembrane region, the site of Lnk recruitment. As for mastocytosis, most Kit point mutations are localized in the kinase domain. In these 2 clinical contexts, our results suggest that Lnk binding to Kit or its expression is lost in these 2 diseases, resulting in constitutive activation of Kit mutants.
The signaling defects observed in Lnk-deficient BMMCs exhibit several similarities with those previously reported for negative regulators Shp1 and SOCS1/6. Shp1−/− BMMCs exhibit an augmented and prolonged SCF-dependent activation of ERK-1, which results in hyperproliferation of these cells.51 Similarly, ectopic expression of SOCS1 or SOCS6 in Ba/F3-Kit cells results in inhibition of SCF-induced cell proliferation. In addition, SOCS6 partially inhibits ERK and p38 activation.7,8 Interestingly, all these negative modulators interact with Kit Y567/Y569. These parallels indicate that Kit-negative regulators exert their function on SCF signaling pathways through binding to these tyrosines. In this regard, it is interesting that mice homozygous for the Kit Y567F (Kit567F/567F) and Y567F/Y569 mutations (KitFF) displayed defects in B-cell and megakaryocyte lineages,9,11 2 hematopoietic lineages also affected in Lnk−/− animals. These results further support a role for Lnk via Kit Y567/569 in the regulation of primary hematopoietic cells.
Expression of Lnk mutant forms in Lnk−/− Lin− progenitor cells showed the contribution of the different domains and Y536 of Lnk to its inhibitory function during proliferation of these cells. Together with our results from primary mast cells, these data confirm the key role of Lnk in regulating SCF-induced growth of undifferentiated (Lin−) and differentiated (mast) hematopoietic cells, both important target populations for SCF.
In conclusion, the results presented in this study provide new evidence that Lnk plays an important regulatory role in signaling emanating from the juxtamembrane domain of Kit in progenitor and primary mast cells. In addition, our findings demonstrate that Lnk through its SH2 domain, down-modulates specific SCF-induced signaling pathways involved in proliferation and migration of these cells. Further identification and analysis of Lnk effectors and signaling pathways in hematopoietic progenitor cells will contribute to the understanding of the regulatory function of Lnk in the expansion and migration of these cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Marie Claude Gendron for helping with flow cytometry sorting, Remy Letestu for assistance with flow cytometry, Neil Warner and Derek Ceccarelli for technical advice, and Christine Le Roy for helping with the migration assay, discussions, and comments on the manuscript.
This work was supported by grants from the Inserm-Avenir, the Ligue Nationale contre le Cancer (LNCC), and the Association pour la Recherche contre le Cancer (ARC; Paris, France). C.S. is a recipient of an Inserm–Ile de France graduate fellowship. E.D. is supported by a postdoctoral fellowship from Fondation contre la Léucemie (Paris, France). A.C. is a recipient of a Ministère de la Recherche graduate fellowship.
Authorship
Contribution: C.S., and A.C. performed experiments; E.D. performed experiments and made the figures; P.d.S. and T.J.K. contributed vital reagents, designed research, and helped analyze the data; N.V.B. contributed scientific discussion and helped analyze the data; and L.V. designed and performed research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura Velazquez, Departement d'Hématologie, Institut Cochin, Hôpital Cochin, 27 rue du Faubourg Saint-Jacques, 75014 Paris, France; e-mail: laura.velazquez@inserm.fr.
References
Author notes
*C.S. and E.D. contributed equally to this work.


![Figure 3. A functional SH2 domain of Lnk is required for inhibition of Kit-dependent proliferation and MAPK activation. (A,B) BMMCs expressing different Lnk forms were cultured with the indicated concentrations of SCF (A) or IL-3 (B) for 24 hours, and proliferation was measured as [3H]thymidine incorporation. The values are the mean counts per minute plus or minus SD (error bars) of triplicate determinations and are representative of at least 2 independent experiments. (C) Total BMMC lysates were stimulated with SCF for the indicated times and then subjected to immunoblot analysis with anti–phospho-Erk1/2 (p-Erk) and anti-Erk1/2 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/10/10.1182_blood-2008-05-154849/7/m_zh80220826570003.jpeg?Expires=1765933084&Signature=tvmiVgkMnHiKzdYQniMbIZ1iZORG5S76A75Nje52TZwOV5~mKGhJcwKyrvGosA1302N7TLpeIOFFhbgzLpf3-WjnD7Nvm9ArdiSppQ0btvryiVk~vq4aqSMiAPS3~dnuIT1Q3Y6u8izuyGmfJMX-2kkZFSwBQRhcA79KcvulwyTioGFmBbaDYYvKhI5uXR0f316HH3oL-e37OCuja3EhhKuFTJ0dyGhNQwtCcBgHh5BDvyHUfbtkZOhkLioHzoY0VW~Y~GiErSwpUtF3zoL5iPMLz6sZMLadeaGnzrFYNkxP9EInjD-uSnvGJpdTg3VtCJG08BsYBgZc6WmD2blMug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal