Notch signaling establishes boundaries in the thymus by inducing T-cell commitment and inhibiting a B-cell choice. Here, we show a significant 1.6-fold increased generation of B-cell precursors in thymuses from mice deficient for Notch target Hes5 compared with wild-type littermates. We further show that culture of bone marrow–derived progenitors with increasing densities of purified immobilized Notch ligand (Delta1ext-IgG) induced increased expression of Notch targets Hes1 and Hes5, and that although Hes5-deficient progenitors responded appropriately to high densities of ligand, they misread intermediate and low densities. Together, our results suggest that to ensure an appropriate outcome in the thymus in response to a lower threshold of induced Notch signaling, induction of the additional target Hes5 is required.
Introduction
During organogenesis, distinct compartments are often bounded by cells that regulate differentiation in neighboring compartments. Notch signaling plays a pivotal role in establishing these cell boundaries in systems such as Drosophila wing development and in vertebrate somite development.1,2 More recently, a crucial role for Notch signaling in the establishment and maintenance of distinct boundaries in the mammalian thymus has been demonstrated (reviewed in Visan et al3 and Maillard et al4 ). It is generally thought that early after entering the thymus in the corticomedulary junction (CMJ), the majority of early thymic progenitors (ETPs), a subset of the CD4−CD8− (double negative [DN]) thymocyte subset, commit to T-cell differentiation, while some ETPs retain potential for B-cell differentiation in vivo and in vitro. Although the few B cells found in the CMJ might be derived extrathymically, they more likely derive from an ETP that makes a B- or T-cell lineage choice soon after entering the thymus.5,6 Notch signaling is required for this earliest stage of T-cell development, since in the absence of Notch signaling, no T cells form, and ETPs instead adopt the default B-cell differentiation pathway.7 In the normal thymus, B cells are predominantly restricted to the CMJ, suggesting a reduced level of Notch signaling in this area of the thymus.8
Perturbations in Notch signaling levels in the thymus influence not only whether B cells are generated but also the number of B cells generated. For example, mice homozygous for a Notch1 deficiency generate only B cells in the thymus.7 While mice heterozygous for a Notch1 deficiency generate about the same proportion of B cells as wild-type mice, introduction of misexpressed Fringe, a glycosyl transferase that modifies Notch, into heterozygotes results in profoundly increased proportions of B cells in the heterozygous mice, but not in wild-type mice misexpressing Fringe.8,9 These results indicate that a partial change in Notch signaling results in changes in an established border. Furthermore, lowering the level of Notch signaling with a Notch1 deficiency or deficiencies in Notch signaling components such as lunatic fringe and RBPjκ or by increased expression of the Notch inhibitor Deltex also results in increased numbers of B cells in the thymus.10,–12 While Notch signaling levels are clearly involved in the T-cell or B-cell choice in the thymus, the mechanisms that determine the different levels of Notch signaling are largely unknown.
Notch targets Hes1 and Hes5 (hairy enhancer of split) are basic helix-loop-helix (bHLH) proteins that function both as transcriptional repressors and antagonists to other bHLH genes such as MASH1 and MyoD and play an essential role in inducing neurogenesis and myogenesis, respectively.13,,–16 Hes1 and Hes5 are expressed in appropriate thymocyte subsets, making them likely candidates for a role in Notch signaling in the thymus.17 Hes1-deficient mice die soon after birth and Hes1-deficient cells from fetal liver are unable to induce T-cell differentiation after transfer to a Rag1−/− thymus, indicating a Hes1 role in thymus development. Although Hes5-deficient mice appear normal, proportions of thymocyte subsets in these mice have not been examined in sufficient detail to exclude a role in fine-tuning thymocyte types.18,19 It has been shown that overexpression of either Hes1 or Hes5 inhibits B-cell development in the murine bone marrow, further indicating that differential expression of these 2 components might be involved in determining the extent of B-cell differentiation in the thymus.20
We previously showed that incubation of sorted bone marrow hematopoietic progenitors with high or intermediate densities of purified Notch ligand Delta1ext-IgG led to inhibition of B-cell progenitor generation, whereas low ligand densities did not inhibit B-cell progenitor generation.21 Here, we show that Hes5 is required for these progenitors to accurately detect both intermediate and low densities of Delta1ext-IgG. Furthermore, we show that Hes5-deficient thymocytes generate increased proportions of B cells in the thymus, suggesting that Hes5 is required for thymic progenitors to accurately detect Notch-established boundaries.
