To the editor:
We recently reported that using plasma rather than peripheral blood cells improves detection of the JAK2 V617F mutation,1 which is the most important characteristic molecular abnormality in patients with nonchronic myeloid leukemia (non-CML)–myeloproliferative disease (MPD).2-4 Here we report the findings after expanding on our study and testing 634 consecutive paired plasma and cell samples for the V617F mutation and mutations in JAK2 exon 12. All 634 samples were collected from patients who were clinically suspected of having a non-CML MPD. We report that the V617F mutation was detected in 151 (24%) patients when plasma was used and only in 140 (22%) paired cell samples. No patients were positive by cell analysis and negative by plasma analysis while 7% of patients positive by plasma were negative by cells.
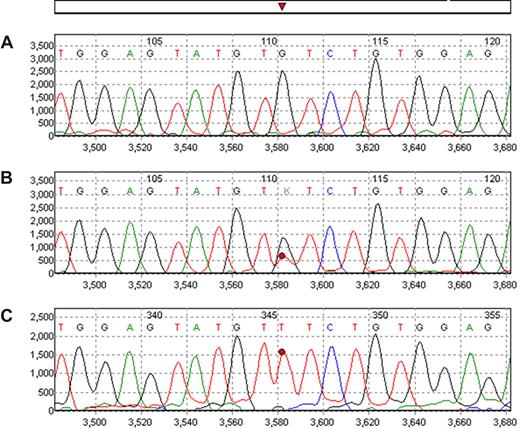
When direct sequencing is used, a dominant (> 80%) mutant (T) peak is usually indicative of homozygous (2 mutant genes) or hemizygous mutation (one mutant gene and one deleted gene).1 Of the 151 V617F-positive samples, 58 (38%) showed homozygous/hemizygous mutation by plasma analysis; but only 36 (24%) were homozygous/hemizygous by cell analysis.
All testing for JAK2 mutations was performed using direct bidirectional sequencing of JAK2 mRNA. Two PCR primers were designed to amplify a 273-bp segment of JAK2 encompassing the codon for amino acid 617. A second set of primers was designed to amplify a 491-bp segment encompassing exons 12, 13, and 14. The primer sequences used were as follows: 5′-GAC TAC GGT CAA CTG CAT GAA A-3′ and 5′-CCA TGC CAA CTG TTT AGC AA-3′ for the 273-bp segment and 5′-tgt aaa acg acg gcc agt CTA AAT GCT GTC CCC CAA AG-3′; and 5′-cag gaa aca gct atg acc CCA TGC CAA CTG TTT AGC AA-3′ (uppercase indicates the actual sequences, and lower case represents the M13 linker) for the 491-bp. The Surveyor software (SoftGenetics, State College, PA) determines the intensity of the mutant peak and the wild-type peak after adjusting relative intensity according to peaks of surrounding similar nucleotides. In patients classified as heterozygous, the G and T peaks were generally equal in plasma samples whereas the T peak was less intense in cell samples (Fig 1). The overall ratios of V617F were significantly higher in plasma (median: 0.61; range: 0.14-1.00) than in cells (median: 0.49, range: 0.00-1.00; P < .001). In cases that were considered heterozygous, the median ratios of mutant:wild-type was 0.51 (range: 0.13-0.80) for plasma and 0.32 (range: 0-0.68) for cells. This also suggests that plasma is enriched with tumor-specific nucleic acid.5-7 Furthermore, for samples determined to be negative by cell analysis but positive by plasma analysis, a considerable ratio (> 13%) was detected in the plasma, also confirming the enrichment of plasma by tumor-specific nucleic acid. None of the 634 tested samples showed any mutation in exon 12. However, additional testing of samples from patients with MPD showed easy detection of exon 12 mutations in plasma.
Example of JAK2 V617F mutation as detected in peripheral blood cells and plasma. Wild-type normal control is shown in (A). In trace B the analysis of the peripheral blood cells shows the mutated peak (T) as heterozygous while in plasma sample from the same patient obtained at the same time (trace C) the mutant peak is clearly homozygous/hemizygous.
Example of JAK2 V617F mutation as detected in peripheral blood cells and plasma. Wild-type normal control is shown in (A). In trace B the analysis of the peripheral blood cells shows the mutated peak (T) as heterozygous while in plasma sample from the same patient obtained at the same time (trace C) the mutant peak is clearly homozygous/hemizygous.
In summary, these data show that plasma is enriched with tumor-specific nucleic acid and should be the sample of choice for testing for JAK2 mutations.
Authorship
Conflict-of-interest disclosure: W.M., X.Z., W.S., A.M.B., I.J., J.G.S., and M.A. work for Quest Diagnostics, which offers plasma testing of JAK2 mutations. The other authors declare no competing financial interests.
Correspondence: Dr Maher Albitar, Quest Diagnostics Nichols Institute, 33608 Ortega Highway, San Juan Capistrano, CA 92675; e-mail: maher.x.albitar@questdiagnostics.com.