Abstract
Selective allodepletion is a strategy to eliminate host-reactive donor T cells from hematopoietic stem cell allografts to prevent graft-versus-host disease while conserving useful donor immune functions. To overcome fluctuations in activation-based surface marker expression and achieve a more consistent and effective allodepletion, we investigated a photodepletion process targeting activation-based changes in p-glycoprotein that result in an altered efflux of the photosensitizer TH9402. Expanded lymphocytes, generated using anti-CD3 and IL-2, were cocultured with responder cells from HLA-matched or -mismatched donors. Optimal results were achieved when cocultured cells were incubated with 7.5 μM TH9402, followed by dye extrusion and exposure to 5 Joule/cm2 light energy at 5 × 106 cells/mL. In mismatched stimulator-responder pairs, the median reduction of alloreactivity was 474-fold (range, 43-fold to 864-fold) compared with the unmanipulated responder. Third-party responses were maintained with a median 1.4-fold (range, 0.9-fold to 3.3-fold) reduction. In matched pairs, alloreactive helper T-lymphocyte precursors were reduced to lower than 1:100 000, while third-party responses remained higher than 1:10 000. This establishes a clinical-scale process capable of highly efficient, reproducible, selective removal of alloreactive lymphocytes from lymphocyte transplant products performed under current Good Manufacturing Practice. This procedure is currently being investigated in a clinical trial of allotransplantation.
Introduction
Overall survival, following allogeneic stem cell transplantation (SCT) for malignant diseases has steadily improved, largely due to reduced transplantation-related mortality.1 In contrast, risk-stratified relapse rates have not changed significantly over the past 3 decades.2 Further improvements in SCT outcome thus await improved control of the malignant disease. One approach is to exploit the unique graft-versus-leukemia (GVL) effect of alloreacting donor immune cells.3-5 Currently GVL is limited by the fact that strategies to prevent graft-versus-host disease (GVHD) with immunosuppression or T-lymphocyte depletion tend to compromise the GvL effect. While T cell–mediated GVHD and GVL depend on similar mechanisms, the antigenic overlap between normal tissue targets of GVHD and between leukemic tissues and normal tissues is not complete. As a consequence, different T-cell populations can distinguish myeloid from lymphoid tissues as well as leukemic from normal cells in vitro.6-9 Thus it should be possible to selectively eliminate GVHD-causing donor lymphocytes from allografts while sparing the valuable T cells exerting GVL and beneficial antimicrobial responses. This approach, usually referred to as selective lymphocyte depletion (SD) or selective allodepletion, uses patient-derived antigen-presenting cells (APCs) for stimulation of donor T cells in an ex vivo coculture. Alloactivated donor lymphocytes are then removed by virtue of their activation status.10 SD has been achieved using immunomagnetic beads or immunotoxin specific for surface markers of early T-cell activation such as CD25,7,9,11-19 CD69,15,19,20 CD71,19 CD137,21 or HLA-DR,19 sorting of nonactivated22 or nonproliferating cells using dye-dilution techniques,23 apoptosis induction24,25 and photodepletion (PD)26,27 targeting the impaired ability of activated T cells to efflux a phototoxic rhodamide-like dye (TH9402) due to changes in their multidrug-resistance pump p-glycoprotein (MDR1). The latter approach was shown to be feasible in an HLA-mismatched, small-scale system both in mice26 and humans.27 Clinical data on SD are limited, but there are promising results from 3 clinical trials using an anti–CD25-immunotoxin for removal of alloactivated T cells.28-30 We found that SD using an anti-CD25-immunotoxin may protect against acute GVHD (aGVHD) both by removal of alloactivated T cells and by allowing regulatory CD4+ T cell (Treg) reconstitution.30,31 The occurrence of residual aGVHD in our series of selectively allodepleted transplantations was associated with low donor Treg numbers31 and poor depletion efficacy.30 Inefficient allodepletion could be due to a down-regulation of CD25 antigen during the coculture period, allowing some alloactivated cells to escape the depletion process. Here, we aimed to improve the efficiency and reliability of SD using a TH9402-based PD method as an alternative strategy to surface marker targeting. We worked with clinical-scale cell volumes under identical conditions used for current Good Manufacturing Practice (cGMP). The use of expanded lymphocytes as antigen-presenting cells (APCs) allowed the generation of large numbers of allodepleted T cells. The established PD process was capable of highly efficient removal of alloreactive lymphocytes from mismatched and matched cocultures and maintained desirable third-party responses including antiviral and antibacterial responses
Methods
Human subjects
Protocols that included procedures for leukapheresis collections from patients with hematologic malignancies, healthy sibling donors, and unrelated, healthy volunteers were approved by the National Heart, Lung, and Blood Institute (NHLBI) Institutional Review Board. Informed consent was obtained from all patients, healthy sibling donors, and healthy volunteers in accordance with the Declaration of Helsinki.
Current Good Manufacturing Practice
Stimulator generation, coculturing, and photodepletion procedures were performed under conditions mimicking cGMP. Using automated instruments with sterile disposable kits, bags, and sterile connecting devices for cell transfer (Terumo, Eschborn, Germany) a semiclosed culture and treatment system was used in anticipation of a clinical trial. All reagents used either were drugs approved by the US Food and Drug Administration (FDA) or had a certificate of analysis certifying safety and characterization requirements. Final cellular product safety testing including sterility, and endotoxin and mycoplasma assays were performed and found to be within acceptable limits for all products.
Stimulator generation
Ex vivo–expanded lymphocytes (ExLy's) were used as stimulator cells. These stimulator cells were generated from either peripheral blood mononuclear cells (PBMCs; Exp's I-III) or CD3-selected T cells (Exp's IV-IX), obtained from a single leukapheresis product (8- to 12-liter blood volume processed; Table 1).
Clinical-scale photodepletion experiments (N = 9)
| Exp no. . | Stim . | HLA-type/Stim . | Resp . | HLA-type/Resp . | HLA disparity . | Stimulator T-cell selection . | PD: TH9402, μM . | Readout assay . | Readout time point(s), days after PD . |
|---|---|---|---|---|---|---|---|---|---|
| I | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,26 B-38,58 DR-04,12 | Mismatched (0/6) | None | 5.0/7.5 | MLR | +1/+4 |
| II | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,29 B-44,44 DR-07,11 | Mismatched (0/6) | None | 5.0/7.5 | MLR | +1/+4 |
| III | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,26 B-38,58 DR-04,12 | Mismatched (0/6) | None | 5.0/7.5 | MLR | +1/+4 |
| IV | PAT | A-02,23 B-44,51 DR-04,07 | MSD | A-02,23 B-44,51 DR-04,07 | Matched (6/6) | CD3+ microbeads | 7.5 | HTLp | +1 |
| V | PAT | A-02,68 B-35,39 DR-04,16 | MSD | A-02,68 B-35,39 DR-04,16 | Matched (6/6) | CD3+ microbeads | 7.5 | HTLp | +1 |
| VI | PAT | A-01,03 B-07,57 DR-15,15 | MSD | A-01,03 B-07,57 DR-15,15 | Matched (6/6) | CD3+ microbeads | 7.5 | HTLp | +4 |
| VII | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-01,25 B-18,35 DR-11,12 | Mismatched (2/6) | CD3+ microbeads | 5.0/7.5 | MLR | +0/+1 |
| VIII | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,29 B-44,44 DR-07,11 | Mismatched (0/6) | CD3+ microbeads | 5.0/7.5 | MLR | +0/+1 |
| IX | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,26 B-38,58 DR-04,12 | Mismatched (0/6) | CD3+ microbeads | 5.0/7.5 | MLR | +0/+1 |
| Exp no. . | Stim . | HLA-type/Stim . | Resp . | HLA-type/Resp . | HLA disparity . | Stimulator T-cell selection . | PD: TH9402, μM . | Readout assay . | Readout time point(s), days after PD . |
|---|---|---|---|---|---|---|---|---|---|
| I | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,26 B-38,58 DR-04,12 | Mismatched (0/6) | None | 5.0/7.5 | MLR | +1/+4 |
| II | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,29 B-44,44 DR-07,11 | Mismatched (0/6) | None | 5.0/7.5 | MLR | +1/+4 |
| III | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,26 B-38,58 DR-04,12 | Mismatched (0/6) | None | 5.0/7.5 | MLR | +1/+4 |
| IV | PAT | A-02,23 B-44,51 DR-04,07 | MSD | A-02,23 B-44,51 DR-04,07 | Matched (6/6) | CD3+ microbeads | 7.5 | HTLp | +1 |
| V | PAT | A-02,68 B-35,39 DR-04,16 | MSD | A-02,68 B-35,39 DR-04,16 | Matched (6/6) | CD3+ microbeads | 7.5 | HTLp | +1 |
| VI | PAT | A-01,03 B-07,57 DR-15,15 | MSD | A-01,03 B-07,57 DR-15,15 | Matched (6/6) | CD3+ microbeads | 7.5 | HTLp | +4 |
| VII | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-01,25 B-18,35 DR-11,12 | Mismatched (2/6) | CD3+ microbeads | 5.0/7.5 | MLR | +0/+1 |
| VIII | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,29 B-44,44 DR-07,11 | Mismatched (0/6) | CD3+ microbeads | 5.0/7.5 | MLR | +0/+1 |
| IX | VUD | A-01,24 B-14,35 DR-14,15 | VUD | A-02,26 B-38,58 DR-04,12 | Mismatched (0/6) | CD3+ microbeads | 5.0/7.5 | MLR | +0/+1 |
Six HLA-mismatched (Exp I-III, VII-IX) and 3 HLA-matched (Exp IV-VI) experiments were performed. Irradiated, expanded lymphocytes were used as allostimulators and either grown from PBMCs (Exp I-III) or from CD3+-selected cells (Exp IV-IX). In mismatch experiments, 2 different doses of TH9402 were used. Readouts were performed by MLR in mismatched pairs and by HTLP in matched pairs at different time points after photodepletion.
Stim indicates stimulator; Resp, responder; VUD, volunteer unrelated donor; PAT, patient; and MSD, matched sibling donor.
For stimulator generation from PBMCs (Exp's I-III), a soft-spin was performed to remove plasma and platelets from apheresed cells, followed by ACK lysing (Cambrex Bio Science, Walkersville, MD) of red cells and 2 washes with 0.9% NaCl (B. Braun Medical, Irvine, CA) with 0.3% tricitrasol (Cytosol Laboratories, Braintree, MA). These PBMCs were cultured in X-VIVO 15 (Cambrex Bio Science) supplemented with 2.2% autologous heat-inactivated plasma and 100 IU/mL interleukin-2 (Proleukin; Chiron, Emeryville, CA) in polyolefin tissue culture bags (Lifecell PL732; Baxter Cellular Therapies, Deerfield, IL). Initially, 100 ng/mL soluble anti-CD3 antibody (OKT3, murumonab-CD3, Orthoclone OKT3; Ortho Biotech, Raritan, NJ) was added to the culture at 0.5 to 1.0 × 106 cells /mL.
For stimulator generation from CD3+-selected T cells (Exp's IV-IX), PBMCs were washed using Miltenyi buffer (Miltenyi Biotech, Bergisch Gladbach, Germany) supplemented with 0.5% human serum albumin (Flexbumin 25%; Baxter Healthcare, West Lake Village, CA), hereafter referred to as “buffer.” Washed cells were resuspended in buffer with 1.5 mg/mL human intravenous immune globulin (Gammagard S/D; Baxter Healthcare) and anti-CD3 microbeads (CliniMACS CD3 microbeads; Miltenyi Biotech) for 30 minutes at room temperature based on the manufacturer's recommendations. Incubated cells were washed twice using buffer and applied to the automated magnetic selection device (CliniMACS System; Miltenyi Biotech). Afterward, CD3+-selected cells were cultured in X-VIVO 20 (Cambrex Bio Science) supplemented with 2.0% autologous heat-inactivated plasma and 100 IU/mL interleukin-2 (Proleukin; Chiron) in gas-permeable polyolefin tissue culture bags (Lifecell PL732; Baxter Cellular Therapies) precoated with OKT3 antibody (murumonab-CD3, Orthoclone OKT3; Ortho Biotech). To precoat bags, 1.05 mg OKT3 was added to 120 mL Hanks balanced salt solution (Cambrex Bio Science) supplemented with 0.1% human serum albumin (Baxter Healthcare) and incubated in culture bags at room temperature for a minimum of 4 hours.
Cells were then incubated at 37°C, 7% CO2, and 90% humidity with fresh medium added to maintain the cell concentration between 0.5 × 106 and 1.5 × 106 cells/mL. Stimulator cells were harvested after 10 to 12 days, using the Fenwal CS3000 Plus blood cell separator (Fenwal, Round Lake, IL) configured to perform continuous flow cell concentration from large-volume tissue cultures. The cells were concentrated in a semiautomated mode, washed with 0.9% NaCl, and subsequently cryopreserved.
Coculture
Responder cells from random HLA-mismatched volunteers and HLA-matched sibling donors were collected by leukapheresis, with 8- to 12-liter blood volume processed on the Fenwal CS3000 Plus blood cell separator (Fenwal). The cells were first incubated with ACK lysing buffer (Cambrex Bio Science) to remove red blood cell contamination, and then washed and resuspended in X-VIVO 20 (Cambrex Bio Science) supplemented with 2.0% autologous heat-inactivated plasma, hereafter referred to as culture media (CM), at a concentration of 5 × 106 cells/mL. Cocultures were carried out in gas-permeable polyolefin tissue culture bags (Lifecell PL732; Baxter Cellular Therapies). Stimulator cells were thawed, gamma irradiated (25 Gy), resuspended in CM at a concentration of 5 × 106 cells/mL, and combined with responder cells at a ratio of 1:1. Responder and irradiated stimulator cells were cocultured for 72 hours at 37°C, 7% CO2. Following the 72-hour coculture, cells were concentrated and immediately forwarded to the photodepletion process.
Photodepletion process
The photodepletion (PD) process is based on 3 consecutive phases: coloration, extrusion, and light exposure. Responder-derived heat-inactivated plasma, hereafter referred to as plasma, collected on the day of responder apheresis and stored at 4°C, was used throughout the PD process. Following the coculture, concentrated cells were resuspended at a concentration of 5 × 106 cells/mL in X-VIVO 10 without phenol red (Cambrex Bio Science) supplemented with 2.5% plasma, and incubated at 37°C with 5.0 and/or 7.5 μM 4,5-dibromorhodamine 123 (TH9402; Kiadis, formerly Celmed, Saint-Laurent, QC). After a 40-minute incubation (coloration phase), cells were centrifuged and resuspended in TH9402-free medium (X-VIVO 10 without phenol red supplemented with 10% plasma) to accelerate the dye efflux (extrusion phase). After 90 minutes of extrusion, cells were exposed to visible light using a fluorescent light-scanning device (Theralux device, Model 514; Kiadis) delivering 5 J/cm2 at a wavelength of 514 nm and rotations of 180 rpm. Coloration was performed in 300- or 600-mL transfer packs (Fenwal). During extrusion and light exposure, 60-, 300-, or 600-mL fluorinated ethylene propylene (FEP) plastic bags (American Fluoroseal, Gaithersburg, MD) were used. The Theralux device accommodates a maximum of 2 600-mL FEP bags at a time allowing the treatment of up to 6 × 109 total viable cells per session. After PD, cells were washed and an automated density gradient separation was performed using a CS3000 apheresis device (Fenwal; only Exp's VII-IX). Afterward, cells were cryopreserved in a final concentration of 5% dimethysulfoxide (DMSO, Cryoserv; Edwards Lifesciences, Irvine, CA) and 6% pentastarch (provided by NIH pharmacy development services), using the standard operating procedures of our cell processing section, and transferred into a liquid nitrogen tank at liquid or vapor phase (−200°C to −150°C) for storage until thaw and distribution.
Cell isolation for experimental readout
Peripheral blood mononuclear cells (PBMCs) from unmanipulated responders, untreated cocultures, and photodepleted products were separated using Ficoll-Hypaque density gradient centrifugation (Organon Teknika, Durham, NC) and subsequently analyzed or frozen in RPMI 1640 complete medium (CM; Life Technologies, Gaithersburg, MD) supplemented with 20% heat-inactivated fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO) according to standard protocols. Before use, frozen cells were thawed, washed, and suspended in RPMI-CM + 10% pooled AB serum (Sigma Chemical, St Louis, MO). As required, CD4+ or CD8+ cell populations were purified with immunomagnetic microbeads and selected with LS columns based on the manufacturer's instructions (Miltenyi Biotech).
Flow cytometry
Cells were phenotypically analyzed by flow cytometry using the following combinations of directly conjugated antibodies (BD Biosciences, San Diego, CA) for surface staining: CD3-APC, CD80-FITC, CD86-PE-Cy5, and HLA-DR-PE; CD3-APC, CD8-FITC, and CD4-PerCP; CD25-PE, CD3-FITC, CD4-PerCP, and CD8-APC-Cy7. Intracellular staining of FOXP3 (eBioscience, San Diego, CA) was performed after surface staining, fixation, and permeabilization according to the manufacturer's recommendation using an APC-conjugated antibody (clone PCH 101) or an APC-conjugated isotype control (rat IgG2A). A total of 100 000 cells were acquired on a LSR II flow cytometer (BD Biosciences) using BD FacsDiva software (BD Biosciences). Flow-Jo software (Tree Star, Ashland, OR) version 7.1.2 was used for graphic presentation and calculation of frequencies.
Tetramer staining
Peptide-MHC class I tetramers were produced as previously.5 CMVpp65–495/HLA-A*0201 and WT1/HLA-A*0201 tetramers conjugated to APC were used as positive and negative controls, respectively. Sample staining was performed using 2 × 106 PBMCs in 50 μL volume. Tetramers (1-2 μg per test with respect to the peptide–MHC class I component) were added for 20 to 30 minutes at 37°C. Cells were washed once and then stained with directly conjugated antibodies including CD3-PE, CD4-FITC, and CD8-PerCP (all from BD Biosciences). For tetramer analysis, a minimum of 1 000 000 total cells were acquired and analyzed as described in “Flow cytometry.”
Mixed lymphocyte reaction (MLR)
Stimulator cells in MLRs were either expanded lymphocytes (ExLy's) or pooled third-party PBMCs (3rd party) from 5 volunteer donors. Responder cells in MLRs were always PBMCs. Stimulators and responders were each adjusted to cell concentration of 106/mL in 10% normal AB serum (NABS) in RPMI 1640 media (Mediatech, Herndon, VA) supplemented with HEPES buffer and gentamicin. Stimulator cells were irradiated with 25 Gy (ExLy) or 50 Gy (3rd party). A volume of 100 μL/well (100 000 cells) of stimulator and responder cells was plated in 96-well round-bottomed microtiter plates in replicates of 6. Stimulator cells alone were plated in replicates of 12. Plates were incubated for 6 days for standard readouts and for 4, 5, and 6 days for proliferation kinetics at 37°C and 5% CO2. 3H-thymidine was added for the last 12 to 18 hours of incubation at 1 μCi (0.037 MBq) in 25 μL RPMI 1640 per well. Afterward, 3H-thymidine uptake was measured by a beta-scintillation counter. Results are expressed as mean and standard deviation of absolute counts per minute (cpm).
Helper T-lymphocyte precursor (HTLp) frequency assay
As previously described,30 this assay determines the frequency at limiting dilution of T-cell progenitors capable of generating an interleukin-2 (IL-2)–producing (T-helper) clone in response to a given and irradiated stimulator. Briefly, stimulator cells (patient PBMCs or pooled third-party PBMCs from 5 volunteer donors) were irradiated with 50 Gy (to block proliferation and cytokine production), adjusted to a concentration of 106/mL, and plated in 100 μL/well in 96-well round-bottomed microtiter plates. Responders were added in 24 replicates of 6 dilutions at concentrations of 10, 8, 6, 4, 2, and 1 × 104 cells/well for HLA-matched pairs and 4, 2, 1, 0.5, 0.25, and 0.125 × 104 cells/well for third-party stimulators. Stimulators alone were plated in 24 replicates. Plates were incubated for 64 hours at 37°C in 5% CO2, frozen at 80°C to stop further proliferation, and then thawed. The prepared IL-2–dependent 9.12 line (generously provided by Dr Charles Shih, Medical College of Wisconsin) was added at 2 × 103 cells/well in 25-μL volumes, and the plates were incubated for a further 20 hours. Then 3H-thymidine was added at 1 μCi (0.037 MBq) per well. Cells were harvested after 12 to 18 hours and 3H-thymidine uptake was measured. The background was defined as the mean control value plus 3 standard deviations of the 3H-thymidine uptake of the 24 control (stimulator only) wells. Test wells greater than this value were considered positive for IL-2 production. From the fraction of negative wells at each dilution, the frequency of IL-2–producing lymphocytes was calculated using the maximum likelihood method. IL-2 dependency of the 9.12 cell line was tested before each assay.
Staphylococcus aureus exotoxin B (SEB) proliferation assay
Responder cells were PBMCs adjusted to cell concentration of 106/mL in 10% normal AB serum (NABS) in RPMI 1640 media (Mediatech) supplemented with HEPES buffer and gentamicin. A volume of 100 μL/well of responder cells was plated in 96-well round-bottomed microtiter plates in replicates of 6. Costimulatory molecules and SEB were added. As a negative control, responder cells were solely incubated with costimulatory molecules. Plates were incubated for 4, 5, 6, and 7 days for proliferation kinetics at 37°C in 5% CO2. 3H-thymidine was added for the last 12 to 18 hours of incubation at 1 μCi (0.037 MBq) in 25 μL RPMI 1640 per well and 3H-thymidine uptake was measured. Results were expressed as stimulation index (stimulation index = cpm [responder + SEB + costim]/cpm [responder + costim]).
Quantitative reverse-transcription–polymerase chain reaction
RNA isolation was performed on magnetically selected CD4 and CD8 cells using RNeasy mini kits (Qiagen, Valencia, CA). Total RNA was eluted with water and stored at − 80°C. For reverse transcription of mRNA and cDNA synthesis, 1 μg total RNA was reverse transcribed and stored at − 20°C until polymerase chain reaction (PCR) analysis. Gene expression for β-actin and FOXP3 was measured using an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) as described previously.32
Measurement of intracellular TH9402
Indirect measurements of TH9402 were performed by flow cytometry detecting the dye in the FITC channel to track each photodepletion process. However, to determine the exact intracellular TH9402 concentrations before, during, and after the photodepletion process, aliquots of 0.5 × 106 viable cells were analyzed by fluorescence spectroscopy. After centrifugation, cell pellets were resuspended and lysed with 400 μL n-butanol (Sigma-Aldrich, Milwaukee, WI). Cell extracts from untreated PBMCs supplemented with defined amounts of TH9402 were used for the preparation of the standard curve and quality controls. For each sample, a total volume of 200 μL was loaded into a well of a black-colored polypropylene 96-well plate (Greiner Labortechnik, Biolynx, Brockville, ON) and analyzed by fluorescence spectroscopy (Gemini-XS spectrofluorometer; Molecular Devices, Sunnyvale, CA) using excitation and emission wavelengths at 514 nm and 568 nm, respectively. TH9402 concentrations were extrapolated from the standard curve and expressed as femtogram per cell.
Statistical analysis
Graphs and statistical analyses were performed with the use of Prism 4.00 for Windows software (GraphPad Software, San Diego, CA). P values of .05 or less were considered significant.
Results
We developed a clinical-scale, semiclosed photodepletion (PD) process enabling the selective allodepletion of large numbers of T cells (Figure 1). We used expanded T lymphocytes as allostimulators and adapted a small-scale, open PD system to the treatment of clinical-scale cell numbers, at high concentration, in a semiclosed culture system. Early experiments showed that high cell concentrations required lower concentrations of the photosensitizer. Optimum conditions for treating 5 × 106 viable cells/mL required 5.0 to 7.5 μM TH9402 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We performed 9 consecutive clinical-scale allodepletions, 6 in HLA-mismatched and 3 in HLA-matched pairs under these conditions (Table 1). In experiments VII to IX an automated, semiclosed Ficoll Hypaque density gradient separation was used to improve final product viability with regard to clinical feasibility. The absolute cell numbers processed in these experiments using a single photodepletion session are presented in Table 2.
Schematic description of the clinical-scale photodepletion approach. Stimulator cells from study subjects were prepared directly from leukapheresed mononuclear cells or from selected CD3+ cells and cultured using anti-CD3 antibody (OKT-3) and 100 IU interleukin-2 (IL-2) for 10 to 12 days, irradiated, and kept frozen thereafter. Donor cells (non–granulocyte–colony-stimulating factor [G-CSF]–mobilized leukapheresis mononuclear cells) were cocultured 1:1 with thawed, irradiated stimulator cells for 72 hours followed by incubation with the photosensitizer TH9402 at 5 × 106/mL cells for 40 minutes. Afterward, cells were transferred to an “extrusion” medium for 90 minutes and exposed to 514 nm visible light in the photodepletion light source at a cell concentration of 5 × 106/mL, in plastic bags. The total light energy delivered was 5 J/cm2. After light exposure, cells were rested for different time periods, separated by Ficoll Hypaque, washed, and used for the readout assays or frozen down for later analysis. *Optional.
Schematic description of the clinical-scale photodepletion approach. Stimulator cells from study subjects were prepared directly from leukapheresed mononuclear cells or from selected CD3+ cells and cultured using anti-CD3 antibody (OKT-3) and 100 IU interleukin-2 (IL-2) for 10 to 12 days, irradiated, and kept frozen thereafter. Donor cells (non–granulocyte–colony-stimulating factor [G-CSF]–mobilized leukapheresis mononuclear cells) were cocultured 1:1 with thawed, irradiated stimulator cells for 72 hours followed by incubation with the photosensitizer TH9402 at 5 × 106/mL cells for 40 minutes. Afterward, cells were transferred to an “extrusion” medium for 90 minutes and exposed to 514 nm visible light in the photodepletion light source at a cell concentration of 5 × 106/mL, in plastic bags. The total light energy delivered was 5 J/cm2. After light exposure, cells were rested for different time periods, separated by Ficoll Hypaque, washed, and used for the readout assays or frozen down for later analysis. *Optional.
Semiclosed, clinical-scale photodepletion and automated Ficoll (N = 3)
| Exp no. . | Stim put in coculture TNCs, ×109 . | Resp put in coculture TNCs, ×109 . | TNCs in coculture at start, ×109 . | TNCs in coculture after 72 h, ×109 . | TVCs in coculture after 72 h, ×109 . | Research sample TVCs, ×109 . | Cells proceeded to PD TVCs, ×109 . | Final SD product after wash and Ficoll TNCs, ×109 . | Viability, % . | CD3+ cells, % . | Total viable CD3+ cells, ×109 . | SD transplantation dose yield for a 70-kg patient, ×107/kg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VII | 5.40 | 5.40 | 10.80 | 7.80 | 5.40 | 0.40 | 5.00 | 1.82 | 91 | 72 | 1.19 | 1.70 |
| VIII | 12.00 | 12.00 | 24.00 | 17.30 | 9.90 | 3.90 | 6.00 | 1.43 | 87 | 81 | 1.01 | 1.44 |
| IX | 9.20 | 9.20 | 18.40 | 15.80 | 9.80 | 3.80 | 6.00 | 0.98 | 85 | 87 | 0.73 | 1.04 |
| Median | 9.20 | 9.20 | 18.40 | 15.80 | 9.80 | — | 6.00 | 1.43 | 87 | 81 | 1.01 | 1.44 |
| Exp no. . | Stim put in coculture TNCs, ×109 . | Resp put in coculture TNCs, ×109 . | TNCs in coculture at start, ×109 . | TNCs in coculture after 72 h, ×109 . | TVCs in coculture after 72 h, ×109 . | Research sample TVCs, ×109 . | Cells proceeded to PD TVCs, ×109 . | Final SD product after wash and Ficoll TNCs, ×109 . | Viability, % . | CD3+ cells, % . | Total viable CD3+ cells, ×109 . | SD transplantation dose yield for a 70-kg patient, ×107/kg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VII | 5.40 | 5.40 | 10.80 | 7.80 | 5.40 | 0.40 | 5.00 | 1.82 | 91 | 72 | 1.19 | 1.70 |
| VIII | 12.00 | 12.00 | 24.00 | 17.30 | 9.90 | 3.90 | 6.00 | 1.43 | 87 | 81 | 1.01 | 1.44 |
| IX | 9.20 | 9.20 | 18.40 | 15.80 | 9.80 | 3.80 | 6.00 | 0.98 | 85 | 87 | 0.73 | 1.04 |
| Median | 9.20 | 9.20 | 18.40 | 15.80 | 9.80 | — | 6.00 | 1.43 | 87 | 81 | 1.01 | 1.44 |
With regard to clinical feasibility and to improve viability of final selectively depleted products, photodepleted cells were washed and separated using a semiclosed, automated Ficoll Hypaque density gradient separation step directly after PD (day +0, Exp VII-IX). PD was performed in a single session using 2 bags (for 5.0 μM and 7.5 μM TH9402) with a maximum cell content of 3 × 109 viable cells per bag. For freshly obtained cell products, total nuclear cell counts were obtained. For cultured or treated products, total viable cell counts were obtained.
Stim indicates irradiated stimulator cell (expanded T lymphocytes); TNCs, total nucleated cells; Resp, responder cell; TVCs, total viable cells; and —, not available.
Generation of T-APCs
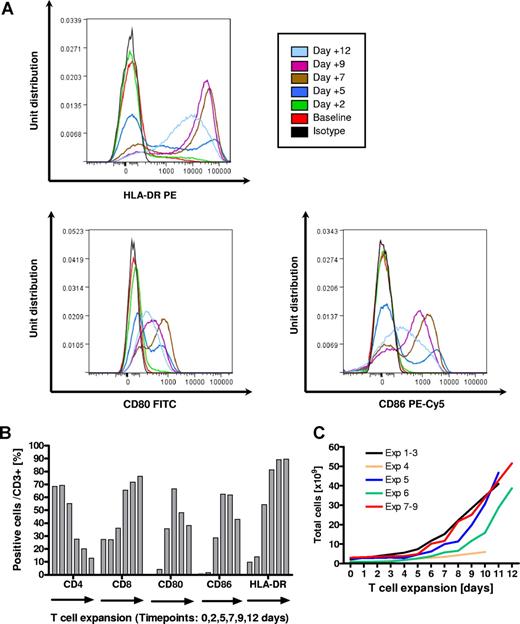
Expanded T lymphocytes (T-APCs) were either grown from unselected PBMCs or magnetically selected CD3+ T cells (Table 1). During expansion, T cells up-regulated expression of costimulatory molecules (CD80 and CD86) and MHC class II (HLA-DR). Median cell expansion was 18.2-fold (range, 8.5- to 40-fold) providing a median of 41.0 ×109 (range, 5.9-51.5 ×109) allostimulators (Figure 2).
Generation of expanded T lymphocytes (T-APCs) as stimulator cells using anti-CD3 antibody (OKT3) and interleukin-2 (IL-2). (A) Overlay histograms of up-regulation of MHC class II and the costimulatory molecules CD80 and CD86 as determined by flow cytometry in gated CD3 cells for the stimulator used in Exp's VII-IX. (B) Fractions of positive cells for CD4, CD8, CD80, CD86, and HLA-DR in CD3+ T cells during the expansion process for the stimulator used in Exp's VII-IX. (C) Numeral expansion of T cells in all experiments performed.
Generation of expanded T lymphocytes (T-APCs) as stimulator cells using anti-CD3 antibody (OKT3) and interleukin-2 (IL-2). (A) Overlay histograms of up-regulation of MHC class II and the costimulatory molecules CD80 and CD86 as determined by flow cytometry in gated CD3 cells for the stimulator used in Exp's VII-IX. (B) Fractions of positive cells for CD4, CD8, CD80, CD86, and HLA-DR in CD3+ T cells during the expansion process for the stimulator used in Exp's VII-IX. (C) Numeral expansion of T cells in all experiments performed.
Depletion efficacy and preservation of 3rd-party alloresponses
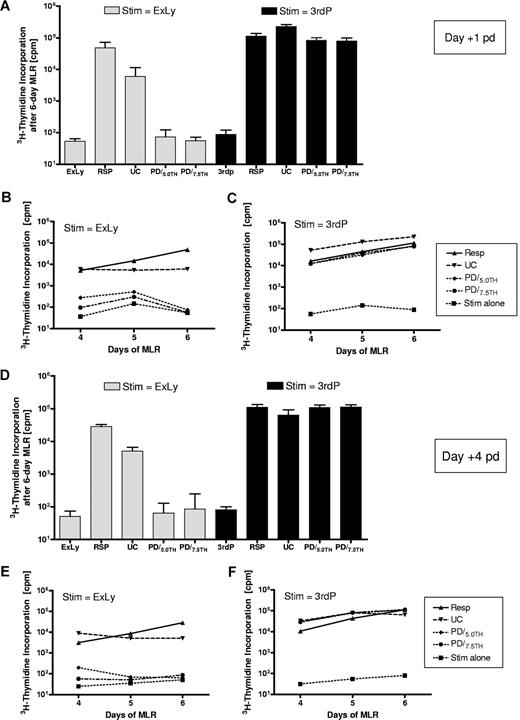
In 3 mismatched experiments (Exp's I-III) the 6-day MLR was performed on days +1 and +4 after PD. Alloreactivity after PD using 5.0 and 7.5 μM TH9402 (PD/5.0TH and PD/7.5TH) was compared with the alloreactivity of unmanipulated responder PBMCs (Resp) and the coculture without photodepletion, referred to as untreated control (UC). Figure 3 illustrates a representative experiment. On day +1 after depletion, mean (± standard deviation) alloreactivity of the selectively depleted products PD/5.0TH (74.2 ± 48.7 cpm) and PD/7.5TH (55.5 ± 16.8 cpm) was reduced compared with the original responder (48 401 ± 23 416 cpm) or the untreated control (5998 ± 5390 cpm) and approached the background alloreactivity of the irradiated stimulator (53.7 ± 10.5 cpm). In contrast, mean (± standard deviation) third-party alloresponses were relatively maintained in the se-lectively depleted products PD/5.0TH (83 945 ± 17 873 cpm) and PD/7.5TH (78 804 ± 20 146 cpm) compared with the original responder (113 654 cpm ± 23 286) or the untreated control (225 826 ± 39 432 cpm). Highly efficient allodepletion against the original stimulator and preservation of 3rd-party alloresponses was maintained in repeat MLRs over 4 to 6 days. In 3 further mismatched experiments (Exp's VII-IX), the functional readout was performed on days 0 and +1 after PD and provided comparable results, confirming that the proliferative capacity of alloactivated cells was inhibited directly after the PD procedure (data not shown).
Depletion efficacy and readout time. The presented experiment (Exp III) is representative for a set of 3 mismatched experiments performed under these conditions. (A,D) Alloreactivity of responder cells, the untreated control and the photodepleted product determined in 6-day mixed lymphocyte reactions against the original stimulator (ExLy) or third-party PBMCs 1 and 4 days after the photodepletion procedure. (B,C,E,F) Alloreactive courses of responder cells, the untreated control, and the photodepleted product determined in 4- to 6-day mixed lymphocyte reactions against the original stimulator (ExLy) or third-party PBMCs 1 and 4 days after the photodepletion procedure. Stim indicates irradiated stimulator cells; Resp, unmanipulated responder PBMCs; 3rdP, pooled third-party PBMCs; UC, untreated control; ExLy, expanded lymphocytes; PD/5.0TH and PD/7.5TH, PD products generated using 5.0 and 7.5 μM TH9402, respectively; cpm, counts per minute; and pd, after depletion.
Depletion efficacy and readout time. The presented experiment (Exp III) is representative for a set of 3 mismatched experiments performed under these conditions. (A,D) Alloreactivity of responder cells, the untreated control and the photodepleted product determined in 6-day mixed lymphocyte reactions against the original stimulator (ExLy) or third-party PBMCs 1 and 4 days after the photodepletion procedure. (B,C,E,F) Alloreactive courses of responder cells, the untreated control, and the photodepleted product determined in 4- to 6-day mixed lymphocyte reactions against the original stimulator (ExLy) or third-party PBMCs 1 and 4 days after the photodepletion procedure. Stim indicates irradiated stimulator cells; Resp, unmanipulated responder PBMCs; 3rdP, pooled third-party PBMCs; UC, untreated control; ExLy, expanded lymphocytes; PD/5.0TH and PD/7.5TH, PD products generated using 5.0 and 7.5 μM TH9402, respectively; cpm, counts per minute; and pd, after depletion.
Results from all 6 mismatched experiments obtained on day +1 after PD are summarized in Figure 4. A depletion index (DI) was used to calculate the reduction of alloreactivity after PD against unmanipulated responder PBMCs or the untreated coculture baselines. DIs were calculated for alloresponses against the original target and 3rd-party stimulators. Using the untreated control baseline, median depletion efficacy was significantly (P = .03) higher for 7.5 μM (DI = 15) compared with 5.0 μM (DI = 10) TH9402. In contrast, the third-party response median DI was not significantly different between 7.5 μM (DI = 1.5) and 5.0 μM TH9402 (DI = 2.2; Figure 4A). Using the original stimulator baseline, median DI was also significantly (P = .03) higher for 7.5 μM (DI = 474) compared with 5.0 μM (DI = 361) TH9402, while third-party responses were not significantly different between 7.5 μM (DI = 1.4) and 5.0 μM (DI = 1.5) TH9402 (Figure 4B). Thus, 7.5 μM TH9402 provided significantly better allodepletion against the original stimulator while conserving 3rd-party alloreactivity. Using this concentration of TH9402, alloreactivity against the original stimulator was significantly (P = .03) lower after PD compared with the original responder. In contrast, alloreactivity against a 3rd-party stimulator was not significantly different (Figure 4C).
Summary of depletion efficacy in 9 consecutive photodepletion (PD) experiments. Displayed P values were obtained using a 2-tailed Wilcoxon matched pair test. (A) Scatterplots (horizontal bars represent the median) of depletion efficacy in 6 mismatched experiments (Exp's I-III, VII-IX) as determined by mixed lymphocyte reactions (MLRs) one day after PD and calculated versus baseline alloreactivity of the untreated primary coculture: Depletion index = [alloreactivity of untreated control]/[alloreactivity of PD product]. (B) Scatterplots (horizontal bars represent the median) of depletion efficacy in 6 mismatched experiments (Exp's I-III, VII-IX) as determined by MLR and calculated versus baseline alloreactivity of unmanipulated responder PBMCs: Depletion index = [alloreactivity of unmanipulated responder]/[alloreactivity of PD product]. (C) Mismatched experiments (Exps I-III, VII-IX) and alloreactivity of MLRs before and after PD (N = 6). (D-F) Matched experiments (Exps IV-VI) and helper T-lymphocyte precursor (HTLp) frequency before and after PD (N = 3). Stim indicates irradiated stimulator cells; Pat, patient PBMCs; Resp, unmanipulated responder PBMCs; 3rdP, pooled third-party PBMCs; UC, untreated control; ExLy, expanded lymphocytes; PD/5.0TH and PD/7.5TH, PD products generated using 5.0 and 7.5 μM TH9402, respectively; DI, depletion index; and cpm, counts per minute.
Summary of depletion efficacy in 9 consecutive photodepletion (PD) experiments. Displayed P values were obtained using a 2-tailed Wilcoxon matched pair test. (A) Scatterplots (horizontal bars represent the median) of depletion efficacy in 6 mismatched experiments (Exp's I-III, VII-IX) as determined by mixed lymphocyte reactions (MLRs) one day after PD and calculated versus baseline alloreactivity of the untreated primary coculture: Depletion index = [alloreactivity of untreated control]/[alloreactivity of PD product]. (B) Scatterplots (horizontal bars represent the median) of depletion efficacy in 6 mismatched experiments (Exp's I-III, VII-IX) as determined by MLR and calculated versus baseline alloreactivity of unmanipulated responder PBMCs: Depletion index = [alloreactivity of unmanipulated responder]/[alloreactivity of PD product]. (C) Mismatched experiments (Exps I-III, VII-IX) and alloreactivity of MLRs before and after PD (N = 6). (D-F) Matched experiments (Exps IV-VI) and helper T-lymphocyte precursor (HTLp) frequency before and after PD (N = 3). Stim indicates irradiated stimulator cells; Pat, patient PBMCs; Resp, unmanipulated responder PBMCs; 3rdP, pooled third-party PBMCs; UC, untreated control; ExLy, expanded lymphocytes; PD/5.0TH and PD/7.5TH, PD products generated using 5.0 and 7.5 μM TH9402, respectively; DI, depletion index; and cpm, counts per minute.