Methods
Generation and immobilization of Delta1ext-IgG
Delta1ext-IgG was prepared and immobilized at different densities as described.22
Mice
Hes5−/− mice (kindly provided by Francois Guillemot, National Institute for Medical Research [NIMR], London, United Kingdom) were maintained and bred at the Fred Hutchinson Cancer Research Center. Hes5−/− and Hes5+/+ pups from crosses of heterozygous parents were genotyped using appropriate primers.
Cell isolation and immunofluorescence studies
Lin−Sca-1+c-kit+ Hoescht side population (LSKSP) cells were enriched from murine bone marrow using fluorescence-activated cell sorting (FACS). Staining and sorting for Lin−Sca-1+c-kit+ have been described.21,23 For staining to detect the Hoescht side population (SP), 106 Lin− cells were resuspended in 1 mL prewarmed (37°C) medium with 5 μg/mL Hoescht 33342 (Calbiochem, La Jolla, CA) and incubated for 90 minutes in a 37°C waterbath. LSKSP cells were obtained using FACS on a Vantage Cell Sorter (Becton Dickinson, Mountain View, CA) and gated as described.24 To assess thymus B-cell and T-cell subsets, dissociated cells were stained with FITC-conjugated CD4, avidin-conjugated CD8, and either PE-conjugated CD19 or CD25 and APC-conjugated B220 or CD44, respectively. Secondary staining with PE-Cy7 was used to detect avidin-conjugated primary antibodies. Cells were stained with DAPI to gate for dead cells. Antibodies were purchased from BD Biosciences (San Jose, CA).
Hematopoietic cell culture
LSKSP cells from mice of the appropriate genotype were cultured with immobilized ligand in Iscove modified Dulbecco medium (IMDM) supplemented with 20% fetal bovine serum (FBS) and 4GF (100 ng/mL each of murine stem cell factor [mSCF], human FLT3L, and human IL-6, and 10 ng/mL human IL-11; PeproTech, Rocky Hill, NJ) as previously described.23 To assess generation of B-cell precursors, LSKSP cells were cultured for 21 days in wells precoated with Delta1ext-IgG, and sorted for expression of B220 as described.21 Isolated B220+ cells were transferred onto an OP-9 monolayer at 3-fold dilutions (8 wells per cell dose) and cultured as described.21 After 6 days of culture, each well was assessed for the presence of B220+CD19+ cells by FACS analysis.
RNA isolation and real-time RT-PCR
Total RNA was extracted using the Absolutely RNA RT-PCR Miniprep Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Single-strand cDNA was synthesized with oligo-dT primer for 45 minutes at 50°C using reagents provided in the ThermoScript RT-PCR System (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using SYBR Green PCR Master Mix on an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) in the following conditions: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 45 seconds; samples were analyzed using SDAsoftware1.9 and DissociationCurves 1.0 (Applied Biosystems). Transcripts were quantitated in duplicate for every sample, and gene expression was normalized to that of the housekeeping gene ribosomal protein L7 (Rpl7). The following primers were designed from sequences of different exons for each gene to prevent amplification of genomic DNA: SYBR-green RT-PCR primers were Hes1 forward primer, 5′-GGCCTCTGAGCACAGAAAAAGT-3′; Hes-1 reverse primer, 5′-GTGTTAACGCCCTCACACG-3′: Hes5 forward primer, 5′-CCAAGGAGAAAAACCGACTG-3′; Rpl7 forward primer, 5′-GAACTCATCTATGAGAAGGC-3′; and Rpl7 reverse primer, 5′-AAGACGAAGGAGCTGCAGAAC-3′. For detection of Hes1 in thymus subsets, Taqman RT-PCR primers for Hes1 (Applied Biosystems) were used and normalized with housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems).
Statistical methods
The Wilcoxon rank-sum test was used for these studies.
Results
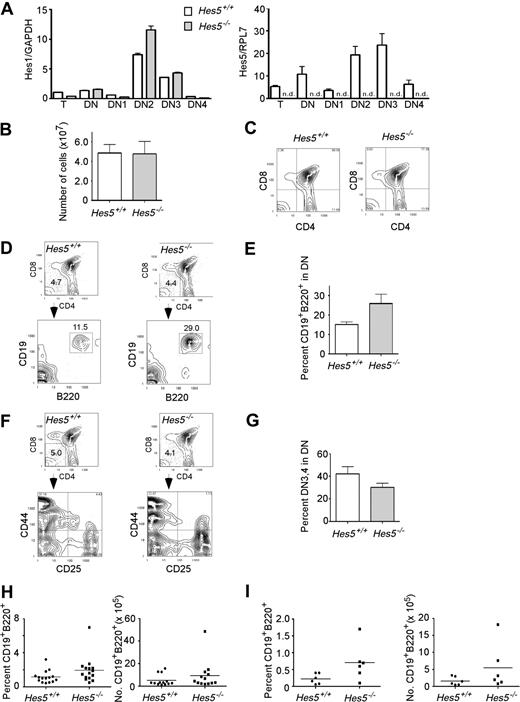
Both Hes1 and Hes5 mRNA are detected in the CD4−CD8− subset (DN) but not the more mature subsets of the murine thymus.17 In the present study, we also detected substantial levels of Hes1 and Hes5 mRNA in the thymus using quantitative RT-PCR. Expression of both Notch targets was about 2-fold higher in the DN subset compared with the whole thymus. Furthermore, when the heterogeneous DN subset was sorted into the DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD25+CD44−), and DN4 (CD25−CD44−) subsets, we found increased relative expression of both Hes1 and Hes5 in the DN2 and DN3 subsets compared with whole thymus, indicating a similar expression pattern to Notch1 (Figure 1A).25
Despite significant Hes5 expression in thymus and thymus subsets, Hes5-deficient thymus contains normal cell numbers and normal proportions of T-cell subsets, but shows a higher percentage of B cells in the thymus. (A) RNA was prepared from whole thymus (T), and sorted thymus subsets DN (CD4−CD8−) and DN gated subsets DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−). Hes1 and Hes5 mRNA levels were quantitated using RT-PCR. □ depicts mRNA levels in wild-type Hes5+/+ thymus; ▩, mRNA levels in Hes5−/− thymus. Values are means of 3 replicates plus or minus SEM. ND indicates not detected. (B) Mean (± SEM) total number of thymus cells from 13-Hes5 deficient mice and their littermates from 6 separate litters. (C) Surface expression of CD4 and CD8 antigens on thymus cells from a representative Hes5−/− mouse and Hes5+/+ littermate. Numbers in the corners represent the percentage of cells within the respective quadrant. (D,F) Top contour plots, showing surface staining for CD4 and CD8 antigens, depict the gated DN subset for representative Hes5−/− and Hes5+/+ littermates, while bottom contour plots depict CD19 and B220 antigen expression (D) and CD25 and CD44 antigen expression (F) by the gated DN population. (E,G) Bar graphs depict the mean percentage (± SEM) of gated DN cells expressing B220 and CD19 (E), CD44−CD25+ (DN3), and CD44−CD25− (DN4) (G) for 11 Hes5−/− mice and their Hes5+/+ littermates. (H) Scatter plots depict the individual percentage (± SEM) and number of B220+CD19+ cells. (I) Scatter plots depict the mean percentage (± SEM) and number of B220+CD19+ cells in 3 Rag1−/− recipients 4 weeks after transplantation of 2 × 105 bone marrow cells from 6 Hes5−/− and 6 Hes5+/+ littermates.
Despite significant Hes5 expression in thymus and thymus subsets, Hes5-deficient thymus contains normal cell numbers and normal proportions of T-cell subsets, but shows a higher percentage of B cells in the thymus. (A) RNA was prepared from whole thymus (T), and sorted thymus subsets DN (CD4−CD8−) and DN gated subsets DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−). Hes1 and Hes5 mRNA levels were quantitated using RT-PCR. □ depicts mRNA levels in wild-type Hes5+/+ thymus; ▩, mRNA levels in Hes5−/− thymus. Values are means of 3 replicates plus or minus SEM. ND indicates not detected. (B) Mean (± SEM) total number of thymus cells from 13-Hes5 deficient mice and their littermates from 6 separate litters. (C) Surface expression of CD4 and CD8 antigens on thymus cells from a representative Hes5−/− mouse and Hes5+/+ littermate. Numbers in the corners represent the percentage of cells within the respective quadrant. (D,F) Top contour plots, showing surface staining for CD4 and CD8 antigens, depict the gated DN subset for representative Hes5−/− and Hes5+/+ littermates, while bottom contour plots depict CD19 and B220 antigen expression (D) and CD25 and CD44 antigen expression (F) by the gated DN population. (E,G) Bar graphs depict the mean percentage (± SEM) of gated DN cells expressing B220 and CD19 (E), CD44−CD25+ (DN3), and CD44−CD25− (DN4) (G) for 11 Hes5−/− mice and their Hes5+/+ littermates. (H) Scatter plots depict the individual percentage (± SEM) and number of B220+CD19+ cells. (I) Scatter plots depict the mean percentage (± SEM) and number of B220+CD19+ cells in 3 Rag1−/− recipients 4 weeks after transplantation of 2 × 105 bone marrow cells from 6 Hes5−/− and 6 Hes5+/+ littermates.
Cells from Hes1-deficient mice reportedly generate reduced numbers of T cells compared with cells from normal mice after transfusion into sublethally irradiated Rag1−/−-deficient mice.18 To examine the role of Hes5 in lymphoiesis, we compared the proportions of T- and B-cell subsets present in thymus from Hes5−/− mice to those of Hes5+/+ littermates. Cell numbers and proportions of T-cell subsets were similar in both the Hes5−/− or Hes5+/+ thymus, indicating that Hes5, unlike Notch1 or Hes1, is not required to generate a normal number of cells in the thymus (Figure 1B,C; Table 1). However, there was a significant 1.6-fold increase in the proportion of B220+CD19+ cells within the DN subset (P = .03; Figure 1E), although there was no difference in the proportion of cells in the DN CD4−CD8− subset (Table 1). This was further reflected in a significant 1.7-fold increase in the mean percentage of B cells in the Hes5−/− thymus compared with Hes5+/+ littermates (P = .04; Figure 1H). Although there were slightly fewer cells found in the Hes5−/− thymus, there was a suggestive 1.6-fold increased mean number of B cells (P = .10; Figure 1H). There was no effect on the proportion or number of B220+CD19− cells within the DN subset in Hes5−/− thymus compared with Hes5+/+, suggesting Hes5 acts on a more mature progenitor (data not shown). However, although not significant, there was a decreased proportion of DN3 plus DN4 subsets, suggesting that the increased proportion of B cells is at the expense of generation of these subsets (P = .1; Figure 1F,G). We further found that Hes1 mRNA levels are similar in Hes5−/− mice and Hes5+/+ thymus in all thymocyte subsets with the possible exception of the DN2 subset, indicating that the phenotype we observe in Hes5−/− mice is likely due to loss of Hes5, and that Hes1 does not substantially compensate for the defects observed with the Hes5 deletion (Figure 1A).
Distribution of thymus-derived precursors
| . | Total number of cells, mean × 107 ± SEM . | . | Percentage, mean ± SEM . | . | |
|---|---|---|---|---|---|
| CD8−CD4− | CD8−CD4+ | CD8+CD4− | CD8+CD4+ | ||
| Hes5+/+ | 4.2 ± 0.8 | 4.6 ± 0.3 | 19.1 ± 2.3 | 2.1 ± 0.2 | 74.1 ± 2.1 |
| Hes5−/− | 3.1 ± 0.5 | 6.0 ± 0.8 | 16.0 ± 2.6 | 2.8 ± 0.3 | 76.2 ± 2.4 |
| P | .34 | .31 | .26 | .06 | .39 |
| . | Total number of cells, mean × 107 ± SEM . | . | Percentage, mean ± SEM . | . | |
|---|---|---|---|---|---|
| CD8−CD4− | CD8−CD4+ | CD8+CD4− | CD8+CD4+ | ||
| Hes5+/+ | 4.2 ± 0.8 | 4.6 ± 0.3 | 19.1 ± 2.3 | 2.1 ± 0.2 | 74.1 ± 2.1 |
| Hes5−/− | 3.1 ± 0.5 | 6.0 ± 0.8 | 16.0 ± 2.6 | 2.8 ± 0.3 | 76.2 ± 2.4 |
| P | .34 | .31 | .26 | .06 | .39 |
Hes5 is required autonomously
To determine whether the requirement for Hes5 in inhibiting B-cell differentiation resides in the signaling cell (nonautonomous) as opposed to the receptive cell (autonomous), bone marrow stem cells from Hes5−/− mice were transplanted into sublethally irradiated Rag1−/− recipients and examined 4 weeks later for reconstitution of both T-cell and B-cell subsets. Rag1−/− mice cannot generate mature T or B cells, but do provide a fairly normal thymus anlage for establishing appropriate donor T or B lymphopoiesis. As with the thymus of Hes5−/− mice, Rag1−/− thymus transplanted with Hes5−/− cells also showed a significant 3.2-fold higher mean percentage of B cells after infusion of Hes5−/− bone marrow cells as opposed to Rag1−/− thymus transplanted with Hes5+/+ cells (P = .04), and a significant 3.4-fold increase in B-cell numbers (P = .03), indicating that Hes5 is required autonomously in the donor cells to limit the generation of B cells in the thymus (Figure 1I).
Increasing doses of Notch ligand differentially induce specific Notch targets
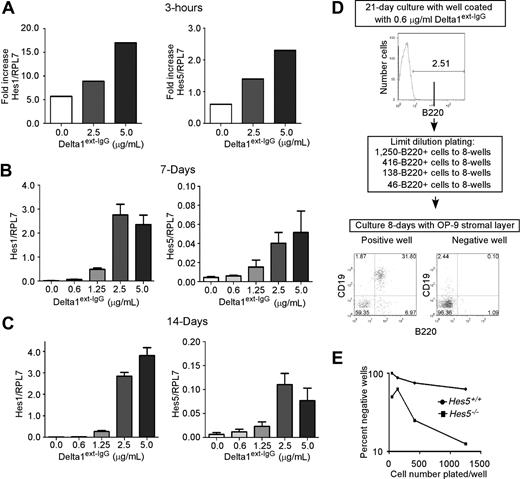
We assessed Hes1 and Hes5 mRNA expression after incubation of LSKSP cells with high and intermediate Delta1ext-IgG densities. In our previous studies, we showed that Delta1ext-IgG must be immobilized to the plastic surface of the tissue culture dish to induce Notch signaling in hematopoietic progenitor cells, and that different densities can be applied by preincubating separate plastic tissue culture wells with known concentrations of Delta1ext-IgG solution. After 3 hours of culture with Delta1ext-IgG, LSKSP cells incubated in wells precoated with a high density of Delta1ext-IgG expressed a 2- to 4-fold increased amount of Hes1 mRNA and higher Hes5 expression compared with wells coated with control human IgG, whereas decreased amounts of Hes1 and undetectable amounts of Hes5 were found at lower Delta1ext-IgG densities. Hes1, but not Hes5, expression is increased without the addition of Delta1ext-IgG, suggesting that other known inducers of Hes1, such as Wnt, may be present in our cultures. However, after 7 and 14 days of culture with increasing doses of Delta1ext-IgG, an increased expression of both Hes1 and Hes5 are seen, with a 2- and 10-fold increased expression at low and high doses, respectively (Figure 2B,C).
Delta1 dose-dependent induction of Notch target genes Hes1 and Hes5 and Hes5 is required to accurately detect different levels of Notch signaling induced by different densities of Delta1ext-IgG. (A-C) RNA was prepared from uncultured LSKSP cells or from LSKSP cells incubated for 3 hours (A), 7 days (B), and 14 days (C) in wells precoated with Delta1ext-IgG solutions (ranging from 10 μg/mL) or human IgG1 solutions (10 μg/mL; depicted as 0). Hes1 and Hes5 mRNA levels were determined by SyBR green quantitative RT-PCR. Results are normalized to the housekeeping gene Rpl7 and are shown either as fold-increase (A) in cultured cells versus uncultured cells or as absolute values. Each bar represents the mean (± SEM) of 3- replicates. (B,C) Generated slopes are significantly different from 0.0 (P < .05). (D) After incubation of sorted LSKSP from Hes5+/+ or Hes5−/− littermates with different densities of Delta1ext-IgG, sorted B220+ cells were transferred to OP-9 stromal layers. Wells were designated positive if more than 1% of the cells growing on OP-9 stromal layers were B220+CD19+. (E) Plot showing results of a single dose using limit dilution to calculate a frequency for B-cell progenitors. In this representative example, the calculated frequency of B-cell progenitors in Hes5+/+ is 490 per 106 B220+ starting cells and for Hes5−/− is 3205 per 106 B220+ starting cells.
Delta1 dose-dependent induction of Notch target genes Hes1 and Hes5 and Hes5 is required to accurately detect different levels of Notch signaling induced by different densities of Delta1ext-IgG. (A-C) RNA was prepared from uncultured LSKSP cells or from LSKSP cells incubated for 3 hours (A), 7 days (B), and 14 days (C) in wells precoated with Delta1ext-IgG solutions (ranging from 10 μg/mL) or human IgG1 solutions (10 μg/mL; depicted as 0). Hes1 and Hes5 mRNA levels were determined by SyBR green quantitative RT-PCR. Results are normalized to the housekeeping gene Rpl7 and are shown either as fold-increase (A) in cultured cells versus uncultured cells or as absolute values. Each bar represents the mean (± SEM) of 3- replicates. (B,C) Generated slopes are significantly different from 0.0 (P < .05). (D) After incubation of sorted LSKSP from Hes5+/+ or Hes5−/− littermates with different densities of Delta1ext-IgG, sorted B220+ cells were transferred to OP-9 stromal layers. Wells were designated positive if more than 1% of the cells growing on OP-9 stromal layers were B220+CD19+. (E) Plot showing results of a single dose using limit dilution to calculate a frequency for B-cell progenitors. In this representative example, the calculated frequency of B-cell progenitors in Hes5+/+ is 490 per 106 B220+ starting cells and for Hes5−/− is 3205 per 106 B220+ starting cells.
Hes5 is required to prevent generation of progenitors capable of B-cell formation in OP-9 at intermediate densities of Delta1ext-IgG
LSK bone marrow progenitors incubated in wells coated with low concentrations of Delta1ext-IgG (1.25 μg/mL) generate B220+ precursors capable of becoming CD19+B220+ B cells after transfer to a supportive stromal layer, whereas LSK progenitors incubated with intermediate to high densities of Delta1ext-IgG failed to generate these precursors (2.5-10 μg/mL).21 Moreover, use of the high densities of Delta1ext-IgG (10 μg/mL) yields a greater number of T-cell progenitors than that obtained with intermediate densities (2.5 and 5 μg/mL), as indicated by the more efficient reconstitution of peripheral blood T cells by LSK cells cultured with high densities.26 To determine whether hematopoietic precursors require Hes5 in distinguishing the different densities of Delta1ext-IgG, LSKSP cells from Hes5−/− mice or from their Hes5+/+ littermates were incubated for 21 days in plastic tissue-culture wells containing specific densities of Delta1ext-IgG. Most of the cells at lower densities of Delta are expressing myeloid antigens GR1 and F480. However, 1% to 5% express the B-cell antigen B220, whereas none of the cells express CD19 (Figure 2D). Preliminary studies indicated that B-cell precursors, defined as a cultured cell capable of generating CD19+B220+ cells after transfer to an OP-9 stromal layer, are highly enriched in the B220+ fraction of these populations, but not the B220− fraction (data not shown). Hence, B220+ cultured cells were sorted and transferred to culture wells containing an OP-9 stromal layer and the cytokines SCF, FLT3L, and IL-7, and examined 7 to 9 days later for the generation of B220+CD19+ B cells using flow cytometry. To directly measure the number of B-cell precursors generated, we performed limiting dilution analysis (Figure 2D). After 21 days of culture with Delta, we sorted B220+ cells and distributed 3-serial dilutions into 6 to 8 separate wells containing an OP-9 monolayer. After 7 to 9 days of culture with OP-9 stromal layers, cells from culture wells containing a B-cell precursor generated cells coexpressing B220 and CD19. We determined the frequency of B-cell precursors based on the number of positive wells per number of wells plated (Figure 2E).
In 4 out of 5 experiments, cultures containing Hes5-deficient cells generated B-cell precursors at a higher Delta1ext-IgG density than did cells derived from the normal littermates (Table 2). Both Hes5+/+ and Hes5−/− LSKSP cells consistently generated B-cell precursors at the 2 lowest densities of Delta1ext-IgG (0.6 and 1.25 μg/mL), with Hes5+/+-derived precursors in 7 of 10 wells, and Hes5−/−-derived precursors in 9 of 10 wells (Table 2). However, only Hes5−/− LSKSP cells consistently generated precursors at the intermediate densities of Delta1ext-IgG (2.5 and 5.0 μg/mL), with 5 of 10 wells containing B-cell precursors compared with only 1 of 10 for Hes5+/+ cells. No B-cell precursor formation was seen at the highest Delta1ext-IgG densities (10 μg/mL) in either Hes5−/− or Hes5+/+ cultures, suggesting that Hes1 is sufficient to prevent B-cell precursor generation at these densities. Furthermore, at the lowest 2 densities of Delta, we found significantly more B-cell precursors generated in the Hes5-deficient mice. Results from the 5 separate experiments showed that Hes5−/− cells cultured at the 2 lowest permissible Delta1ext-IgG density wells generated a mean 210 plus or minus 120 B-cell precursors per 106 cells, whereas Hes5+/+ cultured cells generated a mean 70 plus or minus 50 B-cell precursors (means ± SEM). The number of precursors generated was highly variable between the 5 experiments, but Hes5−/− cultures consistently generated a significant mean 3.2 plus or minus 0.3-fold higher frequency (P = .01) of B-cell precursors compared with Hes5+/+ cultures. Furthermore, increased total numbers of cells were generated at these doses of ligand with cells derived from Hes5−/− mice. Therefore, the approximately 3-fold higher B-cell precursor frequency reflected an average 9-fold increased number of B-cell precursors. Together, these experiments demonstrate that Hes5 is required for hematopoietic cells to accurately assess the Delta1ext-IgG density because B-cell precursor generation in our culture conditions in the absence of Hes5 expression was significantly higher than in its presence.
Individual experiments showing culture wells with different Delta1ext-IgG densities containing (+) or lacking (-) B-cell progenitors defined as cells capable of generating B220+CD19+ cells following transfer to an OP-9 stromal layer
| Genotype . | Delta1ext-IgG plating concentration . | No. positive higher-density Delta1ext-IgG wells from Hes5−/− LSKSP compared with Hes5+/+ LSKSP . | |||||
|---|---|---|---|---|---|---|---|
| 0 μg/mL . | Low . | Intermediate . | High . | ||||
| 0.6 μg/mL . | 1.25 μg/mL . | 2.5 μg/mL . | 5.0 μg/mL . | 10.0 μg/mL . | |||
| Experiment 1 | 2 | ||||||
| Hes5+/+ | + | + | − | − | − | − | |
| Hes5−/− | − | + | + | + | − | − | |
| Experiment 2 | − | 1 | |||||
| Hes5+/+ | − | + | + | + | − | − | |
| Hes5−/− | − | + | + | + | + | − | |
| Experiment 3 | − | 0 | |||||
| Hes5+/+ | − | + | + | − | − | − | |
| Hes5−/− | − | + | + | − | − | − | |
| Experiment 4 | 1 | ||||||
| Hes5+/+ | − | + | − | − | − | − | |
| Hes5−/− | + | + | + | − | − | − | |
| Experiment 5 | 2 | ||||||
| Hes5+/+ | + | − | + | − | − | − | |
| Hes5−/− | − | − | + | + | + | − | |
| Total no. positive | 6 | ||||||
| Hes5+/+ | 2 | 5 | 3 | 1 | 0 | 0 | |
| Hes5−/− | 1 | 5 | 5 | 3 | 2 | 0 | |
| Genotype . | Delta1ext-IgG plating concentration . | No. positive higher-density Delta1ext-IgG wells from Hes5−/− LSKSP compared with Hes5+/+ LSKSP . | |||||
|---|---|---|---|---|---|---|---|
| 0 μg/mL . | Low . | Intermediate . | High . | ||||
| 0.6 μg/mL . | 1.25 μg/mL . | 2.5 μg/mL . | 5.0 μg/mL . | 10.0 μg/mL . | |||
| Experiment 1 | 2 | ||||||
| Hes5+/+ | + | + | − | − | − | − | |
| Hes5−/− | − | + | + | + | − | − | |
| Experiment 2 | − | 1 | |||||
| Hes5+/+ | − | + | + | + | − | − | |
| Hes5−/− | − | + | + | + | + | − | |
| Experiment 3 | − | 0 | |||||
| Hes5+/+ | − | + | + | − | − | − | |
| Hes5−/− | − | + | + | − | − | − | |
| Experiment 4 | 1 | ||||||
| Hes5+/+ | − | + | − | − | − | − | |
| Hes5−/− | + | + | + | − | − | − | |
| Experiment 5 | 2 | ||||||
| Hes5+/+ | + | − | + | − | − | − | |
| Hes5−/− | − | − | + | + | + | − | |
| Total no. positive | 6 | ||||||
| Hes5+/+ | 2 | 5 | 3 | 1 | 0 | 0 | |
| Hes5−/− | 1 | 5 | 5 | 3 | 2 | 0 | |
Discussion
We and others have shown that high and intermediate levels of Notch signaling prevent B-cell progenitor differentiation, whereas only the highest levels induce T-cell differentiation. Here, we show that Notch signaling induced by intermediate levels of immobilized Delta1ext-IgG are accompanied by reduced Hes1 and Hes5 expression levels compared with either one at the higher density. We also show that Hes5 is required at the intermediate densities of Delta1ext-IgG, but not the highest densities, to prevent B-cell precursor differentiation, suggesting that the additional expression of Hes5 at the intermediate levels of Notch signaling imparts either a quantitatively increased inhibitory signal or a more efficient signal compared with Hes1 alone. This process might underlie the capacity for subthreshold levels of Notch signaling to produce distinct populations. Hes1 induced at high levels might impart a particular cell fate, whereas at the intermediate levels of Notch signaling where Hes1 levels are reduced, additional expression of other targets such as Hes5 might induce alternative cell fates.
Both Hes1 and Hes5 inhibit B-cell differentiation when overexpressed in hematopoietic stem cells, and furthermore both have been suggested to equally inhibit expression of inducers of B-cell differentiation, such as E47; however, neither Hes1 nor Hes5 alone completely inhibits B-cell differentiation as effectively as Notch1 overexpression, suggesting full blockage of differentiation requires higher levels of either target or simultaneous expression of both targets.20 Consistent with the former suggestion, higher expression levels of either Hes1 or Hes5 inhibited B-cell differentiation to a greater extent than did lower levels of either target.20
We find that at very high densities of Delta1ext-IgG, Hes1 is sufficient to prevent B-cell precursor generation, but that at intermediate levels, both Hes1 and Hes5 are required. These results predict that in boundaries where high levels of Notch signaling direct adjacent compartments, cells would require only expression of Hes1, whereas fine-tuning boundary edges requires additional targets. The high-level expression of Hes1 in regions of the embryonic brain where boundaries are established is consistent with this prediction.27 Furthermore, deletion of Hes1 leads to abnormal boundary establishment in the developing brain, and the additional deletion of either Hes3 or Hes5 results in complete loss of boundary formation, although deletion of either target alone results in no obvious phenotype.27 These findings suggest that while Hes3 and Hes5 are not critical in absolute boundary formation, they are required in resolving the boundary. Our evidence indicates that hematopoietic progenitor cells detect intermediate levels of Notch signaling by detecting differential induction of Notch targets such as Hes5.
The pattern of Notch component expression dictates where boundaries are established. In most cases, expression of either Notch ligand or an inhibitor of Notch signaling is localized, resulting in the establishment of an area with high-level Notch signaling adjacent to an area with reduced Notch signaling. Establishment of the wing boundary in Drosophila entails differential Notch ligand expression coupled with localized expression of Fringe, a glycosyl transferase that modifies Notch receptors and affects their ability to respond to the differentially localized ligands. Notch1, Notch2, and Notch3 are generally expressed by most precursors in the thymus, whereas lunatic-Fringe is expressed by DN precursors but not by double-positive (DP) precursors, presumably resulting in differential induction of Notch signaling in those cells.8,17 Over- or underexpression of lunatic-Fringe also leads to inhibited T-cell and increased B-cell differentiation with visual disruption of the thymus boundaries. Similarly, we observed an increased proportion of B cells after loss of Hes5, indicating a required role for Hes5 in detecting the differential levels of Notch signaling found in the thymus. Together, these findings underscore the importance of quantitative aspects of Notch signaling in establishing boundaries in the thymus. We show that Hes5 is required for hematopoietic precursors to respond appropriately to different levels of Notch signaling induced by intermediate and low densities of ligand during ex vivo culture. These results suggest a mechanism whereby expression of a second, less vital target allows a cell to differentially detect intermediate and low levels of Notch signaling.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cynthia Nourigat, Amanda Egge, Melissa Comstock, Stacey Dozono, and David Flowers for expert technical assistance and Roland Walter for careful reading of the manuscript. The authors also thank Francois Guillemot for providing Hes5-deficient mice.
This work was supported by grants from the National Institutes of Health (P50 HL054881, T32CA09351, K12 CA076930, and P01 HL084205). I.D.B. is an American Cancer Society Clinical Research Professor supported by a generous gift from the F. M. Kirby Foundation.
National Institutes of Health
Authorship
Contribution: B.V.-F., M.H.D., and I.D.B. participated in designing the research; B.V.-F. and K.K. performed experiments; and B.V.-F. wrote the paper. All authors checked the final manuscript.
Conflict-of–interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Varnum-Finney, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-373, Seattle, WA 98109; e-mail: bvarnumf@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal