Abstract
Definitive hematopoietic stem and progenitor cells (HSCs/Ps) originating from the yolk sac and/or para-aorta-splanchno-pleura/aorta-gonad-mesonephros are hypothesized to colonize the fetal liver, but mechanisms involved are poorly defined. The Rac subfamily of Rho GTPases has been shown to play essential roles in HSC/P localization to the bone marrow following transplantation. Here, we study the role of Rac1 in HSC/P migration during ontogeny and seeding of fetal liver. Using a triple-transgenic approach, we have deleted Rac1 in HSCs/Ps during very early embryonic development. Without Rac1, there was a decrease in circulating HSCs/Ps in the blood of embryonic day (E) 10.5 embryos, while yolk sac definitive hematopoiesis was quantitatively normal. Intraembryonic hematopoiesis was significantly impaired in Rac1-deficient embryos, culminating with absence of intra-aortic clusters and fetal liver hematopoiesis. At E10.5, Rac1-deficient HSCs/Ps displayed decreased transwell migration and impaired inter-action with the microenvironment in migration-dependent assays. These data suggest that Rac1 plays an important role in HSC/P migration during embryonic development and is essential for the emergence of intraembryonic hematopoiesis.
Introduction
Hematopoietic ontogeny is a complex developmental process that requires migration of hematopoietic stem and progenitor cells (HSCs/Ps) via embryonic circulation between several anatomic sites, including the yolk sac (YS), aorta-gonad-mesonephros (AGM), and placenta (Pl),1-3 culminating with the colonization of fetal liver (FL) by midgestation. Subsequently, HSCs/Ps migrate to the bone marrow (BM) and spleen beginning in late gestation and shortly after birth. These sites become the predominant hematopoietic organs during postnatal and adult life.
Murine embryonic hematopoiesis begins soon after gastrulation when a population of mesodermal precursors commit to becoming blood cells.2 The first hematopoietic precursors reside in YS within blood islands and produce primitive erythrocytes to address the oxygen and nutrient delivery needs of the fast developing embryo. Primitive erythrocyte precursor colony-forming cells (EryP-CFCs) are first identified in murine YS as early as embryonic day (E) 7.0 and completely disappear by E9.0.4,5
Definitive hematopoietic precursors (HPCs) are detected in the YS soon after generation of the first EryP-CFCs.5,6 Definitive erythropoiesis is distinguishable from primitive erythropoiesis by expression of distinct hemoglobin molecules and by cytokine-induced formation of colonies in soft agar as well as by expression of CD41 and cKit surface markers.7-10 The YS microenvironment stimulates expansion of HPC pool but not full differentiation into mature blood cells.6 It is believed that migration of HPCs via the viteline vein constitutes the first wave of FL colonization between E9.0 and E10.5.11,12
Definitive embryonic hematopoiesis resembles adult hematopoiesis in that HSCs are multipotential and can differentiate into all blood lineages. Studies by Moore and Metcalf postulated that YS is the primary site of definitive hematopoietic development and that HSCs derived from YS seed intraembryonic sites, including FL.2 In contrast, embryo-YS chick/quail chimera experiments provided evidence that definitive hematopoiesis originates from an intraembryonic site rather than YS.13 Characterization of AGM region at E10.5 provided additional evidence that this region is the source of first HSCs in the murine embryo and a major source for the colonization of FL.14 In contrast, cells that can give rise to long-term hematopoiesis in neonatal animals have been demonstrated in YS as early as E9.0.3,15-18 More recent studies by Kumaravelu et al have suggested that AGM by itself cannot supply the proliferating cell numbers present in FL and that HSCs from other sources, likely outside the embryo proper, contribute to the colonization of FL.19 The placenta has also recently been suggested as a significant reservoir of HSCs at midgestation.1,20 Overall, based on previously published studies, it remains unclear whether definitive HSCs originate from mesoderm following similar differentiation programs at multiple intra/extraembryonic locations or that HSCs emerge in the embryo at a unique site then subsequently migrate, proliferate, and differentiate within different microenvironments. Specifically, it remains unknown if the YS is the origin of definitive HSCs for the entire embryo as first hypothesized by early investigators.
While there is increasing understanding of the molecular interactions involved in migration and retention of HSCs into the adult bone marrow,21,22 little to nothing is known about the molecular mechanisms involved in migration of HSCs during ontogeny, including the colonization of FL with HSCs/Ps. Specifically, it is currently unknown whether colonization of FL is an active and chemokine-directed migratory process or, alternatively, whether the developing liver bud traps circulating HSCs/Ps in capillary sinusoids. If colonization of FL is an active process, it may use molecular signals similar to the process of homing and retention of HSCs/Ps to BM during stem cell transplantation.23 In this regard, genetically engineered mice have implicated stromal-derived factor 1-alpha (SDF1-α) and its receptor, CXCR4; stem cell factor (SCF) or its receptor, cKit; CD44; and β1-integrins in HSC homing and engraftment to the marrow.22,24,25
Rho GTPases are signaling molecules that integrate intracellular pathways downstream of CXCR4, cKit, and β1-integrins. Rac members of the Rho GTPase family have been previously implicated in molecular regulation of HSC/P interactions with hematopoietic microenvironment (HM).21,26-29 The Rac family is composed of 3 highly homologous proteins, Rac1, Rac2, and Rac3. Rac1 and Rac2 share unique and overlapping roles in HSCs/Ps.21,26,27 Rac2-deficient HSCs show defective migration and adhesion resulting in reduced retention in the marrow and increased mobilization into circulation.26 Rac1-deficient HSCs/Ps demonstrate decreased homing to BM and impaired endosteal microlocalization upon transplantation. However, if already resident in BM, Rac1 deficiency does not impair steady-state hematopoiesis, suggesting Rac1 is a key molecular switch regulating engraftment.21
In the current study, we use triple-transgenic mice to achieve hematopoietic-specific constitutive deletion of Rac1 very early in embryonic development. YS definitive hematopoiesis was quantitatively normal in the setting of Rac1−/− HSCs/Ps. However, the number of HPCs circulating in the embryonic vasculature was significantly decreased and there was nearly complete absence of HSCs/Ps in embryonic tissues, including FL, in Rac1−/− mice. Rac1−/− HSCs/Ps derived from YS and circulation demonstrated reduced cellular migration and defective performance in migration-dependent, stromal-dependent assays. These data suggest that Rac1 is essential for the initial wave of FL colonization and plays an important role for the emergence of intraembryonic hematopoiesis.