In 3 matched experiments (Exp's IV-VI) alloreactive precursors against the original stimulator were reduced compared with the baseline, and postphotodepletion frequencies were lower than 1:100 000 (one patient already had a baseline frequency lower than 1:100 000). Precursor frequencies against 3rd-party stimulators were also reduced but remained around or higher than a threshold of 1:10 000 in all 3 experiments (Figure 4D-F).
Conservation of specific antiviral and antibacterial immunity
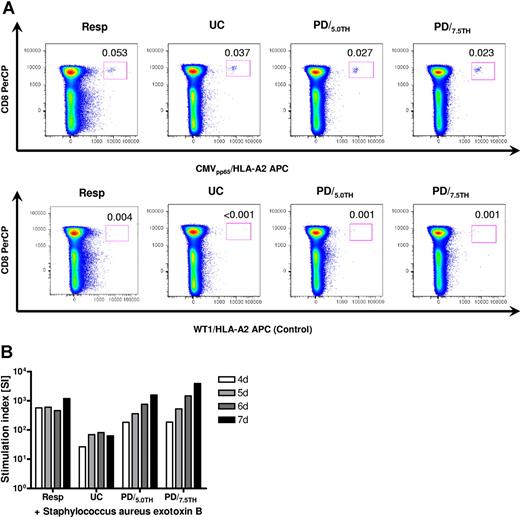
We investigated if specific antiviral and antibacterial immunity was preserved after PD in informative stimulator-responder pairs. In a cytomegalovirus (CMV)–seropositive, HLA-A2–positive responder depleted against a CMV-seropositive HLA-A1–positive stimulator, CMV-specific tetramer-positive CD8+ T cells persisted after selective depletion. The low baseline frequency of 0.053% CMV-specific T cells in CD8+ cells decreased to 0.037% during culture but persisted (0.023% using 7.5 μM TH9402) one day after photodepletion (Figure 5A). Tetramer analysis repeated 4 days after PD provided comparable results (data not shown). Proliferative responses against Staphylococcus aureus exotoxin B (SEB) were measured by 3H-thymidine incorporation. In both experiments, each performed in 2 subsets using 5.0 and 7.5 μM TH9402, proliferative responses were largely maintained after photodepletion compared with the unmanipulated responder and relatively increased compared with the untreated control (Figure 5B).
Maintenance of specific antiviral and antibacterial immunity. (A) Persistence of CMV-specific T cells one day after selective depletion in a CMV-seropositive, HLA-A2–positive responder selectively depleted against a CMV-seropositive, HLA-A1–positive stimulator (Exp VIII). An HLA-A2/WT126 tetramer was used as a negative control. Tetramer staining was repeated 4 days after depletion and provided comparable results. (B) Preservation of proliferative responses against Staphylococcus aureus exotoxin B (SEB) determined by 3H-thymidine incorporation 4 to 7 days after stimulation in a representative experiment (Exp VIII) of 2 performed. Resp indicates unmanipulated responder PBMCs; UC, untreated control (primary coculture); and PD/5.0TH and PD/7.5TH, photodepleted product using 5.0 μM and 7.5 μM TH9402, respectively.
Maintenance of specific antiviral and antibacterial immunity. (A) Persistence of CMV-specific T cells one day after selective depletion in a CMV-seropositive, HLA-A2–positive responder selectively depleted against a CMV-seropositive, HLA-A1–positive stimulator (Exp VIII). An HLA-A2/WT126 tetramer was used as a negative control. Tetramer staining was repeated 4 days after depletion and provided comparable results. (B) Preservation of proliferative responses against Staphylococcus aureus exotoxin B (SEB) determined by 3H-thymidine incorporation 4 to 7 days after stimulation in a representative experiment (Exp VIII) of 2 performed. Resp indicates unmanipulated responder PBMCs; UC, untreated control (primary coculture); and PD/5.0TH and PD/7.5TH, photodepleted product using 5.0 μM and 7.5 μM TH9402, respectively.
Effects of PD on regulatory T cells
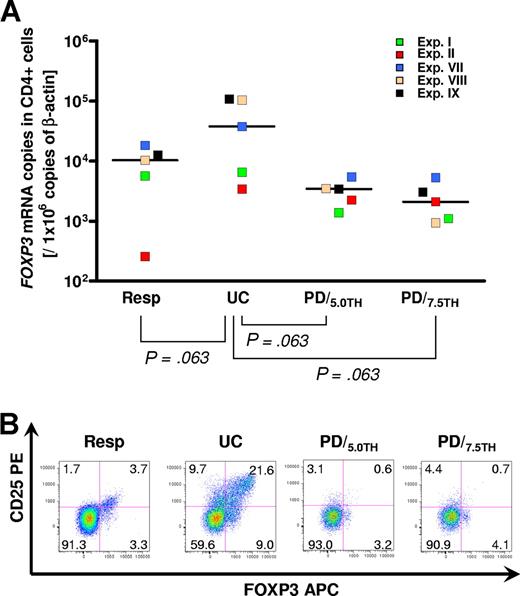
Compared with the unmanipulated responder, median FOXP3+ mRNA expression increased from TH9402 10 378 (range, 259-18 366) to 37 779 (range, 3400-108 399) copies/106 copies of β-actin in the untreated coculture (P = .063). After PD, median FOXP3+ expression was reduced to 3429 (range, 1375-5407) and 2103 (range, 944-5325) copies/106 copies β-actin when using 5.0 (P = .063) and 7.5 (P = .063) μM TH9402, respectively. Intracellular expression of FOXP3 protein showed the same trend: the fraction of FOXP3+ cells in CD4+ cells increased from 7% in the unmanipulated responder to 30.6% in the untreated control and declined to 3.8% and 4.8% after PD with 5.0 and 7.5 μM TH9402, respectively. The majority of FOXP3+CD4+ cells preserved after photodepletion were CD25−FOXP3+CD4+ cells. In addition to these, 3.1% and 4.4% CD25+FOXP3−CD4+ cells persisted 4 days after PD (Figure 6).
A fraction of CD4+FOXP3+ regulatory T cells persists after photodepletion. (A) Scatterplots of quantitative reverse transcription-PCR for FOXP3 mRNA in magnetically selected CD4+ cells performed between day + 2 and day + 4 after photodepletion (N = 5). Horizontal bars represent the median of each group. A 2-tailed Wilcoxon matched pair test was applied. (B) Flow cytometry showing surface staining for CD25 and intracellular staining for FOXP3 protein in gated CD4+ cells performed on day +4 after photodepletion (Exp II). One representative experiment of 5 is displayed. Resp indicates unmanipulated responder PBMCs; UC, untreated control (primary coculture); and PD/5.0TH and PD/7.5TH, photodepleted product using 5.0 μM and 7.5 μM TH9402, respectively.
A fraction of CD4+FOXP3+ regulatory T cells persists after photodepletion. (A) Scatterplots of quantitative reverse transcription-PCR for FOXP3 mRNA in magnetically selected CD4+ cells performed between day + 2 and day + 4 after photodepletion (N = 5). Horizontal bars represent the median of each group. A 2-tailed Wilcoxon matched pair test was applied. (B) Flow cytometry showing surface staining for CD25 and intracellular staining for FOXP3 protein in gated CD4+ cells performed on day +4 after photodepletion (Exp II). One representative experiment of 5 is displayed. Resp indicates unmanipulated responder PBMCs; UC, untreated control (primary coculture); and PD/5.0TH and PD/7.5TH, photodepleted product using 5.0 μM and 7.5 μM TH9402, respectively.
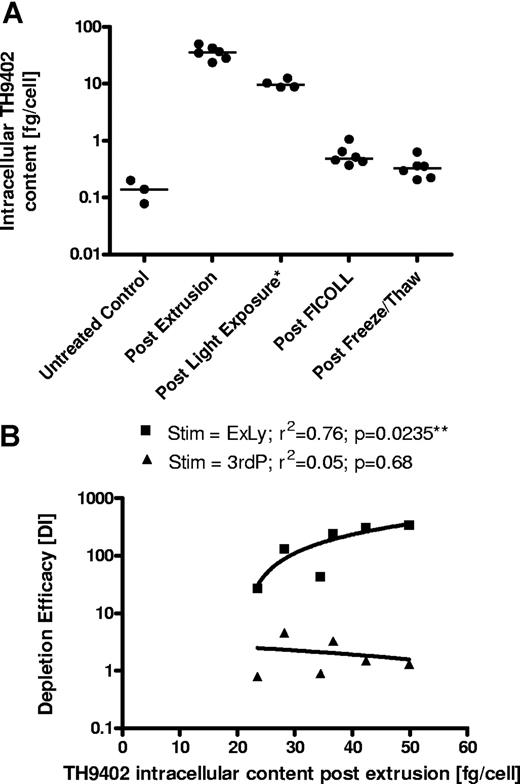
Intracellular TH9402 concentrations and depletion efficacy
The median amount of 0.140 fg/cell (range, 0.078-0.199 fg/cell) TH9402 per cell as obtained in the untreated control represents the analytic background. The median TH9402 amount after the extrusion phase was 35.553 fg/cell (range, 28.138-49.845 fg/cell) decreasing to 9.551 fg/cell (range, 8.702-12.474 fg/cell) after light exposure. After immediate Ficoll separation, the median TH9402 amount fell to 0.484 fg/cell (range, 0.370-1.054 fg/cell). After freezing and thawing, the median TH9402 amount fell to 0.325 fg/cell (range, 0.205-0.628 fg/cell), representing the residual TH9402 content. The intracellular TH9402 amount after extrusion correlated significantly with the depletion efficacy obtained against the original stimulator (r2 = 0.76; P = .024) but not against the 3rd party (r2 = 0.05; P = .68; Figure 7).
Intracellular TH9402 measurement and association with depletion efficacy (N = 6). Data from 3 experiments (Exp's VII-IX) using 2 different TH9402 concentrations (5.0 and 7.5 μM) are summarized. (A) Course of intracellular TH9402 during the depletion process. *In one experiment no sample was available for the post–light exposure analysis for either condition (N = 4). Residual TH9402 concentrations are minimal and just higher than the background of the untreated control (N = 3). (B) The depletion efficacy (measured by the depletion index) against the original stimulator but not against the third party was positively correlated with the residual dye concentration after extrusion (directly before light exposure). The correlation coefficients and P values of the linear regression analysis are presented. **Statistically significant P value.
Intracellular TH9402 measurement and association with depletion efficacy (N = 6). Data from 3 experiments (Exp's VII-IX) using 2 different TH9402 concentrations (5.0 and 7.5 μM) are summarized. (A) Course of intracellular TH9402 during the depletion process. *In one experiment no sample was available for the post–light exposure analysis for either condition (N = 4). Residual TH9402 concentrations are minimal and just higher than the background of the untreated control (N = 3). (B) The depletion efficacy (measured by the depletion index) against the original stimulator but not against the third party was positively correlated with the residual dye concentration after extrusion (directly before light exposure). The correlation coefficients and P values of the linear regression analysis are presented. **Statistically significant P value.
Discussion
Selective allodepletion is at an early stage of develop-ment and while there is accumulating evidence of its feasibility,7,9-13,15,17-23,25-27 the translation of this technically demanding process from bench to bedside remains challenging. The limited clinical experience with SD concerns mainly techniques using an anti–CD25-immunotoxin (CD25-IT).28-30 We previously showed that removal of host-reactive donor T cells from allografts by anti–CD25-IT was clinically feasible and reduced the frequency of severe aGvHD in a group of patients receiving solely low-dose immunosuppression.30 Furthermore, residual aGvHD could be attributed either to low donor Treg contents31 or insufficient removal of alloactivated T cells by the SD process.30 We assumed that the persistence of host-directed alloreactivity as measured by HTLp could have been caused by a down-regulation of CD25 surface expression in some alloactivated donor T cells, allowing them to escape allodepletion. To overcome the instability in surface-marker expression, we established a clinical-scale SD approach where we used TH9402-based PD to target activation-based changes in p-glycoprotein. We aimed to develop a technique to deliver selectively depleted T-cell doses in the range of the T-cell dose in standard bone marrow transplants (107 T cells/kg). Therefore, we adapted an open, small-scale PD system26,27 to treat high cell concentrations in semiclosed culture system and combined this approach with the use of expanded lymphocytes as allostimulators. We found that T-cell expansion was variable depending on apheresis volume, baseline T-cell fraction, and prior chemotherapy of the patient. Nevertheless, in all instances we harvested a minimum of 6 × 109 stimulators after 10 days of expansion allowing the coculturing of 12 × 109 donor cells. Assuming a 50% cell loss during the 3-day coculture period (stimulators are irradiated), around 6 × 109 cells would be submitted to PD resulting in approximately 109 total cells after Ficoll Hypaque density gradient separation and washing. The procedure should therefore reliably provide a minimum of 107 selectively depleted T cells/kg for transplantation using a single PD session.
Since alloactivated cells showed persisting CD25 expression for up to 3 days and nonspecific cell death becomes significant over extended culture times, it was not possible to determine exact depletion efficiency by surface phenotype. Functional readouts were therefore used. Despite the phenotypic persistence of some alloactivated cells, their proliferative capacity was lost immediately after PD. This rapid loss of proliferative function makes it feasible to safely transfuse selectively depleted cells immediately after PD. There was efficient depletion of alloreactivity against the original responder and persistence of 3rd-party alloreactivity in all experiments performed. Persistence of specific anti-infectious immunity was shown for SEB and CMV in a limited set of experiments. However, the ability of selectively depleted allografts to transfer enough antiviral and antifungal immunity to prevent infectious complications after transplantation cannot be answered in an experimental setting and needs to be proven in the ongoing clinical trial. Because baseline alloreactivity of the original responder or the untreated coculture against the original allostimulator varied among different experiments, we observed different levels of allodepletion ranging from less than 1 to almost 3 logs in mismatched donor-recipient constellations. However, in every experiment performed, we depleted all the alloreactivity recruited in the coculture, best reflected by the fact that residual alloreactivity after PD approached the background alloreactivity of the irradiated stimulator alone. The deactivation of p-glycoprotein in activated lymphocytes resulting in higher intracellular TH9402 concentrations after the extrusion phase has been postulated as the main mechanism of allodepletion.27 In support of this we found that the intracellular amount of TH9402 after the extrusion period was directly correlated with specific depletion efficacy. We also found that efficient allodepletion is feasible in HLA-matched pairs. In 3 experiments, 3rd-party helper T-lymphocyte precursors (HTLps) remained higher than 1:10 000, while HTLps against the original stimulator were diminished lower than 1:100 000. Since HTLp frequencies lower than 1:100 000 are associated with a significantly reduced risk for GvHD33,34 we anticipate that this clinical-scale process should prevent GvHD in matched siblings.
Despite the limitations associated with using flow cytometry as a readout for depletion efficacy, we repeatedly observed persistence of CD25 expression 4 days after PD. Guimond et al made similar observations and suggested that these persisting CD25+ cells could be regulatory CD4+ T cells (Tregs).27 Using FOXP3 as a reliable marker for the characterization of Tregs, we confirmed that some Tregs persisted 4 days after PD. These persisting Tregs contributed to a CD25− and CD25+foxp3+ population of CD4+ cells. In addition, some CD25+foxp3− effector CD4+ cells sustained after PD despite sufficient allodepletion as confirmed by functional assays. In our previous clinical trial, we showed that rapid Treg reconstitution can occur following selectively CD25-depleted allografting, either from CD25− Tregs escaping depletion or from residual CD25− and CD25+ Tregs contained in the stem cell product.31 Therefore, small Treg fractions contained in the PD product and transfused into a lymphopenic host leading to their expansion may confer protection against GvHD were allodepletion to be incomplete.
While the efficiency of the SD approach can be determined in vitro with functional assays, they cannot fully predict the clinical outcome that may also be determined by the choice of the APC used for the primary coculture. Thus, despite a fully effective depletion step, the failure of a recipient-derived APC to present GvHD-related antigens to the donor's T cells could result in GvHD. Other groups have used Epstein-Barr virus–transformed lymphoblastoid cell lines11,28 or unmanipulated PBMCs.29 However, an APC expressing myeloid or B-cell antigens could deplete beneficial GvL effects against B-cell or myeloid malignancies. There is accumulating evidence that activated expanded T cells are potent APCs.35 We used T cells as allostimulators as they are available in large numbers, easy to expand, and present T-cell lineage antigens and ubiquitously expressed minor antigens. The lymphocytes expanded for this set of experiments expressed high levels of costimulatory molecules and MHC class II as required for optimal antigen presentation. However, only a clinical trial will determine whether such T-APCs convey sufficient “GvHD message” to allow depletion of GvHD-reactive T-cell clones. One potential advantage of the T-cell APC is that the T-cell selection together with the T-cell expansion reduces the risk of contamination with residual malignant cells, thus protecting donor cells from depletion against B-cell or myeloid-restricted antigens also expressed by malignant cells of these lineages, or leukemia-specific antigens.
In establishing this SD approach, we set out to design a technique that could ultimately be adopted in many cell transplant processing centers. For this purpose, our experiments were all performed with scaled-up cell numbers using sterile bags and tubing systems comparable with a GMP process. Furthermore, we added an automated density gradient separation step allowing us to generate a cell product of high viability that meets the regulatory requirements for quality control and patient safety. This final process was reliably capable of generating at least 107/kg selectively depleted T cells for transfusion using a single PD session. Recent review of our Investigational New Drug application by the FDA and review of our clinical protocol by our Institutional Review Board have been successful, and we have initiated a clinical trial where HLA-identical sibling recipients with hematologic malignancies without a T-cell phenotype are given a CD34-cell selected transplant together with 5 × 106/kg viable selectively depleted donor T cells on day 0, following a radiation-based preparative regimen.
This SD approach has the potential to prevent severe acute GvHD, making it feasible to reduce or completely avoid immunosuppression thereby permitting enhanced residual T-cell responses against the malignant disease. In addition, SD may extend family donor availability by improving the safety of HLA-mismatched transplantations, permitting immune reconstitution without increased risk of acute GvHD.
Clearly a selectively depleted SCT is more costly than a standard unmanipulated transplant product. Specifically, our approach calls for 3 aphereses (one from the recipient, and separate stem cell and lymphocyte products from the donor). In addition, 2 magnetic selection steps are necessary (CD3 selection of the patient, and CD34 selection of the donor collections), and there is a 72-hour culture period, followed by a day-long procedure to prepare the photodepleted product. However, the additional expense of preparing a selectively depleted SCT can ultimately be justified only if SD is associated with less GvHD and reduced relapse rates, resulting in significantly reduced costs of posttransplantation care.
The online version of this article contains a data supplement.
Presented during a poster session at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, 2007.36
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
S.M. received grant support from the Dr-Mildred-Scheel-Stiftung für Krebshilfe, Germany. D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow.
We wish to acknowledge the contribution of Charles S. Carter, our friend and colleague, who supported this study with his unique expertise in the field of large-scale cell processing.
National Institutes of Health
Authorship
Contribution: S.M. conceived and designed the study, designed and performed the experiments, analyzed data, performed statistical analyses, and wrote the paper; R.N. performed experiments; K.R. performed experiments and commented on the paper; V.S.F. performed large-scale cell processing, coculturing, and photodepletion procedures, and commented on the paper; A.V. and S.R.S. were involved in the early initiation of this study; Y.F. performed large-scale magnetic bead selections; E.G. and D.A.P. produced fluorochrome-labeled peptide MHC class I tetrameric complexes; C.S. performed the intracellular TH9402 measurements by fluorescence spectroscopy; E.J.R. supervised the large-scale cell processing and commented on the paper; and A.J.B. supervised the study and wrote the paper.
Conflict-of-interest disclosure: Part of this work was supported by a collaborative research and development agreement (CRADA) between Kiadis (formerly Celmed) Inc and the NHLBI at the NIH. C.S. and A.V. are employed by and hold start-up share options with Kiadis Pharma Inc. C.S. holds start-up shares with Kiadis Pharma Inc. The clinical trial initiated based on the present work is funded in part under a clinical trial agreement (CTA) between Kiadis Inc and the NHLBI/NIH.
Correspondence: Stephan Mielke, Stem Cell Allogeneic Transplant Section, Hematology Branch, NHLBI, NIH, Bldg 10 CRC Rm 3-5288, 10 Center Dr MSC 1202, Bethesda, MD 20892-1202; e-mail: mielkes@nhlbi.nih.gov.

![Figure 1. Schematic description of the clinical-scale photodepletion approach. Stimulator cells from study subjects were prepared directly from leukapheresed mononuclear cells or from selected CD3+ cells and cultured using anti-CD3 antibody (OKT-3) and 100 IU interleukin-2 (IL-2) for 10 to 12 days, irradiated, and kept frozen thereafter. Donor cells (non–granulocyte–colony-stimulating factor [G-CSF]–mobilized leukapheresis mononuclear cells) were cocultured 1:1 with thawed, irradiated stimulator cells for 72 hours followed by incubation with the photosensitizer TH9402 at 5 × 106/mL cells for 40 minutes. Afterward, cells were transferred to an “extrusion” medium for 90 minutes and exposed to 514 nm visible light in the photodepletion light source at a cell concentration of 5 × 106/mL, in plastic bags. The total light energy delivered was 5 J/cm2. After light exposure, cells were rested for different time periods, separated by Ficoll Hypaque, washed, and used for the readout assays or frozen down for later analysis. *Optional.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-08-104471/6/m_zh80020811370001.jpeg?Expires=1769149631&Signature=24I-eDSCm6wIpMRVE9OxLzeTZOJZAnEqwiNnQ0-uqX44M9DpXBC4ZHEubqpCCvBUO3G0jWeoSRbhwJu3XSQsp-Ztgkw4OInzEn0xSfprPfNj34B7ff930m-mH13C6eMhDv7FUOE2RtjyI7Ae4d2FKVuWlyEasZxO9T4Dx~XSqZOg3kl13BzO35MX3gKHEKXc~bA0t7hKpEFlltHDudTPNB17yXxECjxNcnUgwQ~6v0jQaYq5Dr7wzZxvip3uA4vDy4IgJFFnNu4Ij3o2LNWLDy2p7Q4VppVmrAPvclLodG83mXYt0N3rFJSq--TbGIodnb9SYhoxZAWvHIISjeKtmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Summary of depletion efficacy in 9 consecutive photodepletion (PD) experiments. Displayed P values were obtained using a 2-tailed Wilcoxon matched pair test. (A) Scatterplots (horizontal bars represent the median) of depletion efficacy in 6 mismatched experiments (Exp's I-III, VII-IX) as determined by mixed lymphocyte reactions (MLRs) one day after PD and calculated versus baseline alloreactivity of the untreated primary coculture: Depletion index = [alloreactivity of untreated control]/[alloreactivity of PD product]. (B) Scatterplots (horizontal bars represent the median) of depletion efficacy in 6 mismatched experiments (Exp's I-III, VII-IX) as determined by MLR and calculated versus baseline alloreactivity of unmanipulated responder PBMCs: Depletion index = [alloreactivity of unmanipulated responder]/[alloreactivity of PD product]. (C) Mismatched experiments (Exps I-III, VII-IX) and alloreactivity of MLRs before and after PD (N = 6). (D-F) Matched experiments (Exps IV-VI) and helper T-lymphocyte precursor (HTLp) frequency before and after PD (N = 3). Stim indicates irradiated stimulator cells; Pat, patient PBMCs; Resp, unmanipulated responder PBMCs; 3rdP, pooled third-party PBMCs; UC, untreated control; ExLy, expanded lymphocytes; PD/5.0TH and PD/7.5TH, PD products generated using 5.0 and 7.5 μM TH9402, respectively; DI, depletion index; and cpm, counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-08-104471/6/m_zh80020811370004.jpeg?Expires=1769149631&Signature=xa5u~I08yj42mJ4uXOR9DIRoTFi2wIgwP1UPypFldoPOTQt39UVYotmwF-Sn2wI2PyMpop82bsAtAAsm1EAJJgNGHWa-zYr~boGioLXmpZwZXMQbtcEo5Wgli-3IHT~~HvOowjI6DporqVzy7~4HgIY2luVez4NWVBZMRTFas2sd1tcY7lH2s2YJgWiZp5adaRiYW8cobBTWBdE78c5nL6Riyol6iuhD7iID8gYzRueScjXHyAJOdOX0k~UJAxiKPfZ3tfUdz7KzK6D9Hi6En2GLGCtWiOObxy9ty40-R1ku73wgTNK7LZqhM0mtn-fRozn7wo1v5NnoHdrEWC2ZZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal