Abstract
Erythropoiesis requires erythropoietin (Epo) and stem cell factor (SCF) signaling via their receptors EpoR and c-Kit. EpoR, like many other receptors involved in hematopoiesis, acts via the kinase Jak2. Deletion of EpoR or Janus kinase 2 (Jak2) causes embryonic lethality as a result of defective erythropoiesis. The contribution of distinct EpoR/Jak2-induced signaling pathways (mitogen-activated protein kinase, phosphatidylinositol 3-kinase, signal transducer and activator of transcription 5 [Stat5]) to functional erythropoiesis is incompletely understood. Here we demonstrate that expression of a constitutively activated Stat5a mutant (cS5) was sufficient to relieve the proliferation defect of Jak2−/− and EpoR−/− cells in an Epo-independent manner. In addition, tamoxifen-induced DNA binding of a Stat5a–estrogen receptor (ER)* fusion construct enabled erythropoiesis in the absence of Epo. Furthermore, c-Kit was able to enhance signaling through the Jak2-Stat5 axis, particularly in lymphoid and myeloid progenitors. Although abundance of hematopoietic stem cells was 2.5-fold reduced in Jak2−/− fetal livers, transplantation of Jak2−/−-cS5 fetal liver cells into irradiated mice gave rise to mature erythroid and myeloid cells of donor origin up to 6 months after transplantation. Cytokine- and c-Kit pathways do not function independently of each other in hematopoiesis but cooperate to attain full Jak2/Stat5 activation. In conclusion, activated Stat5 is a critical downstream effector of Jak2 in erythropoiesis/myelopoiesis, and Jak2 functionally links cytokine- with c-Kit-receptor tyrosine kinase signaling.
Introduction
Erythropoiesis is a tightly controlled process in bone marrow and spleen of adult mammals and in the fetal liver of embryos that produces highly variable erythrocyte numbers during fetal development and in diseases such as anemia, induced by, for example, hypoxia or blood loss. Erythroid maturation proceeds through burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) stages, the latter cell type dividing 4 to 5 times while maturing into erythrocytes in response to erythropoietin (Epo).
Epo is strictly required for erythropoiesis, promoting survival and late maturation stages.1 Ligand-induced Epo-receptor (EpoR) dimerization triggers activation of the pre-associated kinase Jak2, which then phosphorylates tyrosine residues in the cytoplasmic tail of EpoR. These phosphotyrosines serve as docking sites for SH2-domain containing proteins, leading to activation of various signaling pathways, including phosphatidylinositol 3-kinase (PI3-K),2 mitogen-activated protein kinase,3 protein kinase C,4 and phospholipase C-γ.5 A central pathway in EpoR signaling, however, is the activation of the transcription factor known as signal transducer and activator of transcription 5 (Stat5).6-8 Upon phosphorylation, Stat5 dimers translocate to the nucleus, bind to cognate elements in various promoters, and activate transcription. Stat5-mediated functions regulate cell proliferation, differentiation, apoptosis, and other processes. Several important Stat5 target genes, such as Pim, c-Myc, OncostatinM, Bcl-xL, SOCS, or D-type cyclins are required for functional erythropoiesis.9-14
The requirement of Epo signaling pathway components for erythropoiesis is evident from mice deficient for Epo, EpoR, or Jak2. All mutant animals die in utero at embryonic day 13.5 (E13.5) because of a failure of erythropoiesis; BFU-E and CFU-E progenitors are completely absent from the fetal liver.15-17 The absolute requirement for Jak2 to transduce EpoR signals was recently substantiated by mutational analyses of EpoR domains: all EpoR mutants unable to bind Jak2 were nonfunctional.18
Knowledge about signaling downstream of Epo is still limited. Exogenous Bcr-Abl rescued the erythroid defect of Jak2−/− fetal liver cells in vitro.19 Likewise, a dominant-active mutant of Akt restored erythroid differentiation in Jak2-deficient erythroid progenitors.20
At first, Stat5 seemed to be nonessential for erythropoiesis, in that original Stat5ab−/− mice were viable and showed no overt erythroid defects.8 Later, these mice were found to display fetal anemia and elevated rates of apoptosis of erythroid cells as a result of a failure in Bcl-xL up-regulation.21,22 A recently generated complete knockout of Stat5ab, however, is perinatally lethal and highly anemic in utero.6 Conversely, mice expressing truncated EpoR-mutants that retain solely the ability to activate Stat5 but lack all other tyrosines critical for activation of other signaling pathways live normally.23 These animals display only mild phenotypes in recovery from erythropoietic stress. Additional mutation of Tyr343 (required for Stat5 activation), however, strongly affected stress erythropoiesis.24
This wide range of erythroid phenotypes of mice mutated in the EpoR/Jak2/Stat5 axis prompted us to clarify the role of Stat5 in erythropoiesis. To this purpose, we introduced a hyperactivatable mutant of Stat5a (cS5, S711F25 ) into EpoR−/− and Jak2−/− hematopoietic cells. Tyrosine-phosphorylated, DNA-bound cS5 complemented the proliferation defect of the mutant cells, enabling self-renewal and erythroid differentiation in the absence of Epo signals. Likewise, 4-hydroxy-tamoxifen (4-OH-T)-induced activation of a Stat5a–estrogen receptor (ER)* fusion construct was sufficient to replace Epo in erythropoiesis. Jak2-deficient fetal liver cells also showed defects in myelopoiesis and massively decreased responses to SCF, SCF + IL-3, or SCF + IL-7. Expression of cS5 in Jak2−/− cells partially corrected these proliferation defects in vitro. Moreover, Jak2−/−-cS5 cells efficiently contributed to the erythroid and myeloid lineages in vivo upon transplantation. cS5-mediated rescue of myeloid and erythroid lineages was strictly dependent on c-Kit signaling and Jak2.
Methods
All animal experiments were performed in accordance with Austrian and European laws and under approval of the ethical and animal protection committees.
Cell culture and retroviral infections
E12.5 fetal liver cells from Jak2−/−, EpoR−/−, and wild-type (WT) embryos were isolated and cultivated as described previously.26 For a detailed description of isolation, retroviral infection, and culture of primary erythroblasts; see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) 293T cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA). Transient transfections were done with Lipofectamine 2000 (Invitrogen). Colony assays of retrovirally transduced cells were performed in triplicate as described17 using MethoCult M3234 (StemCell Technologies, Vancouver, BC). The Bcr-Abl-inhibitor imatinib (Gleevec; inhibiting also c-Kit) was used at 10 μmol/L.
Plasmids
The cS5 mutant of mouse Stat5a (S711F) was used.27 In the murine Stat5a cDNA, however, the serine residue lies at amino acid position 710 instead of 711 as initially reported by Onishi et al.25 For clarity, the initial nomenclature was retained here. For the generation of Stat5-ER* constructs, a point-mutated ligand binding domain of the estrogen receptor28 was fused in frame to the C terminus of Stat5a, or cS5 or Stat5aΔ749,7 after digestion with SacII. The Stat5a-EE/AA and Stat5a-Y694F mutants were described by Wang et al.29 Both mutations were introduced into cS5 by polymerase chain reaction (PCR) mutagenesis or cassette exchange.
All constructs were cloned into the retroviral vector pMSCV-IRES-GFP (Clontech, Mountain View, CA) and verified by sequencing. Ecotropic, replication incompetent gp + E86 producers were generated as described previously27 and selected for high virus titer production by fluorescence-activated cell sorting (FACS).
Transplantation of fetal liver-derived hematopoietic progenitors
Freshly isolated E12.5 WT and Jak2−/− fetal liver cells (C57Bl/6, CD45.2) were cocultured with retrovirus-producing cells in DMEM, 15% FCS, stem cell factor (SCF; 200 ng/mL), interleukin-6 (IL-6, 50ng/mL; R&D Systems, Minneapolis, MN) and IL-3 (25 ng/mL; R&D Systems) for 72 hours. Hematopoietic cells (1-2 × 106) were injected into 4- to 6-week-old mice (B6.SJL-PtprcaPep3b/BoyJ; Ly-5.1; CD45.1) after sublethal irradiation (750 rad) via the tail vein. Engraftment of injected cells was monitored by FACS analysis of peripheral blood starting 4 weeks after transplantation at regular intervals.
Flow cytometry
Cultured erythroblasts or single cell suspensions of spleen and bone marrow cells from mice 6 months after transplantation were stained with fluorescence-conjugated antibodies against Ter-119, CD71, GR-1, Mac-1, c-Kit, Sca-1, CD19, B220, CD4, and CD8 (all from BD Biosciences, San Jose, CA). Annexin V staining was performed according to the manufacturer's instructions (BD Biosciences). Samples were analyzed on a FACScalibur flow cytometer (BD Biosciences).
Cytokine stimulation, Western blot analysis, and DNA binding assays
Cultured erythroblasts were starved for 3 hours in plain DMEM and subsequently stimulated for 10 minutes with Epo (10 U/mL) or SCF (100 ng/mL) or with SCF plus imatinib (10 μmol/L). 293T cells transiently transfected with the murine pXM-EpoR expression vector and different Stat5 constructs were stimulated with Epo (50 U/mL) and/or 4-OH-T (50 nM) for 30 minutes. Sample preparation and Western blotting was performed according to standard techniques. Antibodies used for Western blotting were anti-phospho-Stat5ab (Millipore, Billerica, MA), anti-Stat5ab (BD Biosciences), anti-Jak2 (Cell Signaling Technology, Danvers, MA), anti-EpoR (Santa Cruz Biotechnology, Santa Cruz, CA), anti-extracellular signal-regulated kinase 1/2 (Sigma-Aldrich, St Louis, MO), and anti-Actin (Sigma-Aldrich). Stat5-EMSAs were performed as described previously.7
Reporter gene assays
Self-renewing primary WT and Jak2−/− erythroblasts were transfected in triplicate with 2.5 μg of luciferase reporter constructs containing the β-casein-promoter (β-casein-luc)30 or the promoter of the IL-2R-α gene (IL-2R-α-luc)31 along with 0.5 μg of pRL-TK2 (Promega, Madison, WI) using the Nucleofector technology (program U-08; Amaxa Biosystems, Gaithersburg, MD). Cells were stimulated with Epo (50 U/mL) for 6 hours after transfection or left untreated. Luciferase activity was measured 12 hours after transfection.
Quantitative PCR
RNA was isolated using TRIzol (Invitrogen). RNA integrity was checked with a Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA (2.5 μg) was reverse-transcribed using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed on an Eppendorf RealPlex cycler using RealMasterMix (Eppendorf North America, New York, NY) and SYBR Green. The sequences of primers used can be found in Document S1.
Results
Persistent Stat5 activation complemented the proliferation defect of EpoR−/− and Jak2−/− erythroid cells
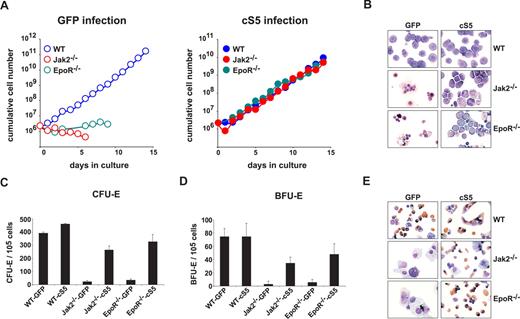
Primary WT erythroblasts cultivated in vitro undergo limited self-renewal in response to SCF, Epo, and dexamethasone (Dex).26 Such expanding progenitors behave like primary erythroid cells from young mice, expressing only adult hemoglobins.32 Fetal liver-derived cells from WT, EpoR−/−, or Jak2−/− E12.5 embryos were tested for outgrowth of immature erythroblasts in the presence of SCF, Epo, and Dex (hence termed “self-renewal conditions”). WT fetal liver erythroblasts proliferated exponentially for 15 days (Figure 1A left), whereas EpoR−/− and Jak2−/− fetal liver cells failed to do so and disintegrated (Figure 1B). To clarify the role of Stat5 in Epo signaling, we used the Stat5a mutant S711F (cS5), which is persistently tyrosine-phosphorylated and exhibits enhanced chromatin binding activity.27 Retroviral transduction of EpoR−/− and Jak2−/− cells with cS5 rescued the severe proliferation defect of mutant cells. All cS5-transduced cell types could be expanded for more than 2 weeks with identical proliferation kinetics (Figure 1A right). WT and cS5-transduced mutant cells showed a typical pro-erythroblast phenotype after 6 days of expansion in cytospins (Figure 1B), whereas uninfected control cells disintegrated. Complementation was confirmed by surface marker analysis after 9 days, which showed coexpression of c-Kit and CD71 together with low levels of Ter119. All cultures contained low numbers of immature progenitors (c-Kit+Sca-1+), whereas T- and B-lymphoid cells were absent (Table S1). Although after 3 days, approximately 65% of immature progenitors were retrovirally infected under the conditions used (data not shown), cS5 did not induce proliferation of multipotent cells, because these would have contributed to both erythroid and other myeloid lineages.
Expression of cS5 rescues proliferation and differentiation of EpoR−/− and Jak2−/− erythroid cells. (A) E12.5 WT, EpoR−/−, and Jak2−/− fetal liver cells infected with retroviruses encoding GFP (left panel) or cS5 (right panel) were cultivated in proliferation medium and cumulative cell numbers calculated after daily determination of growth rates. Data plotted for 1 typical experiment of 4. (B) Cytospins were prepared at day 6 from erythroid cultures shown in (A) and stained for hemoglobin (brownish color) plus histologic dyes. WT, Jak2−/−, and EpoR−/− fetal liver cells expressing cS5 or GFP were subjected to CFU-E- (C) or BFU-E assays (D), and acid benzidine–positive colonies were scored at day 2 (CFU-E) or day 8 (BFU-E), respectively. (E) Cytospins of cells retrieved from the CFU-E assays in (C), stained with hematoxylin/eosin and for hemoglobin (brownish color).
Expression of cS5 rescues proliferation and differentiation of EpoR−/− and Jak2−/− erythroid cells. (A) E12.5 WT, EpoR−/−, and Jak2−/− fetal liver cells infected with retroviruses encoding GFP (left panel) or cS5 (right panel) were cultivated in proliferation medium and cumulative cell numbers calculated after daily determination of growth rates. Data plotted for 1 typical experiment of 4. (B) Cytospins were prepared at day 6 from erythroid cultures shown in (A) and stained for hemoglobin (brownish color) plus histologic dyes. WT, Jak2−/−, and EpoR−/− fetal liver cells expressing cS5 or GFP were subjected to CFU-E- (C) or BFU-E assays (D), and acid benzidine–positive colonies were scored at day 2 (CFU-E) or day 8 (BFU-E), respectively. (E) Cytospins of cells retrieved from the CFU-E assays in (C), stained with hematoxylin/eosin and for hemoglobin (brownish color).
To confirm cS5-dependent erythropoiesis of EpoR−/− and Jak2−/− cells, colony-forming assays for committed erythroid progenitors (BFU-E, CFU-E) were performed. In WT cells, cS5 expression did not alter the numbers of benzidine-positive BFU-E or CFU-E colonies compared with green fluorescent protein (GFP)–vector infected controls (Figure 1C,D). Conversely, the low incidence of CFU-E and BFU-E colonies in EpoR−/− and Jak2−/− cells transduced with a GFP vector control could be raised approximately 10-fold upon expression of cS5, reaching levels comparable with those of WT cultures. Remarkably, Jak2−/−-cS5 and EpoR−/−-cS5 colonies showed the same, typical morphology as WT colonies (Figure S1) and contained mature, enucleated, highly hemoglobinated erythrocytes, similar to WT samples (Figure 1E).
In contrast to cS5, retrovirally transduced WT Stat5a or Bcl-xL failed to induce efficient erythroid colony formation in EpoR−/− and Jak2−/− cells (Figure S2). Thus, activated Stat5 was required and sufficient to overcome the proliferation defect and erythroid colony formation potential of EpoR- and Jak2-deficient cells.
cS5 expression allowed Epo-independent erythropoiesis
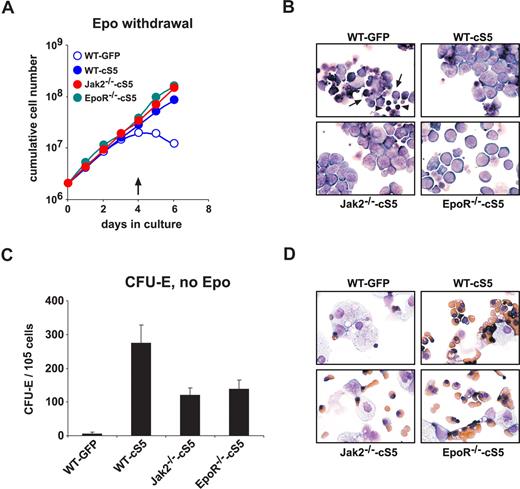
Next we sought to determine whether cS5 activity was sufficient to substitute for Epo signaling in erythropoiesis. WT, EpoR−/−, and Jak2−/− cells expressing cS5 were kept in Epo-free medium for 6 days. cS5-expressing WT, EpoR−/−, or Jak2−/− cells continued to proliferate in the absence of Epo (Figure 2A) without detectable cell disintegration (Figure 2B). WT controls expressing GFP ceased to proliferate after 3 to 4 days (Figure 2A). Dead cells (Figure 2B arrows) and pyknotic nuclei (Figure 2B arrowheads) became visible in cytospins after 4 days without Epo. Withdrawal of SCF rapidly induced terminal differentiation and/or apoptosis in all cell types, indicating that cS5 could not substitute for SCF-signaling (data not shown).
cS5 expression allows erythroid development in the absence of Epo. (A) E12.5 WT, EpoR−/−, and Jak2−/− fetal liver cells were infected with retroviruses encoding GFP or cS5 and cultivated in proliferation medium without Epo for 6 days. Cumulative cell numbers are shown for one representative experiments of 3. (B) Cytospins were prepared from cultures shown in panel A at day 4 ( in A) and stained with hematoxylin/eosin and benzidine.
in A) and stained with hematoxylin/eosin and benzidine.  indicates apoptotic cells; ▶, pyknotic nuclei. (C) WT fetal liver cells expressing cS5 or GFP, or Jak2−/− and EpoR−/− cells expressing cS5, were subjected to CFU-E assays in the absence of Epo and acid benzidine–positive colonies scored at day 2. (D) Cytospins of cells retrieved from the CFU-E assays in (C), stained with hematoxylin/eosin and for hemoglobin (brownish).
indicates apoptotic cells; ▶, pyknotic nuclei. (C) WT fetal liver cells expressing cS5 or GFP, or Jak2−/− and EpoR−/− cells expressing cS5, were subjected to CFU-E assays in the absence of Epo and acid benzidine–positive colonies scored at day 2. (D) Cytospins of cells retrieved from the CFU-E assays in (C), stained with hematoxylin/eosin and for hemoglobin (brownish).
cS5 expression allows erythroid development in the absence of Epo. (A) E12.5 WT, EpoR−/−, and Jak2−/− fetal liver cells were infected with retroviruses encoding GFP or cS5 and cultivated in proliferation medium without Epo for 6 days. Cumulative cell numbers are shown for one representative experiments of 3. (B) Cytospins were prepared from cultures shown in panel A at day 4 ( in A) and stained with hematoxylin/eosin and benzidine.
in A) and stained with hematoxylin/eosin and benzidine.  indicates apoptotic cells; ▶, pyknotic nuclei. (C) WT fetal liver cells expressing cS5 or GFP, or Jak2−/− and EpoR−/− cells expressing cS5, were subjected to CFU-E assays in the absence of Epo and acid benzidine–positive colonies scored at day 2. (D) Cytospins of cells retrieved from the CFU-E assays in (C), stained with hematoxylin/eosin and for hemoglobin (brownish).
indicates apoptotic cells; ▶, pyknotic nuclei. (C) WT fetal liver cells expressing cS5 or GFP, or Jak2−/− and EpoR−/− cells expressing cS5, were subjected to CFU-E assays in the absence of Epo and acid benzidine–positive colonies scored at day 2. (D) Cytospins of cells retrieved from the CFU-E assays in (C), stained with hematoxylin/eosin and for hemoglobin (brownish).
In CFU-E assays, Epo withdrawal prevented colony formation of WT cells. In contrast, cS5-expressing WT, EpoR−/−, and Jak2−/− cells formed erythroid colonies at more than 100-fold higher frequency under the same conditions (Figure 2C). Epo-independent CFU-E formation was more efficient in WT cS5 cells than EpoR−/−-cS5 or Jak2−/−-cS5 cells. Cytospins of cells retrieved from the CFU-E assays revealed the presence of normoblasts and enucleating erythrocytes in cS5 expressing WT, Jak2−/−, and EpoR−/− cells but not in WT GFP cultures (Figure 2D). Apparently, Stat5 activity via cS5 can partially but effectively substitute for Epo signals in WT, EpoR−/−, and Jak2−/− cells during both erythroid progenitor proliferation and terminal differentiation.
cS5 induced Epo-independent Stat5 reporter gene expression but its activity was dependent on tyrosine phosphorylation and DNA-binding
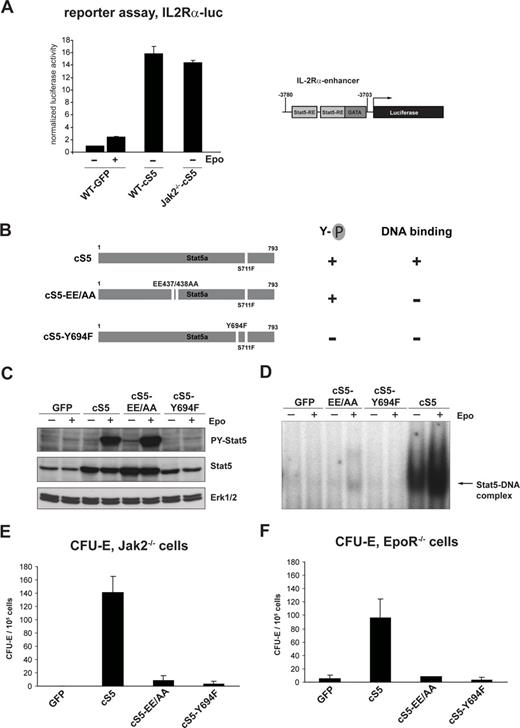
To test whether Epo-independent erythropoiesis in cS5-expressing cells was really due to transcriptional activity of cS5, we performed promoter reporter experiments, using a previously described construct containing a Stat5-responsive part of the IL-2Rα gene enhancer31 (Figure 3A, right). Primary WT GFP, WT cS5, and Jak2−/−-cS5 erythroblasts were transfected with IL-2Rα-luc, and the cells were stimulated with Epo 5 hours before harvest or left untreated. WT GFP cells showed a modest induction of reporter gene expression upon stimulation with Epo (Figure 3A). However, in cS5-expressing WT and Jak2−/− erythroblasts, we observed a much higher induction of the reporter gene expression, even in the absence of Epo stimulation (Figure 3A). Similar results were obtained using a different reporter construct, containing the Stat5-responsive part of the β-casein promoter30 (data not shown). This indicates that cS5 indeed was able to activate gene expression in WT and Jak2−/− erythroid cells.
cS5 allows Epo-independent induction of a Stat5-responsive reporter construct but requires tyrosine phosphorylation and DNA-binding for activity. (A) Self renewing WT GFP and cS5-expressing WT and Jak2−/− erythroblasts were transfected with IL-2R-α-Luc. Before harvesting, cells were stimulated with Epo for 5 hours (+) or left untreated (−). Luciferase expression was measured 12 hours after transfection. Right, schematic representation of the promoter enhancer element from the IL-2R-α gene containing 2 Stat5 response elements (Stat5-RE) fused to the luciferase gene (IL-2R-α-Luc). (B) Schematic representation of the mutants used. 293T cells were transiently transfected with different cS5 constructs, together with a murine EpoR cDNA. Twenty-four hours later, cells were left untreated or treated for 30 minutes with Epo. Extracts were analyzed for tyrosine-phosphorylated Stat5 and total Stat5 by Western blot (C) and DNA-binding of transfected Stat5 constructs by EMSAs on a β-casein-specific promoter sequence (D). Jak2−/− (E) and EpoR−/− (F) fetal liver cells were infected with retroviruses encoding GFP, cS5, cS5-EE/AA, or cS5-Y694F and subjected to CFU-E assays. Acid benzidine–positive colonies were scored at day 2.
cS5 allows Epo-independent induction of a Stat5-responsive reporter construct but requires tyrosine phosphorylation and DNA-binding for activity. (A) Self renewing WT GFP and cS5-expressing WT and Jak2−/− erythroblasts were transfected with IL-2R-α-Luc. Before harvesting, cells were stimulated with Epo for 5 hours (+) or left untreated (−). Luciferase expression was measured 12 hours after transfection. Right, schematic representation of the promoter enhancer element from the IL-2R-α gene containing 2 Stat5 response elements (Stat5-RE) fused to the luciferase gene (IL-2R-α-Luc). (B) Schematic representation of the mutants used. 293T cells were transiently transfected with different cS5 constructs, together with a murine EpoR cDNA. Twenty-four hours later, cells were left untreated or treated for 30 minutes with Epo. Extracts were analyzed for tyrosine-phosphorylated Stat5 and total Stat5 by Western blot (C) and DNA-binding of transfected Stat5 constructs by EMSAs on a β-casein-specific promoter sequence (D). Jak2−/− (E) and EpoR−/− (F) fetal liver cells were infected with retroviruses encoding GFP, cS5, cS5-EE/AA, or cS5-Y694F and subjected to CFU-E assays. Acid benzidine–positive colonies were scored at day 2.
To prove that the cS5-mediated rescue of erythropoiesis in Jak2−/− and EpoR−/− cells was indeed dependent on transcriptional activation of DNA-bound, tyrosine-phosphorylated Stat5 complexes, 2 additional cS5-derived constructs were generated (Figure 3B). In the mutant cS5-EE/AA, 2 glutamic acid residues (Glu437/Glu438) in the DNA-binding domain of cS5 are mutated to alanine, resulting in a tyrosine-phosphorylated cS5 molecule unable to bind to DNA (Figure 3C,D). Conversely, the Y694F mutation in cS5 prevented phosphorylation of the critical tyrosine required for dimerization and DNA binding in transient transfections of 293T cells (Figure 3C,D). Expression of cS5-EE/AA and cS5-Y694F in primary WT fetal liver erythroblasts did not significantly alter CFU-E formation compared with expression of cS5 or GFP (data not shown). In CFU-E assays of Jak2−/− cells expressing GFP, cS5, or the mutants cS5-EE/AA and cS5-Y694F, however, only expression of cS5 resulted in a significant increase in erythroid colony formation (Figure 3E). The same results were obtained using EpoR−/− cells (Figure 3F). Thus, tyrosine-phosphorylation and DNA-binding functions of cS5 are required to allow erythropoiesis of Jak2−/− and EpoR−/− cells.
Induced activation of Stat5 could replace Epo in erythropoiesis
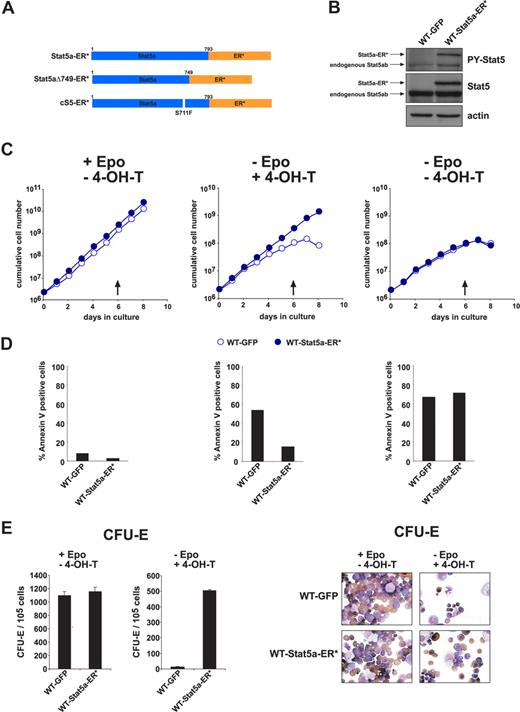
Because cS5 is a leukemogenic protein with enhanced signaling capacity, we generated constructs consisting of Stat5a or cS5 fused to ER*, a point-mutated ligand-binding domain of the estrogen receptor.33 In the respective fusion proteins (Stat5a-ER*, cS5-ER*; Figure 4A), dimerization, nuclear translocation, DNA binding, and transcriptional activity can be triggered by 4-hydroxy-tamoxifen (4-OH-T).28 It is noteworthy that the cS5-dependent hyperactivation of PI3-K signaling34 was not induced by cS5-ER* compared with Stat5a-ER* (Figure S3A). 4-OH-T or Epo induced specific binding of Stat5a-ER* as well as cS5-ER* to a β-casein Stat5 DNA response element, whereas 4-OH-T + Epo strongly enhanced Stat5a-ER* DNA binding (Figure S3B). Retrovirally transduced Stat5a-ER* underwent Epo-induced tyrosine phosphorylation in primary WT fetal liver erythroblasts, as shown by Western blot analysis (Figure 4B). An inducible dominant-negative Stat5-ER* fusion protein (Stat5aΔ749-ER*, Figure 4A7 ) was not tolerated in primary erythroid cells, resulting in strong negative selection against cells expressing this construct (data not shown).
4-Hydroxy-tamoxifen-induced Stat5a-ER* activation replaced Epo in erythropoiesis. (A) Scheme of the 4-OH-T-inducible Stat5-ER* constructs used, encoding fusion proteins of WT Stat5a, cS5, or Stat5aΔ749 with ER* (see “Methods”). (B) Western blot analysis of phosphorylated Stat5 (P-Y-Stat5), and total Stat5 protein in WT fetal liver erythroblasts expressing GFP or Stat5a-ER*. The larger protein recognized by P-Y-Stat5 and Stat5 antibodies corresponds to Stat5a-ER*. Actin, loading control. (C) Cumulative cell numbers (1 representative experiment of 3) of proliferating primary erythroblast cultures expressing Stat5a-ER* or GFP determined in the presence of Epo (+ EPO, −4-OH-T, left, normal self-renewal conditions), the presence of 4-OH-T (5 nM) instead of Epo (−Epo, + 4-OH-T, middle) and without Epo and 4-OH-T (right). (D) Percentage of apoptotic cells of cultures in panel C at day 6 (arrows in panel C) as analyzed by annexin V staining. (E) WT fetal liver cells expressing Stat5a-ER* or GFP were subjected to CFU-E assays in the presence of Epo (left panels) or 4-OH-T (50 nM) instead of Epo (right panels). Acid benzidine–positive colonies were scored at day 2. (F) Cytospins of cells retrieved from the CFU-E assays in panel E and stained with hematoxylin/eosin and for hemoglobin (brownish color).
4-Hydroxy-tamoxifen-induced Stat5a-ER* activation replaced Epo in erythropoiesis. (A) Scheme of the 4-OH-T-inducible Stat5-ER* constructs used, encoding fusion proteins of WT Stat5a, cS5, or Stat5aΔ749 with ER* (see “Methods”). (B) Western blot analysis of phosphorylated Stat5 (P-Y-Stat5), and total Stat5 protein in WT fetal liver erythroblasts expressing GFP or Stat5a-ER*. The larger protein recognized by P-Y-Stat5 and Stat5 antibodies corresponds to Stat5a-ER*. Actin, loading control. (C) Cumulative cell numbers (1 representative experiment of 3) of proliferating primary erythroblast cultures expressing Stat5a-ER* or GFP determined in the presence of Epo (+ EPO, −4-OH-T, left, normal self-renewal conditions), the presence of 4-OH-T (5 nM) instead of Epo (−Epo, + 4-OH-T, middle) and without Epo and 4-OH-T (right). (D) Percentage of apoptotic cells of cultures in panel C at day 6 (arrows in panel C) as analyzed by annexin V staining. (E) WT fetal liver cells expressing Stat5a-ER* or GFP were subjected to CFU-E assays in the presence of Epo (left panels) or 4-OH-T (50 nM) instead of Epo (right panels). Acid benzidine–positive colonies were scored at day 2. (F) Cytospins of cells retrieved from the CFU-E assays in panel E and stained with hematoxylin/eosin and for hemoglobin (brownish color).
Using primary erythroblasts expressing Stat5a-ER* or GFP, we tested whether 4-OH-T–induced activation of WT Stat5 could substitute for Epo in erythroid progenitor expansion. Under self-renewal conditions, both Stat5a-ER*- and GFP-expressing cells proliferated with identical kinetics (Figure 4C left) and equally low rates of apoptosis (annexin V staining, day 6, Figure 4D left). Replacement of Epo with 4-OH-T under the same conditions allowed sustained proliferation of Stat5a-ER* expressing cells, but not of GFP-control cells (Figure 4C middle), consistent with corresponding apoptotic indices (∼10% vs > 50% annexin V–positive cells; Figure 4D middle). In the absence of Epo and 4-OH-T, both cell types gradually ceased to proliferate and underwent cell death (Figure 4C,D right). Cultures of fetal liver cells expressing cS5-ER* instead of Stat5a-ER* behaved similar in these experiments (data not shown).
Induction of terminal erythroid differentiation by 4-OH-T-activated Stat5a-ER* was analyzed in CFU-E assays in the presence of 4-OH-T instead of Epo. Stat5a-ER*– but not GFP-expressing cells formed CFU-E colonies (Figure 4E), which in both cases mainly consisted of mature normoblasts and erythrocytes as seen in cytospins of cells recovered from CFU-E assays and stained for hemoglobin (Figure 4F).
Taken together, 4-OH-T–induced activation of Stat5a-ER* was able to significantly substitute for Epo signaling in erythropoiesis.
A role for Jak2 in c-Kit signaling in hematopoietic progenitors
In addition to EpoR, Jak2 interacts with a variety of other cytokine receptors. Thus, we tested whether cS5 would alleviate Jak2 deficiency also in other lineages. cS5- or GFP-expressing WT or Jak2−/− fetal liver cells were subjected to colony-forming assays in the presence of granulocyte macrophage–colony-stimulating factor (GM-CSF). Jak2−/−-GFP cells yielded 5.2-fold lower colony numbers than WT GFP cells, whereas expression of cS5 increased colony numbers 2.6-fold in Jak2−/− cells (Table 1). Therefore, activation of Stat5 may be sufficient to augment myeloid differentiation in absence of Jak2.
Colony formation ability of fetal liver hematopoietic cells in response to various cytokines and growth factors is dependent on Jak2 and Stat5
| Cytokine . | WT GFP . | WT cS5 . | Jak2−/−-GFP . | Jak2−/−-cS5 . | EpoR−/−-GFP . | EpoR−/−-cS5 . |
|---|---|---|---|---|---|---|
| Epo | 392 ± 8 | 461 ± 5† | 24 ± 8 | 264 ± 29† | 35 ± 9 | 325 ± 57† |
| Epo + IL-3 | 75 ± 12 | 75 ± 20‡ | 3 ± 5 | 35 ± 9† | 5 ± 5 | 48 ± 16† |
| GM-CSF | 293 ± 26 | 432 ± 32† | 56 ± 8 | 144 ± 29† | nd | nd |
| SCF | 304 ± 8 | 352 ± 21* | 109 ± 12 | 173 ± 20† | nd | nd |
| SCF + IL-3 | 448 ± 8 | 528 ± 28† | 139 ± 12 | 219 ± 28† | nd | nd |
| SCF + IL-7 | 360 ± 14 | 408 ± 32‡ | 149 ± 20 | 216 ± 16† | nd | nd |
| None | 0 | 0 ± 1 | 1 | 1 ± 1 | 1 | 0 ± 1 |
| Cytokine . | WT GFP . | WT cS5 . | Jak2−/−-GFP . | Jak2−/−-cS5 . | EpoR−/−-GFP . | EpoR−/−-cS5 . |
|---|---|---|---|---|---|---|
| Epo | 392 ± 8 | 461 ± 5† | 24 ± 8 | 264 ± 29† | 35 ± 9 | 325 ± 57† |
| Epo + IL-3 | 75 ± 12 | 75 ± 20‡ | 3 ± 5 | 35 ± 9† | 5 ± 5 | 48 ± 16† |
| GM-CSF | 293 ± 26 | 432 ± 32† | 56 ± 8 | 144 ± 29† | nd | nd |
| SCF | 304 ± 8 | 352 ± 21* | 109 ± 12 | 173 ± 20† | nd | nd |
| SCF + IL-3 | 448 ± 8 | 528 ± 28† | 139 ± 12 | 219 ± 28† | nd | nd |
| SCF + IL-7 | 360 ± 14 | 408 ± 32‡ | 149 ± 20 | 216 ± 16† | nd | nd |
| None | 0 | 0 ± 1 | 1 | 1 ± 1 | 1 | 0 ± 1 |
SCF-induced colonies represent immature progenitors and mast cells, SCF + IL-3 and SCF + IL-7 induce colony formation of myeloid and lymphoid progenitors (data not shown).
nd indicates not determined.
P < .05.
P < .01.
Not significant.
Immature blood cells of all lineages require c-Kit signaling for proliferation and/or differentiation, mainly visible in cooperation with other hematopoietic cytokines.35-37 We therefore analyzed whether loss of Jak2 would affect colony formation induced by SCF alone or in combination with other cytokines. Jak2-deficient fetal liver cells indeed showed a 2.8-fold reduction in SCF-dependent colony formation compared with WT cells (Figure 5A), in line with previous findings.17,38 Jak2−/−-cS5 cells generated significantly more colonies than Jak2−/−-GFP cells (1.6 fold; Figure 5A). The size of SCF-dependent colonies was strongly reduced in Jak2−/−-GFP cells but massively increased by cS5 in both WT and Jak2 mutant cells (Figure 5B). Colony formation of early myeloid and lymphoid progenitors induced by SCF + IL-3 and SCF + IL-7, respectively, was 3.2- and 2.5-fold reduced in Jak2-deficient cells (Table 1). Again, cS5 expression led to more than 1.6-fold increased colony numbers when expressed in Jak2−/− cells, whereas WT cells showed a similar but weaker increase (Table 1).
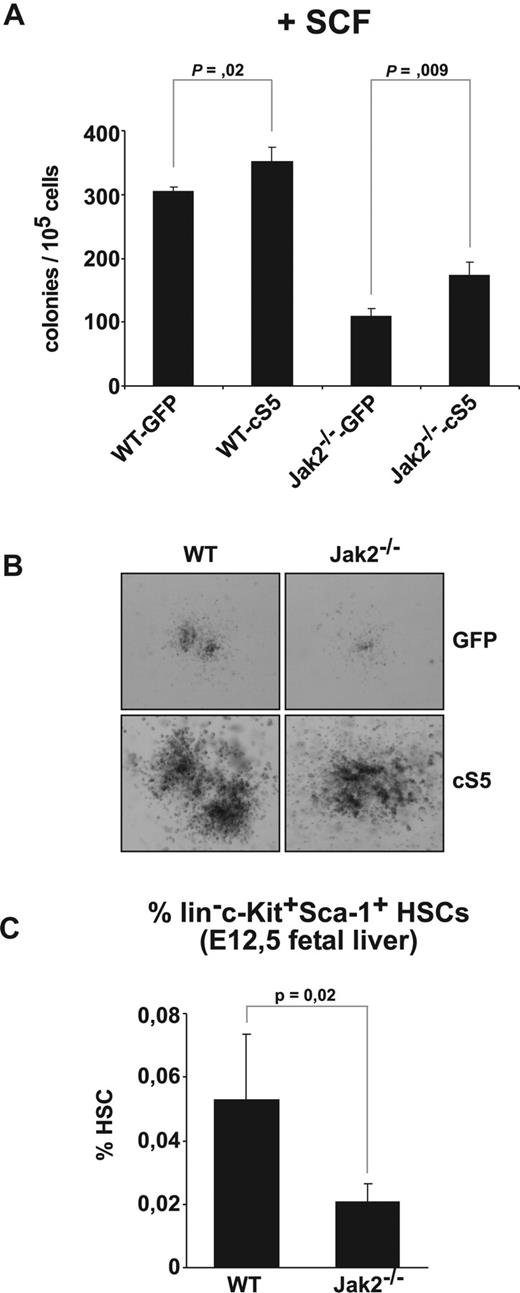
c-Kit signaling depends on Jak2 and is modulated by Stat5. (A) WT and Jak2−/− fetal liver cells transduced with GFP or cS5 were subjected to colony assays supplemented with SCF (50 ng/mL). Colonies were scored at day 8. (B) Photographs of colonies from (A). Representative pictures of 4 for each condition are shown. (C) E12.5 WT and Jak2−/− fetal livers analyzed for HSC content (lin−c-Kit+Sca-1+ cells) by flow cytometry. The percentage of HSCs is depicted (n = 4).
c-Kit signaling depends on Jak2 and is modulated by Stat5. (A) WT and Jak2−/− fetal liver cells transduced with GFP or cS5 were subjected to colony assays supplemented with SCF (50 ng/mL). Colonies were scored at day 8. (B) Photographs of colonies from (A). Representative pictures of 4 for each condition are shown. (C) E12.5 WT and Jak2−/− fetal livers analyzed for HSC content (lin−c-Kit+Sca-1+ cells) by flow cytometry. The percentage of HSCs is depicted (n = 4).
Hematopoietic stem cells (HSCs; lin−c-Kit+ Sca-1+ cells) also depend on SCF for proliferation.39 Because c-Kit function might be linked with Jak2, we tested whether Jak2 deficiency would affect HSC numbers. Indeed, HSC numbers were approximately 70% lower in Jak2−/− fetal livers than in WT fetal livers (n = 4; Figure 5C) as determined by flow cytometry in freshly prepared fetal livers of E12.5 embryos. These observations argue that Jak2 has an essential function in hematopoietic stem cells.
Complementation of Jak2 deficiency in vivo
The finding that Jak2 is not only necessary for erythropoiesis but also important for expansion of c-Kit–responsive immature hematopoietic progenitors and HSCs prompted us to analyze whether Jak2−/− cells expressing cS5 were capable of long-term repopulation of erythromyeloid lineages in vivo. Freshly isolated fetal liver cells from WT and Jak2−/− embryos were transduced with cS5. Equal cell numbers were injected into irradiated recipient mice 72 hours later. To rule out that Jak2−/− fetal livers generated lower numbers of HSCs during the retroviral infection period, we determined the amounts of GFP-positive, long-term (LT) HSCs 72 hours after retroviral infection. Thy1.1low/Flt3− LT-HSCs are the sole cells capable of long-term reconstitution of transplanted mice.40 We found that LT HSCs were efficiently infected by the retrovirus, leading to an equal presence of GFP+ LT HSCs in WT cS5 and Jak2−/−-cS5 cultures (1667 ± 451 GFP+ LT HSC per 106 WT cS5 cells; 1967 ± 351 GFP+ LT HSC per 106 Jak2−/−-cS5 cells; n = 3, data not shown).
Use of congenic mice expressing CD45.1 allowed detection of CD45.2+ donor cells among the host cells.41 Because cS5-expressing fetal liver cells cause leukemia,27 onset of the disease was strongly delayed by transplanting reduced numbers of cS5-transduced fetal liver cells into sublethally rather than lethally irradiated mice. Indeed, all mice in which the transplanted Jak2−/−-cS5 cells were engrafted (n = 6) remained disease-free for more than 6 months and did not develop leukemia.
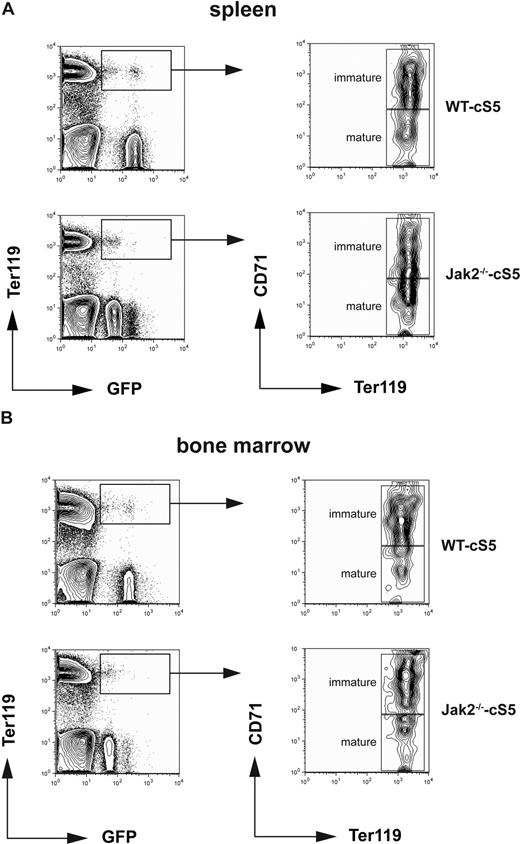
Transplanted mice were regularly monitored for transplant-derived, GFP-CD45.2 double-positive cells in peripheral blood. Animals were killed 6 months after transplantation and assayed for the presence of GFP+Ter119+ erythroid cells in bone marrow and spleen and for GFP+GR-1+ and/or GFP+Mac-1+ myeloid cells in peripheral blood and bone marrow. Significant numbers of transplant-derived, GFP+Ter119+ double-positive cells were observed in spleen and bone marrow of mice receiving WT cS5 cells (9 of 10 animals) as well as Jak2−/−-cS5 (4 of 6 mice, Table 2, Figure 6A,B top and bottom left panels). This indicated that cS5 could partially but efficiently substitute for Jak2 in erythroid differentiation in vivo. To characterize transplant-derived erythroid cells for different stages of maturation, GFP+Ter119+ cells were gated for high versus low CD71 expression, allowing us to distinguish mature (Ter119+CD71low) from more immature erythroid progenitors (Ter119+CD71+). In both spleen and bone marrow, transplanted Jak2−/−-cS5 as well as WT cS5 cells gave rise to multiple stages of erythroid cells in vivo, ranging from CD71+Ter119+ immature, basophilic erythroblasts to almost mature CD71−Ter119+ orthochromatophilic erythroblasts22 (Figure 6A,B right panels).
Hematopoietic repopulation is influenced by Jak2
| Transplant . | Mice engrafted . | Engraftment of GFP+CD45.2+cells in peripheral blood% (range) . | Erythroid(mice withGFP+Ter119+ cells) . | Granulocytic(mice withGFP+GR-1+ cells) . | Macrophage(mice withGFP+Mac-1+ cells) . |
|---|---|---|---|---|---|
| WT-cS5 | 10 (10) | 14.8 (4-40) | 9 (10) | 10 (10) | 5 (10) |
| Jak2−/−-cS5 | 6 (8) | 8.6 (1-23) | 4 (6) | 6 (6) | 2 (6) |
| Transplant . | Mice engrafted . | Engraftment of GFP+CD45.2+cells in peripheral blood% (range) . | Erythroid(mice withGFP+Ter119+ cells) . | Granulocytic(mice withGFP+GR-1+ cells) . | Macrophage(mice withGFP+Mac-1+ cells) . |
|---|---|---|---|---|---|
| WT-cS5 | 10 (10) | 14.8 (4-40) | 9 (10) | 10 (10) | 5 (10) |
| Jak2−/−-cS5 | 6 (8) | 8.6 (1-23) | 4 (6) | 6 (6) | 2 (6) |
GFP+CD45.2+ cells correspond to transplant-derived, cS5-expressing cells. Transplant-derived erythroid cells were scored in spleen and bone marrow, and GFP+ myeloid (GR-1+ and Mac-1+) cells were measured in peripheral blood and bone marrow.
Jak2−/− cells expressing cS5 undergo erythroid differentiation in vivo. Equal numbers of cS5-transduced E12.5 WT and Jak2−/− fetal liver cells were injected into sublethally irradiated mice. Six months after transplantation, spleen (A) and bone marrow (B) of engrafted animals were monitored for GFP-positive erythroid cells. FACS plots for Ter119 and GFP (left) indicate transplant-derived double-positive erythroid cells. Gated cells (boxed) were further analyzed for CD71 and Ter119 (right) to discriminate between immature (CD71highTer119pos) and mature (CD71lowTer119pos) erythroid cells.
Jak2−/− cells expressing cS5 undergo erythroid differentiation in vivo. Equal numbers of cS5-transduced E12.5 WT and Jak2−/− fetal liver cells were injected into sublethally irradiated mice. Six months after transplantation, spleen (A) and bone marrow (B) of engrafted animals were monitored for GFP-positive erythroid cells. FACS plots for Ter119 and GFP (left) indicate transplant-derived double-positive erythroid cells. Gated cells (boxed) were further analyzed for CD71 and Ter119 (right) to discriminate between immature (CD71highTer119pos) and mature (CD71lowTer119pos) erythroid cells.
Likewise, animals successfully engrafted with WT cS5 cells displayed robust contribution of GFP+ cells to mature myeloid lineages (GFP+GR-1+ granulocytes, 10 of 10 mice; GFP+Mac-1+ monocytes/macrophages, 5 of 10 mice; Table 2) in peripheral blood and bone marrow (Figure S4 right panels and data not shown). It is noteworthy that Jak2−/−-cS5 transplanted mice showed a significant contribution to the Mac-1+- (2 of 6 animals with GFP+ cells) and GR-1+-compartments (6 of 6, Table 2; Figure S4 right panels), both in peripheral blood and bone marrow (Table 2 and data not shown). Thus, in GM-CSF-dependent myelopoiesis in vivo, cS5 can substitute for the lack of Jak2 to a significant extent.
It should be noted that overall engraftment was clearly lower in Jak2−/−-cS5 transplanted mice (6 of 8, 8.6% engraftment) than in respective WT cS5 controls (10 of 10, 14.8% engraftment, Table 2). Likewise, contribution to erythroid (4 of 6) and macrophage lineages (2 of 6) was clearly reduced in Jak2−/−-cS5 transplanted animals than in control WT cS5 mice (9 of 10 and 5 of 10, Table 2).
In summary, Jak2−/− cells expressing cS5 contribute to both erythroid and myeloid lineages upon transplantation, albeit with lower efficiency than WT cS5 cells. Furthermore, Jak2−/−-cS5 cells were able to generate mature CD71−Ter119+ erythroid cells. Thus, the severe defects resulting from Jak2 deficiency in erythro- and myelopoiesis can be efficiently but not completely corrected upon expression of cS5 in vivo.
cS5 phosphorylation required Jak2 or c-Kit kinase activity
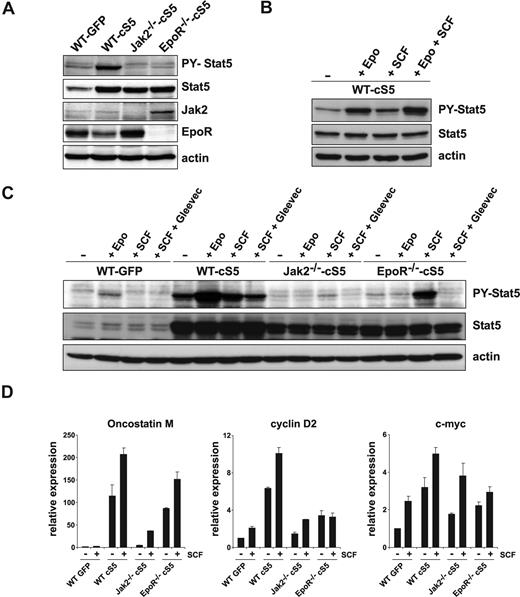
We next tested the tyrosine phosphorylation status of WT Stat5 in self-renewing primary erythroblasts. WT cells expressing GFP exhibited low P-Y-Stat5 levels under self-renewal conditions42 (Figure 7A). As expected, proliferating WT cells expressing cS5 displayed strongly increased P-Y-Stat5 abundance. It is noteworthy that Jak2−/−-cS5 and EpoR−/−-cS5 cells showed only modest P-Y-Stat5 levels, similar to those of WT GFP cells, although cS5 protein was similarly overexpressed in WT, Jak2−/−, and EpoR−/− cells.
Jak kinase(s) and c-Kit cooperate in cS5 activation. (A) Lysates from self-renewing WT GFP, WT cS5, Jak2−/−-cS5, and EpoR−/−-cS5 cultures were analyzed for P-Y-Stat5, total Stat5, Jak2, and EpoR protein levels. Actin, loading control. (B) cS5-expressing WT erythroblasts were starved for 3 hours (−) and subsequently stimulated with Epo, SCF, or Epo + SCF for 10 minutes. Lysates were analyzed for P-Y-Stat5 and total Stat5 protein. Erk1/2, loading control. (C) WT erythroblasts expressing GFP or cS5, and Jak2−/− and EpoR−/− cells expressing cS5 were starved for 3 hours (−) and subsequently stimulated with Epo, SCF, or SCF + imatinib (10 μmol/L) for 10 minutes. Lysates were analyzed for P-Y-Stat5 and total Stat5 protein. Erk1/2, loading control. (D) WT GFP, WT cS5, Jak2−/−-cS5, and EpoR−/−-cS5 cultures were starved for 3 hours (−) and subsequently restimulated with SCF for 2 hours (+). Expression of the Stat5 target genes oncostatinM, cyclin D2, and c-myc was assessed by real-time PCR.
Jak kinase(s) and c-Kit cooperate in cS5 activation. (A) Lysates from self-renewing WT GFP, WT cS5, Jak2−/−-cS5, and EpoR−/−-cS5 cultures were analyzed for P-Y-Stat5, total Stat5, Jak2, and EpoR protein levels. Actin, loading control. (B) cS5-expressing WT erythroblasts were starved for 3 hours (−) and subsequently stimulated with Epo, SCF, or Epo + SCF for 10 minutes. Lysates were analyzed for P-Y-Stat5 and total Stat5 protein. Erk1/2, loading control. (C) WT erythroblasts expressing GFP or cS5, and Jak2−/− and EpoR−/− cells expressing cS5 were starved for 3 hours (−) and subsequently stimulated with Epo, SCF, or SCF + imatinib (10 μmol/L) for 10 minutes. Lysates were analyzed for P-Y-Stat5 and total Stat5 protein. Erk1/2, loading control. (D) WT GFP, WT cS5, Jak2−/−-cS5, and EpoR−/−-cS5 cultures were starved for 3 hours (−) and subsequently restimulated with SCF for 2 hours (+). Expression of the Stat5 target genes oncostatinM, cyclin D2, and c-myc was assessed by real-time PCR.
Thus, although the EpoR-Jak2 axis is the main pathway leading to Stat5 activation, the clearly detectable basal P-Y-Stat5 levels and, more importantly, the resulting strongly Stat5 activation in cells devoid of EpoR or Jak2, must originate from another tyrosine kinase. Because we found a role for Jak2 in c-Kit signaling, we reasoned that c-Kit might be critical for cS5 phosphorylation. cS5-expressing erythroblasts were stimulated with Epo, SCF, or Epo + SCF (see Document S1). Epo induced high P-Y-Stat5 levels, but SCF stimulation was also able to induce significant Stat5 phosphorylation (Figure 7B). Strikingly, the combination of Epo and SCF induced the highest P-Y-Stat5 levels, indicating that both c-Kit and EpoR could contribute to Stat5 activation. To elucidate the molecules involved in SCF-induced Stat5 activation, the previous experiment was repeated with WT GFP, WT cS5, EpoR−/−-cS5, and Jak2−/−-cS5 cells in the presence or absence of the Bcr-Abl inhibitor imatinib, which also inhibits c-Kit kinase activity. Analysis of P-Y-Stat5 levels revealed that Epo but not SCF stimulation induced significant levels of P-Y-Stat5 in WT GFP cells (Figure 7C). In contrast, WT cS5 cells showed high levels of P-Y-Stat5 after starvation, further increased by Epo. Jak2−/−-cS5 cells showed lower expression levels of cS5 protein than WT cS5 or EpoR−/−-cS5 cells. As expected, no significant P-Y-Stat5 was found after starvation or Epo stimulation. SCF stimulation induced significant Stat5-phosphorylation that could be blocked by imatinib treatment. In EpoR−/−-cS5 cells, basal P-Y-Stat5 levels were unexpectedly high (similar to that in Jak2−/−-cS5 cells after SCF stimulation) and did not disappear upon starvation. SCF strongly increased P-Y Stat5 levels, which were reduced back to basal levels by imatinib, whereas, as expected, Epo had no effect. Thus, SCF-activated c-Kit was able to induce strong tyrosine-phosphorylation of cS5 in the absence of EpoR, suggesting that Jak2 contributed to both basal and c-Kit-enhanced cS5 phosphorylation in EpoR−/−-cS5 cells.
To confirm that c-Kit could functionally activate cS5, we determined SCF-dependent gene expression of known Stat5 target genes. Self-renewing WT GFP, WT cS5, EpoR−/−-cS5, and Jak2−/−-cS5 cells were starved for 3 hours and subsequently restimulated with SCF or left untreated. In the absence of SCF, oncostatin M (OSM), cyclin D2, and c-myc mRNAs were already expressed at significantly elevated levels in WT cS5, EpoR−/−-cS5, and Jak2−/−-cS5 cells compared with WT GFP cells. It is noteworthy that all cS5-expressing cell types showed a further up-regulation of Stat5 target genes in response to SCF (Figure 7D).
These data provide direct molecular evidence that the SCF-activated c-Kit tyrosine kinase was able to cause cS5 phosphorylation and target gene transcription, which to a large extent depends on the presence of Jak2.
Discussion
Activated Stat5 modulates diverse cellular processes, including induction of proliferation, suppression of apoptosis, and promotion or inhibition of differentiation, particularly in cells of the hematopoietic lineage. This output can vary extensively, depending on cell type and stages of maturity. Here, we showed that expression of activated Stat5 was sufficient to allow erythropoiesis in vitro and in vivo, both upon ablation of the EpoR or Jak2 or in the absence of Epo-signaling (Figure S5A). Moreover, our data clearly implicate the c-Kit pathway in Jak2/Stat5 activation in immature hematopoietic cells (Figure S5B).
Activated Stat5 was an essential target of Epo signaling in erythroid cells
The role of Stat5 in promoting erythropoiesis is well recognized but remained controversial because of different phenotypes of Stat5-deficient mouse models, either retaining a hypomorphic Stat5 allele21,22,42 or representing a complete knockout.6 Until now, however, it remained unclear whether or not Stat5 would be an essential downstream target of EpoR and Jak2 in erythropoiesis.
To test this possibility, we expressed cS5, a persistently activated Stat5a mutant (S711F,27 ), in primary fetal liver–derived hematopoietic progenitors of WT, Jak2−/−, and EpoR−/− embryos. cS5 allowed proliferation and terminal differentiation of erythroid cells from Jak2- and EpoR-deficient embryos in vitro. Both EpoR−/−-cS5 and Jak2−/−-cS5 cells were able to produce mature CFU-E and BFU-E colonies of normal appearance. The observed effect of cS5 was completely dependent on tyrosine-phosphorylation and DNA-binding ability. cS5 did not require endogenous Stat5 proteins for constitutive activity, because cS5 readily transformed Stat5 mutant cells (Moriggl et al27 and data not shown). In line, only the cS5 protein was persistently tyrosine-phosphorylated in the absence of cytokines in cS5-expressing WT cells, whereas stimulation with IL-3 induced activation of both endogenous WT Stat5 as well as exogenous cS5 proteins.34
Remarkably, cS5 was reported to cause activation of PI3-K signaling via binding to the adaptor protein Gab2.34 PI3-K signaling, in turn, is required for erythroid renewal.43 To rule out such possible indirect effects of cS5 on erythropoiesis, we used a 4-OH-T–inducible WT Stat5a-ER* construct, which has little effect on PI3-K activation. Consistent with a direct role for Stat5 proteins in promoting erythropoiesis, primary erythroblasts expressing WT Stat5a-ER* were able to proliferate and terminally differentiate upon replacement of Epo with 4-OH-T. Thereby it could be excluded that the observed genetic complementation of Jak2−/− and EpoR−/− cells with cS5 was due to effects caused by the particular mutation in cS5. We therefore concluded that tyrosine-phosphorylated, transcriptionally active Stat5 was essential and sufficient to enable terminal erythropoiesis.
The initially reported Stat5a/b double-knockout mice (Stat5ΔN) showed surprisingly mild hematopoietic phenotypes, especially with respect to Epo-signaling.8 These mice still expressed a hypomorphic, N-terminally truncated Stat5 allele.42,44 In contrast, complete ablation of Stat5a/b (Stat5null) resulted in embryonic lethality.6 On a mixed Sv129 × C57Bl/6 genetic background, however, approximately 1% of animals survived up to 6 weeks.45 Thus, even the complete lack of Stat5 proteins resulted in a less severe erythroid phenotype than shown by EpoR−/− or Jak2−/− mice.6 This could be due to additional signaling pathways, activated by Epo-EpoR-Jak2 besides Stat5 activation. Alternatively, other Stat protein family members might compensate for the absence of Stat5.46 In Stat5null but not WT erythroblasts, we indeed observed strong Stat1- and Stat3 tyrosine phosphorylation and DNA-binding (F.G. and M.A.K., unpublished observations). A final proof for redundancy among Stat family members will have to await additional experiments, including erythroid-specific Stat1/3/5 knockout mouse models.
One important Stat5 target in erythroid cells is the antiapoptotic protein Bcl-xL.21,22 Bcl-xL–deficient mice display severe erythroid defects,12 whereas Bcl-xL overexpression in erythroid cells allows Epo-independent maturation.47,48 It is likely, however, that there are additional functions of the EpoR/Jak2/Stat5 axis in erythropoiesis. Recently, roles for Epo regulation of cell cycle progression49 and cell adhesion50 were indeed proposed. In line with these findings, cS5 but not Bcl-xL rescued renewal and alleviated Epo-dependence of primary erythroid cells of WT as well as EpoR−/− or Jak2−/− fetal livers. Likewise, expression of another hyperactive Stat5 variant was sufficient to allow Epo-independent colony formation of human erythroid cells,48 and human CD34+ cells expressing a constitutive active Stat5 mutant showed enhanced self-renewal with increased erythroid commitment of transduced cells.51 Besides their requirement for Epo-signaling in erythropoiesis, Jak2 and Stat5 are similarly essential in other hematopoietic lineages. Constitutive activation of Stat5 was recently shown to abrogate cytokine dependence for proliferation of several hematopoietic lineages.34,52 Here, we show that GM-CSF–dependent myelopoiesis decreased more than 5-fold in Jak2-deficient versus WT fetal liver cells in vitro, whereas cS5 expression in Jak2−/− fetal liver cells robustly increased GM-CSF–dependent colony formation. This suggests that P-Y-Stat5 is necessary to allow functional myeloid differentiation in absence of Jak2. Together with the ability of activated Stat5 to promote renewal and differentiation of erythroid cells in the absence of Epo/EpoR/Jak2 signaling, this suggests a considerable amount of conservation among distinct cytokine receptor/Jak/Stat5 signaling pathways.
Jak2 was required for efficient c-Kit signaling during hematopoietic development
Hematopoietic progenitors and HSCs depend on SCF/c-Kit signaling for proliferation. In addition, erythropoiesis depends on both Epo/EpoR/Jak2- and SCF/c-Kit signals. This raised the question whether or not Jak2 participated in c-Kit signaling. Direct activation of Jak kinases by the tyrosine kinase activity of c-Kit remains controversial,53,54 although a direct connection between EpoR and c-Kit signaling modules was demonstrated.55-57 A role for Jak2 in SCF-dependent colony formation and differentiation was also described in mast cells.17,38 We consistently observed that Jak2-deficient fetal liver cells were impaired for SCF-dependent colony formation in several immature hematopoietic lineages. Jak2−/− cells not only failed to efficiently respond to SCF alone (favoring immature hematopoietic and mast cells) but were also less responsive to SCF + IL-3 and SCF + IL-7, promoting myeloid and lymphoid progenitor development, respectively. Again, cS5 expression partially reversed these defects in cytokine-dependent colony formation of Jak2−/− cells. These data suggest that c-Kit–dependent Stat5 activation via Jak2 mainly occurs in immature, primary hematopoietic cells (Figure S5B). SCF alone induced robust Stat5 phosphorylation in WT cS5 cells, which was further enhanced by the combination of Epo and SCF. Furthermore, Stat5 activation was also induced upon SCF stimulation in EpoR−/−-cS5 and Jak2−/−-cS5 cells. SCF-dependent activation of cS5 was completely dependent on the kinase activity of c-Kit, because it was blocked by the c-Kit inhibitor imatinib. The contribution of (other) Jak kinases in this process is likely because EpoR−/−-cS5- and Jak2−/−-cS5 cells were clearly more susceptible to a pan-Jak inhibitor than WT GFP and WT cS5 cells (data not shown). Alternatively, the c-Kit-dependent cS5 activation could occur in a complex “EpoR-c-Kit-signalosome,” harboring different scaffold proteins and signal transducers.
Together, these interpretations are consistent with a model in which EpoR and c-Kit cooperate to activate the Jak-Stat pathway (Figure S5B), perhaps by interaction of the respective signaling modules upon receptor activation.55,56,58 Such interactions could reflect the in vivo situation more closely than anticipated.
The role of Jak2 in c-Kit signaling might also have mechanistic implications for the biology of the Jak2V617F mutation in patients with polycythemia vera.59-62 Because cells from respective patients respond more strongly to SCF,63 it is conceivable that Jak2V617F is more susceptible to SCF signaling and thus also to Stat5 activation.
cS5 compensated for loss of Jak2 in mouse hematopoiesis in vivo
It is noteworthy that Jak2−/−-cS5 cells gave rise to mature erythroid and myeloid cells in vivo upon transplantation of freshly transduced fetal liver cells in mice. To avoid development of the aggressive leukemia induced by cS5,27 we used competitive repopulation conditions (ie, low amounts of fetal liver cells injected into sublethally irradiated mice). Under those conditions, all mice stayed healthy for longer than 6 months. Transplantation of higher cell numbers into lethally irradiated mice failed, because Jak2−/−-cS5 cells did not provide radioprotection, whereas transplanted WT cS5 cells induced the expected leukemia.
It is noteworthy that Jak2−/−-cS5 cells differentiated into mature CD71−Ter119+ erythroid cells in vivo. This is of particular interest because erythroid cells of this stage were undetectable in fetal livers of Jak2−/− embryos. Attempts of transplanting Jak2−/−-GFP cells failed because of the inability of those mutant cells to proliferate in vitro. Thus, we never obtained sufficient numbers of viable cells for transplantation experiments after the retroviral infection period (data not shown).
Despite the observed functional erythropoiesis of Jak2−/−-cS5 cells in vivo, we observed a profound long-term repopulation defect of the Jak2 mutant cells. In line with this, transplantation of equal numbers of GFP+Jak2−/−-cS5 versus WT cS5 LT HSCs always resulted in a lower degree of chimerism in animals receiving Jak2−/−-cS5 cells. Although all animals infused with WT cS5 cells maintained abundant GFP+ cells in their peripheral blood, 75% of mice receiving Jak2−/−-cS5 cells were successfully engrafted, yet showed lower degrees of chimerism; some even lacked erythroid and/or macrophage-like cells.
Here we demonstrated that activation of Stat5 allowed in vitro and in vivo erythropoiesis and myelopoiesis in the absence of EpoR or Jak2. Note that the receptor tyrosine kinase c-Kit exhibited a significant degree of cross-talk with the Jak2-Stat5 axis to promote hematopoiesis (Figure S5B). Our data suggest that contributions from cytokine and growth factor signaling pathways converge on Jak2-Stat5 activation. This may represent a principle shared by different hematopoietic lineages or even multipotent progenitors and HSCs, to ensure efficient hematopoiesis as well as its strict regulation under different physiologic or pathologic conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank G. Stengl for FACS and M. von Lindern for critical reading of the manuscript.
This work was supported by grants WK-001 (F.G. and M.A.K.) and SFB F028 (M.M,. H.B., R.M., E.W.M.) from the Austrian Research Foundation (Fonds zur Förderung der wissenschaftlichen Forschung); the Herzfelder Family Foundation (E.W.M.); and BM_WFa GZ200.112/1-VI/1/2004 (M.M.) from the Austrian Federal Ministry for Science and Research.
Authorship
Contribution: F.G., R.M., and H.B. designed research. F.G., M.A.K., B.K., R.M., and H.D. performed research and collected and analyzed data. T.K., V.B., U.K., K.P., and M.M. provided mouse lines and fetal liver cells. F.G. wrote the manuscript with the help of E.W.M., H.B., and R.M.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Ernst W. Müllner, Max F. Perutz Laboratories, Medical University of Vienna, Dr Bohr-Gasse 9, Vienna 1030, Austria; e-mail: ernst.muellner@univie.ac.at.
References
Author notes
*E.W.M. and R.M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal