Abstract
Dyskeratosis congenita (DC) is an inherited bone marrow (BM) failure syndrome associated with mutations in telomerase genes and the acquisition of shortened telomeres in blood cells. To investigate the basis of the compromised hematopoiesis seen in DC, we analyzed cells from granulocyte colony-stimulating factor mobilized peripheral blood (mPB) collections from 5 members of a family with autosomal dominant DC with a hTERC mutation. Premobilization BM samples were hypocellular, and percentages of CD34+ cells in marrow and mPB collections were significantly below values for age-matched controls in 4 DC subjects. Directly clonogenic cells, although present at normal frequencies within the CD34+ subset, were therefore absolutely decreased. In contrast, even the frequency of long-term culture-initiating cells within the CD34+ DC mPB cells was decreased, and the telomere lengths of these cells were also markedly reduced. Nevertheless, the different lineages of mature cells were produced in normal numbers in vitro. These results suggest that marrow failure in DC is caused by a reduction in the ability of hematopoietic stem cells to sustain their numbers due to telomere impairment rather than a qualitative defect in their commitment to specific lineages or in the ability of their lineage-restricted progeny to execute normal differentiation programs.
Introduction
Dyskeratosis congenita (DC) is an inherited multisystem disorder of premature aging characterized by early bone marrow (BM) failure.1,2 The 2 major forms of this disease, X-linked and autosomal dominant (AD), are associated with mutations in specific genes that are components of the telomerase complex. In the X-linked form, DKC1 gene mutations have been found that affect dyskerin expression, a small nucleolar ribonucleoprotein involved in ribosomal RNA processing that is also involved in the processing and stabilization of the RNA component of telomerase, hTERC.3,4 In AD DC, several different mutations have been identified in hTERC.5,6 Relevant to the current study, a large multigenerational AD DC family was identified in Iowa whose affected members have a deletion of the last 74 base pairs of the 3′ end of one hTERC allele.6 Recently, mutations in the reverse-transcriptase component of telomerase hTERT have also been found in at least one AD DC family.7 An autosomal recessive form of this disorder also exists, with mutations of NOP-10 being identified.8 Of note, homozygous hTERT mutations have also been found in 2 distinct consanguineous families, further accounting for other incompletely defined cases of autosomal recessive DC.9 Somatic cells from DC patients have markedly shortened telomeres, which is presumed to be a consequence of their telomerase deficiency.1,4,10,11 The clinical triad of DC consists of abnormal skin pigmentation, nail dystrophy, and mucosal leukoplakia.12 Morbidity is often associated with the development of cytopenia, and most X-linked DC patients do not survive past 30 years of age due to complications of marrow aplasia.13 It has been suggested that X-linked disease has a more severe phenotype than the AD form.14 However, the primary determinant of disease onset and severity does not appear to be telomerase levels per se but telomere length at birth, and ongoing observations of the multigeneration family with AD DC from Iowa support a model of disease anticipation15 in which later-generation subjects with hTERC mutations manifest clinical symptoms earlier than their parents.
Telomere length and telomerase play an essential, although not fully understood, role in normal hematopoiesis, and telomere length is clearly involved in the pathogenesis of DC.16-19 Previous studies on the Iowa AD DC family showed that haploinsufficiency of hTERC leads to telomere shortening of hematopoietic-derived cells compared with age-matched controls.20 While some subjects had peripheral cytopenias, nearly all had immune abnormalities, including marked B lymphopenia, decreased immunoglobulin M (IgM) levels, and overexpression of senescent markers on T cells.21 Of note, short telomeres were also observed in noncarrier siblings of those with hTERC mutations, although none had clinical or laboratory evidence of DC. This observation suggests that only below a certain threshold does telomere length affect the function of hematopoietic-derived cells. Shortened telomeres have been reported in other BM failure disorders, including Shwachman-Diamond syndrome (SDS)22 and aplastic anemia,23 while mutations and polymorphisms within genes encoding telomerase components have been noted in “idiopathic aplastic anemia.”5,24,25 However, it remains unknown whether the degree of cytopenia or onset of BM aplasia correlates with telomere length in hematopoietic cells or may be a function of defects in the microenvironment.26 It is also unclear whether the defects observed in BM function in DC are derivative of a compromised hematopoietic stem cell (HSC) population, and if so, whether this is primarily due to qualitative or quantitative perturbations of this compartment. These questions are of major significance to understanding the hematopoietic defect in DC and will also be important for developing future strategies to treat DC patients effectively. To this end, we undertook studies to phenotypically and functionally characterize the circulating CD34+ cells that were mobilized by granulocyte colony-stimulating factor (G-CSF) administration to DC patients.
Methods
Subjects and controls
All DC subjects enrolled on these studies signed informed consents that were approved by the University of Iowa Children's Hospital Institutional Review Board and in accordance with the Declaration of Helsinki. The diagnosis of AD DC was confirmed by mutational analysis, demonstrating a 74–base pair deletion in a single hTERC allele, as previously described.6 Eligibility requirements at the time of mobilized peripheral blood (mPB) collections allowed for the presence of mild cytopenias in DC subjects. Minimal values were as follows: hemoglobin level higher than 10 g/dL absolute neutrophil count (ANC) higher than 1000/mm3, and a platelet count higher than 50 000/mm3. In addition, precollection BM aspirations were analyzed to ensure subjects had no evidence of leukemic transformation or myelodysplasia. Control cells, mPB, and BM were obtained from clinically discarded samples at the University of Iowa Hematopoietic Stem Cell Processing Laboratory that had been isolated from healthy donors and were no longer required.
BM analysis
BM aspirates were collected and the CD34+ cell content was measured according to the International Society of Hematotherapy and Graft Engineering guidelines.27 Hematoxylin and eosin staining was used to assess morphology and cellularity. BM aspirates were also sent for standard cytogenetics, fluorescent in situ hybridization (FISH) analyses, and diepoxybutane (DEB)–induced chromosome breakage studies.28,29
Collection of mPB cells
Prior to collecting the mPB cells by leukapheresis, all subjects received 10 μg/kg G-CSF (Amgen, Thousand Oaks, CA) subcutaneously daily for 5 days, with the last 2 injections occurring in the morning just prior to leukapheresis. DC subjects were required to weigh at least 40 kg such that peripheral intravenous catheters were of sufficient gauge to allow apheresis collections to be undertaken. The collection protocol aimed to collect a minimum of 2 × 106 CD34+ cells/kg. Complete blood counts (CBCs) with differential and CD34+ analyses from peripheral blood (PB) were obtained prior to harvesting, but the collection was allowed to proceed independent of the pre-apheresis CD34+ counts. Peripheral intravenous catheters were placed bilaterally into the antecubital veins, and 5 blood volume collections (3.5-6 liters) were performed over 3 to 4 hours in the University of Iowa Hospitals and Clinics DeGowin Blood Center on 2 consecutive days using a Cobe Spectra instrument (Gambro BCT, Lakewood, CO). Total nucleated cell counts, mononuclear cell counts, and CD34+ cell counts were obtained on each apheresis collection. Each collection was cryopreserved using a rate controlled freezer (Custom Biogenics Systems, Shelby Township, MI). Ten percent of each collection was separately cryopreserved to allow laboratory studies to be performed.
Immunophenotyping
Immunophenotyping of CD34+ cells from leukapheresis collections was performed on fresh and frozen/thawed products from DC subjects and a range of age-matched controls. Briefly, mononuclear cells were stained with a combination of fluorescein- or phycoerythrin-conjugated antibodies to CD34, CD33, CD38, CD19, CD7, CD71, and CD41 (Becton Dickinson, San Jose, CA) as previously described.30 Cells were analyzed on a FACSscan flow cytometer (Becton Dickinson) using the International Society of Hematotherapy and Graft Engineering gating strategies,27 and data analysis used Cellquest V3.2 software (Becton Dickinson).
In vitro assays
Populations enriched in CD34+ cells were isolated immunomagnetically (Easy Sep; StemCell Technologies, Vancouver BC) from thawed DC and control cells according to the manufacturer's instructions (purity of DC samples = 2%-38%; purity of normal controls 5%-82%). To determine the frequency of colony-forming cells (CFCs) within the CD34+ compartment of these samples, aliquots were then plated in standard MethoCult H4434 (StemCell Technologies) that contains interleukin-3 (IL-3), IL-6, steel factor (SF), and erythropoietin (EPO). Erythroid colonies (from erythroid burst-forming units [BFU-Es]), granulopoietic colonies (from granulocyte-macrophage colony-forming units [CFU-GMs]), and multilineage colonies (from granulocyte, erythrocyte, monocyte, macrophage colony-forming units [CFU-GEMMs]) were scored by direct visualization using an inverted microscope (40×) 14 days later using standard criteria. These values were then added together to obtain total CFC counts and expressed per 103 actual CD34+ cells plated. The average number and types of mature cells in the colonies generated in these assays was determined by harvesting the cells from diluted 14-day-old cultures and then performing cell counts and fluorescence-activated cell sorting (FACS) analyses (using anti–glycophorin A, anti-CD15/66b, anti-CD33, and anti-CD41a antibodies). Long-term culture-initiating cell (LTC-IC) frequencies were determined as previously described.31 In brief, a defined number of CD34+ test cells were cocultured with irradiated mouse fibroblasts engineered to make human IL-3, G-CSF, and SF for 6 weeks at 37°C, and then the total number of CFCs present was determined on the harvested cells. LTC-IC numbers were then calculated assuming each LTC-IC will have produced an average of 25 CFCs detectable at that time. Photomicrographs of colonies used a Canon EOS digital Rebel XT camera (Tokyo, Japan) mounted on a Zeiss microscope model 475638 (Carl Zeiss, Heidelberg, Germany), with magnification of objective lens 2.5/.08 and eyepiece magnification of 12.5×/20W.
Telomere length measurements: flow-FISH, Q-FISH, and STELA
Telomere length measurements using flow-fluorescence in situ hybridization (flow-FISH) was performed as described.32 Briefly, white blood cells (WBCs) were isolated by osmotic lysis of erythrocytes in whole blood samples using NH4Cl. The WBCs were then mixed with bovine thymocytes of known telomere length (internal control), denatured in formamide at 87°C, and hybridized with a fluorescein-conjugated (CCCTAA)3 peptide nucleic acid (PNA) probe specific for telomere repeats and counterstained with LDS751 DNA dye. The fluorescence intensity in WBCs, granulocytes, and total lymphocytes relative to internal control cells was measured on a FACSCalibur instrument (Becton Dickinson) to calculate telomere length.
Quantitative fluorescence in situ hybridization (Q-FISH) with Cy-3–labeled (CCCTAA)3 peptide nucleic acid (PNA) and analysis of telomere length from digital images were performed as described.33 Briefly, Cy-3–labeled PNA probe (CCCTAA)3 specific for mammalian telomeres (Applied Biosystems, Foster City, CA) was hybridized to metaphase chromosomes obtained from 4-day-old suspension cultures initiated with CD34+ mPB cells cultured in StemSpan (StemCell Technologies) containing IL-3, IL-6, SF, and EPO. To remove nonhybridized PNA, slides were washed twice for 15 minutes each time with wash solution I (70% formamide, 0.1% BSA, and 10 mM Tris) and then 3 times for 5 minutes each time with wash solution II (0.1 M Tris, 0.15 M NaCl, 0.08% Tween 20, pH = 7.0-7.5). Slides were counterstained with the DNA dye DAPI and examined using a Zeiss Axioplan II fluorescence microscope (Carl Zeiss). For each DC sample, 15 individual metaphase nuclei were analyzed, and the mean fluorescence intensity (MFI) was correlated to telomere length as measured relative to plasmid standards with fixed numbers of telomere repeats.33 The fluorescent signal was then quantified and analyzed with software for Q-FISH image analysis (Flintbox, http://www.flintbox.com).
Single telomere analysis (STELA) was conducted as described previously.34 Briefly, enriched CD34+ mPB cells were added directly into tubes containing 1 × SSC and 1 × 105 SF9 insect cells. Since only a small number of enriched CD34+ cells were tested, inclusion of SF9 cells acted as both a cellular and genomic carrier. DNA was extracted from enriched populations by standard proteinase K, RNase A, and phenol/chloroform protocols. DNA was ligated at 35°C for 12 hours with 0.001 μM telorette linker 806 that anneals to the telomeric 3′ G-rich overhang and ligates directly to the 5′ end of the telomere. DNA was diluted to 50 genome equivalents per microliter, based on cell numbers obtained from sorting and added to a polymerase chain reaction (PCR) reaction containing oligonucleotides 800 and 810, specific for the XpYp subtelomeric region and a sequence on the linker 806, respectively. A total of 24 rounds of amplification were undertaken, and samples were resolved on 0.7% LE agarose, Southern blotted, and hybridized to a probe specific to the XpYp subtelomeric region (generated by PCR using primers 800 and 801).
Results
Clinical characteristics and BM findings on DC subjects
Initial clinical data on subjects identified as having AD DC were previously published, and laboratory studies on these subjects have also been recently reported.6,21 Over the past 6 years, subjects have been followed on a yearly basis, and CBCs obtained over that period have shown a slight decline in platelet counts in several individuals, specifically those in the third generation (UPNs 7 and 9). Due to the possibility of progressive BM failure, subjects were offered the opportunity to participate in a study designed to collect and store mPB cells for potential later use as autologous transplants. Five subjects, comprising 2 third-generation subjects (ages 11 and 22 years) and 3 second-generation subjects (ages 36-50 years) were enrolled. All had clinical and laboratory features of DC, including erythrocyte macrocytosis and mild cytopenia (Table 1).
Clinical characteristics of DC subjects
| Subject . | UPN 2 . | UPN 5 . | UPN 6 . | UPN 7 . | UPN 9 . | Normal range . |
|---|---|---|---|---|---|---|
| Age/generation/sex | 52/II/M | 40/II/M | 33/II/M | 21/III/F | 11/III/M | N/A |
| Weight, kg | 86 | 89 | 70 | 57 | 45 | N/A |
| Clinical findings | S,L,N | S,L,N | S,L,N | N | N | N/A |
| Hemoglobin, g/dL | 12.9 | 14.4 | 13.7 | 13.4 | 12.9 | 12.7-17.0 |
| MCV, fL | 93 | 95 | 104 | 102 | 92 | 77-90 |
| Platelet count, ×103/mm3 | 264 | 175 | 105 | 49 | 138 | 150-400 |
| WBC count, ×103/mm3 | 4.6 | 3.3 | 3.4 | 3.4 | 4.7 | 3.7-10.5 |
| ANC, ×103/mm3 | 1.426 | 1.400 | 1.360 | 1.894 | 2.350 | 1.550-6.100 |
| Subject . | UPN 2 . | UPN 5 . | UPN 6 . | UPN 7 . | UPN 9 . | Normal range . |
|---|---|---|---|---|---|---|
| Age/generation/sex | 52/II/M | 40/II/M | 33/II/M | 21/III/F | 11/III/M | N/A |
| Weight, kg | 86 | 89 | 70 | 57 | 45 | N/A |
| Clinical findings | S,L,N | S,L,N | S,L,N | N | N | N/A |
| Hemoglobin, g/dL | 12.9 | 14.4 | 13.7 | 13.4 | 12.9 | 12.7-17.0 |
| MCV, fL | 93 | 95 | 104 | 102 | 92 | 77-90 |
| Platelet count, ×103/mm3 | 264 | 175 | 105 | 49 | 138 | 150-400 |
| WBC count, ×103/mm3 | 4.6 | 3.3 | 3.4 | 3.4 | 4.7 | 3.7-10.5 |
| ANC, ×103/mm3 | 1.426 | 1.400 | 1.360 | 1.894 | 2.350 | 1.550-6.100 |
MCV indicates mean corpuscular volume; WBC, white blood cell; ANC, absolute neutrophil count; S, skin dyspigmentation; L, leukoplakia; and N, nail dystrophy.
Bone marrow aspirations were performed prior to apheresis collections and showed an overall decrease in BM cellularity in all DC subjects. This was most striking in the youngest third-generation subjects (UPNs 7 and 9, Table 2). However, there was no evidence of dyspoiesis, myelodysplasia, or leukemia, and the myeloid-erythroid ratios were within the normal range, as were the percentages of hematopoietic intermediates, although the percentages of lymphocytes in the BM smears were increased in 3 of the 5 cases. Of note, decreased numbers of megakaryocytes were observed in all BM except that from UPN 5. Strikingly, the percentage of CD34+ cells in the BM aspirates was lowest in the 2 third-generation subjects (0.05% and 0.16% compared with 0.5%-1% in the healthy controls). Cytogenetic and FISH analyses of the BM cells showed no gross abnormalities, although the possibility of unresolved mutations would not be precluded by these analyses. No increase in chromatin breakage was found in DEB-supplemented cultures of DC lymphocytes suggesting no gross defects in DNA repair (data not shown).
BM findings in DC subjects
| UPN . | CD34, % . | Cellularity . | Marrow differential, % . | M/E ratio . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ep . | pm . | my . | mmy . | band . | segs . | baso . | lymph . | pro-lymph . | mono . | pro-mono . | plasma cells . | blasts . | eos . | ||||
| 2 | 0.57 | 5-15* | 27 | 9 | 12 | 9 | 12 | 14 | 1 | 12 | 0 | 0 | 0 | 1 | 1 | 2 | 2:1 |
| 5 | 0.24 | 5-30* | 20 | 4 | 6 | 9 | 12 | 15 | 0 | 24 | 0 | 4 | 0 | 2 | 0 | 4 | 1.7:1 |
| 6 | 0.40 | 20-30* | 36 | 2 | 5 | 8 | 12 | 19 | 0 | 14 | 0 | 0 | 0 | 2 | 0 | 2 | 2.2:1 |
| 7 | 0.05 | 5-15* | 26 | 0 | 7 | 4 | 13 | 16 | 0 | 25 | 0 | 4 | 0 | 7 | 1 | 1 | 2.4:1 |
| 9 | 0.16 | 10-30* | 21 | 1 | 2 | 8 | 15 | 19 | 0 | 24 | 0 | 5 | 0 | 2 | 1 | 2 | 2.8:1 |
| ctrl | 0.5-1 | 50-80 | 18-32 | 2-6 | 3-7 | 5-9 | 10-16 | 18-28 | 0-1 | 9-19 | 0-3 | 1-5 | 0-3 | 0-1 | 0-2 | 1-5 | 1.5-3:1 |
| UPN . | CD34, % . | Cellularity . | Marrow differential, % . | M/E ratio . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ep . | pm . | my . | mmy . | band . | segs . | baso . | lymph . | pro-lymph . | mono . | pro-mono . | plasma cells . | blasts . | eos . | ||||
| 2 | 0.57 | 5-15* | 27 | 9 | 12 | 9 | 12 | 14 | 1 | 12 | 0 | 0 | 0 | 1 | 1 | 2 | 2:1 |
| 5 | 0.24 | 5-30* | 20 | 4 | 6 | 9 | 12 | 15 | 0 | 24 | 0 | 4 | 0 | 2 | 0 | 4 | 1.7:1 |
| 6 | 0.40 | 20-30* | 36 | 2 | 5 | 8 | 12 | 19 | 0 | 14 | 0 | 0 | 0 | 2 | 0 | 2 | 2.2:1 |
| 7 | 0.05 | 5-15* | 26 | 0 | 7 | 4 | 13 | 16 | 0 | 25 | 0 | 4 | 0 | 7 | 1 | 1 | 2.4:1 |
| 9 | 0.16 | 10-30* | 21 | 1 | 2 | 8 | 15 | 19 | 0 | 24 | 0 | 5 | 0 | 2 | 1 | 2 | 2.8:1 |
| ctrl | 0.5-1 | 50-80 | 18-32 | 2-6 | 3-7 | 5-9 | 10-16 | 18-28 | 0-1 | 9-19 | 0-3 | 1-5 | 0-3 | 0-1 | 0-2 | 1-5 | 1.5-3:1 |
ctrl indicates control; ep, erythrocyte precursor; pm, promyelocytes; my, myelocytes; mmy, metamyelocytes; segs, segmented neutrophils; baso, basophils; mono, monocytes; pro-mono, promonocytes; and eos, eosinophils.
Markedly hypocellular relative to age-matched controls.
mPB collections
All subjects tolerated 5 daily subcutaneous injections of G-CSF without significant toxicity and, while mild discomfort was reported, none required analgesia for myalgias or bone pain. HSC collections proceeded without incident and, per routine, oral calcium supplements were administered throughout to prevent hypocalcemia. Hemograms were obtained prior to initial collections and, as noted in Table 3, the post–G-CSF/pre-apheresis ANC and total WBC count increased only moderately in all DC subjects except UPN 2, where a more typical increase was observed (WBC count = 38 900/mm3). Following 2 days of mPB cell collections, hemoglobin and WBC counts remained fairly close to preapheresis values. In contrast, platelet counts were markedly decreased from baseline values. Of note, no subject required transfusion support nor had any bleeding complications. When subjects were seen for routine follow up 6 months later, platelet counts had returned to preapheresis levels (data not shown). Although the target was to collect 1 to 2 × 108 CD34+ cells, based partially upon subject weight and the number of cells routinely used in transplantation protocols, this number was met for only 2 subjects (UPNs 2 and 5). Only 3.4 × 107 CD34+ cells (∼ 0.5 × 106 CD34+ cells/kg) were collected from the other second-generation subject, UPN 6. This subject had experienced significant thrombocytopenia over the previous 10 years and had a more pronounced phenotype than the other second-generation subjects. Yet the lowest yield was in the third-generation subject, UPN 7, from whom only 3.2 × 107 CD34+ cells were collected. Of note, this subject also had the lowest percentage of CD34+ cells in both the mPB product and in the BM. She also had a long-standing history of thrombocytopenia. The youngest subject, UPN 9, had a several year history of mild thrombocytopenia, and the collection yielded a total of 9.7 × 107 CD34+ cells.
mPB collections from DC subjects
| Name . | UPN 2 . | UPN 5 . | UPN 6 . | UPN 7 . | UPN 9 . |
|---|---|---|---|---|---|
| Age, y | 52 | 40 | 33 | 21 | 11 |
| Precollection CBC* | |||||
| Hgb level, g/dL | 12.8 | 13.8 | 13.2 | 12.7 | 12.1 |
| Platelet count, ×103/mm3 | 213 | 177 | 90 | 39 | 107 |
| WBC count, ×103/mm3 | 38.9 | 12.1 | 9.6 | 5.9 | 14.8 |
| ALC, ×103/mm3 | 2.8 | 1.9 | 1.3 | 1.3 | 1.8 |
| ANC, ×103/mm3 | 33.6 | 9.1 | 7.5 | 3.9 | 11.9 |
| Postcollection CBC | |||||
| Hgb level, g/dL | 11.2 | 13.1 | 11.6 | 10.7 | 10.7 |
| Platelet count, ×103/mm3 | 40 | 69 | 29 | 11 | 48 |
| WBC count, ×103/mm3 | 29.2 | 16.8 | 12.1 | 5.8 | 10.1 |
| ALC, ×103/mm3 | 0.7 | 1.1 | 0.8 | 0.7 | 1.2 |
| ANC, ×103/mm3 | 26.4 | 14.6 | 10.1 | 4.8 | 7.9 |
| mPB collection averages | |||||
| % CD34+† | 0.320 | 0.090 | 0.044 | 0.050 | 0.140 |
| No. CD34+‡ | 2.31E+08 | 9.38E+07 | 1.61E+07 | 1.66E+07 | 5.88E+07 |
| TNCs | 1.43E+11 | 1.12E+11 | 6.77E+10 | 6.43E+10 | 1.03E+11 |
| MNCs | 1.46E+09 | 9.14E+08 | 8.81E+08 | 9.67E+08 | 1.30E+09 |
| Total CD34+ cells/kg§ | 6.16E+06 | 1.93E+06 | 4.80E+05 | 5.60E+05 | 2.15E+06 |
| Total CD34+ cells | 5.30E+08 | 1.75E+08 | 3.37E+07 | 3.21E+07 | 9.72E+07 |
| Name . | UPN 2 . | UPN 5 . | UPN 6 . | UPN 7 . | UPN 9 . |
|---|---|---|---|---|---|
| Age, y | 52 | 40 | 33 | 21 | 11 |
| Precollection CBC* | |||||
| Hgb level, g/dL | 12.8 | 13.8 | 13.2 | 12.7 | 12.1 |
| Platelet count, ×103/mm3 | 213 | 177 | 90 | 39 | 107 |
| WBC count, ×103/mm3 | 38.9 | 12.1 | 9.6 | 5.9 | 14.8 |
| ALC, ×103/mm3 | 2.8 | 1.9 | 1.3 | 1.3 | 1.8 |
| ANC, ×103/mm3 | 33.6 | 9.1 | 7.5 | 3.9 | 11.9 |
| Postcollection CBC | |||||
| Hgb level, g/dL | 11.2 | 13.1 | 11.6 | 10.7 | 10.7 |
| Platelet count, ×103/mm3 | 40 | 69 | 29 | 11 | 48 |
| WBC count, ×103/mm3 | 29.2 | 16.8 | 12.1 | 5.8 | 10.1 |
| ALC, ×103/mm3 | 0.7 | 1.1 | 0.8 | 0.7 | 1.2 |
| ANC, ×103/mm3 | 26.4 | 14.6 | 10.1 | 4.8 | 7.9 |
| mPB collection averages | |||||
| % CD34+† | 0.320 | 0.090 | 0.044 | 0.050 | 0.140 |
| No. CD34+‡ | 2.31E+08 | 9.38E+07 | 1.61E+07 | 1.66E+07 | 5.88E+07 |
| TNCs | 1.43E+11 | 1.12E+11 | 6.77E+10 | 6.43E+10 | 1.03E+11 |
| MNCs | 1.46E+09 | 9.14E+08 | 8.81E+08 | 9.67E+08 | 1.30E+09 |
| Total CD34+ cells/kg§ | 6.16E+06 | 1.93E+06 | 4.80E+05 | 5.60E+05 | 2.15E+06 |
| Total CD34+ cells | 5.30E+08 | 1.75E+08 | 3.37E+07 | 3.21E+07 | 9.72E+07 |
Hgb indicates hemoglobin (g/dL); Plt, platelet count; WBC, white blood cell; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; TNCs, total nucleated cells; and MNCs, mononuclear cells.
Precollection CBC values were obtained just prior to the first apheresis, and postcollection CBC values were obtained 30 minutes following the first apheresis.
Percentage CD34+ represents average values from both collections.
Represents number of cells collected from first collection.
Average number of cells collected from healthy HSC apheresis volunteers (n=11) at University of Iowa DeGowin Blood Center = 9.13×106 CD34+ cells/kg.
Phenotypic characterization of DC cells
Immunophenotyping experiments carried out on mPB cells from DC subjects and age-matched controls showed that the representation of subsets of CD34+ cells coexpressing erythroid (CD71), B- and T-lymphoid (CD19 and CD7), megakaryocytic (CD41), and granulopoietic (CD33) markers, or that had a very primitive phenotype (were CD38−) was similar to that seen in age-matched controls and, in almost all cases, values for DC subjects fell within the control range. The only exception was for UPN 9, where the percentage of CD34+CD38− cells was higher than normal. A similar finding was also noted in the BM sample from this patient (data not shown).
Functional characterization of primitive DC cells
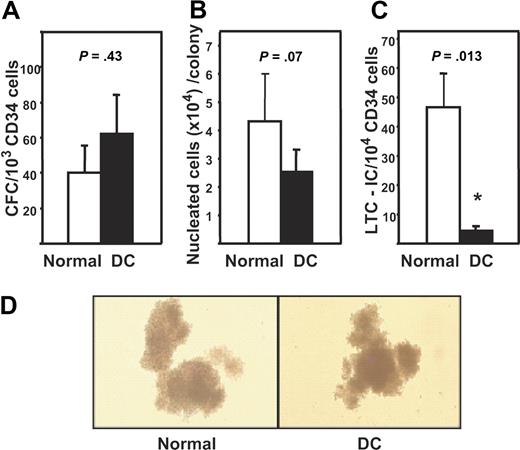
CFC assays performed on CD34+-enriched mPB cells from all 5 DC subjects revealed the frequencies of granulopoietic (CFU-GM), erythroid (CFU-E + BFU-E), and multilineage (CFU-GEMM) progenitors to be similar to the frequencies of these progenitors in comparable populations isolated from mPB harvests from 5 healthy donors. Figure 1A shows the results for the total CFC numbers per 103 CD34+ cells. Comparison of the total size and distribution of different types of colonies produced by the normal and DC cells in these assays indicated these were also indistinguishable (Figure 1B,D and data not shown), and this was confirmed by more detailed immunophenotyping analyses of the different lineages present within the pooled colonies from the CFC assays (number of nucleated glycophorin A+ cells generated in CFC assays per 103 normal CD34+ mPB cells plated was 33, 34, 119, 210, and 359 and corresponding values for DC assays were 17, 48, 92, 146, and 153; P > .3. Parallel values for CD16 and/or CD66b+ cells were 2, 4, 5, 5, and 6 for the normal cells and 1, 6, 7, 14, and 125 for the DC cells, P > .3). However, given the markedly reduced frequency of CD34+ cells in the BM of the DC subjects and the associated reduced absolute numbers of CD34+ cells that could be collected from them, it can be inferred that the total CFC numbers in the DC subjects were significantly reduced.
CFC and LTC-IC activity of DC mPB cells. (A) Mean plus or minus SEM frequency of CFCs expressed per 103 CD34+ cells in the 5 DC mPB samples (black bar) and in 5 healthy donors (□). Values shown are the sum of the total erythroid (BFU-E), granulopoietic (CFU-GM), and multilineage (CFU-GEMM) progenitors detected in these assays. (B) Measurements of the average size (nucleated cell content) of the colonies produced in panel A. Values shown are the mean plus or minus SEM. Each value was calculated by dividing the total number of cells measured in the 2-week CFC assay cultures (as described in “Methods”) by the total number of colonies scored in those assay cultures. (C) Mean plus or minus SEM of the number of LTC-ICs expressed per 104 DC (■) and normal (□) CD34+ mPB cells assayed. (D) Photomicrographs of typical colonies detected in the CFC assays of normal (left panel) and DC (right panel) cells.
CFC and LTC-IC activity of DC mPB cells. (A) Mean plus or minus SEM frequency of CFCs expressed per 103 CD34+ cells in the 5 DC mPB samples (black bar) and in 5 healthy donors (□). Values shown are the sum of the total erythroid (BFU-E), granulopoietic (CFU-GM), and multilineage (CFU-GEMM) progenitors detected in these assays. (B) Measurements of the average size (nucleated cell content) of the colonies produced in panel A. Values shown are the mean plus or minus SEM. Each value was calculated by dividing the total number of cells measured in the 2-week CFC assay cultures (as described in “Methods”) by the total number of colonies scored in those assay cultures. (C) Mean plus or minus SEM of the number of LTC-ICs expressed per 104 DC (■) and normal (□) CD34+ mPB cells assayed. (D) Photomicrographs of typical colonies detected in the CFC assays of normal (left panel) and DC (right panel) cells.
LTC-IC assays performed on the same 5 DC and 5 control mPB samples revealed an overall 10-fold reduction (P < .05) in the frequency of LTC-ICs within the CD34+ subset of DC cells with DC values ranging from 2- to 40-fold below the minimum normal value measured (Figure 1C). Given the further reduction in the total number of CD34+ cells in the DC subjects, the absolute decrease in their LTC-IC compartment would be very profound.
Telomere length analysis of DC cells
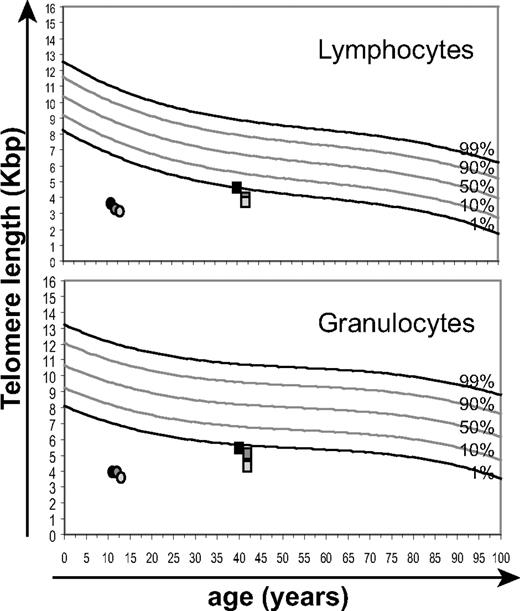
Telomere length analysis was performed by flow-FISH on PB samples over a period of a year and a half from 2 selected second- and third-generation AD DC subjects (UPNs 5 and 9, respectively). Both subjects showed telomere lengths below the first percentile of the normal distribution irrespective of age (Figure 2). Measurements at the first time point were performed on both PB and apheresis samples with similar results (data not shown). Telomeres were shorter in lymphocytes than in granulocytes (UPN 5: 4.6 to 3.7 Kb for lymphocytes vs 5.5 to 4.3 Kb for granulocytes; UPN 9: 3.6 to 3.1 Kb for lymphocytes vs 3.9 to 3.5 Kb for granulocytes), and these values appeared to decrease over the course of the study period, although this trend did not reach significance. These results provide further support for a mechanism of disease anticipation in this AD DC family where the symptoms and disease phenotype of the third-generation subject (UPN 9) were associated with earlier and more pronounced telomere length impairment compared with the previous generation (UPN 5).
Telomere length analysis of DC PB. Longitudinal data were obtained over a period of 20 months for 2 of the DC subjects, at 1, 15, and 20 months, respectively from the second generation (UPN 5, filled circles) and third generation (UPN 9, squares) of the DC family. These analyses of mean telomere lengths of PB lymphocytes and granulocytes are shown relative to normal telomere distribution curves derived from best-fit analysis for 400 healthy individuals (Baerlocher and P.M.L., unpublished data, August 2001).
Telomere length analysis of DC PB. Longitudinal data were obtained over a period of 20 months for 2 of the DC subjects, at 1, 15, and 20 months, respectively from the second generation (UPN 5, filled circles) and third generation (UPN 9, squares) of the DC family. These analyses of mean telomere lengths of PB lymphocytes and granulocytes are shown relative to normal telomere distribution curves derived from best-fit analysis for 400 healthy individuals (Baerlocher and P.M.L., unpublished data, August 2001).
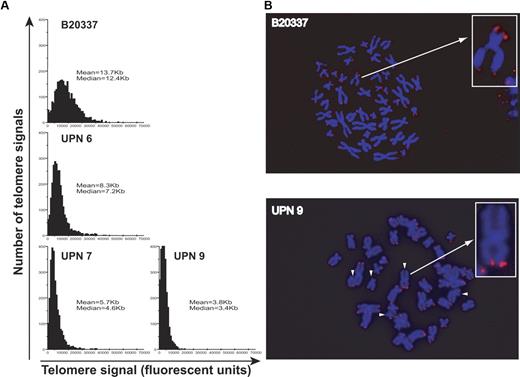
Telomere length and distribution measurements were also performed on 4-day cultured CD34+ mPB cells by quantitative telomere FISH (Q-FISH). In the second-generation AD DC subject (UPN 6), telomere length was reduced compared with an age-matched control: median telomere length of 7.2 Kb versus 12.4 Kb, respectively (Figure 3A). This finding was further accentuated in the CD34+ mPB cells from third-generation subjects (median telomere lengths of 4.6 Kb [UPN 7] and 3.4 Kb [UPN 9]) with the tightest telomere length distribution indicating that most telomere ends were affected. Since more signal-free telomere ends (scored as 0, Figure 3A and indicated by white arrows, Figure 3B) were detected by Q-FISH in AD DC subjects compared with controls, PCR-based XpYp chromosome-specific STELA assays were performed. As shown in Figure 4A, these measurements demonstrated that the telomere length distribution at the XpYp telomeres from DC CD34+ cells was relatively short overall with the majority of detected telomere ends below 5 Kb, compared with a mPB CD34+ control (IWN1) where the majority of telomere ends were detected above 5 Kb in length (note: control and DC samples were run on separate gels to ensure optimal resolution). Furthermore, detectable outlier or ultrashort telomeres defined as more than 2 standard deviations below the mean (represented as black bars, Figure 4B) could be readily detected in all samples. These comprised up to 9.5% of the XpYp telomeres detected for UPN 7. However since the overall length distribution was low in DC, the frequency of short telomeres below 1.5 Kb in length is increased in DC mPB CD34+ cells.
Telomere length analysis of DC CD34+ cells. (A) Quantitative FISH analysis of telomere length distribution and mean or median telomere length was measured in 15 individual metaphases (or 2750 individual telomeres) for day-4 cultured CD34+ cells from a control mPB sample (B20337, 39 years old), a second-generation AD DC patient (UPN 6), and 2 third-generation patients (UPNs 7 and 9). (B) Representative Q-FISH metaphase spreads (and inset enlarged chromosomes) for day-4 CD34+ cells from a control mPB sample showing detectable telomere spots for each chromosome end and from a DC sample, showing detectable signal-free ends indicated by the white arrows.
Telomere length analysis of DC CD34+ cells. (A) Quantitative FISH analysis of telomere length distribution and mean or median telomere length was measured in 15 individual metaphases (or 2750 individual telomeres) for day-4 cultured CD34+ cells from a control mPB sample (B20337, 39 years old), a second-generation AD DC patient (UPN 6), and 2 third-generation patients (UPNs 7 and 9). (B) Representative Q-FISH metaphase spreads (and inset enlarged chromosomes) for day-4 CD34+ cells from a control mPB sample showing detectable telomere spots for each chromosome end and from a DC sample, showing detectable signal-free ends indicated by the white arrows.
Telomere length measurements in DC CD34+ cells by STELA. (A) Representative STELA blot. Multiple PCR replicates for each DC sample of day-4 cultured CD34+ mPB cells were amplified at an estimated concentration of 10 molecules per well, loaded blind and resolved on 0.7% agarose, Southern blotted, and detected with an XpYp-specific probe. Amplicons were counted into size bins to estimate average telomere length. (B) Distribution of telomere lengths in CD34+ cells of one control (IWN1) and 3 DC patients. The average telomere length for each patient (± SEM) and the number of telomeres analyzed are given. Black portions of the graph represent ultrashort telomeres, defined by their length relative to the majority of telomere molecules.
Telomere length measurements in DC CD34+ cells by STELA. (A) Representative STELA blot. Multiple PCR replicates for each DC sample of day-4 cultured CD34+ mPB cells were amplified at an estimated concentration of 10 molecules per well, loaded blind and resolved on 0.7% agarose, Southern blotted, and detected with an XpYp-specific probe. Amplicons were counted into size bins to estimate average telomere length. (B) Distribution of telomere lengths in CD34+ cells of one control (IWN1) and 3 DC patients. The average telomere length for each patient (± SEM) and the number of telomeres analyzed are given. Black portions of the graph represent ultrashort telomeres, defined by their length relative to the majority of telomere molecules.
Discussion
DC is a premature aging disorder of telomere attrition characterized by both progressive telomere shortening and BM failure. This disease thus serves as an excellent model to investigate the effects of telomerase deficiency on different cellular functions, and in particular, those of the hematopoietic system. A major question is whether increased telomere shortening represents a causal link between HSC turnover, replicative senescence, and/or the induction of genetic instability in DC hematopoietic cells and possibly other acquired BM failure disorders. Furthermore, one could hypothesize that BM failure in DC reflects a qualitative impairment of the normal ability of HSCs and their progeny to execute a variety of differentiation programs and/or an altered ability to sustain HSC numbers. To begin to address these questions, we undertook a characterization of the CD34+ cells present in the BM and G-CSF–mobilized product harvested from a family of DC patients.
Two lines of evidence were obtained to indicate that the HSC compartment of DC subjects is profoundly reduced, even before the onset of severe symptoms of BM failure or transformation. The first was the reduced frequency of CD34+ cells in the preapheresis hypocellular BM aspirates studied and the poor yields of CD34+ cells in the G-CSF–mobilized products collected from the DC patients. These data are similar to that reported for Fanconi anemia and aplastic anemia patients, where BM CD34+ cell frequencies were also significantly lower than in healthy donors.35,36 The reduced absolute numbers of CD34+ cells in vivo would also mean that the absolute numbers of CFCs were correspondingly reduced in DC patients, even though their frequency in the CD34+ subset was not different from normal. The second result indicative of a reduced HSC compartment in DC patients was the further dramatic reduction within the mobilized CD34+ cells of more primitive cells identified functionally as LTC-ICs. LTC-ICs represent a cell type that is closely related to HSCs. Evidence of a more pronounced deficiency of the most primitive hematopoietic cells was also noted in other BM failure syndromes, including idiopathic aplastic anemia and paroxysmal nocturnal hemoglobinuria.37-39
At the same time, we obtained data indicating that the differentiation program of primitive hematopoietic cells from DC subjects is not grossly perturbed. This included the finding that differentiating CD34+ cells coexpressing various lineage markers were represented at normal frequencies within the total CD34+ population isolated directly from patients. Similarly, more primitive progenitors detected by their clonogenic activity were also present within the CD34+ cells at normal frequencies and were found to display the same proliferative and myeloid differentiation activity in vitro as their counterparts in mPB samples obtained from healthy donors.
Mounting evidence suggests a relationship between HSC turnover and the shortening of WBC telomeres (particularly granulocytes that have a very short lifespan), during both normal ontogeny and after transplantation,40-44 and may even limit HSC regenerative ability.45 It is therefore likely that telomere shortening also plays a causal role in DC, BM failure disorders, and different forms of aplastic anemia,23 all of which are associated with shorter telomeres in both mature WBCs and in their more primitive precursors. In the present study, telomere lengths in PB and apheresis samples were found to be shorter than normal in both patients who were assessed at the time of the aphereses. Therefore, it is inviting to speculate that this may reflect a higher turnover rate of the HSC population in DC patients. However, the best human HSC purification strategies applied to normal BM or mPB yield very limited numbers of cells and even these are not pure suspensions of HSCs. Therefore in DC samples where the HSC compartment appears markedly reduced, it is not possible to analyze their telomere lengths directly. Nevertheless we were able to perform telomere measurements by Q-FISH and STELA in CD34+ mPB cells after a very short period in culture and confirmed that these also have reduced telomere lengths. Furthermore, we detected telomere free ends in metaphases from CD34+ hematopoietic progenitors (Figure 3); however, no end to end fusions were found, suggesting at least partial integrity of telomere function in the CD34+ cells that were examined. Further proliferation of these cells would be expected to lead to further telomere loss followed by telomere dysfunction and growth arrest due to a DNA damage response. These findings thus provide support for the possibility that BM failure in DC subjects may be a consequence of HSCs and progenitor cells entering a state of proliferative arrest or death in third-generation subjects due to an accumulated shortening of their telomeres over multiple generations.
In addition to the proposed intrinsic effect of telomere length and levels of telomerase on replicative exhaustion of the HSC pool in vivo, the stroma of DC subjects may also be compromised. This hypothesis has been suggested as a possible cause of BM failure in SDS. Not only were there lower numbers of CD34+ cells in the BM of SDS patients, but SDS stromal cells failed to support hematopoiesis in vitro.39 More recently, using a telomerase knockout mouse model, additional data have been obtained to indicate that telomere dysfunction may extrinsically impair HSC function by affecting mesenchymal and stromal cell functions.26
From a clinical perspective, it has been difficult to predict either the time of onset or the degree of BM failure in DC, and clear differences exist based upon the inheritance pattern of DC. This is of critical importance because unlike other forms of aplastic anemia, DC is associated with a rather poor outcome with respect to treatment with allogeneic HSC-containing transplants. Treatment with immunosuppressive drugs, growth factors, and anabolic steroids has also been largely unsuccessful. The poor prognosis for DC patients has led investigators to consider alternative therapies including reduced-intensity myeloablation and umbilical cord blood transplantations46-48 to circumvent potential increased cellular sensitivity to alkylating agents or radiation in DC subjects.49-51 Excessive toxicities associated with chemotherapy or myeloablative therapy are also limiting in other congenital BM failure syndromes, including Fanconi anemia and SDS.52,53 Preemptive collection of HSCs in BM failure disorders has long been considered a theoretic option based on the idea that these cells might provide a source of autologous HSCs in the setting of marrow aplasia, or serve as vehicles for potential gene transfer strategies. Importantly, our results suggest that HSCs from DC patients are not functionally impaired in their ability to differentiate into specific lineages, although it may be difficult to collect sufficient HSCs to allow permanent repopulation unless coupled with a method for expanding their genetically corrected HSCs ex vivo. However, since DC is a heterogeneous disorder and the phenotype is influenced by the specific gene mutation, further investigations are warranted to establish whether our findings may be applicable to all forms of DC.
In summary, our study provides new evidence that telomerase insufficiency and short telomeres at least initially cause a quantitative change in HSC numbers rather than a qualitative change in their ability to differentiate. These findings point to the need for new approaches to study the effects of telomere shortening directly in HSCs and support the concept of collecting autologous HSCs early in the course of disease for potential future use in marrow rescue strategies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Elisabeth Chavez and Irma Vulto for excellent technical assistance. We also thank Justin Fishbaugh for his technical assistance in FACS, David Tatman for his administrative assistance, and Shiva Patel for providing data on DEB-induced chromosome breakage analyses.
Work in the Goldman laboratory was supported by Aiming for a Cure Foundation and the Canadian Institutes of Health Research (GMH79042). Work in the Lansdorp and Eaves laboratories was supported by grants from the National Institutes of Health (AI29524), the Canadian Institutes of Health Research (MOP38075 and GMH79042), and the National Cancer Institute of Canada (with support from the Terry Fox Run).
National Institutes of Health
Authorship
Contribution: F.D.G. directed the project and contributed to the writing of the paper; G.A. was co–first author, performed experiments, and also contributed equally to the writing of the paper; A.J.K. contributed to the interpretation of the data and the writing of the paper; M.H., S.R.C., W.S.H., and K.L. performed experiments; A.J.S. assisted in the interpretation of the data and writing of the paper; C.J.E. interpreted data and contributed to the writing of the paper; P.M.L. helped conceptualize and supervise the project and contributed to the writing of the paper.
Conflict-of-interest disclosure: P.M.L. is a founding shareholder in Repeat Diagnostics Inc, a company specializing in leukocyte telomere length measurements using flow-FISH. All other authors declare no competing financial interests.
Correspondence: Fred Goldman, University of Iowa Children's Hospital, Department of Pediatrics, Division of Hematology Oncology, 200 Hawkins Dr, 2538 JCP, Iowa City, IA 52242; e-mail: frederick-goldman@uiowa.edu.
References
Author notes
*F.D.G. and G.A. contributed equally to this work.