Methods
Mice, tissue dissection, and hematopoietic cell isolation
Vav1Cre transgenic mice were bred to Rac1wt/null mice.30 Vav1Cre-positive Rac1wt/null females were identified by polymerase chain reaction (PCR) analysis of genomic DNA as described.31 These mice were used for timed pregnancies with Rac1flox/flox males.27 The morning of vaginal plug was considered E0.5 and pregnant females were killed at specified times. Pregnant uterine horns were removed from the peritoneal cavity and washed several times with phosphate buffer saline (PBS; Cellgro, Herndon, VA) containing 1000 U/mL penicillin/streptomycin (P/S; Cellgro) and 20 U/mL heparin sodium (American Pharmaceutical Partners, Los Angeles, CA), termed dissection buffer. Embryos were separated from the uterine horns and Reichter membrane and deciduas were removed. Traces of maternal blood were removed by several washings with dissection buffer, and embryos were individually deposited in 3 mL dissection buffer in a non–tissue culture 6-well plate (Becton Dickinson Labware [BD Labware], Franklin Lakes, NJ). The specific developmental stage was confirmed by comparing the morphologic features of each embryo with their corresponding stage from the Edinburgh Mouse Atlas Project (EMAP).32 The YS was removed and the heart and large vessels of the neck were dissected so that blood could flow freely into the collection dish. Fetal liver was separated from the embryonic remnants. PBS containing embryonic blood was collected, passed through 40-μm nylon filter (BD Labware), pelleted (1600g, 5 minutes. at 4°C), and used for additional assays. To obtain single-cell suspensions from YS, FL, and embryonic remnants (embryo after removal of YS, FL, and blood), respective tissues were incubated with PBS, 20% fetal calf serum (FCS; HyClone, Logan, UT), 1000 U/mL P/S, and 0.1% collagenase (Sigma-Aldrich, St Louis, MO), at 37°C for 20 minutes. The resulting cell suspensions were passed through a 27½-G needle using a 1-mL syringe. Single-cell suspensions obtained were washed once with PBS and used for subsequent assays.
To demonstrate the presence of Cre-mediated recombination during early embryonic development, Vav1Cre mice were bred with either ROSA26GFP or ROSA26LacZ reporter mice33 (The Jackson Laboratories, Bar Harbor, ME). To determine Cre-mediated recombination using ROSA26GFP reporter mice, embryos were harvested at E9.5 and single-cell suspensions of their YS were obtained. The cells were then incubated for 30 minutes at 4°C with PBS and 0.5 μg/mL anti-CD41 antibody (BD PharMingen, San Jose, CA) previously labeled with Alexa647 (Molecular Probes, Eugene, OR) and subsequently analyzed by flow cytometry (FACSCalibur; Becton Dickinson). To determine Cre-mediated recombination using ROSA26LacZ reporter mice, embryos were harvested at E7.5 and fixed by incubation with acetone for 10 minutes. After washing with PBS, fixed embryos were incubated with a X-gal solution (0.1% X-gal [Roche, Indianapolis, IN], 5 mM potassium ferricyanide crystalline, 5 mM potassium ferricyanide trihydrate, 2 mM magnesium chloride [all from Sigma-Aldrich] in PBS) for 12 hours at 37°C.
Light microscopy
Embryos were dissected at specified stages as described in “Mice, tissue dissection, and hematopoietic cell isolation” and fixed using 4% paraformaldehyde (PFA; Fisher Scientific, Fair Lawn, NJ). Sections of 8 μm were prepared from paraffin-embedded embryos starting at the level of forelimbs and proceeding sequentially through the entire caudal region of the embryo, including the AGM. The sections were stained with hematoxylin and eosin for tissue identification and examined using a Nikon Optiphot-2 microscope (Nikon Instech, Kawasaki, Japan) equipped with RT Color Spot camera (Diagnostic Instruments, Sterling Heights, MI).
Hematopoietic progenitor assay
To determine the progenitor content of each tissue, single-cell suspensions were plated in colony-forming unit (CFU) assays as previously described.28 Briefly, cells were plated in CFUs in triplicate in methylcellulose (MethocultM3100; StemCell Technologies, Vancouver, BC) in the presence of 30% FCS, 100 ng/mL stem cell factor (SCF; Amgen, Thousands Oaks, CA), 100 ng/mL interleukin-3 (IL3; Peprotech, Rocky Hill, NJ), 4 U/mL erythropoietin (EPO; Amgen), 100 ng/mL granulocyte colony-stimulating factor (G-CSF; Amgen), and 100 ng/mL megakaryocytic growth and differentiation factor (MGDF; Amgen). Cultures were incubated in 5% CO2 at 37°C and the number of colonies was determined using an inverted microscope 7 days later.
Migration and adhesion
Non–tissue culture 6-well plates (BD Labware) were coated with recombinant fibronectin (FN) fragments (CH-296, which contains α4β1-integrin– and α5β1-integrin–binding sites and the high-affinity heparin-binding site)34 at 8 μg/cm2 or bovine serum albumin (BSA; Roche; as a control) for 2 hours at 37°C. All plates were subsequently blocked with 2% BSA for 30 minutes at 37°C. Blood content of each embryo was washed with PBS and resuspended in 2 mL StemSpan SFEM (StemCell Technologies). The cells were allowed to adhere to the coated plates for 1 hour at 37°C. After incubation, nonadherent cells were collected by gently rinsing the plates 3 to 4 times with PBS. Adherent cells are harvested by incubation with cell dissociation buffer (Gibco, Carlsbad, CA) and vigorously rinsing the plates with PBS. The adherent and nonadherent cells were separately washed with PBS, resuspended in 400 μL StemSpan SFEM, and plated in CFU assay.
For directed migration (chemotaxis), blood cells collected from each embryo were washed once with PBS, resuspended in 1.5 mL StemSpan SFEM and added to the upper chamber of a transwell culture plate containing 8-μm pore size filter (Costar, Cambridge, MA) previously coated with FN CH296 as described above. StemSpan SFEM (2.6 mL) containing 100 ng/mL SDF-1α (Peprotech) and 100 ng/mL SCF (used as chemoattractants) was placed in the lower chamber. After 4-hour incubation at 37°C in 5% CO2, the upper chamber was carefully removed and cells from the upper chamber as well as the cells adherent to the transwell membrane were harvested by washing with PBS and incubation with trypsin (Sigma-Aldrich) for 5 minutes at 37°C. The cells from the bottom chamber were harvested by washing with PBS. The cells from the upper and lower chambers were separately washed once with PBS, resuspended in 400 μL StemSpan SFEM, and plated in CFU assay.
Apoptosis and proliferation
To study apoptosis of definitive HPCs at E10.5, staged embryos were harvested and single-cell suspensions from YS, blood, and embryo proper were obtained as described in “Mice, tissue dissection, and hematopoietic cell isolation” and then washed once with ice-cold PBS. The cells were then incubated with anti-CD41 antibody as described above. After washing with PBS, the cells were incubated for 20 minutes at room temperature with annexinV staining solution (5 μg/mL 7amino-actinomycinD [7AAD; Molecular Probes], 5 μg/mL annexinV–fluorescein isothiocyanate [FITC; BD PharMingen] in 10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and then analyzed by flow cytometry.
To study proliferation, E10.5 pregnant females were injected intravenously with 200 μL PBS containing 2.5 mg/mL bromodeoxyuridine (BrdU; BD PharMingen). The embryos were harvested one hour later and single-cell suspensions from YS and embryo proper were obtained as described in “Mice, tissue dissection, and hematopoietic cell isolation.” The cells were stained with antiCD41-Alexa488 as described in “Mice, tissue dissection, and hematopoietic cell isolation,” and subsequently anti-BrdU staining was performed. Briefly, cells were fixed and permeabilized by incubation with Cytoperm/Cytofix buffer (BD PharMingen) for 30 minutes, incubated with CytopermPlus Buffer (BD PharMingen) for 10 minutes, and then refixed by incubation with Cytoperm/Cytofix buffer (BD PharMingen) for 5 minutes; all steps were performed on ice. The cells were then treated with 100 μL PBS, 300 μg/mL DNase I (BD PharMingen) for 1 hour at 37°C in the dark. After washing with Perm/Wash buffer (BD PharMingen), the cells were stained with allophycocyanin (APC)–labeled anti-BrdU antibody for 20 minutes at room temperature in the dark and washed with Perm/Wash buffer. DNA was stained using 7AAD solution (BD PharMingen) and analyzed by flow cytometer.
Cobblestone area–forming assay
Cobblestone area–forming cell (CAFC) frequency analysis was performed as described previously.35 Single-cell suspensions of AFT024 FL stromal cells were irradiated with 20 Gy using a 137Cs irradiator (Mark-I-68; JL Shepherd and Associates, San Fernando, CA) and 2 × 104 cells were plated in a flat-bottom tissue culture–treated 96-well plate (BD Labware). Two days later, single-cell suspensions of E10.5 YS was overlaid on this stroma layer in 2-fold dilutions, 15 replicates per condition to allow limiting dilution analysis. CAFCs were scored after 7 days in culture. Wells were scored positive if at least one phase-dark hematopoietic clone (cobblestone, containing 5 or more cells) was observed. The frequency of CAFCs was then calculated using Poisson statistics as described previously.36
Hanging drop, fetal organ culture
Single-cell solutions of each E10.5 YS were suspended in 120 μL StemSpan SFEM, 5 × 10−5 M β-mercapto-ethanol (β-ME; Sigma-Aldrich), 100 U/mL P/S, 2 mM l-glutamine (Cellgro). Thirty microliters of cell suspension (containing 1/4 of each YS) was mixed with 15 μL StemSpan SFEM containing 104 AFT024 cells and deposited on the inner part of a 24-well plate lid. The lid was then carefully turned over and deposited on top of a 24-well plate containing 300 μL PBS for humidification. Cultures were maintained for 48 hours at 37°C in 5% CO2 incubator. After the culture period, the lid was carefully turned over and the cells from each drop were collected and dissociated using a 1-mL syringe and 27½-G needle. The single-cell suspension was washed with PBS and used for methylcellulose-based CFU assay as described above.
Protein expression analysis
Cells were isolated from individual hematopoietic colonies and washed with PBS. The expression levels of Rac1 and β-actin proteins were determined by immunoblot analysis as previously described.28 Briefly, the cells were incubated with 25 μL protein lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 10% glycerol, 50 mM NaF, 1 mM Na3VO4, 1 mM dithiothreitol, 0.1% NonidetP-40, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride, 10 μg/mL aprotinin and leupeptin) for 30 minutes on ice. The lysate was cleared by centrifugation for 30 minutes at more than 9200g in a refrigerated centrifuge (AllegraTMX-22R; Beckman Coulter, Palo Alto, CA) and incubated with Laemmli Sample Buffer (Bio-Rad Laboratories, Hercules, CA) for 5 minutes at 95°C. Aliquots of cell lysates were then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) separation and immunoblots were probed with anti-Rac antibody (1:1000; Upstate, Waltham, MA) and specific monoclonal antibody anti–β-actin (1:10 000; Sigma-Aldrich) for loading control.
Results
Rac1 expression is required in hematopoietic cells during fetal development
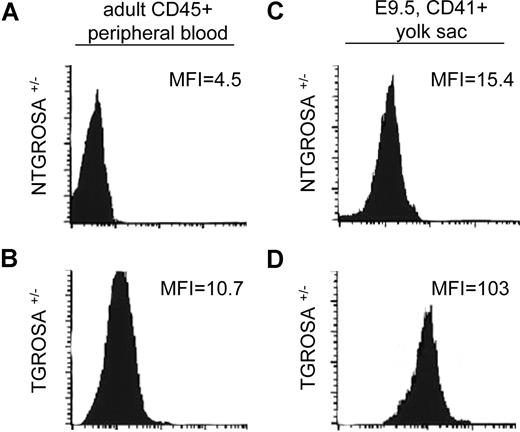
In the mouse embryo, Rac1 GTPase is essential for the formation and survival of the extraembryonic mesoderm and homozygous Rac1null mice show embryonic lethality at gastrulation.37 To study the role of Rac1 GTPase in the ontogeny of definitive hematopoiesis, a tissue-specific conditional knockout approach was used. To achieve lineage-specific deletion of Rac1 during fetal development, we used a floxed Rac1 allele27 and Vav1-Cre transgenic mice.31 Mice expressing Cre recombinase (Cre) under the control of a Vav1 promoter-enhancer cassette have been shown to display recombination in hematopoietic stem and progenitor cells and a very restricted subset of endothelial cells during adult life.31 To determine the developmental stage at which Cre is expressed and the fidelity of expression in hematopoietic tissues from the Vav1Cre transgene, transgenic Vav1Cre+/− females were bred with Rosa26GFP reporter mice. Under these experimental conditions, expression of Cre should induce recombination of Rosa26 locus and subsequent GFP expression. When cells from adult double-transgenic mice were analyzed via flow cytometry (Figure 1 left column), a 2-fold shift in the mean fluorescence intensity (MFI) of the transgenic leukocytes (TGs, lower left histogram) in comparison with leukocytes from nontransgenic mice (NTG, top left histogram) was observed. The expression of GFP in these mice was associated with complete recombination of Rosa26GFP locus as determined by genomic PCR analysis (data not shown). Next, we analyzed CD41+ definitive hematopoietic progenitor cells at E9.5 (Figure 1 right column). CD41+ cells harvested from YS of Vav1Cre:RosaGFP TG animals showed 7-fold increase in GFP MFI (Figure 1 lower right histogram) in comparison with cells from NTG animals. These results demonstrate that Vav1 promoter-enhancer elements can drive Cre-mediated recombination of definitive hematopoietic progenitor cells as early as E9.5. Using a Rosa26LacZ mouse reporter line, presence of Vav1Cre-mediated recombination was observed in YS as early as E7.5 in a bandlike pattern similar to the distribution of Flk1dimCD41dim cells at this stage of embryonic development8 (data not shown).
Expression profile of Vav1-driven Cre-mediated recombination. Flow cytometric analysis of recombination status of RosaGFP locus in CD41+ definitive hematopoietic progenitors from E9.5 yolk sac (right column) and CD45+ adult peripheral blood leukocytes (left column). As a negative control, a Vav1Cre nontransgenic (NTG) ROSA heterozygous mouse is presented in the top row. The mean fluorescence intensity (MFI) of the GFP channel is depicted in each panel. Data are representative of 3 independent specimens analyzed for each genotype (N = 3).
Expression profile of Vav1-driven Cre-mediated recombination. Flow cytometric analysis of recombination status of RosaGFP locus in CD41+ definitive hematopoietic progenitors from E9.5 yolk sac (right column) and CD45+ adult peripheral blood leukocytes (left column). As a negative control, a Vav1Cre nontransgenic (NTG) ROSA heterozygous mouse is presented in the top row. The mean fluorescence intensity (MFI) of the GFP channel is depicted in each panel. Data are representative of 3 independent specimens analyzed for each genotype (N = 3).
To further enhance the probability of complete deletion of genomic Rac1 sequences for the experiments described, triple-transgenic mice (Vav1Cre, Rac1null,30 and Rac1flox) were used (Figure S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article). Mice transgenic for Vav1Cre (TG) were bred with Rac1wt/null mice, and TGRac1wt/null F1 female mice were subsequently bred with Rac1flox/flox males. Genotype analysis of F2 mice revealed no triple-transgenic animals (TGRac1Δ/null, Table 1) suggesting that lack of expression of Rac1 in hematopoietic-restricted fashion was associated with embryonic lethality. To determine the stage at which TGRac1Δ/null mice were dying in utero, pregnant females were killed at various stages of gestation after timed pregnancies. The distribution of embryos from F2 generation of 4 possible genotypes was normal at E9.5 and E10.5 (E9.5: 27/105 vs 26/105 and E10.5: 39/179 vs 45/179, observed vs expected frequency of TGRac1Δ/null, P > .6; Table 1). However, by E12.5 only 2 of 59 embryos of TGRac1Δ/null genotype were observed compared with an expected ratio of 15 of 59 (P < .01). In conclusion, Rac1 expression in hematopoietic cells appears essential for fetal survival after E11.5.
Distribution of genotypes in the F2 population
| Age . | Genotype (%) . | P . | |||
|---|---|---|---|---|---|
| NTGRac1Flox/Wt . | NTGRac1Flox/null . | TGRac1Flox/Wt . | TGRac1Flox/null . | ||
| E9.5 | 23/105 (22) | 29/105 (27) | 26/105 (25) | 27/105 (26) | .870 |
| E10.5 | 51/179 (28) | 46/179 (26) | 43/179 (24) | 39/179 (22) | .634 |
| E11.5 | 17/57 (30) | 15/57 (26) | 18/57 (32) | 7/57 (12) | .155 |
| E12.5 | 21/59 (36) | 19/59 (32) | 17/59 (29) | 2/59 (3) | .002 |
| E14.5 | 20/61 (33) | 18/61 (29) | 23/61 (38) | 0/61 (0) | .001 |
| Age . | Genotype (%) . | P . | |||
|---|---|---|---|---|---|
| NTGRac1Flox/Wt . | NTGRac1Flox/null . | TGRac1Flox/Wt . | TGRac1Flox/null . | ||
| E9.5 | 23/105 (22) | 29/105 (27) | 26/105 (25) | 27/105 (26) | .870 |
| E10.5 | 51/179 (28) | 46/179 (26) | 43/179 (24) | 39/179 (22) | .634 |
| E11.5 | 17/57 (30) | 15/57 (26) | 18/57 (32) | 7/57 (12) | .155 |
| E12.5 | 21/59 (36) | 19/59 (32) | 17/59 (29) | 2/59 (3) | .002 |
| E14.5 | 20/61 (33) | 18/61 (29) | 23/61 (38) | 0/61 (0) | .001 |
Data are n/N (%).
NTG indicates nontransgenic; and TG, Vav1-Cre transgenic.
Rac1 is essential for establishment of fetal liver hematopoiesis
To determine the cause of midgestation embryonic lethality of TGRac1Δ/null mice, we analyzed in detail embryos at E11.5 (Figure 2A). TGRac1Δ/null embryos demonstrated pallor associated with very small fetal livers (see area delineated in Figure 2A). TGRac1Δ/null embryos also displayed hemorrhaging in the cephalic area and the dorsal region underlying the spinal cord (Figure 2A arrowheads), a phenotype previously reported in several murine models of defective FL hematopoiesis.38,39 These studies suggested diminished FL hematopoiesis in TGRac1Δ/null mice.
Phenotypic analysis of embryos at E11.5. (A,B) Gross appearance of surviving TGRac1Flox/null embryos and littermate controls at E11.5. The limbs from the right side of the embryos were removed for clarity of presentation. The appearance of the littermate controls was similar for NTGRac1Flox/Wt, NTGRac1Flox/null, and TGRac1Flox/Wt (hereafter referred to as “control”). The anatomic region of the fetal liver is delineated and noted by arrows. The arrowheads point to accumulation of blood in cephalic regions as well as areas underlying the spinal cord, a phenotype previously noted in mutant animals deficient in hematopoietic cell production at the fetal liver stage. (C) Quantitative analysis of fetal liver hematopoiesis as analyzed by CFU/FL of F2 embryos at E11.5. Data represent mean (± SD) of at least 3 embryos analyzed for each genotype. *P < .01, TGRac1Flox/null versus all other genotypes.
Phenotypic analysis of embryos at E11.5. (A,B) Gross appearance of surviving TGRac1Flox/null embryos and littermate controls at E11.5. The limbs from the right side of the embryos were removed for clarity of presentation. The appearance of the littermate controls was similar for NTGRac1Flox/Wt, NTGRac1Flox/null, and TGRac1Flox/Wt (hereafter referred to as “control”). The anatomic region of the fetal liver is delineated and noted by arrows. The arrowheads point to accumulation of blood in cephalic regions as well as areas underlying the spinal cord, a phenotype previously noted in mutant animals deficient in hematopoietic cell production at the fetal liver stage. (C) Quantitative analysis of fetal liver hematopoiesis as analyzed by CFU/FL of F2 embryos at E11.5. Data represent mean (± SD) of at least 3 embryos analyzed for each genotype. *P < .01, TGRac1Flox/null versus all other genotypes.
To more rigorously analyze FL hematopoiesis, the number of HPCs was quantified by enumerating definitive hematopoietic progenitor-derived colonies. Fetal livers from littermate control animals yielded 7496 (± 2096) colonies (mean ± SD; Figure 2B) and no significant difference in number of colonies was observed between the 3 littermate control genotypes in which at least one functional Rac1 allele was present. The number of progenitor colonies was decreased 30-fold in FL of TGRac1Δ/null mice (235 ± 103 vs 7496 ± 2096, TGRac1Δ/null vs littermate controls, P < .01). Thus, embryonic lethality observed in mice lacking Rac1 is associated with nearly absent FL hematopoiesis at E11.5.
Rac1 is not necessary for development of YS definitive hematopoiesis
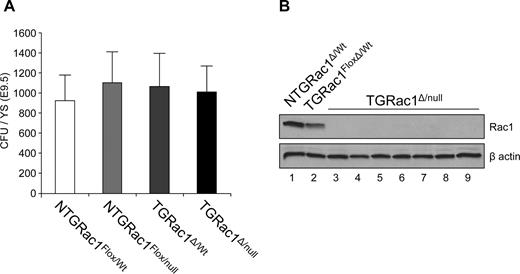
To more precisely determine the developmental stage and anatomic site at which Rac1 expression is required for ontogeny of definitive hematopoiesis, YS hematopoiesis was analyzed beginning at E9.5. At this stage of embryonic development, most definitive hematopoietic activity resides in YS with hematopoietic progenitor activity minimal in FL bud and throughout embryo proper. The gross appearance of TGRac1Δ/null embryos at this stage was indistinguishable from littermate controls (data not shown). As shown in Figure 3A, the number of HPCs in the YS of TGRac1Δ/null embryos was similar to littermate control genotypes (1011 ± 257 vs 1096 ± 297, TGRac1Flox/null vs littermate controls, P = .92). There was no significant differences in the distribution of blast-forming unit–erythroids (BFU-Es) and colony-forming unit–macrophages (CFU-Ms) between TGRac1Flox/null embryos and littermate control embryos (BFU-Es: 451 ± 101 vs 322 ± 122; CFU-Ms: 705 ± 103 vs 603 ± 178; TGRac1Flox/null vs littermate control, Figure S2).
Definitive hematopoiesis in the yolk sac at E9.5. (A) Quantitative analysis of definitive hematopoietic progenitor cells as analyzed by CFU/YS of F2 embryos at E9.5. Data represent mean (± SD) of at least 7 embryos analyzed for each genotype. There was no significant difference among genotypes. (B) Immunoblot analysis of Rac1 protein expression level of individual hematopoietic progenitor colonies from the yolk sac of TGRac1Flox/null mice (lanes 3-9) and control mice (lanes 1-2) at E9.5. β-Actin is shown as a loading control. Note intensity of Rac1 band in lane 2 versus lane 1, representing expression of Rac1 in the heterozygous versus WT genotype. These results are representative of 3 independent experiments with similar results.
Definitive hematopoiesis in the yolk sac at E9.5. (A) Quantitative analysis of definitive hematopoietic progenitor cells as analyzed by CFU/YS of F2 embryos at E9.5. Data represent mean (± SD) of at least 7 embryos analyzed for each genotype. There was no significant difference among genotypes. (B) Immunoblot analysis of Rac1 protein expression level of individual hematopoietic progenitor colonies from the yolk sac of TGRac1Flox/null mice (lanes 3-9) and control mice (lanes 1-2) at E9.5. β-Actin is shown as a loading control. Note intensity of Rac1 band in lane 2 versus lane 1, representing expression of Rac1 in the heterozygous versus WT genotype. These results are representative of 3 independent experiments with similar results.
To confirm that Rac1 was effectively deleted from E9.5 YS definitive HPCs, expression of Rac1 was determined by immunoblot in multiple HPC-derived colonies from YS (Figure 3B). All (> 20) HPC-derived colonies isolated from YS of multiple TGRac1Δ/null animals demonstrated absence of Rac1 protein expression (Figure 3B lanes 3-9). The level of Rac1 protein in NTGRac1Flox/Wt colonies was approximately 2-fold higher compared with colonies containing one functional allele of Rac1 (TGRac1Δ/wt, Figure 3B lane 2). In summary, these data show that TGRac1Δ/null embryos have normal definitive YS hematopoiesis at E9.5 in spite of complete absence of Rac1 protein.
Intraembryonic hematopoiesis is abnormal without Rac1
Fetal hematopoietic activity has been demonstrated in both extraembryonic, YS, and multiple intraembryonic sites by E10.5. To determine the role of Rac1 in the development of intraembryonic sites of definitive hematopoiesis, we next studied circulating blood, YS, FL, and embryonic tissues for hematopoietic colony-forming activity at E10.5. As shown in Figure 4A, in agreement with the results obtained from E9.5, the number of definitive HPCs found in the YS of TGRac1Δ/null embryos at E10.5 was similar to that of littermate control genotypes (721 ± 178 vs 719 ± 166, TGRac1Δ/null vs littermate controls, P = .97). However the number of progenitor cells circulating in the peripheral blood of TGRac1Δ/null animals was significantly decreased compared with control littermates (Figure 4B, 253 ± 74 vs 489 ± 116, TGRac1Δ/null vs littermate controls, P < .01). After careful removal of extraembryonic membranes, FL, and circulating blood, HPCs were enumerated from embryonic remnants. Hematopoietic progenitors in embryonic remnants of TGRac1Δ/null mice were significantly decreased compared with littermate controls (Figure 4C, 74 ± 62 vs 825 ± 214, TGRac1Δ/null vs littermate controls, P < .01). In agreement with previously presented results from E11.5, the number of definitive HPCs was significantly decreased in FL of TGRac1Δ/null embryos compared with littermate control animals (Figure 4D, 40 ± 32 vs 432 ± 112, TGRac1Δ/null vs littermate controls, P < .01).
Definitive hematopoiesis of embryos at E10.5. (A-D) Quantitative analysis of definitive hematopoiesis at E10.5 by determination of number of CFUs in (A) yolk sac; (B) blood; (C) embryonic remnants (embryonic tissue after removal of yolk sac and amniotic membranes, blood, fetal liver, and tail bud, used for genotyping); and (D) fetal liver. Data represent means (± SD) of 8 or more F2 embryos for each genotype, *P < .01, TGRac1Flox/null versus all other genotypes. (E,F) Histologic analysis of the aortic clusters at E10.5. Punctuated squares delineate the area shown at higher magnification in the upper-right corner. The figures are representative of all animals analyzed for each genotype. Data are summated in Table 2. Arrow in panel E shows hematopoietic cells on the ventral wall of dorsal aorta. Arrowheads in panel F show circulating primitive red blood cells. No TGRac1Flox/null mice demonstrated aortic clusters. Images acquired as described in “Light microscopy,” with a 40×/0.5 numeric aperture objective.
Definitive hematopoiesis of embryos at E10.5. (A-D) Quantitative analysis of definitive hematopoiesis at E10.5 by determination of number of CFUs in (A) yolk sac; (B) blood; (C) embryonic remnants (embryonic tissue after removal of yolk sac and amniotic membranes, blood, fetal liver, and tail bud, used for genotyping); and (D) fetal liver. Data represent means (± SD) of 8 or more F2 embryos for each genotype, *P < .01, TGRac1Flox/null versus all other genotypes. (E,F) Histologic analysis of the aortic clusters at E10.5. Punctuated squares delineate the area shown at higher magnification in the upper-right corner. The figures are representative of all animals analyzed for each genotype. Data are summated in Table 2. Arrow in panel E shows hematopoietic cells on the ventral wall of dorsal aorta. Arrowheads in panel F show circulating primitive red blood cells. No TGRac1Flox/null mice demonstrated aortic clusters. Images acquired as described in “Light microscopy,” with a 40×/0.5 numeric aperture objective.
We next examined the presence and number of aortic clusters in developing embryo of each genotype. Aortic clusters, morphologically defined cellular structures found intraluminally attached to the ventral floor of the dorsal aorta in AGM region, are also believed to contain definitive hematopoietic progenitor activity.40 No aortic clusters were found in TGRac1Δ/null embryos at E10.5 (Table 2), while 7 to 12 aortic clusters per embryo were identified in littermate control embryos (arrow in Figure 4E). This correlated with the presence of CD31+ intraluminal clusters in control embryos. No CD31+ clusters were present in TGRac1Δ/null embryos (data not shown). Taken together, these data demonstrate that without Rac1 definitive hematopoiesis is severely impaired in multiple intraembryonic sites even though definitive YS hematopoiesis is not significantly affected in these embryos.
Apoptosis and proliferation of definitive HPCs is not affected by the absence of Rac1
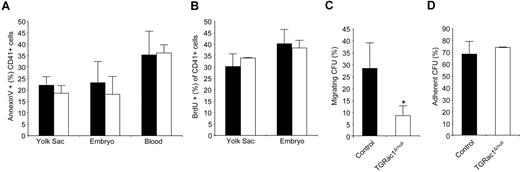
Rac GTPases have been previously implicated in adult HPC apoptosis and proliferation when analyzed in vitro using specific agonists.27 In these studies, Rac1 appears to regulate cell-cycle progression, while Rac2 maintains cell survival in the presence of SCF, a chemokine known as a survival factor for HSCs/Ps. To determine whether deficiency of definitive HPCs in embryonic tissues of TGRac1Δ/null mice was due to altered proliferation or survival in vivo, we next analyzed apoptosis and proliferation of definitive HPCs isolated from YS, embryo proper, and blood. There was no significant difference in apoptosis as measured by 7AAD/annexinV staining between TGRac1Δ/null animals and littermate controls (Figure 5A)
Apoptosis, proliferation, adhesion, and migration of definitive hematopoietic progenitors at E10.5. (A) Level of apoptosis of CD41+ definitive hematopoietic progenitors from different embryonic tissues at E10.5 analyzed by annexinV/7AAD staining and flow cytometry. Figure shows one representative experiment of 3 with similar results. Data represent means (± SD) of at least 3 embryos analyzed for each genotype. Differences between genotypes were not significant. (B) BrdU incorporation of definitive hematopoietic progenitors isolated from the yolk sac or the embryo proper of F2 embryos at E10.5. Figure shows one representative experiment of 3 with similar results. Data represent means (± SD) of at least 3 embryos analyzed for each genotype. Differences among genotypes were not significant. (C) SCF/SDF1-α induced directed migration on FN CH296-coated transwell filters of circulating E10.5 definitive hematopoietic progenitors. Data represent means (± SD) of 3 independent experiments. (D) β1-integrin–mediated adhesion to FN CH296 of circulating E10.5 definitive hematopoietic progenitors assayed by CFU. Data represent means (± SD) of 3 independent experiments. There was no significant difference among groups. *P < .05, TGRac1Flox/null versus control (N = 3). (■ indicates littermate control; □, TGRac1Flox/null).
Apoptosis, proliferation, adhesion, and migration of definitive hematopoietic progenitors at E10.5. (A) Level of apoptosis of CD41+ definitive hematopoietic progenitors from different embryonic tissues at E10.5 analyzed by annexinV/7AAD staining and flow cytometry. Figure shows one representative experiment of 3 with similar results. Data represent means (± SD) of at least 3 embryos analyzed for each genotype. Differences between genotypes were not significant. (B) BrdU incorporation of definitive hematopoietic progenitors isolated from the yolk sac or the embryo proper of F2 embryos at E10.5. Figure shows one representative experiment of 3 with similar results. Data represent means (± SD) of at least 3 embryos analyzed for each genotype. Differences among genotypes were not significant. (C) SCF/SDF1-α induced directed migration on FN CH296-coated transwell filters of circulating E10.5 definitive hematopoietic progenitors. Data represent means (± SD) of 3 independent experiments. (D) β1-integrin–mediated adhesion to FN CH296 of circulating E10.5 definitive hematopoietic progenitors assayed by CFU. Data represent means (± SD) of 3 independent experiments. There was no significant difference among groups. *P < .05, TGRac1Flox/null versus control (N = 3). (■ indicates littermate control; □, TGRac1Flox/null).
To determine the proliferative status of hematopoietic progenitors in vivo, we performed BrdU labeling of developing embryos by intravenous injection of BrdU into pregnant dams at E10.5. CD41+ cells derived from YS or embryo proper of TGRac1Δ/null embryos showed similar levels of BrdU staining measured by flow cytometry compared with littermate control genotypes (Figure 5B, YS: 30.65% ± 0.07% vs 30.03% ± 5.67%; embryo: 38.25% ± 3.23% vs 40.25% ± 6.24%; TGRac1Δ/null vs littermate controls, P > .45). In summary, TGRac1Δ/null hematopoietic progenitors in YS or embryo proper have similar levels of apoptosis and proliferation in vivo compared with littermate control animals.
Rac1-deficient circulating HPCs demonstrate altered cellular migration
The FL bud is believed to be colonized by HSCs/Ps migrating from intraembryonic or extraembryonic sites (or both) via the fetal circulation. Because Rac1 GTPases have been previously shown to regulate HSC migration/engraftment in adult hematopoietic organs, we next analyzed the potential role of Rac1 in migration of embryonic HPCs. To study migration of definitive HPCs, in vitro transwell migration assays were developed. SCF and SDF-1α have both been shown to be potent chemoattractants for HPCs, including those isolated from FL.41 However, no previous studies have defined chemotactic factors for circulating fetal HSCs/Ps. Of multiple chemoattractant factors tested, the combination SCF/SDF1α consistently induced migration of approximately 30% of circulating wild-type HPCs. HPCs from TGRac1Δ/null mice showed significantly defective migration in response to SCF/SDF-1α compared with HPCs from control embryos (Figure 5C, 8.7% ± 4.1% vs 28.4% ± 10.8%, TGRac1Δ/null vs littermate controls, P < .05). This altered migration was not due to defective β1-integrin–mediated adhesion because a high percentage of definitive HPCs adhered to the recombinant fibronectin fragments (CH296) regardless of Rac1 status (Figure 5D, 73.2% ± 0.6% vs 67.8% ± 10.5%, TGRac1Flox/null vs littermate controls). In con-clusion, definitive HPCs isolated from peripheral blood of TGRac1Δ/null embryos have significantly decreased SCF/SDF1-α-–directed transwell migration in spite of normal α4β1-integrin– and α5β1-integrin–mediated adhesion.
Definitive HPCs isolated from YS of TGRac1Δ/null embryos demonstrate normal adhesion to stroma but reduced CAFC formation associated with defective migration
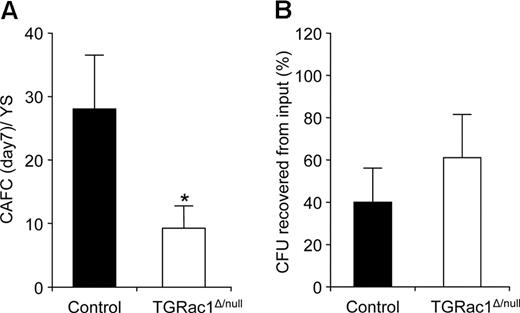
The interaction between HSCs/Ps and HM within the putative “stem cell niche” is crucial for normal hematopoiesis in adult animals.42,43 Rac GTPases have been shown to be critical regulators of the interaction of adult HSCs/Ps with HM and for colocalization and retention of HSCs/Ps in the bone marrow.21,22 To determine whether Rac1 is critical for the interaction of HPCs with fetal HM, we performed a CAFC assay modified to test fetal hematopoietic cells. YS cells isolated from E10.5 embryos were analyzed for adherence and migration on a preformed confluent layer of FL-derived stromal cell line, AFT024. Cobblestone areas formed within 7 days of plating were enumerated as readout of successful adhesion, migration, survival, and proliferation of HPCs. There was 3-fold decrease in number of CAFCs derived from YS of TGRac1Δ/null embryos compared with control littermates in this assay (9.2 ± 3.4 vs 27.7 ± 8.3, TGRac1Δ/null vs littermate controls, P < .05, Figure 6A).
In vitro characterization of interaction between yolk sac–derived E10.5 definitive hematopoietic progenitors and stromal cells. (A) Total number of CAFCs per yolk sac of E10.5 F2 embryos. Data represent means (± SD) of 3 independent experiments. *P < .05, TGRac1Flox/null versus littermate controls (N = 3). (B) Survival of definitive hematopoietic progenitors from the yolk sac at E10.5 in a hanging drop organ culture with AFT024 stroma cells (“Methods”). Data represent means (± SD) of 3 independent experiments (N = 3). There was no significant difference between genotypes. ■ indicates littermate control; □, TGRac1Flox/null.
In vitro characterization of interaction between yolk sac–derived E10.5 definitive hematopoietic progenitors and stromal cells. (A) Total number of CAFCs per yolk sac of E10.5 F2 embryos. Data represent means (± SD) of 3 independent experiments. *P < .05, TGRac1Flox/null versus littermate controls (N = 3). (B) Survival of definitive hematopoietic progenitors from the yolk sac at E10.5 in a hanging drop organ culture with AFT024 stroma cells (“Methods”). Data represent means (± SD) of 3 independent experiments (N = 3). There was no significant difference between genotypes. ■ indicates littermate control; □, TGRac1Flox/null.
Because we had previously demonstrated normal apoptosis and proliferation of Rac1−/− HPCs in vivo, we hypothesized that defective migration could explain the observed decrease in formation of cobblestone areas. Because migration was reduced in the presence of normal adhesion of Rac1−/− HPCs, we next mixed AFT024 stroma cells directly with YS cells in “hanging drop culture,” which required no migration of input cells. HPCs were enumerated in colony assay after 48 hours of incubation. As shown in Figure 6B, the number of definitive HPCs expressed as percentage of input HPCs was similar in TGRac1Δ/null mice compared with littermates control (60.9% ± 21.1% vs 40.4% ± 13.9%, TGRac1Δ/null vs control group, P = .25). Taken together, these data suggest that definitive HPCs lacking Rac1 have defective capacity to migrate across stroma, but can survive in response to stromal-derived signals if migration is not required.
Discussion
The development of definitive hematopoiesis in adult animals is the result of a complex process presumably involving migration of a defined population of mesoderm-derived hematopoietic stem cells during embryonic development.44 HSCs capable of maintaining life-long hematopoiesis in the adult animal are generated early in the fetal life, potentially in both extraembryonic and intraembryonic sites, ultimately seeding the fetal liver. Previous studies have shown that HSCs/Ps appear in the liver bud soon after circulation is established in the mouse embryo.11,12 The origin of first definitive HSCs/Ps to seed the liver bud is controversial and has alternatively been postulated to involve both the YS and the AGM.
Rac1 GTPase, a member of small Rho GTPases family, has been shown to control F-actin polymerization and actin cytoskeleton reorganization. Without Rac1, HSCs/Ps display delayed retraction of uropods and decreased SDF1α-directed migration.26,27 HSCs/Ps from adult mice deficient in Rac1 are defective in microlocalization in the bone marrow endosteal niche after transplantation into recipient mice resulting in decreased engraftment of these cells.21,27 In contrast, deletion of Rac1 after HSCs/Ps are already localized to the bone marrow does not affect steady-state hematopoiesis, as measured in competitive repopulation assays.21 Based on these findings in adult mice, we hypothesized that hematopoiesis would develop normally (within defined developmental boundaries) without Rac1 in tissues that produce de novo hematopoiesis and that seeding of secondary hematopoietic sites within the developing embryo would be impaired.
We provide evidence that Rac1 regulates migration of HSCs/Ps and seeding of the fetal liver during embryonic development. Without Rac1, fetal liver hematopoiesis is significantly reduced at E10.5 and E11.5, while definitive YS hematopoiesis appears normal. This phenotype could be explained by decreased migration of HSCs/Ps from the embryonic bloodstream into the fetal liver and other embryonic sites because adhesion, survival, and proliferation of fetal HSCs/Ps was not affected by Rac1 deficiency and Rac1−/− HSCs/Ps demonstrated significantly decreased migration as determined by in vitro assays. It has previously been hypothesized that definitive HPC pool from YS serves as the source of initial seeding of the fetal liver.2 In this scenario, HPCs would leave YS and migrate via the vitelline veins to reach the intrahepatic circulation. The data presented here support this view of the origin of definitive hematopoiesis, because Rac1-deficient HSCs/Ps are shown to be defective in migration, present in the YS, and absent in all intraembryonic tissues examined, including in the FL.
In contrast to YS, other anatomic sites of intraembryonic hematopoiesis were disrupted without Rac1. The AGM and aortic clusters were significantly deficient of HSCs/Ps in Rac1−/− embryos. Overall, the total number of hematopoietic progenitor cells in the embryo proper (after removal of fetal liver and circulating blood) was significantly reduced without Rac1. One interpretation of these data are that hematopoiesis in AGM region is dependent on migration of hematopoietic precursor cells to this region. As it relates to the lack of aortic clusters in Rac1−/− mice, recent data from cell tracking techniques have demonstrated that cells originating from YS colonize the luminal surface of intraembryonic vessels, including the aorta.45 Alternatively, North et al46 and Bertrand et al10 suggest that aortic clusters are generated by hematopoietic precursor cells migrating from the mesoderm adjacent to aorta and subaortic patches, respectively. Without Rac1, these precursors would display impaired local migration through the endothelium into the vessels with the resulting observed absence of aortic clusters. A third possibility is that differentiation of HSCs/Ps within the AGM region is profoundly affected in the absence of Rac1 and YS hematopoiesis is completely independent of this mechanism. To date, we and others have not observed any significant qualitative or quantitative effect on differentiation of HSCs/Ps without Rac1 alone. However, as it relates to this possibility, Hadland et al have postulated that Notch1 is required for intraembryonic definitive hematopoiesis but not for YS definitive hematopoiesis, suggesting developmental or location-specific dependence on unique signaling pathways.47
We observed normal YS hematopoiesis at E9.5 and E10.5 without Rac1. These data suggest that definitive hematopoiesis arises de novo in the YS niche. This observation is in agreement with the results of studies published by Palis et al that showed the presence of definitive HPCs in the YS before establishment of circulation.5,6 The absence of increased numbers of YS HSCs/Ps and the presence of circulating HSCs/Ps suggest that Rac1 is not essential for migration of these cells from the YS. In this case, while Rac1 plays a critical role in migration of HSCs/Ps into embryonic sites, including FL, a different member of Rho GTPase family may control the emigration of these cells from YS.
In summary, we demonstrate that Rac1 controls migration of HSCs/Ps during embryonic development, a process essential for the seeding of FL and the development of intraembryonic hematopoiesis but not required for YS hematopoiesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of our laboratory; Drs Yi Zheng, Chris Wylie, and Wally Ip for helpful discussions; and Eva Meunier and Kiesha Steward for administrative assistance. We thank Dr Andrew Roberts for providing Vav1Cre mice.
This work was supported by National Institutes of Health grants 1R01HL63169 (M.C.Y.), 1P01HL69974, and 1R01DK062757 (D.A.W.).
National Institutes of Health
Authorship
Contribution: G.G. designed and performed research, contributed vital new reagents/analytical tools, analyzed data, and wrote the paper; M.J.F., M.D.M., D.W., and M.C.Y. contributed vital new reagents/analytical tools; J.B. performed research; J.A.C. analyzed data and wrote the paper; D.A.W. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Williams, Children's Hospital Boston, 300 Longwood Ave, Boston, MA 02115; e-mail: dawilliams@childrens.harvard.edu.