To the editor:
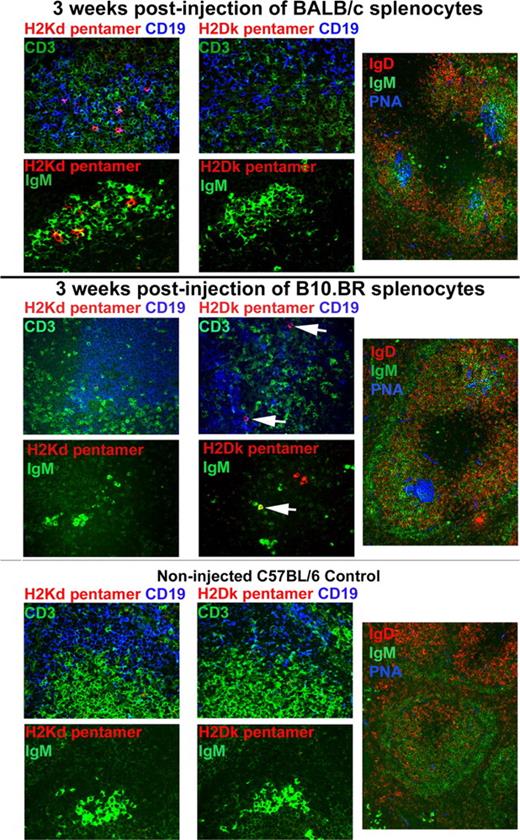
We recently published a report in Blood documenting that, in allosensitized murine recipients, preformed antibody is the initial barrier to bone marrow engraftment.1 We now demonstrate a novel method to detect alloantibody secreting cells in situ in our allosensitization model using immunohistochemistry and H2 pentamers. Nonirradiated 10-week-old female C57BL/6 (H2b) mice were injected intravenously with 20 × 106 BALB/c (H2d) splenocytes (red-cell depleted) and electively killed 3 weeks later. Spleen cryosections were cut 6-μm thick and fixed in acetone for 5 minutes at room temperature. Figure 1 (top panels) shows sections that were 3-color stained using biotinlyated H2Kd-pentamer (peptide sequence GYKDGNEYI, ProImmune, Oxford, United Kingdom) used at 0.5 μg/mL PBS containing 1% normal donkey serum (1 hour at room temperature) followed by Streptavidin-Cy3 (Jackson ImmunoResearch, West Grove, PA) shown in red, rat anti–mouse CD19 (BD PharMingen, San Jose, CA) with Cy5-labeled antirat (Jackson Immunoresearch) shown in blue and rabbit anti–mouse CD3 (BD PharMingen) with Alexa488-labeled antirabbit antibody (Molecular Probes, Eugene, OR) shown in green. Sections were analyzed by confocal microscopy using an Olympus BX51 FluoView 500 confocal microscope and FluoView software. Fuschia-colored cells represent pentamer binding, CD19+ B cells. As a staining specificity control, serial sections were stained with H2Dk pentamer (peptide sequence RRLGRTLLL, ProImmune) and were negative. As a specificity control for the alloresponse, C57BL/6 (H2b) mice were injected intravenously with 20 × 106 B10.BR (H2k) splenocytes and electively killed 3 weeks later. The middle panels in Figure 1 show that H2Dk (white arrows), instead of H2Kd, pentamer-binding B cells are present. Further corroborating evidence for pentamer-binding B cells was obtained by staining serial cryosections with biotinylated H2 pentamers with Streptavidin-Cy3 shown in red and FITC-labeled anti-IgM (BD PharMingen) shown in green. Pentamer+, IgM+ cells are orange-yellow in intensely IgM+ areas typical of germinal centers (GC). We did not detect any pentamer+ cells in the spleens of noninjected C57BL/6 controls (Figure 1 bottom panels). This allosensitization model induced numerous, robust GCs in the spleen as illustrated in splenic cryosections (Figure 1 right panels) stained with FITC-labeled anti-IgM (green), PE-labeled anti-IgD (BD PharMingen; red) and biotinylated PNA (Vector Laboratories, Burlingame, CA) with Streptavidin-Cy5 (blue). GCs are shown as aqua-blue because GC B cells are IgM+, IgD−, PNA+. No, or rare, GCs were found in the spleens of noninjected C57BL/6 controls.
Allospecific B cells can be detected in situ in allosensitized murine spleen using pentamers. Colors for the different markers are indicated in colored text. Magnification is 400× (left and middle panels) and 200× (right panels).
Allospecific B cells can be detected in situ in allosensitized murine spleen using pentamers. Colors for the different markers are indicated in colored text. Magnification is 400× (left and middle panels) and 200× (right panels).
There are recent reports of MHC or HLA class I tetramers being used to detect allospecific B cells by flow cytometry in mice2 and in humans.3-5 To our knowledge, this is the first report of pentamers being used for this purpose, and the first time that allospecific B cells have been detected in situ. We view this as a significant advance because it now paves the way for one to ask site-specific questions to obtain spatial and geographical information concerning the sites of alloantibody production and the cellular interactions required for this sensitization in this rodent model. In addition, this technique is applicable to studies of tissue samples, blood, and bone marrow cells from human allosensitized recipients.
Authorship
We would like to acknowledge the technical assistance of Mike Ehrhardt and the use of the confocal microscope made available through an National Center for Research Resources (NCRR) Shared Instrumentation Grant (#1 S10 RR16851).
This work was funded by National Institutes of Health R01 HL63452 (B.R.B.).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Panoskaltsis-Mortari, Department of Pediatrics, Division of Blood and Marrow Transplantation and the Cancer Center, University of Minnesota, 420 Delaware St SE, MMC 366, Minneapolis, MN 55455; e-mail: panos001@umn.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal