Abstract
Interleukin-21 (IL-21) is a recently identified γ-chain receptor cytokine family member that promotes B-cell apoptosis as well as activation of innate immune system. Based on this, we hypothesized that IL-21 might enhance the apoptosis induced by fludarabine and rituximab and also play a role in augmenting immune-mediated clearance of the chronic lymphocytic leukemia (CLL) cells. Our studies demonstrate that the majority of CLL patients have surface IL-21 receptor-α, and its expression correlates with apoptosis, tyrosine phosphorylation of STAT1, and up-regulation of the proapoptotic BH3 domain protein BIM. IL-21–induced BIM up-regulation is critical for apoptosis because inhibition of BIM expression using small interfering RNA prevented IL-21–induced apoptosis. IL-21 treatment of CLL cells but not normal T cells with fludarabine or rituximab additively enhanced the direct cytotoxic effect of these therapies. In addition to its proapoptotic effect, IL-21 promoted STAT1 and STAT5 phosphorylation in natural killer cells with concurrent enhanced antibody-dependent cellular cytotoxicity against rituximab-coated CLL cells in vitro. These data provide justification for combination studies of IL-21 with fludarabine and rituximab in CLL and suggest that BIM up-regulation might serve as relevant pharmacodynamic end point to measure biologic effect of this cytokine in vivo.
Introduction
Chronic lymphocytic leukemia (CLL) is one of the more common types of adult leukemia. The majority of patients are asymptomatic at diagnosis, and therapy is usually initiated when symptoms develop because studies comparing early versus delayed chlorambucil demonstrated no survival benefit with immediate treatment.1 Although alkylator therapy was commonly used in the past to treat CLL, randomized phase 3 studies have demonstrated that fludarabine is superior.2-4 Addition of rituximab to fludarabine-based therapy has produced promising preliminary results, but the treatment goal for CLL remains palliative.5 Introduction of new therapies that both augment the direct and immunologic cytotoxic effect of traditional CLL therapies is highly desired. Therapeutic use of recombinant interleukin-21 (IL-21) may represent such a therapy
IL-21 is a member of the cytokine-receptor γ-chain family that includes IL-2, IL-4, IL-7, IL-9, and IL-15.6 IL-21 has a 4-helix bundle type fold that signals distinctly through type 1 cytokine receptor IL-21 receptor (IL-21R) in conjunction with the γ-chain common to this family. IL-21 has pleiotropic effects on cytotoxic T cells and natural killer (NK) cells, in which it effectively augments antitumor activity of antibodies and vaccines in several preclinical models including non-Hodgkin lymphoma.7,8 In vitro studies of murine IL-21 on normal B cells have demonstrated this cytokine promotes caspase-dependent apoptosis.9 Application of IL-21 to different B-cell malignancies has only been minimally explored. In multiple myeloma, one study has demonstrated that IL-21 promotes resistance to apoptosis.10 Two studies have demonstrated that CLL cells express a modest amount of IL-21Rα that increases with CD40 ligand or cytosine-phosphate-guanosine (CpG) oligonucleotide treatment.11,12 Both of these previously published CLL studies have examined the combination of CD40 ligand or CpG in combination with IL-21 on CLL cells. To date, no studies have examined the influence of the single agent IL-21 on CLL cells or the combination with therapeutic agents clinically available to promote apoptosis (fludarabine) or recruit innate immune effector cell cytotoxicity (rituximab). Herein, we describe the favorable preclinical features of IL-21 in CLL, for which it promotes a direct apoptotic signal involving BIM up-regulation, sensitizes CLL cells to cell death by common agents used to treat this disease, and enhances innate immune antibody-dependent cytotoxicity. These results together provide justification for the use of IL-21 in combination with chemoimmunotherapy approaches for CLL.
Methods
Approval from The Ohio State University Institutional Internal Review Board was obtained for use of the clinical samples used in this study.
CLL and normal cell isolation
Blood was collected from patients with CLL under a protocol approved by the hospital internal review board. Informed consent was obtained in accordance with the Declaration of Helsinki. All patients examined in this series had immunophenotypically defined CLL as outlined by the modified 1996 National Cancer Institute criteria.13 CLL B cells were isolated from freshly donated blood using ficoll density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare, Little Chalfont, United Kingdom). Enriched CLL fractions were prepared using MACs negative selection kit by Miltenyi Biotech (Auburn, CA) or by “Rosette-Sep” kit from StemCell Technologies (Vancouver, BC) according to manufacturer instructions. Human NK cells (> 95% CD56+) or CD3+ cells (> 95% CD3+) derived from CLL patients were isolated directly from fresh whole blood by 30-minute incubation with Rosette-Sep cocktail (StemCell Technologies) before ficoll hypaque density gradient centrifugation (Sigma-Aldrich, St Louis, MO). The monocytes were isolated from the peripheral blood mononuclear cells (PBMCs) by negative selection using the monocytes isolation kit (Miltenyi Biotech). PBMCs were treated with the cocktail of biotin-conjugated antibodies (biotin-conjugated CD3, CD7, CD16, CD19, CD56, CD123, and CD235a antibodies) followed by MACs anti-biotin magnet beads and then passed through the MACs column. The collected effluent are more than 95% rich in monocytes. Cells were incubated in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 2 mM l-glutamine (Invitrogen, Carlsbad, CA), and penicillin (100 U/mL)/streptomycin (100 μg/mL; Sigma-Aldrich) at 37°C in an atmosphere of 5% CO2. For signaling studies, the cells were incubated with the Hybridoma serum-free media (SFM) supplemented with 10% human serum, 2 mM l-glutamine, and penicillin (100 U/mL)/streptomycin (100 μg/mL) at 37°C in an atmosphere of 5% CO2.
Cytokines and antibodies
Recombinant human IL-21 and biotin-labeled IL-21Rα was kindly provided by ZymoGenetics (Seattle, WA), and IL-2 was purchased from PeproTech (Rocky Hill, NJ). Polyclonal human immunoglobulin G (IgG) was purchased from Sigma-Aldrich. Rabbit anti-human phospho-STAT1Y-701 (p-STAT1Y-701), p-STAT2Y-690, and p-STAT5Y-694 were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti-human p-STAT3Y-705 monoclonal antibody (mAb) was purchased from Upstate Biotechnology (Charlottesville, VA). Rabbit anti–human Bim was purchased from Cell Signaling Technology, and mouse anti–human Mcl-1 and rabbit anti–human Bcl-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti–human HLA-DR fluorescein isothiocyanate (FITC) and mouse anti–human CD80 and CD86 were purchased from BD PharMingen (San Diego, CA), and mouse anti-human CD40 phycoerythrin (PE) was purchased from Beckman Coulter (Fullerton, CA). FITC-labeled annexin V and propidium iodide (PI) were purchased from BD PharMingen. Alemtuzumab was produced by Ilex Pharmaceuticals (San Antonio, TX) and purchased commercially. Rituximab and trastuzumab were produced by Genentech (South San Francisco, CA) and purchased commercially. Goat anti–human IgG antibody (Fc gamma fragment specific) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Assessment of apoptosis by flow cytometry
The apoptosis of cells after incubation with antibodies was measured using annexin V–FITC/PI staining followed by FACS analysis. Cells cultured either in 12-well plates or culture tubes with indicated treatments were stained with 5 μL annexin V (BD PharMingen) and 5 μL PI (BD PharMingen) [5 × 105 cells in 200 μL binding buffer (BD PharMingen)], and kept in dark, at room temperature, for 15 minutes before resuspension with 300 μL binding buffer and analysis by flow cytometry. Unstained cell sample and cells stained with annexin V or PI only were also processed for compensation. Results were represented as percentage of total positive cells over media control [(% annexin V and/or PI-positive cells in the treated sample) − (% total annexin V and/or PI-positive cells in the media control)].
All flow cytometric analyses were performed using a Beckman Coulter EPICS XL flow cytometer (Beckman Coulter). Ten thousand events were collected from each sample, and data were acquired in list mode and analyzed by System II software package (Beckman Coulter).
Assessment of STAT1 and STAT3 signaling by flow cytometry
The CD19+ B–CLL cells or NK cells (2.5 × 105) were incubated at 37°C for 15 minutes after stimulation with phosphate-buffered saline (PBS), IL-2 (10 ng/mL), IL-21 (10 ng/mL), or interferon-α (IFN-α; 104 U/mL). The staining was performed using fix/perm kit from Caltag Laboratories (Burlingame, CA) according to manufacturer instructions.14 Briefly, 100 μL Reagent A was added and incubated at room temperature in dark for 2 to 3 minutes followed by 3 to 4 mL methanol fixation. Cells were incubated for 10 minutes at room temperature, washed in flow buffer (PBS with 5% FBS), and permeabilized with 100 μL Reagent B. Appropriate amounts of rabbit anti–human p-STAT1 antibody (Tyr 701), rabbit anti–human p-STAT2Y-690 (Cell Signaling Technology), mouse anti–human p-STAT3Y705 antibodies (Upstate Biotechnology) or appropriate isotype controls were added and incubated at room temperature for 30 minutes. The cells are then washed and incubated with Alexa Fluor 488–conjugated goat antirabbit secondary antibodies for 30 minutes and washed with flow buffer and fixed in 1% formalin and stored at 4°C. The flow cytometic analysis was performed using Beckman Coulter EPICS XL flow cytometer (Beckman Coulter).
Western blotting
Whole cell lysates were prepared and kept in −80°C as described previously15 with the addition of the protease and phosphatase inhibitors (2 mM sodium orthovanadate, 0.004 μg/mL microcystin LR, 1 mM phenylmethanesulfonyl fluoride, 1 mM benzamidine, and 1.04 mM (4-(2-aminoethyl) benzenesulfonyl fluoride, 15 μM pepstatin A, 14 μM E-64, 40 μM bestatin, 20μM leupeptin and 0.8μM aprotinin; all from Sigma-Aldrich). Protein concentration in the lysates was quantified by the bicin choninic acid method (Pierce Chemical, Rockford, IL). Lysates with 20 μg total protein were loaded to each lane in sodium dodecyl sulfate-polyacrylamide gels (10% for p-STAT1 and p-STAT3), and transferred to 0.2 μm nitrocellulose membranes (Whatman Schleicher and Schuell, Keene, NH) after electrophoresis. Horseradish peroxidase (HRP)–conjugated goat anti–rabbit IgG (Bio-Rad, Hercules, CA) for STAT1 and HRP-conjugated goat anti–rabbit IgG for STAT3 were used as secondary antibodies. Detection was made with chemiluminescent substrate (SuperSignal; Pierce Chemical).
Antibody-dependent cellular cytotoxicity assay
Antibody-dependent cellular cytotoxicity (ADCC) activity was determined by standard 4-hour 51Cr-release assay as described previously.16 Indicated 51Cr-labeled target cells (B–CLL cells) were placed in 96-well plates, and indicated concentrations of antibodies were added to wells. Effector cells (PBMCs or NK cells from CLL patient donors either autologous or allo donors) were then added to the plates at indicated effector to target (E:T) ratios. After 4-hour incubation, supernatants were removed and counted in a gamma counter. The percentage of specific cell lysis was determined by % lysis = 100 × (ER-SR)/(MR-SR), where ER, SR, and MR represent experimental, spontaneous, and maximum 51Cr-release, respectively.
Monocyte ADCC
Monocytes were cultured in RPMI containing 10% FBS and 2 mM l-glutamine (Invitrogen), penicillin (100 U/mL)/streptomycin (100 μg/mL; Sigma-Aldrich) at 37°C in an atmosphere of 5% CO2 supplemented with either IL-21 at 10 or 100 ng/mL or media alone for 18 hours, and standard ADCC assay as described above was performed with Cr-labeled target cells (Raji) in the presence of indicated antibodies with the exception that the targets and the effectors are cocultured for 24 hours for the maximum lysis.
Transfection of small interfering RNA into CLL cells
The small interfering RNA to BIM or nonspecific control RNA were obtained from Dharmacon RNA Technologies (Lafayette, CO). Amaxa nucleofector apparatus and programs T16 or L-17 were used per manufacturer instruction. Briefly, 7 × 106 CLL cells were resuspended in nucleofector solution (Nuclofector Kit R; Amaxa Biosystems, Cologne, Germany) with 2.7 μg Bim small interfering RNA (siRNA) or nonsense siRNA. Immediately after transfection, cells were transferred to the Hybridoma SFM, and after 18 to 20 hours, the transfected CLL cells were treated with media or IL-21 (100 ng/mL) for 72 hours.
Statistical analysis
All the analyses were performed by statisticians in the Center for Biostatistics, The Ohio State University. SAS software (version 9.1; SAS Institute, Cary, NC) was used for all the statistical analysis. For the multiple factor experiments, analysis of variance models were used to estimate main effects and interactions. For cell donor experiments, mixed linear models were used to account for correlated responses within individual patients' cells.
Results
IL-21Rα expression in CLL cells is variable and correlates with direct apoptosis by IL-21
As reported previously, CLL cells have variable expression of the IL-21Rα subunit as shown in the Table. Expression of IL-21Rα varied from less than 10% to 80% of cells, with 13 of the 16 (81%) patients expressing IL-21Rα on 20% or more of the cells. The relative mean fluorescence intensity (MFI) of IL-21Rα in CD19+ CLL cells varied considerably among patients (median MFI 4.32; range 1.85-22.1). Overall, these findings suggest that the majority of CLL patients express the IL-21Rα even in the absence of modulation with CpG oligonucleotides or CD40 ligand treatment. Similar to previous reports by others, we did note effective up-regulation after CLL treatment with CpG oligonucleotides or CD40 ligand (data not shown). Given the lack of noninvestigational, clinically available CpG oligonucleotide or CD40 ligand, we performed all subsequent experiments only with IL-21 to best model what could be translated easily to clinical trials in patients with CLL.
IL-21R expression and clinical characteristics of CLL patient samples
| No . | Age . | Previous therapy . | Interphase cytogenetics . | % CD38+ . | % IL-21Rα expression . | RMFI of IL-21Rα+cells . |
|---|---|---|---|---|---|---|
| 1 | 55 | 5 | Nullisomy 13q- (39%), | 6 | 46 | 3.89 |
| 2 | 66 | 1 | 17P- (76%) | 0 | 27.37 | 22.2 |
| 3 | 55 | 9 | 11q22.3- (93%) | 11 | 80 | 19.1 |
| 4 | 68 | 1 | Normal | 1 | 67 | 1.85 |
| 5 | 45 | 0 | 13q- (68%) | 0 | 65 | 2 |
| 6 | 50 | 0 | 11q22.3-(90%), 13q- (93%) | 22 | 60 | 4.32 |
| 7 | 57 | 10 | 11q22.3- (93%) | 5 | 19.4 | 6.07 |
| 8 | 67 | 0 | Normal | 0 | 38 | 16.8 |
| 9 | 54 | 4 | 11q22.3 (83.4%), 13q14- (92%) | 48 | 53.6 | 4.24 |
| 10 | 64 | 4 | Normal | 0 | 64.5 | 24 |
| 11 | 77 | 4 | Normal | — | 34 | 3.89 |
| 12 | 76 | 7 | 13q14- (97%), 11q22.3 (94.3%) | 13 | 49 | 21 |
| 13 | 76 | 1 | 11q22.3 (93.3%) | 95 | 30.3 | 3.5 |
| 14 | 46 | 0 | 13q14 (28.2%) | 1 | 15 | 2.1 |
| 15 | 53 | 5 | 11q23 (10%) | — | 0.31 | 4.32 |
| 16 | 72 | 5 | 11q22.3 (7.8%) | 7 | 27.1 | 21.1 |
| 17p- (7.8%) |
| No . | Age . | Previous therapy . | Interphase cytogenetics . | % CD38+ . | % IL-21Rα expression . | RMFI of IL-21Rα+cells . |
|---|---|---|---|---|---|---|
| 1 | 55 | 5 | Nullisomy 13q- (39%), | 6 | 46 | 3.89 |
| 2 | 66 | 1 | 17P- (76%) | 0 | 27.37 | 22.2 |
| 3 | 55 | 9 | 11q22.3- (93%) | 11 | 80 | 19.1 |
| 4 | 68 | 1 | Normal | 1 | 67 | 1.85 |
| 5 | 45 | 0 | 13q- (68%) | 0 | 65 | 2 |
| 6 | 50 | 0 | 11q22.3-(90%), 13q- (93%) | 22 | 60 | 4.32 |
| 7 | 57 | 10 | 11q22.3- (93%) | 5 | 19.4 | 6.07 |
| 8 | 67 | 0 | Normal | 0 | 38 | 16.8 |
| 9 | 54 | 4 | 11q22.3 (83.4%), 13q14- (92%) | 48 | 53.6 | 4.24 |
| 10 | 64 | 4 | Normal | 0 | 64.5 | 24 |
| 11 | 77 | 4 | Normal | — | 34 | 3.89 |
| 12 | 76 | 7 | 13q14- (97%), 11q22.3 (94.3%) | 13 | 49 | 21 |
| 13 | 76 | 1 | 11q22.3 (93.3%) | 95 | 30.3 | 3.5 |
| 14 | 46 | 0 | 13q14 (28.2%) | 1 | 15 | 2.1 |
| 15 | 53 | 5 | 11q23 (10%) | — | 0.31 | 4.32 |
| 16 | 72 | 5 | 11q22.3 (7.8%) | 7 | 27.1 | 21.1 |
| 17p- (7.8%) |
Surface staining of B-CLL cells: primary cell samples from 16 B-CLL patients were stained with PE-labeled mouse mAb to human IL-21Rα or a nonbinding isotype control and analyzed by flow cytometry. The percentage of cells expressing receptor was correlated with interphase cytogenetics, CD38 expression, and direct cytotoxicity mediated by IL-21, as examined by annexin+/PI+cells.
RMFI indicates relative mean fluorescent intensity; and —, not detected.
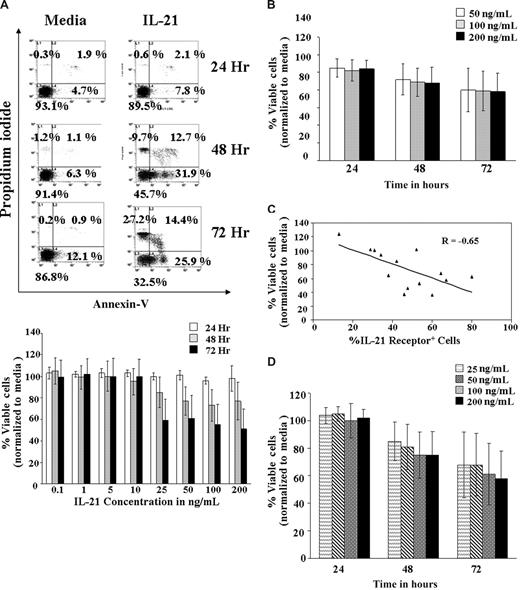
The IL-21 cytokine has been known to play an important role in the homeostasis of the normal B cells.17 To determine whether CLL cells responded similar to murine B cells when treated with IL-21, purified CD19+ CLL cells were treated with increasing concentrations of IL-21 (0.1, 1, 5, 10, 25, 50, 100, and 200 ng/mL) in vitro and apoptosis examined at 24, 48, and 72 hours (Figure 1A). IL-21 induced a statistically significant decrease of 1.8% viable cells for every fold increase in IL-21 concentration (95% confidence interval [CI], −3.6%, −0.04%; P = .046). However, it is apparent that there exists a significant nonlinear trend in IL-21 dose response (P < .001), indicating a leveling of response at 25 ng/mL. The effect of IL-21 relative to media control on 16 freshly isolated CLL patient B cells is summarized in Figure 1B. IL-21 induced significant levels of apoptosis in 12 of the 16 patient samples examined, with an overall average of 33% (±7%) apoptotic cells at 72 hours (P < .007). An average of 41% (±5%) apoptosis was observed in the 12 responder samples (excluding the 4 nonresponder samples, in which no apoptosis was observed).
IL-21–induced cell death in B-CLL cells. (A) Dose kinetics. B-CLL cells isolated from 7 patients were left untreated in media or treated with IL-21 at 0.1, 1, 5, 10, 25, 50, 100, and 200 ng/mL for 72 hours. At 24, 48, and 72 hours, cells were stained with FITC-annexin V and PI and analyzed by flow cytometry. Direct cell death was evaluated by normalizing annexin V/PI–negative cells with the respective media control. The top panel shows a dot plot of one of the representative experiments. The bottom panel shows summarized average of results from the 7 independent experiments. The SD of the mean within the population is shown as error bars (P = .046, implying a slight decrease in the percentage of live cells with dose increases beyond 25 ng/mL). Number on plots are percentages of total cells. (B) Time kinetics. CD19+ B-CLL cells were treated with 50, 100, or 200 ng/mL of IL-21 in media, and the direct cell death caused by IL-21 at different time points were assessed by annexin V/PI staining. Direct cell death was evaluated by normalizing annexin V/PI cells with the respective media control. Error bars indicate SD of mean in 16 B-CLL patient cell samples. (C) IL-21–mediated direct cytotoxicity correlates with receptor expression. Primary cell samples from 14 B-CLL patients were stained with PE-labeled mouse mAb to human IL-21R or a nonbinding isotype control and analyzed by flow cytometry. The percentage of the CLL cells expressing IL-21R and percentage of viable CLL cells (annexin/PI compared with media) were analyzed by multiple regression (n = 16; P = .009; R = Pearson correlation). (D) IL-21–mediated direct cytotoxicity in responding patients-dose and time dependence. B-CLL cells were left untreated (media) or treated with IL-21 at 25, 50, 100, and 200 ng/mL for 72 hours. Cells were stained with FITC–annexin V and PI. Direct cell death was evaluated by normalizing annexin V/PI cells with the respective media control. Error bars represent SD of mean in 9 B-CLL patient cell samples (n = 9; P < .01 compared with media control).
IL-21–induced cell death in B-CLL cells. (A) Dose kinetics. B-CLL cells isolated from 7 patients were left untreated in media or treated with IL-21 at 0.1, 1, 5, 10, 25, 50, 100, and 200 ng/mL for 72 hours. At 24, 48, and 72 hours, cells were stained with FITC-annexin V and PI and analyzed by flow cytometry. Direct cell death was evaluated by normalizing annexin V/PI–negative cells with the respective media control. The top panel shows a dot plot of one of the representative experiments. The bottom panel shows summarized average of results from the 7 independent experiments. The SD of the mean within the population is shown as error bars (P = .046, implying a slight decrease in the percentage of live cells with dose increases beyond 25 ng/mL). Number on plots are percentages of total cells. (B) Time kinetics. CD19+ B-CLL cells were treated with 50, 100, or 200 ng/mL of IL-21 in media, and the direct cell death caused by IL-21 at different time points were assessed by annexin V/PI staining. Direct cell death was evaluated by normalizing annexin V/PI cells with the respective media control. Error bars indicate SD of mean in 16 B-CLL patient cell samples. (C) IL-21–mediated direct cytotoxicity correlates with receptor expression. Primary cell samples from 14 B-CLL patients were stained with PE-labeled mouse mAb to human IL-21R or a nonbinding isotype control and analyzed by flow cytometry. The percentage of the CLL cells expressing IL-21R and percentage of viable CLL cells (annexin/PI compared with media) were analyzed by multiple regression (n = 16; P = .009; R = Pearson correlation). (D) IL-21–mediated direct cytotoxicity in responding patients-dose and time dependence. B-CLL cells were left untreated (media) or treated with IL-21 at 25, 50, 100, and 200 ng/mL for 72 hours. Cells were stained with FITC–annexin V and PI. Direct cell death was evaluated by normalizing annexin V/PI cells with the respective media control. Error bars represent SD of mean in 9 B-CLL patient cell samples (n = 9; P < .01 compared with media control).
Interestingly, IL-21–mediated direct apoptosis correlated with expression of IL-21Rα is shown in Figure 1C (P = .01). IL-21–mediated apoptosis (> 20% compared with media) was observed in B-CLL cells expressing more than 20% of IL-21Rα in 9 patient samples. In these 9 patients, responses to higher doses of IL-21 were not significantly different (estimated difference in response for every fold increase of IL-21 concentration −1.56; 95% CI, −7.08, 3.96; P = .574). In addition, the response was time dependent (P < .001; Figure 1D). Direct cytotoxicity was not related to activation of CLL cells because markers of activation including surface expression of CD40, HLA-DR, and CD86, were not altered at 24- or 72-hour time points and after exposure to different concentrations of IL-21 (data not shown).
Requirement of BIM induction in IL-21–mediated direct apoptosis in CLL B cells
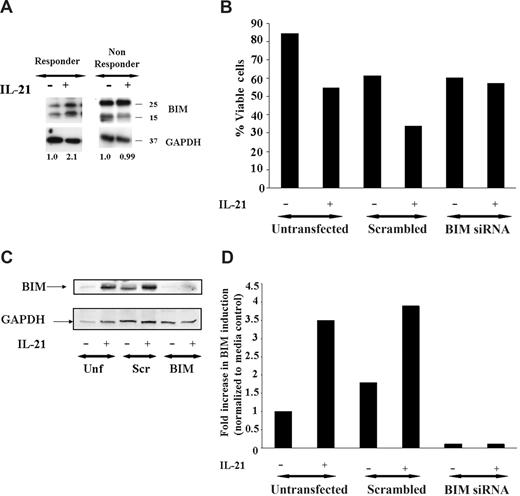
Studies in normal murine B cells have demonstrated that IL-21–mediated apoptosis occurs in part through up-regulation of the BH3 only family member Bim.17 Figure 2A demonstrates that similar to that observed in murine B cells, IL-21 also causes up-regulation of BIM in a patient with sensitivity to IL-21, whereas a nonresponder does not have similar induction. Indeed, in 5 of 5 patients who responded to IL-21–mediated direct cytotoxicity greater than 20%, we observed similar BIM up-regulation, whereas in 3 nonresponders, Bim induction was not observed. This finding suggests that BIM up-regulation by IL-21 may contribute to apoptosis mediated by this cytokine in CLL.
Induction of BIM is required for direct apoptosis induced by IL-21. (A) IL-21 induces BIM induction. CD19+ B-CLL cells were stimulated with media, IL-21 (100 ng/mL) for 24 hours. Cells were lysed in appropriate buffers and analyzed by Western blotting using specific mAb for Bim and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described in Methods. Figure shows data from a representative responding and nonresponding patient. Numbers below the lanes represent the fold increase in Bim levels in IL-21–stimulated CLL cells relative to unstimulated cells from the same patient and normalized to GAPDH levels. Data are expressed in relative densitometric units. (B) BIM siRNA protects CD19+ B-CLL cells from IL-21–mediated direct cytotoxicity. CD19+ B-CLL cells were mock transfected (no siRNA) or were transfected with nonsense siRNA or BIM siRNA (n = 3). The CLL cells transfected with Bim siRNA are resistant to the IL-21–mediated direct cytotoxicity compared with scrambled or mock-transfected cells. (C) Western blot analysis of BIM protein expression in nonsense siRNA, BIM-specific siRNA, and mock-transfected CLL cells. The CLL cells mock transfected or nonsense siRNA or BIM siRNA and treated with IL-21 or media therapy for 72 hours and analyzed by immunoblot using BIM specific antibody. GAPDH panel represents the loading control. (D) The bar graph representing the fold increase in the BIM with and without IL-21 is shown. Results shown are representative of 3 independent patients.
Induction of BIM is required for direct apoptosis induced by IL-21. (A) IL-21 induces BIM induction. CD19+ B-CLL cells were stimulated with media, IL-21 (100 ng/mL) for 24 hours. Cells were lysed in appropriate buffers and analyzed by Western blotting using specific mAb for Bim and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described in Methods. Figure shows data from a representative responding and nonresponding patient. Numbers below the lanes represent the fold increase in Bim levels in IL-21–stimulated CLL cells relative to unstimulated cells from the same patient and normalized to GAPDH levels. Data are expressed in relative densitometric units. (B) BIM siRNA protects CD19+ B-CLL cells from IL-21–mediated direct cytotoxicity. CD19+ B-CLL cells were mock transfected (no siRNA) or were transfected with nonsense siRNA or BIM siRNA (n = 3). The CLL cells transfected with Bim siRNA are resistant to the IL-21–mediated direct cytotoxicity compared with scrambled or mock-transfected cells. (C) Western blot analysis of BIM protein expression in nonsense siRNA, BIM-specific siRNA, and mock-transfected CLL cells. The CLL cells mock transfected or nonsense siRNA or BIM siRNA and treated with IL-21 or media therapy for 72 hours and analyzed by immunoblot using BIM specific antibody. GAPDH panel represents the loading control. (D) The bar graph representing the fold increase in the BIM with and without IL-21 is shown. Results shown are representative of 3 independent patients.
To directly determine the importance of Bim modulation in IL-21–induced direct cytotoxicity, siRNA directed against BIM was used. As shown in Figure 2B, whereas IL-21 induced direct cytotoxicity in untransfected and scrambled-control siRNA-transfected primary B CLL B cells by 72 hours tested, cells transfected with BIM-specific siRNA exhibited resistance to IL-21–induced cytotoxicity. The specific modulation of BIM protein expression in CLL B cells transfected with BIM siRNA but not control-scrambled siRNA or untransfected cells treated with media or IL-21 is shown in Figure 2C,D. These data highlight the importance of the role of proapoptotic protein BIM in IL-21–mediated cytotoxicity.
IL-21 enhances apoptosis mediated by fludarabine and rituximab in CLL
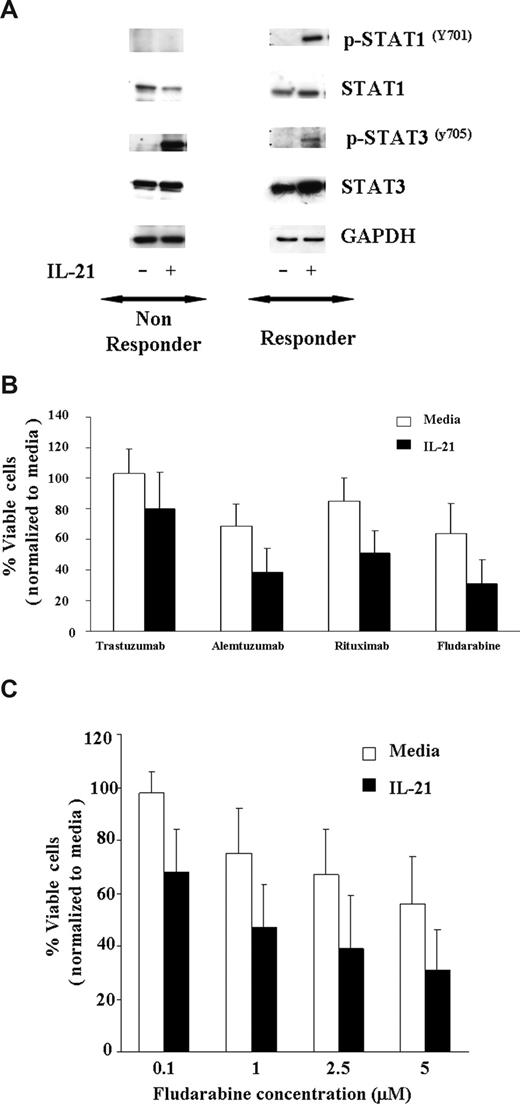
Fludarabine is currently the most successful front-line treatment in CLL, often used in combination with rituximab. Previous studies have demonstrated that other cytokines, such as IFN-γ, that activate STAT1 enhance apoptosis induced by cytotoxic therapy. As shown in Figure 3A, IL-21–induced tyrosine phosphorylation of STAT1Y701 and STAT3Y705 in 7 CLL patients' cells susceptible to IL-21 induced direct cytotoxicity, whereas the phosphorylation of STAT1Y701 was not observed in 3 CLL patients' cells that were not susceptible to IL-21–mediated direct cytotoxicity. As reported in multiple myeloma cells and normal B cells, IL-21 induced STAT3Y705 phosphorylation in 6 of 7 responders and 2 of 3 nonresponders. Given the potential of STAT1 activation to enhance apoptosis and STAT3 to antagonize this same process, we sought to determine the effect of IL-21 treatment on apoptosis induced by rituximab or fludarabine. CLL cells pretreated with IL-21 (100 ng/mL) or media control for 18 hours were treated with rituximab, alemtuzumab (alternative CLL antibody), trastuzumab (control antibody), or fludarabine. Figure 3B demonstrates that compared with the media control, IL-21–treated responder cells have significantly greater apoptosis averaged across all antibodies (P = .018). In particular, IL-21 significantly increased the effect of rituximab by 24% (95% CI, 8.4, 39.2; P = .004) and fludarabine effect by 23% (95% CI, 8.1, 37.9; P = .004) on percentage cell death. Thus, in each of these experiments, IL-21 did not appear to prevent death induced by rituximab, alemtuzumab, or fludarabine. There was no significant interaction between IL-21 with rituximab, alemtuzumab, or fludarabine, suggesting an additive effect of the combination. Next, we evaluated the IL-21–induced sensitivity to fludarabine titration on the CLL cells. When responder CLL cells were treated with the fixed concentration of IL-21 (100 ng/mL) and exposed to the increasing concentration of fludarabine (0.1-5 μM), as shown in Figure 3C, there was a significant effect of IL-21 on the percentage of live cells across all the concentrations of fludarabine (P = .01). There was no significant interaction between IL-21 and fludarabine (P = .95), suggesting an additive effect of the combination. In a separate experiment, concentrations of both IL-21 (range 0.5-500 ng/mL) and fludarabine (range 0.08-20 ng/mL) were varied. No significant interaction in dose effects was found (P = .77), again suggesting an additive effect of the combination. Of note, IL-21 did not increase the effects of fludarabine or the antibodies in CLL cells from patients who were insensitive to the direct effects of IL-21 (data not shown).
IL-21 treatment of CLL cells enhances direct cytotoxicity mediated by antibodies and fludarabine. (A) IL-21 mediates tyrosine phosphorylation of STAT1Y701 and STAT3Y705 in C19+ B-CLL cells. CD19+ B-CLL cells were stimulated with media or IL-21 (100 ng/mL) for 24 hours. Cells were lysed in appropriate buffers and analyzed by Western blotting using mAb specific for indicated STAT proteins and GAPDH. Shown are the results of a representative responding and nonresponding patient. (B) IL-21 enhances additively antibody and fludarabine-mediated cytotoxicity. CD19+ B-CLL cells were treated with media or IL-21 at 100 ng/mL concentration for 18 hours followed by incubation with cross-linker alone (media), trastuzumab with cross-linker (Trastuzumab), rituximab with cross-linker (Rituximab), alemtuzumab with cross-linker (Alemtuzumab), and fludarabine. At 48 hours, direct cytotoxicity by antibody exposure and fludarabine was analyzed by annexin V/PI staining. Shown here are the results from 7 consecutive experiments. Error bars are SD among samples. (C) IL-21 enhances fludarabine-induced cytotoxicity. Dose kinetics. CD19+ B-CLL cells were treated with IL-21 at 100 ng/mL concentration of IL-21 or media alone for 18 hours, and the pretreated cells were incubated with fludarabine at 0.1, 1, 2.5, and 5 μM concentration. At 48 hours, direct cytotoxicity by fludarabine was analyzed by annexin V/PI staining. Shown here are the results from 7 consecutive experiments. Error bars are SD between samples.
IL-21 treatment of CLL cells enhances direct cytotoxicity mediated by antibodies and fludarabine. (A) IL-21 mediates tyrosine phosphorylation of STAT1Y701 and STAT3Y705 in C19+ B-CLL cells. CD19+ B-CLL cells were stimulated with media or IL-21 (100 ng/mL) for 24 hours. Cells were lysed in appropriate buffers and analyzed by Western blotting using mAb specific for indicated STAT proteins and GAPDH. Shown are the results of a representative responding and nonresponding patient. (B) IL-21 enhances additively antibody and fludarabine-mediated cytotoxicity. CD19+ B-CLL cells were treated with media or IL-21 at 100 ng/mL concentration for 18 hours followed by incubation with cross-linker alone (media), trastuzumab with cross-linker (Trastuzumab), rituximab with cross-linker (Rituximab), alemtuzumab with cross-linker (Alemtuzumab), and fludarabine. At 48 hours, direct cytotoxicity by antibody exposure and fludarabine was analyzed by annexin V/PI staining. Shown here are the results from 7 consecutive experiments. Error bars are SD among samples. (C) IL-21 enhances fludarabine-induced cytotoxicity. Dose kinetics. CD19+ B-CLL cells were treated with IL-21 at 100 ng/mL concentration of IL-21 or media alone for 18 hours, and the pretreated cells were incubated with fludarabine at 0.1, 1, 2.5, and 5 μM concentration. At 48 hours, direct cytotoxicity by fludarabine was analyzed by annexin V/PI staining. Shown here are the results from 7 consecutive experiments. Error bars are SD between samples.
IL-21 does not potentiate fludarabine-mediated cytotoxicity in T cells
A major limitation of treatment with fludarabine in CLL is cellular immune suppression observed with this agent. Given the enhanced direct cell death observed with IL-21 and fludarabine in primary CLL cells, a primary concern could be that this same cytotoxicity will occur in T cells expressing IL-21Rα. To examine this effect, we examined CD3+ selected T cells from patients with CLL to determine the influence of IL-21 treatment when combined with fludarabine. Unlike CLL cells, in which additive cytotoxicity was observed from these agents, IL-21 did not enhance the cytotoxic effect of fludarabine when examined at various concentrations (0.08-20 ng/mL; P = .74). Averaging across doses of fludarabine, the IL-21 effect on T cells was not significant (estimated slope difference was 0.16; 95% CI, −0.78, 1.10; P = .19; Figure 4). These data suggest that IL-21 addition to augmenting chemoimmunotherapy in CLL should not enhance cellular immune suppression associated with this treatment.
IL-21 treatment of T cells does not enhance direct cytotoxicity of fludarabine. CD3+ T cells from CLL patients were treated with IL-21 at 100 ng/mL concentration or media alone and at indicated concentration of fludarabine ranging from 0.08 to 20μM. At 72 hours, direct cytotoxicity by fludarabine was analyzed by annexin V/PI staining. Shown here is the result from 5 consecutive experiments. Error bars are SD between samples.
IL-21 treatment of T cells does not enhance direct cytotoxicity of fludarabine. CD3+ T cells from CLL patients were treated with IL-21 at 100 ng/mL concentration or media alone and at indicated concentration of fludarabine ranging from 0.08 to 20μM. At 72 hours, direct cytotoxicity by fludarabine was analyzed by annexin V/PI staining. Shown here is the result from 5 consecutive experiments. Error bars are SD between samples.
IL-21 enhances ADCC of autologous NK cells against rituximab-coated CLL cells
Previous studies have demonstrated that both IL-2 and IL-12 potentiate the cytolytic function of the NK cells to antibody-coated target cells in vitro.18 Unfortunately, both of these cytokines also promote proliferation and/or inhibit apoptosis of CLL cells. Previous studies have shown that IL-21 enhances ADCC of breast cancer cell lines coated with antibodies, although no such studies have been performed against primary tumor cells.19 Freshly isolated NK cells from patients with CLL were cultured with media alone or IL-21 at 100 ng/mL individually for 18 hours. Figure 5A demonstrates that IL-21 treatment of NK cells derived from patients with CLL significantly enhanced basal STAT1 and STAT5 tyrosine phosphorylation, an indicator of increased NK cell activity. This finding was corroborated by enhanced ADCC, as shown in Figure 5B. Here, we demonstrate resting autologous NK cells to mediate ADCC against autologous CLL cells coated with rituximab. Furthermore, compared with untreated NK cells, IL-21 treatment significantly enhances rituximab-mediated cytotoxicity of autologous NK cells (P < .001 at all E:T ratios shown). Concomitant with demonstrated ADCC, we also observed enhanced STAT1 and STAT5 signaling in NK cells treated with IL-21 (results from a representative patient sample shown in Figure 5A). Interestingly, IL-21 enhanced STAT signaling and ADCC in NK cells from all patients tested, including patients whose CLL cells were insensitive to direct IL-21–induced apoptosis. In addition, IL-21 treatment significantly increased rituximab-mediated ADCC mediated by HLA-mismatched NK cells against primary CLL cells (P = .04 at the 25:1 E/T ratio) without cytokine (Figure 5C). IL-21 did not enhance alemtuzumab-mediated ADCC against CLL cells with either autologous or allogeneic NK cells. Similar studies examining IL-21 activation of normal monocytes did not demonstrate evidence of phagocytosis or ADCC (data not shown). Overall, these data demonstrate that IL-21 can enhance NK cell–directed ADCC against primary CLL cells in vitro.
IL-21 enhances NK-mediated ADCC of rituximab-coated CLL cells. (A) IL-21 mediates tyrosine phosphorylation of STAT1 and STAT5 in NK cells. NK cells (CLL patient derived) were stimulated with media, IL-21 (10 ng/mL), IL-2 (10 U), or IFN-α (104 U/mL). Cells were permeabilized and stained for p-STAT1 and p-STAT5 with appropriate controls as described in “Assessment of STAT and STAT3 signaling by flow cytometry” and analyzed by flow cytometry. Shown is a representative histogram of 3 independent experiments. (B) IL-21 enhances autologous NK cell–mediated ADCC of rituximab-coated CLL cells. 51Cr-labeled freshly isolated B-CLL cells were incubated with alemtuzumab, rituximab, or isotype control trastuzumab. CLL patient–derived NK cells treated with media alone (M) or 100 ng/mL of IL-21 (E) were incubated with autologous B-CLL cells at 37°C for 18 hours. Percentages of relative cytotoxicity were measured after 4 hours as described in “Antibody-dependent cellular cytotoxicity assay.” Data shown here are summary of 3 patient samples, and error bars represent SD between patients. IL-21 plus rituximab significantly enhances ADCC compared with rituximab alone (P < .001 at all E:T ratios). (C) IL-21 enhances allogeneic NK cell–mediated ADCC of rituximab-coated CLL cells. Freshly isolated CD19+ B-CLL cells were incubated with alemtuzumab, rituximab, or isotype control trastuzumab. Donor CLL patient–derived NK cells were treated with media alone or 100 ng/mL of IL-21 (18 hours) and incubated with B-CLL cells at 37°C. Percentages of relative cytotoxicity was measured after 4 hours as described above. Data shown here are a summary of 3 patient samples, and error bars represent SD between patients (n = 3). IL-21 plus rituximab significantly enhances ADCC compared with rituximab alone (P = .04 at an E:T ratio of 25:1).
IL-21 enhances NK-mediated ADCC of rituximab-coated CLL cells. (A) IL-21 mediates tyrosine phosphorylation of STAT1 and STAT5 in NK cells. NK cells (CLL patient derived) were stimulated with media, IL-21 (10 ng/mL), IL-2 (10 U), or IFN-α (104 U/mL). Cells were permeabilized and stained for p-STAT1 and p-STAT5 with appropriate controls as described in “Assessment of STAT and STAT3 signaling by flow cytometry” and analyzed by flow cytometry. Shown is a representative histogram of 3 independent experiments. (B) IL-21 enhances autologous NK cell–mediated ADCC of rituximab-coated CLL cells. 51Cr-labeled freshly isolated B-CLL cells were incubated with alemtuzumab, rituximab, or isotype control trastuzumab. CLL patient–derived NK cells treated with media alone (M) or 100 ng/mL of IL-21 (E) were incubated with autologous B-CLL cells at 37°C for 18 hours. Percentages of relative cytotoxicity were measured after 4 hours as described in “Antibody-dependent cellular cytotoxicity assay.” Data shown here are summary of 3 patient samples, and error bars represent SD between patients. IL-21 plus rituximab significantly enhances ADCC compared with rituximab alone (P < .001 at all E:T ratios). (C) IL-21 enhances allogeneic NK cell–mediated ADCC of rituximab-coated CLL cells. Freshly isolated CD19+ B-CLL cells were incubated with alemtuzumab, rituximab, or isotype control trastuzumab. Donor CLL patient–derived NK cells were treated with media alone or 100 ng/mL of IL-21 (18 hours) and incubated with B-CLL cells at 37°C. Percentages of relative cytotoxicity was measured after 4 hours as described above. Data shown here are a summary of 3 patient samples, and error bars represent SD between patients (n = 3). IL-21 plus rituximab significantly enhances ADCC compared with rituximab alone (P = .04 at an E:T ratio of 25:1).
Discussion
The introduction of chemoimmunotherapy with fludarabine and rituximab has produced promising complete and overall response rates with extended progression-free survival compared with historic controls using fludarabine monotherapy. Herein, we provide preclinical evidence that the γ-receptor chain family member cytokine IL-21 mediates apoptosis directly in a subset of CLL patients through up-regulation of BIM. Our data also demonstrate that IL-21 also activates STAT1, and, similar to γ-IFN, enhances the direct cytotoxic activity of both cytotoxic therapies and therapeutic antibodies with demonstrated augmentation of direct cytotoxicity even at subtherapeutic concentrations of fludarabine. Furthermore, IL-21 enhanced both autologous and HLA-mismatched NK cell–mediated rituximab-dependent cytotoxicity against primary CLL cell targets. The favorable direct cytotoxic properties of IL-21 appear to be B cell specific because this cytokine does not enhance fludarabine cytotoxicity toward CD3+ T cells derived from patients with CLL. These findings provide support for initiation of combination studies of IL-21 with fludarabine- and rituximab-based therapy in CLL.
Our study supports the results of 2 other studies in which we have shown that the CLL cells express IL-21R.11,12 The observed direct effect of IL-21 in promoting apoptosis and enhancing the cytotoxic effect of other agents varied and was dependent on the amount of IL-21R expression on CLL cells. Previously published studies with CD40 ligand11 or CpG oligonucleotides12 have demonstrated that the IL-21Rα can be elevated with either of these agents, and apoptosis can be enhanced. Interestingly, apoptosis with CD40 ligand–incubated CLL cells occurred through a delayed, extrinsic pathway of apoptosis, similar to what has been described when CD40 ligand–treated cells are exposed to FAS ligand or tumor necrosis factor–related apoptosis-inducing ligand. Similarly, a second study examining IL-21 and CpG oligonucleotides noted enhanced expression of the IL-21Rα with CpG treatment and enhanced apoptosis.12 Interestingly, they demonstrated that this combination therapy and that of CpG oligonucleotides and B-cell receptor ligation promoted CLL and normal B cells to produce granzyme B, which contributed to the cytotoxicity observed. Although of great interest, both of these reports rely on the use of therapeutic agents such as CD154 gene therapy or CpG oligonucleotide therapy that are in early-phase 1/2 trials as investigational agents. Our data contrast with this because we examine the influence of IL-21 as a single agent and provide data that are clinically translatable to therapeutic agents such as fludarabine and rituximab, which are currently used in the treatment of CLL. These data provide an immediate pathway to translate IL-21 to early clinical trials in CLL with agents commonly used as initial treatment in this disease.
To date, the influence of single-agent IL-21 on CLL has not been described. In normal murine B cells, IL-21 treatment promotes caspase-dependent apoptosis that is dependent on protein and mRNA synthesis with BIM induction.17 Our studies demonstrate similar results in CLL cells, in which BIM induction occurs in all patients responding to IL-21 therapy. Further, the abrogation of IL-21–mediated apoptosis and lack of induction of proapoptotic protein BIM in the CLL cells transfected with BIM siRNA in contrast to the scrambled-control siRNA confirms the importance of BIM in the IL-21–mediated direct cytotoxicity. The apoptosis induced by IL-21 is dependent on the surface expression of IL-21Rα in CLL cells and is associated with phosphorylation of STAT1, which differs from multiple myeloma cells, which also possess IL-21R; but when treated with IL-21, they actually become more resistant to apoptosis concurrent with tyrosine phosphorylation of STAT3 but not STAT1. Our studies of CLL responders demonstrated IL-21–mediated STAT1 and STAT3 tyrosine phosphorylation. The importance of the STAT family member activated after IL-21 remains to be established. STAT1 generally generates a proapoptotic signal and sensitizes to chemotherapy.20 In contrast, activation of STAT3 can be a transforming event that mediates resistance to apoptosis and can actually antagonize the proapoptotic effect of STAT1 in other systems. The relative contribution of STAT1 and STAT3 activation to IL-21–mediated apoptosis in CLL and related diseases has not been examined and warrants future study. The mechanism involved in IL-21–induced BIM up-regulation is not known. Although it is possible that IL-21–induced STAT1 and STAT3 regulate BIM expression, detailed promoter analysis of BIM and its regulation in response to IL-21 stimulation remain to be tested, and such studies are ongoing in our laboratory at this time.
To date, IL-21 has been pursued in a single-agent phase 1 study in solid tumor patients for whom a 30 μg/kg per day intravenous dose daily for 5 days every 21 days was well tolerated. Thirty-four patients with malignant melanoma and renal cell carcinoma were treated with an acceptable toxicity profile that included pyrexia, fatigue, chills, headache, and rash. The majority of patients developed lymphocytopenia that persisted throughout therapy. Recently, a phase 1 study of IL-21 with rituximab was initiated in CD20+ B-cell malignancies. Providing safety of this combination is confirmed, our data provide support for extending this to a fludarabine and rituximab combination in CLL. In addition, our data prove support for strategies to up-regulate IL-21α on CLL to enhance the antitumor activity of this agent.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the T32 grant (T-32-5CA009338), National Cancer Institute (P01 CA95426; CLL Research Consortium P01 CA81534-02), the Leukemia and Lymphoma Society, and the D. Warren Brown Foundation.
National Institutes of Health
Authorship
Contribution: A.G. performed the majority of the in vitro research, analyzed the data, and wrote the initial draft of the paper. J.R., S.-R.A.H., A.R., T.J., S.S., and C.C. performed some of the in vitro research and reviewed the drafts of the paper. D.J., X.Z., and A.L. performed the statistical work and reviewed the drafts of the paper. W.K. contributed reagents and reviewed drafts of the paper. M.A.C. and W.E.C. provided intellectual input into the NK cell studies and reviewed drafts of the paper. S.T. provided intellectual input into the monocyte studies and reviewed drafts of the paper. N.M. and J.C.B. together designed the research, reviewed all data, participated in analysis of data, and modified the initial drafts of the paper. N.M. and J.C.B. contributed equally to the work described herein.
Conflict-of-interest disclosure: W.K. is an employee of ZymoGenetics and has financial interest in IL-21. All other authors declare no competing financial interests.
Correspondence: John C. Byrd, MD, B302 Starling-Loving Hall, 320 W 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu; or Natarajan Muthusamy, DVM, PhD, 455E, OSUCCC, 410 W 12th Ave, Columbus, OH 43210; e-mail: raj.muthusamy@osumc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal