Abstract
An understanding of the prognostic factors associated with the various forms of induction mortality in patients with acute promyelocytic leukemia (APL) has remained remarkably limited. This study reports the incidence, time of occurrence, and prognostic factors of the major categories of induction failure in a series of 732 patients of all ages (range, 2-83 years) with newly diagnosed APL who received all-trans retinoic acid (ATRA) plus idarubicin as induction therapy in 2 consecutive studies of the Programa de Estudio y Tratamiento de las Hemopatias Malignas (PETHEMA) Group. Complete remission was attained in 666 patients (91%). All the 66 induction failures were due to induction death. Hemorrhage was the most common cause of induction death (5%), followed by infection (2.3%) and differentiation syndrome (1.4%). Multivariate analysis identified specific and distinct pretreatment characteristics to correlate with an increased risk of death caused by hemorrhage (abnormal creatinine level, increased peripheral blast counts, and presence of coagulopathy), infection (age >60 years, male sex, and fever at presentation), and differentiation syndrome (Eastern Cooperative Oncology Group [ECOG] score >1 and low albumin levels), respectively. These data furnish clinically relevant information that might be useful for designing more appropriately risk-adapted treatment protocols aimed at reducing the considerable problem of induction mortality in APL.
Introduction
Since the routine introduction of all-trans retinoic acid (ATRA) in front-line therapy of acute promyelocytic leukemia (APL), significant improvements in patient outcomes were achieved. A number of studies conducted during the past decade have contributed to the optimizing of the antileukemic efficacy of ATRA, especially when it is combined with chemotherapy.1-3 In fact, the current standard for induction therapy is the simultaneous combination of ATRA with anthracycline-based chemotherapy, which results in extremely high antileukemic efficacy, achieving a 90% to 95% complete remission (CR) rate. Although leukemia resistance to therapy has become an uncommon cause of remission induction failure, death during induction from hemorrhage, infection, and differen-jtiation syndrome (formerly retinoic acid syndrome) has re-mained the main problem during the early treatment phase. The frequency of induction death from medical complications has probably not changed during recent years. The relative incidence and time of occurrence of each of these categories of induction failure, as well as their pretreatment characteristics (prognostic factors), have been investigated critically and in detail in rare studies only.4
The present study reports the incidence, time of occurrence, and prognostic factors of the major categories of induction failure in a large series of 732 patients with newly diagnosed APL who received ATRA plus idarubicin (AIDA)5 as induction therapy in 2 consecutive studies of the Programa de Estudio y Tratamiento de las Hemopatias Malignas (PETHEMA) Group (LPA96 and LPA99).
Methods
Eligibility
Patients enrolled in the consecutive PETHEMA LPA96 and LPA99 trials were required to have a diagnosis of de novo APL with demonstration of the t(15;17) or PML/RARα rearrangements, normal hepatic and renal function, no cardiac contraindications to anthracycline chemotherapy, and an Eastern Cooperative Oncology Group (ECOG) performance status of less than 4. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The protocol was approved by the Research Ethics Board of each participating hospital. A list of the participating institutions and clinicians can be found in Document S1 (available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article).
Induction therapy
Induction therapy consisted of oral ATRA (45 mg/m2 per day) divided into 2 daily doses, which was maintained until complete hematologic remission or for a maximum of 90 days, and idarubicin (12 mg/m2 per day) given as an intravenous bolus on days 2, 4, 6, and 8 (AIDA regimen). For patients 20 years of age or younger, the ATRA was adjusted to 25 mg/m2 per day. Since November 1999 (LPA99 trial), the dose of idarubicin on day 8 has been omitted for patients older than 70 years of age. Treatment with ATRA was started as soon as a diagnosis of APL was made by morphologic criteria.6,7 For patients in whom the diagnosis was not confirmed by genetic studies, ATRA treatment was withdrawn, and alternative chemotherapy was given at the physician's discretion.
Supportive measures
Management of coagulopathy.
Platelet transfusions were given to maintain a platelet count of more than 30 × 109/L until resolution of the coagulopathy. Once the coagulopathy was under control, platelet transfusions were only given for patients with infectious or hemorrhagic manifestations, or when the platelet count dropped below 20 × 109/L. Patients with active coagulopathy were treated with fresh frozen plasma, cryoprecipitate, or fibrinogen to maintain a fibrinogen level higher than 1.5 mg/L and hemostatic levels of coagulation factors. Heparin prophylaxis was not recommended, except for the treatment of thrombotic events. Since November 1999 (LPA99 study), patients with platelet counts less than 50 × 109/L or evident clinical-biologic signs of coagulopathy have received tranexamic acid (100 mg/kg per day) by continuous intravenous infusion until the disappearance of signs of coagulopathy and the platelet count was higher than 50 × 109/L.
Management of differentiation syndrome.
At the first signs of suspected differentiation syndrome (DS), patients were given 10 mg dexamethasone every 12 hours. ATRA was discontinued only in case of progression to severe DS. In the LPA96 trial, patients with a white blood cell (WBC) count greater than 5 × 109/L received prophylaxis with dexamethasone (10 mg/12 h intravenously for 7 days), whereas in the LPA99 study, all patients received DS prophylaxis with prednisone (0.5 mg/kg per day orally) from days 1 to 15.
Other supportive measures.
Patients were admitted in single or double conventional rooms with no specific isolation measures. Management of febrile episodes was not standardized and varied with the practice of each institution. Treatment with granulocyte colony stimulating factors was not recommended. For patients with extreme hyperleukocytosis, hydroxyurea and leukapheresis were given at the physician's discretion. Packed red cell transfusions were recommended to maintain blood hemoglobin greater than 9 g/dL.
Definitions and study endpoints
Remission induction response was assessed according to the recently revised criteria by Cheson et al8 A morphologic CR designation requires less than 5% blasts and atypical promyelocytes in an aspirate sample and an absolute neutrophil count of more than 109/L and platelets of more than 100 × 109/L. Hemoglobin concentration or hematocrit has no bearing on remission status, although the patient must be independent of transfusions. Treatment failure includes those patients for whom treatment has failed to achieve a CR. The causes of treatment failure were classified as follows.
Resistant disease.
In APL, at variance with other subtypes of acute myeloblastic leukemia, leukemia resistance can be assessed only after sufficient time has passed to allow for full terminal differentiation of the malignant promyelocytes. The bone marrow aspirate performed too early after induction therapy may reveal a hypercellular specimen with residual blasts and atypical promyelocytes and may give the misleading impression of response failure, occasionally even after some weeks after the start of treatment (up to 40-50 days).
Death during induction therapy.
Causes of induction deaths include the following categories.
Infection.
When death is due to a clinical, radiologic, or microbiologically documented infection.
Hemorrhage.
When a major bleeding occurs in a vital organ (central nervous system, lungs). Gastrointestinal tract hemorrhage requires massive melena or hematemesis accompanied by fall in blood pressure.
Differentiation syndrome.
That is, death occurring in patients with definitely present DS and not explained by infection, hemorrhage, or any other cause. Definitely present DS is defined as the presence of at least 4 of the characteristic signs or symptoms described by Frankel et al9 : fever, dyspnea, pleural or pericardial effusion, pulmonary infiltrates, renal failure, hypotension, and unexplained weight gain greater than 5 kg.
Other.
That is, any other cause not classified as infection, hemorrhage, or DS that was the cause of death.
Duration of neutropenia and thrombocytopenia was defined as the time, in days, from the end of chemotherapy until the day of the first measurement of absolute neutrophil count greater than 109/L and platelets greater than 50 × 109/L, respectively.
Prognostic factors
Twenty-two patient and disease characteristics documented at initial evaluation were examined in the prognostic factor analysis to establish their relation to induction response. Basic demographic data and clinical characteristics at presentation included age, sex, ECOG score, fever, total body surface, as well as liver and spleen enlargement. Serum biochemical parameters were creatinine, uric acid, and albumin. Peripheral blood features included hemoglobin level and platelet, WBC, and blast cell counts. Bone marrow aspirate parameters evaluated were cellularity, peroxidase reactivity, French-American-British subtype, cytogenetics, and PML/RARA isoforms. The protocol, fibrinogen level, and the presence of coagulopathy were also included. The latter was defined as a prolonged prothrombin time or activated partial thromboplastin time, in addition to hypofibrinogenemia or increased levels of fibrin degradation products or D-dimers.
Statistical analysis
The chi-square test with Yates correction and Fisher exact test were used for statistical analyses. P values were calculated using the 2-tailed test. Characteristics selected for inclusion in the multivariate analysis were those for which there was some indication of a significant association with induction failure in univariate analysis (P < .1) and, if available, those that prior studies had suggested a possible relation. Multivariate analysis was performed using a logistic regression model.10 Missing data were substituted by the mean values from patients in whom data were available.11 Computations were performed using the programs from the BMDP statistical library (BMDP Statistical Software, Los Angeles, CA).
Results
Accrual and patient characteristics
Between November 1996 and June 2005, 792 consecutive patients with genetic diagnosis of APL were registered from 82 institutions from Spain, The Netherlands, Argentina, Uruguay, and the Czech Republic. A total of 42 patients (5%) were considered not eligible for the treatment because of severe clinical condition contraindicating the administration of chemotherapy, 8 (4%) and 34 (6%) in the LPA96 and LPA99 trials, respectively. Thus, 750 patients met the previously defined entry criteria and were enrolled in the LPA96 and LPA99 studies. Eighteen additional patients were not evaluated because of protocol violations during induction therapy (8 of 181 in the LPA96 trial and 10 of 570 in the LPA99 trial). Causes of protocol violation were the addition of cytarabine (10 patients), premature administration of salvage therapy because of inappropriate assessment for response as resistant leukemia (6 patients), and lost to follow-up because of transfer to another hospital during induction therapy (2 patients). The 6 patients inappropriately assessed for response had been evaluated during pancytopenia on days 18, 20, 23, 26, 30, and 33 after chemotherapy and 4 of them had developed a definitely present DS. Five patients achieved CR after second-line therapy. Details about noneligible and nonevaluable patients are shown in Table 1.
Reasons for the exclusion from the study
| . | No. of patients . |
|---|---|
| Noneligible patients | |
| Poor performance status/severe active infection or hemorrhage | 28 |
| Intracranial hemorrhage | 15 |
| Pulmonary hemorrhage | 4 |
| Septic shock (bacteremia) | 3 |
| Pneumonia | 1 |
| Encephalitis | 1 |
| Severe thromboembolism | 3 |
| Unfit for chemotherapy | 14 |
| Very elderly patients (75, 78, 78, 82, 88, 90 y) | 6 |
| ECOG score of 4 | 1 |
| Cardiac contraindications | 3 |
| Abnormal renal or hepatic function | 2 |
| Serious psychiatric illness | 2 |
| Hemiplegia after stroke | 1 |
| Nonevaluable patients (protocol violations) | 18 |
| Addition of cytarabine | 10 |
| Premature administration of salvage therapy because of inappropriate assessment for response | 6 |
| Lost to follow-up because of transfer to another hospital | 2 |
| . | No. of patients . |
|---|---|
| Noneligible patients | |
| Poor performance status/severe active infection or hemorrhage | 28 |
| Intracranial hemorrhage | 15 |
| Pulmonary hemorrhage | 4 |
| Septic shock (bacteremia) | 3 |
| Pneumonia | 1 |
| Encephalitis | 1 |
| Severe thromboembolism | 3 |
| Unfit for chemotherapy | 14 |
| Very elderly patients (75, 78, 78, 82, 88, 90 y) | 6 |
| ECOG score of 4 | 1 |
| Cardiac contraindications | 3 |
| Abnormal renal or hepatic function | 2 |
| Serious psychiatric illness | 2 |
| Hemiplegia after stroke | 1 |
| Nonevaluable patients (protocol violations) | 18 |
| Addition of cytarabine | 10 |
| Premature administration of salvage therapy because of inappropriate assessment for response | 6 |
| Lost to follow-up because of transfer to another hospital | 2 |
ECOG indicates Eastern Cooperative Oncology Group.
The main clinical and biologic characteristics of the 732 patients evaluable for induction are shown in Table 2.
Demographic and baseline characteristics of the study population
| Characteristic . | LPA96 . | LPA99 . | P . | Total . | |||
|---|---|---|---|---|---|---|---|
| Median (range) . | No. (%) . | Median (range) . | No. (%) . | Median (range) . | No. (%) . | ||
| Overall | — | 172 (24) | — | 560 (76) | — | — | 732 (100) |
| Age, y | 39 (2-78) | — | 40 (2-83) | — | — | 40 (2-83) | — |
| 15 or younger | — | 11 (6) | — | 48 (9) | .83 | — | 59 (8) |
| 16 to 40 | — | 75 (43) | — | 240 (43) | — | — | 315 (43) |
| 41 to 60 | — | 57 (33) | — | 171 (30) | — | — | 228 (31) |
| 61 to 70 | — | 19 (11) | — | 68 (12) | — | — | 87 (12) |
| 71 or older | — | 10 (6) | — | 33 (6) | — | — | 43 (6) |
| Sex | |||||||
| Male | — | 102 (59) | — | 270 (48) | .01 | — | 372 (51) |
| Female | — | 70 (41) | — | 290 (52) | — | — | 360 (49) |
| ECOG score | 1 (0-3) | — | 1 (0-3) | — | — | 1 (0-3) | — |
| 0 to 1 | — | 130 (81) | — | 377 (73) | .07 | — | 507 (75) |
| 2 | — | 20 (13) | — | 102 (20) | — | — | 122 (18) |
| 3 | — | 10 (6) | — | 36 (7) | — | — | 46 (7) |
| Fever | |||||||
| No | — | 108 (63) | — | 333 (60) | .57 | — | 441 (61) |
| Yes | — | 64 (37) | — | 220 (40) | — | — | 284 (39) |
| WBC count, × 109/L | 2.0 (0.3-210) | — | 2.2 (0.2-460) | — | — | 2.2 (0.2-460) | — |
| Less than 5 | — | 108 (62) | — | 336 (60) | .79 | — | 444 (61) |
| 5 to 10 | — | 21 (13) | — | 84 (15) | — | — | 105 (14) |
| 10 to 50 | — | 31 (18) | — | 99 (18) | — | — | 130 (18) |
| 50 or higher | — | 12 (7) | — | 40 (7) | — | — | 52 (7) |
| PB blast count, × 109/L | 0.6 (0-210) | — | 0.7 (0-179) | 0.7 (0-210) | |||
| Less than 30 | — | 151 (93) | — | 459 (90) | .35 | — | 610 (91) |
| 30 or higher | — | 12 (7) | — | 50 (10) | — | — | 62 (9) |
| Platelet count, × 109/L | 20 (1-161) | — | 22 (1-207) | — | — | 22 (1-207) | — |
| Less than 40 | — | 132 (77) | — | 431 (77) | .37 | — | 563 (77) |
| 40 or higher | — | 40 (23) | — | 128 (23) | — | — | 168 (23) |
| Hemoglobin level, g/dL | 9.4 (4.3-15.2) | — | 9.2 (3-16.9) | — | — | 9.3 (3-16.9) | — |
| Less than 10 | — | 104 (60) | — | 361 (65) | .26 | — | 465 (64) |
| 10 or higher | — | 68 (40) | — | 198 (35) | — | — | 266 (36) |
| Creatinine level, mg/dL | 0.9 (0.2-1.7) | — | 0.9 (0.2-2.4) | — | — | 0.9 (0.2-2.4) | — |
| Less than 1.4 | — | 171 (99) | — | 525 (98) | .22 | — | 696 (98) |
| 1.4 or higher | — | 1 (1) | — | 13 (2) | — | — | 14 (2) |
| Coagulopathy | |||||||
| No | — | 51 (30) | — | 141 (28) | .61 | — | 192 (28) |
| Yes | — | 121 (70) | — | 369 (72) | — | — | 490 (72) |
| Uric acid level, mg/dL | 3.8 (1-9.6) | — | 3.9 (0.29-12.7) | — | — | 3.8 (0.29-12.7) | — |
| Less than 7 | — | 152 (95) | — | 442 (94) | .65 | — | 594 (94) |
| 7 or higher | — | 8 (5) | — | 28 (6) | — | — | 36 (6) |
| Fibrinogen level, mg/dL | 160 (0-784) | — | 158 (0-862) | — | — | 158 (0-862) | — |
| Less than 170 | — | 91 (30) | — | 285 (28) | .89 | — | 376 (28) |
| 170 or higher | — | 76 (70) | — | 232 (72) | — | — | 308 (72) |
| Albumin level, g/dL | 4.1 (2.2-6.2) | — | 4 (1.7-6.7) | — | — | 4 (1.7-6.7) | — |
| Less than 3.5 | — | 22 (14) | — | 107 (24) | .01 | — | 129 (22) |
| 3.5 or higher | — | 129 (86) | — | 333 (76) | — | — | 462 (78) |
| Morphologic subtype | |||||||
| Hypergranular | — | 143 (83) | — | 450 (82) | .7 | — | 593 (82) |
| Microgranular | — | 29 (17) | — | 100 (18) | — | — | 129 (18) |
| PML/RARα isoform | |||||||
| BCR1/BCR2 | — | 91 (56) | — | 286 (59) | .42 | — | 377 (58) |
| BCR3 | — | 73 (44) | — | 198 (41) | — | — | 271 (42) |
| Characteristic . | LPA96 . | LPA99 . | P . | Total . | |||
|---|---|---|---|---|---|---|---|
| Median (range) . | No. (%) . | Median (range) . | No. (%) . | Median (range) . | No. (%) . | ||
| Overall | — | 172 (24) | — | 560 (76) | — | — | 732 (100) |
| Age, y | 39 (2-78) | — | 40 (2-83) | — | — | 40 (2-83) | — |
| 15 or younger | — | 11 (6) | — | 48 (9) | .83 | — | 59 (8) |
| 16 to 40 | — | 75 (43) | — | 240 (43) | — | — | 315 (43) |
| 41 to 60 | — | 57 (33) | — | 171 (30) | — | — | 228 (31) |
| 61 to 70 | — | 19 (11) | — | 68 (12) | — | — | 87 (12) |
| 71 or older | — | 10 (6) | — | 33 (6) | — | — | 43 (6) |
| Sex | |||||||
| Male | — | 102 (59) | — | 270 (48) | .01 | — | 372 (51) |
| Female | — | 70 (41) | — | 290 (52) | — | — | 360 (49) |
| ECOG score | 1 (0-3) | — | 1 (0-3) | — | — | 1 (0-3) | — |
| 0 to 1 | — | 130 (81) | — | 377 (73) | .07 | — | 507 (75) |
| 2 | — | 20 (13) | — | 102 (20) | — | — | 122 (18) |
| 3 | — | 10 (6) | — | 36 (7) | — | — | 46 (7) |
| Fever | |||||||
| No | — | 108 (63) | — | 333 (60) | .57 | — | 441 (61) |
| Yes | — | 64 (37) | — | 220 (40) | — | — | 284 (39) |
| WBC count, × 109/L | 2.0 (0.3-210) | — | 2.2 (0.2-460) | — | — | 2.2 (0.2-460) | — |
| Less than 5 | — | 108 (62) | — | 336 (60) | .79 | — | 444 (61) |
| 5 to 10 | — | 21 (13) | — | 84 (15) | — | — | 105 (14) |
| 10 to 50 | — | 31 (18) | — | 99 (18) | — | — | 130 (18) |
| 50 or higher | — | 12 (7) | — | 40 (7) | — | — | 52 (7) |
| PB blast count, × 109/L | 0.6 (0-210) | — | 0.7 (0-179) | 0.7 (0-210) | |||
| Less than 30 | — | 151 (93) | — | 459 (90) | .35 | — | 610 (91) |
| 30 or higher | — | 12 (7) | — | 50 (10) | — | — | 62 (9) |
| Platelet count, × 109/L | 20 (1-161) | — | 22 (1-207) | — | — | 22 (1-207) | — |
| Less than 40 | — | 132 (77) | — | 431 (77) | .37 | — | 563 (77) |
| 40 or higher | — | 40 (23) | — | 128 (23) | — | — | 168 (23) |
| Hemoglobin level, g/dL | 9.4 (4.3-15.2) | — | 9.2 (3-16.9) | — | — | 9.3 (3-16.9) | — |
| Less than 10 | — | 104 (60) | — | 361 (65) | .26 | — | 465 (64) |
| 10 or higher | — | 68 (40) | — | 198 (35) | — | — | 266 (36) |
| Creatinine level, mg/dL | 0.9 (0.2-1.7) | — | 0.9 (0.2-2.4) | — | — | 0.9 (0.2-2.4) | — |
| Less than 1.4 | — | 171 (99) | — | 525 (98) | .22 | — | 696 (98) |
| 1.4 or higher | — | 1 (1) | — | 13 (2) | — | — | 14 (2) |
| Coagulopathy | |||||||
| No | — | 51 (30) | — | 141 (28) | .61 | — | 192 (28) |
| Yes | — | 121 (70) | — | 369 (72) | — | — | 490 (72) |
| Uric acid level, mg/dL | 3.8 (1-9.6) | — | 3.9 (0.29-12.7) | — | — | 3.8 (0.29-12.7) | — |
| Less than 7 | — | 152 (95) | — | 442 (94) | .65 | — | 594 (94) |
| 7 or higher | — | 8 (5) | — | 28 (6) | — | — | 36 (6) |
| Fibrinogen level, mg/dL | 160 (0-784) | — | 158 (0-862) | — | — | 158 (0-862) | — |
| Less than 170 | — | 91 (30) | — | 285 (28) | .89 | — | 376 (28) |
| 170 or higher | — | 76 (70) | — | 232 (72) | — | — | 308 (72) |
| Albumin level, g/dL | 4.1 (2.2-6.2) | — | 4 (1.7-6.7) | — | — | 4 (1.7-6.7) | — |
| Less than 3.5 | — | 22 (14) | — | 107 (24) | .01 | — | 129 (22) |
| 3.5 or higher | — | 129 (86) | — | 333 (76) | — | — | 462 (78) |
| Morphologic subtype | |||||||
| Hypergranular | — | 143 (83) | — | 450 (82) | .7 | — | 593 (82) |
| Microgranular | — | 29 (17) | — | 100 (18) | — | — | 129 (18) |
| PML/RARα isoform | |||||||
| BCR1/BCR2 | — | 91 (56) | — | 286 (59) | .42 | — | 377 (58) |
| BCR3 | — | 73 (44) | — | 198 (41) | — | — | 271 (42) |
ECOG indicates Eastern Cooperative Oncology Group; WBC, white blood count; PB, peripheral blood; and —, not applicable.
Induction therapy
Response and induction mortality.
Six hundred sixty-six of the 732 evaluable patients achieved morphologic CR (91%; 95% CI, 89.9%-92.1%). The median time intervals to CR were 36 days (range, 21-78 days) in the LPA96 trial and 37 days (range, 21-77 days) in the LPA99 trial. The median time to reach neutrophil counts greater than 109/L and platelet counts greater than 50 × 109/L was 23 days (range, 0-60 days) and 19 days (range, 0-50 days), respectively. All the 66 induction failures were attributed to death during induction.
Hemorrhage and infection accounted for most of the deaths during induction therapy (37 and 17 patients, respectively). Differentiation syndrome and acute myocardial infarction were contributing causes of death in 10 and 2 patients, respectively.
Factors predicting induction death.
We first set out to evaluate overall induction mortality, and in the subsequent sections we evaluated distinct types of death separately, ie, hemorrhagic death, infection-related death, and death associated with the differentiation syndrome, respectively.
The univariate analysis of prognostic factors (Table 3) identified the following characteristics predicting induction mortality: older age, with 60 years as the most significant cutoff point (P < .001); male sex (P = .003); ECOG score 2 to 3 (P = .01); fever at presentation (P = .02); increased WBC and peripheral blast counts at presentation, with 10 × 109/L and 30 × 109/L as the most statistically significant cutoff points, respectively (P < .001); as well as abnormal levels of serum creatinine (P < .001), low levels of albumin (P = .003), microgranular subtype (P = .002), and presence of coagulopathy (P = .02). After multivariate analysis, the following factors remained for their independent predictive significance for induction mortality: abnormal creatinine level (P < .001), peripheral blast count of more than 30 × 109/L (P < .001), age older than 60 years (P < .001), male sex (P < .001), and WBC count of more than 10 × 109/L (P = .04; Table 4).
Univariate analysis according to the type of induction remission failure
| Characteristic . | Overall deaths during induction . | Failure rates according to the cause of death . | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) . | P . | Hemorrhage . | Infection . | DS . | ||||
| No. (%) . | P . | No. (%) . | P . | No. (%) . | P . | |||
| Overall | 66 (9.0) | — | 37 (5.0) | — | 17 (2.3) | — | 10 (1.4) | — |
| Protocol | ||||||||
| LPA96 | 16 (9.3) | .88 | 9 (5.2) | .90 | 5 (2.9) | .77 | 2 (1.2) | 1 |
| LPA99 | 50 (8.9) | — | 28 (5.0) | — | 12 (2.1) | — | 8 (1.4) | — |
| Age, y | ||||||||
| 15 or younger | 5 (8.5) | <.001 | 3 (5.1) | .06 | 0 (0.0) | <.001 | 2 (3.4) | .08 |
| 16 to 40 | 14 (4.4) | — | 11 (3.5) | — | 3 (1.0) | — | 0 (0.0) | — |
| 41 to 60 | 19 (8.3) | — | 11 (4.8) | — | 3 (1.3) | — | 5 (2.2) | — |
| 61 to 70 | 17 (19.5) | — | 6 (6.9) | — | 7 (8.0) | — | 2 (2.3) | — |
| 71 or older | 11 (25.6) | — | 6 (14.0) | — | 4 (9.3) | — | 1 (2.3) | — |
| Sex | ||||||||
| Male | 45 (12.1) | .003 | 23 (6.2) | .16 | 14 (3.8) | .02 | 6 (1.6) | .79 |
| Female | 21 (5.8) | — | 14 (3.9) | — | 3 (0.8) | — | 4 (1.1) | — |
| ECOG score | ||||||||
| 0 to 1 | 38 (7.5) | .01 | 24 (4.7) | .2 | 11 (2.2) | .66 | 3 (0.6) | .01 |
| 2 | 15 (12.3) | — | 6 (4.9) | — | 3 (2.5) | — | 4 (3.3) | — |
| 3 | 9 (19.6) | — | 5 (10.9) | — | 2 (4.3) | — | 2 (4.3) | — |
| Fever | ||||||||
| No | 31 (7.0) | .02 | 19 (4.3) | .31 | 5 (1.1) | .01 | 6 (1.4) | .999 |
| Yes | 34 (12.0) | — | 17 (6.0) | — | 12 (4.2) | — | 4 (1.4) | — |
| WBC count, × 109/L | ||||||||
| Less than 5 | 23 (5.2) | <.001 | 12 (2.7) | <.001 | 7 (1.6) | .23 | 4 (0.9) | .21 |
| 5 to 10 | 10 (9.5) | — | 5 (4.8) | — | 3 (2.9) | — | 2 (1.9) | — |
| 10 to 50 | 21 (16.2) | — | 11 (8.5) | — | 4 (3.1) | — | 4 (3.1) | — |
| 50 or higher | 12 (23.1) | — | 10 (17.3) | — | 3 (5.8) | — | 0 (0.0) | — |
| PB blast count, × 109/L | ||||||||
| Less than 30 | 44 (7.2) | <.001 | 24 (3.9) | <.001 | 13 (2.1) | .11 | 7 (1.1) | .999 |
| 30 or higher | 17 (27.4) | — | 10 (16.1) | — | 4 (6.5) | — | 1 (1.6) | — |
| Platelet count, × 109/L | ||||||||
| Less than 40 | 55 (9.8) | .2 | 32 (5.7) | .16 | 13 (2.3) | .999 | 8 (1.4) | .999 |
| 40 or higher | 11 (6.5) | — | 5 (3.0) | — | 4 (2.4) | — | 2 (1.2) | — |
| Hemoglobin level, g/L | ||||||||
| Less than 10 | 43 (9.2) | .79 | 26 (5.6) | .39 | 11 (2.4) | .999 | 6 (1.3) | .999 |
| 10 or higher | 23 (8.6) | — | 11 (4.1) | — | 6 (2.3) | — | 4 (1.5) | — |
| Creatinine level, mg/dL | ||||||||
| Less than 1.4 | 52 (7.5) | <.001 | 27 (3.9) | <.001 | 15 (2.2) | .04 | 8 (1.1) | .43 |
| 1.4 or higher | 10 (71.4) | — | 7 (50.0) | — | 2 (14.3) | — | 1 (7.1) | — |
| Coagulopathy | ||||||||
| No | 10 (5.2) | .02 | 3 (1.6) | .01 | 3 (1.6) | .57 | 3 (1.6) | .999 |
| Yes | 53 (10.8) | — | 33 (6.7) | — | 13 (2.7) | — | 6 (1.2) | — |
| Uric acid level, mg/dL | ||||||||
| Less than 7 | 45 (7.6) | .44 | 21 (3.5) | .07 | 13 (2.2) | .77 | 9 (1.5) | .98 |
| 7 or higher | 4 (11.1) | — | 4 (11.1) | — | 0 (0) | — | 0 (0.0) | — |
| Fibrinogen level, mg/dL | ||||||||
| Less than 170 | 32 (8.5) | .58 | 18 (4.8) | .999 | 9 (2.4) | .999 | 4 (1.1) | .52 |
| 170 or higher | 30 (9.7) | — | 15 (4.9) | — | 8 (2.6) | — | 6 (1.9) | — |
| Albumin level, mg/dL | ||||||||
| Less than 3.5 | 19 (14.7) | .003 | 9 (7.0) | .13 | 5 (3.9) | .34 | 5 (3.9) | .02 |
| 3.5 or higher | 30 (6.5) | — | 16 (3.5) | — | 9 (1.9) | — | 3 (0.6) | — |
| Morphologic subtype | ||||||||
| Hypergranular | 45 (7.6) | .002 | 26 (4.4) | .09 | 11 (1.9) | .11 | 8 (1.3) | .999 |
| Microgranular | 21 (16.3) | — | 11 (8.5) | — | 6 (4.7) | — | 2 (1.6) | — |
| PML/RARα isoform | ||||||||
| BCR1/BCR2 | 27 (7.2) | .11 | 14 (3.7) | .09 | 8 (2.1) | .91 | 5 (1.3) | .75 |
| BCR3 | 29 (10.7) | — | 19 (7.0) | — | 7 (2.6) | — | 2 (0.7) | — |
| Characteristic . | Overall deaths during induction . | Failure rates according to the cause of death . | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) . | P . | Hemorrhage . | Infection . | DS . | ||||
| No. (%) . | P . | No. (%) . | P . | No. (%) . | P . | |||
| Overall | 66 (9.0) | — | 37 (5.0) | — | 17 (2.3) | — | 10 (1.4) | — |
| Protocol | ||||||||
| LPA96 | 16 (9.3) | .88 | 9 (5.2) | .90 | 5 (2.9) | .77 | 2 (1.2) | 1 |
| LPA99 | 50 (8.9) | — | 28 (5.0) | — | 12 (2.1) | — | 8 (1.4) | — |
| Age, y | ||||||||
| 15 or younger | 5 (8.5) | <.001 | 3 (5.1) | .06 | 0 (0.0) | <.001 | 2 (3.4) | .08 |
| 16 to 40 | 14 (4.4) | — | 11 (3.5) | — | 3 (1.0) | — | 0 (0.0) | — |
| 41 to 60 | 19 (8.3) | — | 11 (4.8) | — | 3 (1.3) | — | 5 (2.2) | — |
| 61 to 70 | 17 (19.5) | — | 6 (6.9) | — | 7 (8.0) | — | 2 (2.3) | — |
| 71 or older | 11 (25.6) | — | 6 (14.0) | — | 4 (9.3) | — | 1 (2.3) | — |
| Sex | ||||||||
| Male | 45 (12.1) | .003 | 23 (6.2) | .16 | 14 (3.8) | .02 | 6 (1.6) | .79 |
| Female | 21 (5.8) | — | 14 (3.9) | — | 3 (0.8) | — | 4 (1.1) | — |
| ECOG score | ||||||||
| 0 to 1 | 38 (7.5) | .01 | 24 (4.7) | .2 | 11 (2.2) | .66 | 3 (0.6) | .01 |
| 2 | 15 (12.3) | — | 6 (4.9) | — | 3 (2.5) | — | 4 (3.3) | — |
| 3 | 9 (19.6) | — | 5 (10.9) | — | 2 (4.3) | — | 2 (4.3) | — |
| Fever | ||||||||
| No | 31 (7.0) | .02 | 19 (4.3) | .31 | 5 (1.1) | .01 | 6 (1.4) | .999 |
| Yes | 34 (12.0) | — | 17 (6.0) | — | 12 (4.2) | — | 4 (1.4) | — |
| WBC count, × 109/L | ||||||||
| Less than 5 | 23 (5.2) | <.001 | 12 (2.7) | <.001 | 7 (1.6) | .23 | 4 (0.9) | .21 |
| 5 to 10 | 10 (9.5) | — | 5 (4.8) | — | 3 (2.9) | — | 2 (1.9) | — |
| 10 to 50 | 21 (16.2) | — | 11 (8.5) | — | 4 (3.1) | — | 4 (3.1) | — |
| 50 or higher | 12 (23.1) | — | 10 (17.3) | — | 3 (5.8) | — | 0 (0.0) | — |
| PB blast count, × 109/L | ||||||||
| Less than 30 | 44 (7.2) | <.001 | 24 (3.9) | <.001 | 13 (2.1) | .11 | 7 (1.1) | .999 |
| 30 or higher | 17 (27.4) | — | 10 (16.1) | — | 4 (6.5) | — | 1 (1.6) | — |
| Platelet count, × 109/L | ||||||||
| Less than 40 | 55 (9.8) | .2 | 32 (5.7) | .16 | 13 (2.3) | .999 | 8 (1.4) | .999 |
| 40 or higher | 11 (6.5) | — | 5 (3.0) | — | 4 (2.4) | — | 2 (1.2) | — |
| Hemoglobin level, g/L | ||||||||
| Less than 10 | 43 (9.2) | .79 | 26 (5.6) | .39 | 11 (2.4) | .999 | 6 (1.3) | .999 |
| 10 or higher | 23 (8.6) | — | 11 (4.1) | — | 6 (2.3) | — | 4 (1.5) | — |
| Creatinine level, mg/dL | ||||||||
| Less than 1.4 | 52 (7.5) | <.001 | 27 (3.9) | <.001 | 15 (2.2) | .04 | 8 (1.1) | .43 |
| 1.4 or higher | 10 (71.4) | — | 7 (50.0) | — | 2 (14.3) | — | 1 (7.1) | — |
| Coagulopathy | ||||||||
| No | 10 (5.2) | .02 | 3 (1.6) | .01 | 3 (1.6) | .57 | 3 (1.6) | .999 |
| Yes | 53 (10.8) | — | 33 (6.7) | — | 13 (2.7) | — | 6 (1.2) | — |
| Uric acid level, mg/dL | ||||||||
| Less than 7 | 45 (7.6) | .44 | 21 (3.5) | .07 | 13 (2.2) | .77 | 9 (1.5) | .98 |
| 7 or higher | 4 (11.1) | — | 4 (11.1) | — | 0 (0) | — | 0 (0.0) | — |
| Fibrinogen level, mg/dL | ||||||||
| Less than 170 | 32 (8.5) | .58 | 18 (4.8) | .999 | 9 (2.4) | .999 | 4 (1.1) | .52 |
| 170 or higher | 30 (9.7) | — | 15 (4.9) | — | 8 (2.6) | — | 6 (1.9) | — |
| Albumin level, mg/dL | ||||||||
| Less than 3.5 | 19 (14.7) | .003 | 9 (7.0) | .13 | 5 (3.9) | .34 | 5 (3.9) | .02 |
| 3.5 or higher | 30 (6.5) | — | 16 (3.5) | — | 9 (1.9) | — | 3 (0.6) | — |
| Morphologic subtype | ||||||||
| Hypergranular | 45 (7.6) | .002 | 26 (4.4) | .09 | 11 (1.9) | .11 | 8 (1.3) | .999 |
| Microgranular | 21 (16.3) | — | 11 (8.5) | — | 6 (4.7) | — | 2 (1.6) | — |
| PML/RARα isoform | ||||||||
| BCR1/BCR2 | 27 (7.2) | .11 | 14 (3.7) | .09 | 8 (2.1) | .91 | 5 (1.3) | .75 |
| BCR3 | 29 (10.7) | — | 19 (7.0) | — | 7 (2.6) | — | 2 (0.7) | — |
ECOG indicates Eastern Cooperative Oncology Group; WBC, white blood count; PB, peripheral blood; and —, not applicable.
Multivariate analysis according to type of induction death
| Covariate . | Unfavorable category . | Induction death . | Cause of death . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemorrhage . | Infection . | Differentiation syndrome . | |||||||
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||
| Creatinine level | Abnormal levels | 23.8 (6.3-89.2) | <.001 | 24.3 (7.5-78.1) | <.001 | — | .29 | — | .22 |
| WBC count, × 109/L | 10 or higher | 2.2 (1.1-4.6) | .04 | — | .12 | — | .21 | — | .49 |
| PB blast count, × 109/L | 30 or higher | 3.3 (1.4-7.9) | <.001 | 4.4 (1.9-10.2) | <.001 | — | .13 | — | .85 |
| Age, y | 60 years or older | 4.4 (2.4-8.1) | <.001 | — | .14 | 11.0 (3.9-31.4) | <.001 | — | .56 |
| Sex | Male | 2.8 (1.5-5.2) | <.001 | — | .16 | 5.1 (1.4-18.3) | .003 | — | .61 |
| Coagulopathy at presentation | Yes | — | .21 | 3.3 (0.97-11.6) | .03 | — | .78 | — | .88 |
| Fever at presentation | Yes | — | .49 | — | .85 | 3.9 (1.3-11.7) | .009 | — | .47 |
| ECOG score | At least 2 | — | .45 | — | .86 | — | .98 | 4.5 (1.2-17.2) | .009 |
| Albumin level | Abnormal levels | — | .11 | — | .62 | — | .52 | 4.1 (1.1-16.1) | .045 |
| Covariate . | Unfavorable category . | Induction death . | Cause of death . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemorrhage . | Infection . | Differentiation syndrome . | |||||||
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||
| Creatinine level | Abnormal levels | 23.8 (6.3-89.2) | <.001 | 24.3 (7.5-78.1) | <.001 | — | .29 | — | .22 |
| WBC count, × 109/L | 10 or higher | 2.2 (1.1-4.6) | .04 | — | .12 | — | .21 | — | .49 |
| PB blast count, × 109/L | 30 or higher | 3.3 (1.4-7.9) | <.001 | 4.4 (1.9-10.2) | <.001 | — | .13 | — | .85 |
| Age, y | 60 years or older | 4.4 (2.4-8.1) | <.001 | — | .14 | 11.0 (3.9-31.4) | <.001 | — | .56 |
| Sex | Male | 2.8 (1.5-5.2) | <.001 | — | .16 | 5.1 (1.4-18.3) | .003 | — | .61 |
| Coagulopathy at presentation | Yes | — | .21 | 3.3 (0.97-11.6) | .03 | — | .78 | — | .88 |
| Fever at presentation | Yes | — | .49 | — | .85 | 3.9 (1.3-11.7) | .009 | — | .47 |
| ECOG score | At least 2 | — | .45 | — | .86 | — | .98 | 4.5 (1.2-17.2) | .009 |
| Albumin level | Abnormal levels | — | .11 | — | .62 | — | .52 | 4.1 (1.1-16.1) | .045 |
OR indicates odds ratio; WBC, white blood cell; PB, peripheral blood; ECOG, Eastern Cooperative Oncology Group; and —, not applicable.
Deaths as a result of hemorrhage
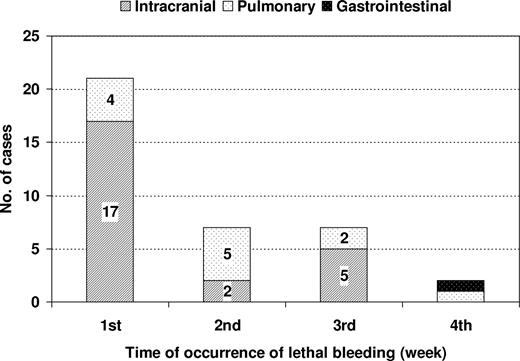
Overall, 37 deaths were attributable to hemorrhages. The mortality rates resulting from hemorrhage were similar in both the LPA96 and LPA99 studies (9 of 175, 5.1% and 28 of 561, 5%, respectively), despite using tranexamic acid prophylaxis in the latter study. Hemorrhagic mortalities were almost exclusively caused by intracranial (24 patients, 65%) and pulmonary hemorrhages (12 patients, 32%). Of the 24 patients with intracranial bleeding, 2 developed the hemorrhage over an extensive cerebral thrombosis, and 2 additional patients had concomitant pulmonary hemorrhage. One case of fatal gastrointestinal tract bleeding was registered. The median time interval from start of treatment to development of intracranial and pulmonary hemorrhage was 6 days (range, 1-21 days) and 9 days (range, 1-23 days), respectively. Most lethal hemorrhages occurred during the first week (21 patients, 57%). There were 7 deaths (19%) each during the second and third week, and 2 deaths (5%) were noted during the fourth week (Figure 1). No lethal hemorrhages were recorded beyond the fourth week. Seventeen (71%) of 24 cerebral hemorrhages and 4 (33%) of 12 pulmonary hemorrhages occurred during the first week of starting induction (P = .07). Twenty-five of (69%) 36 patients who died from cerebral (19 of 24) or pulmonary hemorrhages (6 of 12) had a fulminant course, with death occurring within 24 hours from the onset of lethal bleeding.
Chronology and site of lethal hemorrhages occurring during induction therapy with the AIDA regimen.
Chronology and site of lethal hemorrhages occurring during induction therapy with the AIDA regimen.
Factors predicting fatal hemorrhage
The univariate analysis (Table 3) identified the following prognostic factors for hemorrhagic mortality: older age, with 70 years as the most significant cutoff point (P = .02); increased WBC and peripheral blast counts at presentation, with 10 × 109/L and 30 × 109/L as the most statistically significant cutoff points, respectively (P < .001); as well as abnormal levels of serum creatinine (P < .001) and the presence of coagulopathy (P = .01), whereas there was a trend for microgranular subtype (P = .09) and short PML/RARα isoform (bcr3; P = .09). After multivariate analysis, the following factors remained significant: abnormal creatinine level (P < .001), peripheral blast count of more than 30 × 109/L (P < .001), and presence of coagulopathy (P = .03; Table 4).
Deaths as a result of infection
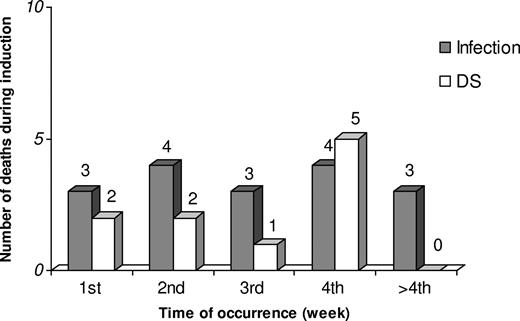
Overall, 17 deaths were attributable to infection. Infection-associated mortality was due to pneumonia (9 patients, 53%), septicemia (6 patients, 35%), orbital cellulitis (n = 1), and clinically and radiologically suspected but not microbiologically documented hepatosplenic candidiasis (n = 1). Eleven patients had a microbiologically documented infection. Eight were bacterial infections (4 Gram-positive and 4 Gram-negative), 2 fungal infections (pulmonary aspergillosis), and 1 mixed infection (pulmonary infection by Aspergillus spp and Klebsiella pneumoniae). Deaths resulting from infection occurred at a median time of 21 days (range, 3-39 days), at a constant rate during the induction period (3-4 deaths each week; Figure 2).
Chronology of deaths because of infection and differentiation syndrome (DS) occurring during induction therapy with the AIDA regimen.
Chronology of deaths because of infection and differentiation syndrome (DS) occurring during induction therapy with the AIDA regimen.
Factors predicting fatal outcome of infection
The following prognostic factors (Table 3) for infectious mortality appeared from univariate analysis: older age, with 60 years as the most significant cutoff point (P < .001); male sex (P = .02); fever at presentation (P = .01); and abnormal serum creatinine level (P = .04). Among them, multivariate analysis identified the following independent prognostic parameters: age older than 60 years (P < .001), male sex (P = .003), and fever at presentation (P = .009; Table 4).
Deaths as a result of differentiation syndrome
Ten deaths attributable to DS occurred at a median of 17 days of starting induction (range, 1-26 days). The time of occurrence of deaths resulting from DS is shown in Figure 2. The mortality rates because of DS were similar in both the LPA96 and LPA99 studies (1.1% and 1.4%, respectively), despite prednisone prophylaxis in the latter. Renal and respiratory failures, either combined or separately, were implicated in all deaths (8 combined, 1 renal, and 1 respiratory failure). Two pa-tients underwent hemodialysis, 2 mechanical ventilation, and 3 both procedures.
Factors predicting fatal outcome of DS
The following prognostic factors (Table 3) correlated with death associated with DS after univariate analysis: ECOG score 2 to 3 (P = .01), low level of albumin (P = .02), whereas there was a trend for younger age, with 15 years as the most significant cutoff point (P = .08). Multivariate analysis identified ECOG score of at least 2 and low level of albumin (P = .045) as the only significant independent prognostic factors (Table 4).
Other causes of death
In the absence of history of cardiac events and coronary risk factors, 2 male patients of 65 and 68 years of age developed a fatal acute myocardial infarction at days 15 and 33 of induction therapy, respectively. Both were diagnosed by chest pain consistent with ongoing myocardial ischemia with concomitant typical changes in electrocardiogram and creatine kinase levels.
Discussion
Remission induction deaths continue to represent one of the major stumbling blocks in modern therapy of APL. This study shows that hemorrhage is the single most common cause of death (5%) during induction therapy, followed by infection (2.3%) and differentiation syndrome (1.4%) in patients with APL receiving ATRA and idarubicin (AIDA regimen). Typically, the majority of lethal hemorrhages occurred early during induction, whereas infection and DS caused deaths at a somewhat later time. Multivariate analysis identified pretreatment characteristics associated with an increased risk of death, which were different to predict fatal hemorrhage (abnormal creatinine level, increased peripheral blast counts, and presence of coagulopathy), death because of infection (age older than 60 years, male sex, and fever at presentation), and death because of DS (ECOG score ≥ 2 and abnormal albumin levels). Thus, apparently these different types of death seem subject to different and specific risk factors. This may provide clinically relevant information that in the future may be useful for designing more appropriately risk-adapted treatment protocols and improving treatment outcome in this type of leukemia.
A number of studies conducted during the past decade have contributed to the optimizing of the antileukemic efficacy of ATRA when used in combination with anthracycline chemotherapy for induction therapy.1-3 In fact, this combination shows an extremely high antileukemic efficacy, leading to complete remission rates in 90% to 95% of cases, and leukemia resistance has been only reported in infrequent cases.4,12-14
It should be noted that 4 of the 6 nonevaluable patients who were registered by their physicians in our study as resistant leukemia and then administered salvage therapy had developed a definitely present DS, and all of them were assessed prematurely for response, that is, during pancytopenia. This is contradictory to the current recommendations of response assessment in APL.8 Unfortunately, the hasty administration of salvage therapy to all these patients with delayed clearance of blasts did not allow us to verify whether they were really chemotherapy resistant or rather only exhibited a delay in terminal differentiation of blasts. In case of any doubt about the achievement of CR, it is recommended to repeat another bone marrow assessment after an additional interval of 2 to 3 weeks and meanwhile refrain from new therapeutic interventions. Since the introduction of this policy, no case of resistant leukemia was recorded among the most recent 350 patients enrolled in the PETHEMA studies.
Apart from a virtual absence of leukemia resistance and a lower mortality rate observed in APL compared with other subtypes of acute myeloblastic leukemia (AML), the causes of induction deaths showed a characteristic pattern and time of occurrence that also differ from those in AML.15 Although infection is the predominant cause of death in AML, hemorrhage is the principal cause of death during induction therapy in APL. However, few data are available about the clinical features of this complication, such as time of onset and time interval between hemorrhage and death, and also little is still known about the prognostic factors of this particular complication. As far as we know, only a study by Kantarjian et al16 that was performed in 60 patients with morphologically diagnosed APL treated between 1973 and 1984 has previously addressed the analysis of the prognostic factors associated with induction failure because of hemorrhage, as in the present study. Two studies of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) group,4,17 one of them in the pre-ATRA era, have also analyzed the prognostic factors associated with early hemorrhagic death, but it was restricted to deaths occurring within the first 10 days of induction therapy. Finally, 2 additional studies of the ATRA era,18,19 with 3 and 8 hemorrhagic deaths, respectively, have analyzed the prognostic factors associated with the development of severe hemorrhage but not those factors associated with an increased risk of death because of hemorrhage.
In addition to an increased WBC count, which has been recognized as an independent prognostic factor of response to induction therapy in other studies,12-14,20 we also found that the presence of coagulopathy and abnormal levels of creatinine were significantly associated with a higher risk of mortality and, most particularly, of hemorrhagic mortality during induction remission. It should be noted that the inclusion of peripheral blast counts in multivariate analysis, which was also recognized as independent prognostic factor of early death (defined as death occurring within the first 10 days of induction treatment) by the Italian GIMEMA group,4,17 prompted that WBC count was removed from the regression model. The reason for the association between an elevated creatinine value and death of hemorrhage is not clear, although one may speculate that it might be a reflective sign of the disseminated intravascular coagulopathy compromising the glomerular microcirculation. However, this speculation is an apparent contrast with the presence of coagulopathy as an independent prognostic factor. Whatever the explanation, it may also be noted that an abnormal level of creatinine was already found to predict poor response to induction therapy in a study reported by us in the pre-ATRA era.21 The prognostic value of the hemorrhagic score defined by the GIMEMA group,22 and also recognized as independent prognostic factor,4 was not analyzed in the present study because clinical assessment of our patients did not include the use of this score.
It should be noted that the reported hemorrhagic mortality was observed despite a generalized and early aggressive supportive care given to all patients, regardless of prognostic factors. In this context, the acknowledgment of a particular set of prognostic factors can be useful to identify high-risk patients as a potential target population to explore experimental or novel approaches to minimize the risk of death because of hemorrhage. For the systematic use of tranexamic acid prophylaxis in the LPA99 trial, a historical comparison with the LPA96 trial, without tranexamic acid prophylaxis, showed no effect in decreasing hemorrhagic mortality. However, there was a trend toward a higher incidence of thrombosis.23 Therefore, a potential benefit of the use of tranexamic acid in this setting is not supported by this study.
The type of infections associated with mortality in the present study appears similar to that seen during induction therapy in AML. However, the incidence of infectious deaths (2.3%) was lower than generally reported in other subtypes of AML. Apart from the apparently shorter time to hematologic recovery in APL compared with AML, the relatively low median age of patients and the lack of use of cytarabine, among others, might explain the low infectious mortality rate observed in our series. As far as we know, a study of prognostic factors of remission failure because of infection in APL has not been reported previously. Although age is generally recognized as a risk factor for death during induction therapy because of the greater “vulnerability” to chemotherapy toxicity of older patients, we have found 2 additional factors that contribute to a higher risk of infection-related death, ie, male sex and fever at presentation. It is presently unclear why we observed a lower mortality rate among women. We can speculate on a better tolerance to the side effects of chemotherapy linked to better organ function in female patients, particularly at older age, which would be in line with the somewhat greater life expectancy of women in general. This factor was already noted in a previous study of our group concerned with elderly patients.24 The greater risk of death because of infection that was apparent in patients presenting with fever at time of diagnosis may be related with an intrinsic susceptibility to infections but also to a more prolonged use of antibiotics, leading to a higher risk of breakthrough bacterial and fungal infections.
It should be noted that the characteristics predisposing to lethal DS, such as ECOG of at least 2 and low levels of serum albumin, should be cautiously interpreted because of low number of events observed (10 of 732 patients at risk). These characteristics were not recognized as independent prognostic factors of remission failure, probably because of the low contribution of DS-associated mortality on the overall mortality. In this regard it is also of note that the systematic use of prednisone prophylaxis in the LPA99 trial, showed no effect on reducing mortality because of DS compared with a selective use of dexamethasone prophylaxis in patients with WBC count greater than 5 × 109/L in the LPA96 trial.
In summary, our study of a large series of patients homogeneously treated for induction with ATRA and idarubicin shows a characteristic pattern of causes of induction failure, as well as a specific set of prognostic variables that can be applied to predict separate types of induction failure. These predictive models, based on readily available baseline characteristics, may be useful for designing more appropriately risk-adapted treat-ment protocols aimed at reducing mortality from hemorrhage, infection, or DS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Miguel Priego for data collection and management.
This study was supported in part by the Fundación para la Investigación Hospital Universitario La Fe-Ayudas Bancaja (grant 2006/0137), Red Temática de Investigación Cooperativa en Cáncer (RD06/0020/0031).
Authorship
Contribution: J.d.l.S., M.A.S., and P.M. conceived the study and analyzed and interpreted the data; J.d.l.S., M.A.S., P.M., and B.L. wrote the paper; P.M. performed the statistical analyses; E.V., C. Rayón, R.P., A.L., J.E., J.M.B., G.M., G.D., C. Rivas, M.G., M.T., J.D.-M., J.D.G., S.N., E.A., and S.B. included data of patients treated in their institutions, reviewed the manuscript, and contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of the members of the Programa de Estudio y Tratamiento de las Hemopatias Malignas study, see Document S1.
Correspondence: Dr Miguel A. Sanz, Servicio de Hematología, Hospital Universitario La Fe, Avenida Campanar 21, 46019 Valencia, Spain; e-mail: msanz@uv.es.