High-dose methotrexate (MTX) has been extensively used for treatment of acute lymphoblastic leukemia (ALL). To determine the optimal dose of MTX in childhood relapsed ALL, the ALL Relapse Berlin-Frankfurt-Münster (ALL-REZ BFM) Study Group performed this prospective randomized study. A total of 269 children with a first early/late isolated (n = 156) or combined (n = 68) bone marrow or any isolated extramedullary relapse (n = 45) of precursor B-cell (PBC) ALL (excluding very early marrow relapse within 18 months after initial diagnosis) were registered at the ALL-REZ BFM90 trial and randomized to receive methotrexate infusions at either 1 g/m2 over 36 hours (intermediate dose, ID) or 5 g/m2 over 24 hours (high dose, HD) during 6 (or 4) intensive polychemotherapy courses. Intensive induction/consolidation therapy was followed by cranial irradiation, and by conventional-dose maintenance therapy. Fifty-five children received stem-cell transplants. At a median follow-up of 14.1 years, the 10-year event-free survival probability was .36 (± .04) for the ID group (n = 141), and .38 (± .04) for the HD group (n = 128, P = .919). The 2 groups did not differ in terms of prognostic factors and other therapeutic parameters. In conclusion, methotrexate infusions at 5 g/m2 per 24 hours, compared with 1 g/m2 per 36 hours, are not associated with increased disease control in relapsed childhood PBC acute lymphoblastic leukemia.
Introduction
Long-term event-free survival (EFS) can be achieved by aggressive polychemotherapy in a substantial proportion of children with acute lymphoblastic leukemia (ALL) who relapse after successful frontline induction and consolidation therapy.1,,,–5 A second remission is achieved by more continuous elements using a 4-drug induction with corticosteroids, Vinca alkaloids, anthracyclines, asparaginase, and intrathecal therapy in some groups,2,6,7 or by more intensive short-course elements containing high-dose methotrexate and high-dose cytarabine, as used by the ALL Relapse Berlin-Frankfurt-Münster (ALL-REZ BFM) group.8,9 Various regimens for reinduction/consolidation have been used including more continuous elements, rapid alternating 2-drug elements, or again intensive short-course multidrug elements as used by the ALL-REZ BFM group.2,6,–8 For treatment or prevention of CNS leukemia, intrathecal therapy is given during intensive treatment, and CNS irradiation is administered after the end of intensive chemotherapy.10,–12 In the case of testicular relapse, local irradiation or orchiectomy is performed.13,–15 Less intensive maintenance therapy is given for up to 2 years.8,9 Despite heterogeneous approaches, rather comparable results are achieved. Remission and long-term survival rates depend more on clinical and biologic characteristics of the leukemia than on the specific treatment regimen. The time point and the site of relapse as well as the immunophenotype of the leukemia and a variety of translocation-associated fusion genes have been identified as the most important prognostic determinants.8,16 Patients with poor prognostic features such as early time point of relapse, bone marrow involvement, T-lineage disease, or BCR/ABL fusion transcript could not be salvaged even with intensive multidrug chemotherapy and cranial radiation therapy and have been allocated to allogeneic stem-cell transplantation (SCT). Whereas superior results could be achieved with stem-cell transplant from HLA-identical siblings compared with chemotherapy/radiotherapy in patients with bone marrow re-lapse, the benefit of stem-cell transplant from unrelated donors could be confirmed for high-risk patients only. Furthermore, the role of allogeneic SCT in patients with extramedullary relapse remains controversial.17,,–20
Methotrexate (MTX) is one of the most active agents for the treatment of ALL, and as such is a component of most modern ALL frontline treatment worldwide. In contrast to other antineoplastic drugs, the cytotoxic effects of MTX can be antagonized by a specific antidote, activated folic acid (leucovorin). This allows for the use of escalating doses of MTX, which are associated with greater antileukemic effects in randomized trials and upfront window studies, as assessed by peripheral blast cytoreduction.21,22 In addition, high dosages of MTX offer the advantage of targeting extramedullary leukemia by producing cytotoxic concentrations in sanctuary sites where low-dose MTX does not readily distribute (eg, testes, cerebrospinal fluid).10,23 However, the optimal dose of MTX, the adequate duration of the drug infusion, and the adequate folinic acid rescue remain controversial.1,24,,,–28 While one randomized trial indicated that patients at increased risk of relapse treated initially with high-dose MTX (4 g/m2) had significantly better event-free survival (EFS) rates compared with patients treated with low-dose MTX (40 mg/m2),24 ultra-high-dose MTX (12 g/m2) at a 4-hour infusion and an intensive folinic acid rescue was not found to be beneficial in relapsed ALL children, compared with intermediate-dose MTX (1 g/m2) at a 36-hour infusion and a reduced folinic acid rescue.1,29 Since higher dosages of parenteral MTX are associated with significant toxicity, including long-term neurologic sequelae, and higher costs,30,–32 its optimal use is an important question of clinical research. In 1990, the Berlin-Frankfurt-Münster (BFM) Relapse Study Group set out to address this issue in a prospective randomized fashion. The long-term results of this trial, ALL-REZ BFM 90, are the subject of the following report.
Methods
Patients
Between July 1990 and June 1995, a total of 374 children and adolescents up to 18 years of age with a first relapse of precursor B-cell ALL, including any isolated extramedullary relapse irrespective of the time point of relapse and bone marrow (BM) relapses occurring at least 18 months after initial diagnosis, were enrolled in the cooperative trial ALL-REZ BFM 90, conducted in 80 hospitals located in Germany, Austria, Switzerland, The Netherlands, Denmark, and Russia. Patients with very early bone marrow relapse were excluded from the study and enrolled to experimental trials. All treatment protocols had been approved by the local ethics committee of each participating institution (listed in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Informed consent was obtained from the guardians and also patients if applicable in accordance with the Declaration of Helsinki.
The diagnosis of a combined relapse was defined as the presence of at least 5% blast cells in the bone marrow with the presence of extramedullary disease, and isolated bone marrow relapse was defined by the presence of at least 25% blast cells in the bone marrow without evidence of extramedullary disease. Wright-Giemsa–stained BM and peripheral blood smears and cytocentrifuge preparations were subject to central review. Flow cytometric immunophenotyping according to EGIL classification33 and polymerase chain reaction–based detection of bcr-abl fusion transcripts were performed in central laboratories.
Relapses were considered very early if they occurred within 18 months after initial diagnosis, early if they occurred after 18 and within 6 months after cessation of frontline therapy, or late if they occurred 6 months after elective cessation of frontline treatment. Patients with early isolated or combined BM relapse were stratified to the treatment group A; those with late isolated or combined BM relapse, to group B; and those with any isolated extramedullary relapse, to group C.
Treatment
After confirmation of diagnosis, children were centrally randomized to receive 1 g/m2 methotrexate (MTX) over 36 hours (ID-MTX) or 5 g/m2 over 24 hours (HD-MTX) by continuous intravenous infusion during subsequent chemotherapy courses (R1 and R2). Therapy was started with prednisone (100 mg/m2) for 5 days, followed by alternating courses of polychemotherapy (R1, R2, R3), which are outlined in Table 1. Children with early (group A) or late (group B) isolated or combined BM relapse were scheduled to receive a total of 9 courses (ie, 6 R1/R2 courses containing ID-MTX or HD-MTX), whereas patients with isolated extramedullary relapse (group C) received 6 courses (ie, 4 R1/R2 courses containing ID-MTX or HD-MTX). Scheduled intervals between the start of the first 2 courses were 2 weeks, and between all subsequent courses were 3 weeks (Figure 1).
Drugs and dosing of alternating polychemotherapy courses R1-3
| Drug . | Dose . | Route . | Days drug given . |
|---|---|---|---|
| Course R1 | |||
| Dexamethasone | 20 mg/m2 | PO | 1-5 |
| 6-Mercaptopurine | 100 mg/m2 | PO | 1-5 |
| Vincristine | 1.5 mg/m2 | IV | 1, 6 |
| Methotrexate* | 1 or 5 g/m2 | IV | 1 |
| Methotrexate | 12 mg | IT | 1 |
| Cytarabine | 30 mg | IT | 1 |
| Prednisone | 10 mg | IT | 1 |
| Cytarabine | 2 g/m2 q 12 h | IV | 5 |
| l-asparaginase | 25 000 U/m2 | IM/IV | 6 |
| Course R2 | |||
| Dexamethasone | 20 mg/m2 | PO | 1-5 |
| 6-Thioguanine | 100 mg/m2 | PO | 1-5 |
| Vindesine | 3 mg/m2 | IV | 1 |
| Methotrexate* | 1 or 5 g/m2 | IV | 1 |
| Methotrexate | 12 mg | IT | 1 (and 5)† |
| Cytarabine | 30 mg | IT | 1 (and 5)† |
| Prednisone | 10 mg | IT | 1 (and 5)† |
| Daunorubicin | 50 mg/m2 | IV | 5 |
| Ifosfamide | 400 mg/m2 | IV | 1-5 |
| l-asparaginase | 25 000 U/m2 | IM/IV | 6 |
| Course R3 | |||
| Dexamethasone | 20 mg/m2 | PO | 1-5 |
| Cytarabine | 2 g/m2 q 12 h | IV | 1, 2 |
| Etoposide | 150 mg/m2 | IV | 3-5 |
| Methotrexate | 12 mg | IT | 5 |
| Cytarabine | 30 mg | IT | 5 |
| Prednisone | 10 mg | IT | 5 |
| l-asparaginase | 25 000 U/m2 | IM/IV | 6 |
| Drug . | Dose . | Route . | Days drug given . |
|---|---|---|---|
| Course R1 | |||
| Dexamethasone | 20 mg/m2 | PO | 1-5 |
| 6-Mercaptopurine | 100 mg/m2 | PO | 1-5 |
| Vincristine | 1.5 mg/m2 | IV | 1, 6 |
| Methotrexate* | 1 or 5 g/m2 | IV | 1 |
| Methotrexate | 12 mg | IT | 1 |
| Cytarabine | 30 mg | IT | 1 |
| Prednisone | 10 mg | IT | 1 |
| Cytarabine | 2 g/m2 q 12 h | IV | 5 |
| l-asparaginase | 25 000 U/m2 | IM/IV | 6 |
| Course R2 | |||
| Dexamethasone | 20 mg/m2 | PO | 1-5 |
| 6-Thioguanine | 100 mg/m2 | PO | 1-5 |
| Vindesine | 3 mg/m2 | IV | 1 |
| Methotrexate* | 1 or 5 g/m2 | IV | 1 |
| Methotrexate | 12 mg | IT | 1 (and 5)† |
| Cytarabine | 30 mg | IT | 1 (and 5)† |
| Prednisone | 10 mg | IT | 1 (and 5)† |
| Daunorubicin | 50 mg/m2 | IV | 5 |
| Ifosfamide | 400 mg/m2 | IV | 1-5 |
| l-asparaginase | 25 000 U/m2 | IM/IV | 6 |
| Course R3 | |||
| Dexamethasone | 20 mg/m2 | PO | 1-5 |
| Cytarabine | 2 g/m2 q 12 h | IV | 1, 2 |
| Etoposide | 150 mg/m2 | IV | 3-5 |
| Methotrexate | 12 mg | IT | 5 |
| Cytarabine | 30 mg | IT | 5 |
| Prednisone | 10 mg | IT | 5 |
| l-asparaginase | 25 000 U/m2 | IM/IV | 6 |
PO indicates by mouth; IV, intravenously; IT, intrathecally; q, every; and IM, intramuscularly.
MTX 1 g/m2 and 5 g/m2 at a 36- and 24-hour infusion, respectively.
Children with overt meningeal leukemia.
Design of study ALL-REZ BFM 90. A indicates early isolated/combined bone marrow relapse; B, late isolated/combined bone marrow relapse; C, isolated extramedullary relapse (A/B/C, strategic groups); CP, cytoreductive prophase with prednisone; R1/R2/R3, multiagent chemotherapy courses (as described in Table 1); ↓, cranial radiation therapy; and M 12/24, maintenance therapy duration in months. *Continuation of the next chemotherapy strictly according to the time schedule, as long as no remission was achieved. After achievement of a second CR, the following criteria were mandatory to proceed with the next course: leukocyte count higher than 2 × 109/L; neutrophil count higher than .5 × 109/L; and platelet count higher than 80 × 109/L.
Design of study ALL-REZ BFM 90. A indicates early isolated/combined bone marrow relapse; B, late isolated/combined bone marrow relapse; C, isolated extramedullary relapse (A/B/C, strategic groups); CP, cytoreductive prophase with prednisone; R1/R2/R3, multiagent chemotherapy courses (as described in Table 1); ↓, cranial radiation therapy; and M 12/24, maintenance therapy duration in months. *Continuation of the next chemotherapy strictly according to the time schedule, as long as no remission was achieved. After achievement of a second CR, the following criteria were mandatory to proceed with the next course: leukocyte count higher than 2 × 109/L; neutrophil count higher than .5 × 109/L; and platelet count higher than 80 × 109/L.
Ten percent of the MTX dose was administered intravenously over a period of 30 minutes, and the remaining 90% was given during the subsequent 23.5 or 35.5 hours in children receiving 5 g/m2 or 1 g/m2, respectively. Forty-two or 48 hours after initiation of MTX infusion, respectively, activated folic acid was given as rescue at a dosage of 15 mg/m2 every 6 hours 3 or 2 times, respectively. The dosage was adjusted in cases of inappropriate excretion of MTX. No additional rescue was given when MTX serum concentrations had dropped to less than 0.25 μmol/L 54 hours or later after the end of MTX infusion.
To treat subclinical meningeal leukemia, triple intrathecal therapy was administered during each course, consisting of MTX (12 mg), cytarabine (30 mg), and prednisone (10 mg). Children with overt meningeal leukemia received initially 1 to 3 doses of intrathecal therapy until the CSF was cleared from leukemic blasts, and additional triple intrathecal injections at the end of each R2 course. The intensive polychemotherapy courses were followed by cranial irradiation (12 Gy) in children with BM relapse; in patients with overt meningeal leukemia, craniospinal irradiation at a dose of 18 Gy was recommended (doses were reduced in children younger than 2 years or with previous cranial irradiation). Any testis with overt disease was subjected to orchiectomy or local radiotherapy at a dose of 24 Gy; a contralateral clinically not involved testis was irradiated at a dose of 18 Gy, or 15 Gy if leukemic involvement was excluded by biopsy.
Maintenance therapy consisted of daily 6-thioguanine (50 mg/m2) and every other week intravenous MTX (50 mg/m2) given for 1 year in isolated extramedullary disease and for 2 years in patients with BM relapse (Figure 1).
Stem-cell transplant from an HLA-identical sibling was recommended for patients with isolated or combined BM relapse within 4 years after initial diagnosis after 3 to 5 courses of polychemotherapy. In increased-risk patients without a sibling donor, HLA-matched unrelated donors, HLA mismatched family donors, or autologous transplants were considered as experimental alternative stem-cell sources during the course of the study. The preferred conditioning regimen was total body irradiation (TBI) 12 Gy and VP16 60 mg/kg; graft-versus-host disease (GVHD) prophylaxis after allogeneic transplantations consisted of short-course MTX, and cyclosporin A in most patients.
Statistical analysis
In a blinded fashion, enrolled patients were centrally randomized by the trial coordination center (Department of Pediatric Oncology/Hematology, Charité, Berlin) using a randomization list with equal probabilities for the 2 protocols. Randomization was stratified according to the treatment groups A/B/C.
Differences in the distribution of variables among patient groups were assessed by the χ2 test for categoric variables, and the Mann-Whitney U or Kruskal-Wallis tests for continuous variables. Kaplan-Meier life table analysis was used to estimate the probability of event-free survival (pEFS) from the start of salvage therapy until the date of death in remission, a second relapse, or a second malignant neoplasm, whichever was first. Patients who were not in complete remission (CR) after 3 treatment courses (less than 5% blast cells in an otherwise normocellular marrow) were considered induction failures and censored at time zero. Children lost to follow-up were censored at the date of last contact. For the calculation of relapse-free survival times, the analysis was restricted to children achieving second CR, and all events were censored except for second relapses. Subgroups were compared by the 2-sided log-rank test. In all tests, 2-sided P at .05 or higher was regarded as not significant (NS). A sample size calculation indicated a recruitment of 133 patients in each randomized arm to detect a superiority of the HD-MTX arm of 15% at an expected EFS rate of 35% of the ID-MTX arm with a power of 0.8 and a level of significance of .05. Multivariate Cox stepwise forward conditional regression analysis was performed to determine statistically significant independent indicators of outcome, including SCT as time-dependent covariate.
Results
Of the 374 children recruited during the 5-year trial, 269 children (72%) were randomized to ID-MTX (n = 141) or HD-MTX (n = 128). Four patients randomized to the HD-MTX arm and included into the intention-to-treat analysis nevertheless received ID-MTX due to parents' decision. Lack of the parents' consent to subject their children to randomization resulted in 51 children to receive ID-MTX, and 54 children to receive HD-MTX out of the investigative protocol. The age of the randomized children did not differ between the ID-MTX and the HD-MTX group (median/range: 8.5/1.8-17.3 vs 8.8/3.3-18.0 years, P = .554), nor was there any significant difference with respect to the patients' sex, time or site of relapse,1 peripheral blast count at relapse,4 immunophenotype,33 detection of bcr-abl fusion transcripts,34 or frontline treatment by BFM35 or CoALL36 protocols, as well as stem-cell transplantation and cranial irradiation at relapse (Table 2). Pro-B immunophenotype was more frequent in the ID-MTX than the HD-MTX group (10 vs 2 patients). Excluding patients with pro-B immunophenotype does not influence the results with respect to EFS.
Baseline patient characteristics and therapeutic parameters of the 269 randomized patients receiving intermediate-dose methotrexate (ID-MTX) or high-dose methotrexate (HD-MTX)
| . | Total . | ID-MTX . | HD-MTX . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Total | 269 | (100) | 141 | (52) | 128* | (48) | |
| Clinical parameters | |||||||
| Sex | .611 | ||||||
| Male | 174 | 65 | 89 | 63 | 85 | 66 | |
| Female | 95 | 35 | 52 | 37 | 43 | 34 | |
| Age at relapse | .610 | ||||||
| Younger than 5 y | 26 | 10 | 13 | 9 | 13 | 10 | |
| 5 y or older and younger than 10 y | 143 | 53 | 79 | 56 | 64 | 50 | |
| 10 y and older | 100 | 37 | 49 | 35 | 51 | 40 | |
| Time point of relapse† | .313 | ||||||
| Very early | 10 | 4 | 7 | 5 | 3 | 2 | |
| Early | 112 | 42 | 62 | 44 | 50 | 39 | |
| Late | 147 | 55 | 72 | 51 | 75 | 59 | |
| Site | .587 | ||||||
| Bone marrow isolated | 156 | 58 | 85 | 60 | 71 | 56 | |
| BM combined | 68 | 25 | 32 | 23 | 36 | 28 | .156 |
| Plus CNS | 35 | 52 | 21 | 66 | 14 | 39 | |
| Plus testis | 26 | 38 | 9 | 28 | 17 | 47 | |
| Plus CNS/testis | 2 | 3 | 0 | 0 | 2 | 6 | |
| Plus CNS/other | 1 | 2 | 0 | 0 | 1 | 3 | |
| Plus other | 4 | 6 | 2 | 6 | 2 | 6 | |
| Isolated extramedullary | 45 | 17 | 24 | 17 | 21 | 16 | .538 |
| CNS | 18 | 40 | 9 | 38 | 9 | 43 | |
| Testis | 25 | 56 | 14 | 58 | 11 | 52 | |
| CNS/testis | 1 | 2 | 1 | 4 | 0 | 0 | |
| Other | 1 | 2 | 0 | 0 | 1 | 5 | |
| Peripheral blast cell count | .673 | ||||||
| Less than 1/μL | 93 | 35 | 49 | 35 | 44 | 35 | |
| 1 to less than 10 000/μL | 142 | 53 | 72 | 41 | 70 | 55 | |
| 10 000/μL or more | 32 | 12 | 19 | 15 | 13 | 10 | |
| No data | 2 | (.7) | 1 | (.7) | 1 | (.8) | |
| BCR/ABL fusion transcript | .746 | ||||||
| Positive | 11 | 9 | 6 | 8 | 5 | 11 | |
| Negative | 115 | 91 | 73 | 92 | 42 | 89 | |
| No data | 143 | (53) | 62 | (44) | 81 | (63) | |
| Immunophenotype‡ | .082 | ||||||
| Pro-B | 12 | 5 | 10 | 7 | 2 | 2 | |
| Common ALL | 191 | 73 | 95 | 69 | 96 | 77 | |
| Pre-B | 58 | 22 | 33 | 24 | 25 | 20 | |
| Biphenotypic | 1 | .4 | 0 | 0 | 1 | .8 | |
| No data | 7 | (3) | 3 | (2) | 4 | (3) | |
| Treatment | |||||||
| Frontline protocol | .887 | ||||||
| ALL-BFM | 179 | 67 | 98 | 70 | 81 | 63 | |
| COALL | 31 | 12 | 16 | 12 | 15 | 12 | |
| Other | 59 | 22 | 27 | 19 | 32 | 25 | |
| Irradiation at relapse | .792 | ||||||
| None | 88 | 33 | 47 | 34 | 41 | 32 | |
| Cranial | 94 | 36 | 45 | 33 | 49 | 38 | |
| Craniospinal | 37 | 14 | 21 | 15 | 16 | 13 | |
| TBI | 46 | 17 | 24 | 18 | 22 | 17 | |
| No data | 4 | (2) | 4 | (3) | 0 | 0 | |
| Stem-cell transplantation in CR2 | |||||||
| None | 214 | 80 | 110 | 78 | 104 | 81 | .426 |
| Matched family donor | 32 | 12 | 17 | 12 | 15 | 12 | |
| Unrelated donor | 9 | 3 | 7 | 5 | 2 | 2 | |
| Mismatched family donor | 4 | 2 | 1 | 1 | 3 | 2 | |
| Autologous | 10 | 4 | 6 | 4 | 4 | 3 | |
| . | Total . | ID-MTX . | HD-MTX . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Total | 269 | (100) | 141 | (52) | 128* | (48) | |
| Clinical parameters | |||||||
| Sex | .611 | ||||||
| Male | 174 | 65 | 89 | 63 | 85 | 66 | |
| Female | 95 | 35 | 52 | 37 | 43 | 34 | |
| Age at relapse | .610 | ||||||
| Younger than 5 y | 26 | 10 | 13 | 9 | 13 | 10 | |
| 5 y or older and younger than 10 y | 143 | 53 | 79 | 56 | 64 | 50 | |
| 10 y and older | 100 | 37 | 49 | 35 | 51 | 40 | |
| Time point of relapse† | .313 | ||||||
| Very early | 10 | 4 | 7 | 5 | 3 | 2 | |
| Early | 112 | 42 | 62 | 44 | 50 | 39 | |
| Late | 147 | 55 | 72 | 51 | 75 | 59 | |
| Site | .587 | ||||||
| Bone marrow isolated | 156 | 58 | 85 | 60 | 71 | 56 | |
| BM combined | 68 | 25 | 32 | 23 | 36 | 28 | .156 |
| Plus CNS | 35 | 52 | 21 | 66 | 14 | 39 | |
| Plus testis | 26 | 38 | 9 | 28 | 17 | 47 | |
| Plus CNS/testis | 2 | 3 | 0 | 0 | 2 | 6 | |
| Plus CNS/other | 1 | 2 | 0 | 0 | 1 | 3 | |
| Plus other | 4 | 6 | 2 | 6 | 2 | 6 | |
| Isolated extramedullary | 45 | 17 | 24 | 17 | 21 | 16 | .538 |
| CNS | 18 | 40 | 9 | 38 | 9 | 43 | |
| Testis | 25 | 56 | 14 | 58 | 11 | 52 | |
| CNS/testis | 1 | 2 | 1 | 4 | 0 | 0 | |
| Other | 1 | 2 | 0 | 0 | 1 | 5 | |
| Peripheral blast cell count | .673 | ||||||
| Less than 1/μL | 93 | 35 | 49 | 35 | 44 | 35 | |
| 1 to less than 10 000/μL | 142 | 53 | 72 | 41 | 70 | 55 | |
| 10 000/μL or more | 32 | 12 | 19 | 15 | 13 | 10 | |
| No data | 2 | (.7) | 1 | (.7) | 1 | (.8) | |
| BCR/ABL fusion transcript | .746 | ||||||
| Positive | 11 | 9 | 6 | 8 | 5 | 11 | |
| Negative | 115 | 91 | 73 | 92 | 42 | 89 | |
| No data | 143 | (53) | 62 | (44) | 81 | (63) | |
| Immunophenotype‡ | .082 | ||||||
| Pro-B | 12 | 5 | 10 | 7 | 2 | 2 | |
| Common ALL | 191 | 73 | 95 | 69 | 96 | 77 | |
| Pre-B | 58 | 22 | 33 | 24 | 25 | 20 | |
| Biphenotypic | 1 | .4 | 0 | 0 | 1 | .8 | |
| No data | 7 | (3) | 3 | (2) | 4 | (3) | |
| Treatment | |||||||
| Frontline protocol | .887 | ||||||
| ALL-BFM | 179 | 67 | 98 | 70 | 81 | 63 | |
| COALL | 31 | 12 | 16 | 12 | 15 | 12 | |
| Other | 59 | 22 | 27 | 19 | 32 | 25 | |
| Irradiation at relapse | .792 | ||||||
| None | 88 | 33 | 47 | 34 | 41 | 32 | |
| Cranial | 94 | 36 | 45 | 33 | 49 | 38 | |
| Craniospinal | 37 | 14 | 21 | 15 | 16 | 13 | |
| TBI | 46 | 17 | 24 | 18 | 22 | 17 | |
| No data | 4 | (2) | 4 | (3) | 0 | 0 | |
| Stem-cell transplantation in CR2 | |||||||
| None | 214 | 80 | 110 | 78 | 104 | 81 | .426 |
| Matched family donor | 32 | 12 | 17 | 12 | 15 | 12 | |
| Unrelated donor | 9 | 3 | 7 | 5 | 2 | 2 | |
| Mismatched family donor | 4 | 2 | 1 | 1 | 3 | 2 | |
| Autologous | 10 | 4 | 6 | 4 | 4 | 3 | |
Missing values are not included in the calculation of percentages and P values.
BM indicates bone marrow; BFM, Berlin-Frankfurt-Münster Study Group; CNS, central nervous system; COALL, Cooperative ALL Study Group; CR2, second complete remission; and TBI, total body irradiation.
Four patients randomized to treat with HD-MTX received ID-MTX due to parental decision.
Very early indicates within 18 month after primary diagnosis; early, after 18 months after primary diagnosis and within 6 months after elective cessation of frontline therapy; and late, later than 6 months after elective cessation of therapy.
Immunophenotype classified according to EGIL.33
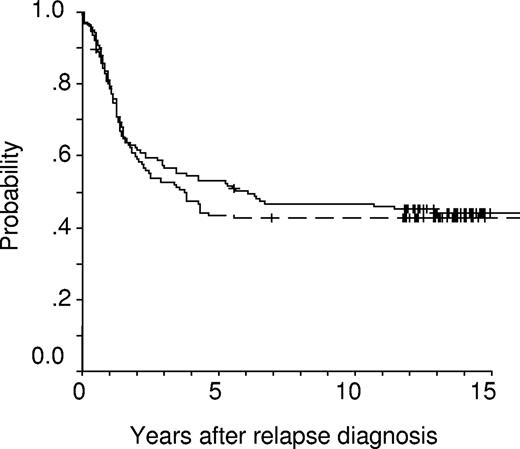
Events occurring after initiation of relapse treatment did not differ between the 2 groups (Table 3). This was also true for the rates of second CR (ID-MTX: 94%, HD-MTX: 89%, P = .197). Although the rate of subsequent isolated extramedullary relapses seems to be higher in patients treated with ID-MTX, this difference is not significant with respect to frequencies, or cumulative incidences of subsequent CNS, testicular, or any isolated and/or combined extramedullary relapses. Children of the 2 groups had virtually identical 10-year event-free survival (.36 ± .04 [standard error; ID-MTX] vs .38 ± .04 [HD-MTX], P = .919) and overall survival (OS) probabilities (.47 ± .04 [ID-MTX] vs .43 ± .04 [HD-MTX], P = .633). Kaplan-Meier plots for EFS probability revealed a highly similar temporal pattern of treatment failures in both groups (Figure 2). In contrast, OS probability (pOS) is approximately 10% higher in the ID-MTX group at 5 years, whereas at 15 years the OS probabilities approach nearly the identical level (Figure 3). Regarding SCT as censored event, EFS estimates between both groups were also not significantly different (ID-MTX: pEFS = .39 ± .05; HD-MTX: pEFS = .40 ± .05; P = .957). When data of children were analyzed for received treatment irrespective of the randomization, EFS rates (ID-MTX: n = 196, censored = 73, pEFS = .37 ± .04; HD-MTX: n = 178, censored = 62, pEFS = .35 ± .04; P = .564) did not differ significantly. Three secondary malignancies occurred in the population randomized to the ID-MTX arm: 2 patients suffered an osteosarcoma 5.7 and 7.3 years after syngeneic and allogeneic SCT, respectively. A 17-year-old girl suffered a myelodysplastic syndrome (MDS) 2.6 years after relapse diagnosis. Of 71 patients of the ID-MTX arm and 58 patients of the HD-MTX arm with subsequent relapse, 11 and 6 patients, respectively, are alive in third CR (P = .455).
Treatment results in 269 randomized patients receiving intermediate-dose methotrexate (ID-MTX) or high-dose methotrexate (HD-MTX)
| . | Total . | ID-MTX . | HD-MTX . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Total | 269 | (100) | 141 | (52) | 128 | (48) | .275 |
| Induction death | 8 | 3 | 4 | 3 | 4 | 3 | — |
| Nonresponse | 15 | 6 | 5 | 4 | 10 | 8 | |
| Complete remission achieved | 246 | 91 | 132 | 94 | 114 | 89 | .197 |
| Lost to follow-up | 2 | 1 | 0 | 0 | 2 | 2 | — |
| Therapy-related death | 15 | 6 | 8 | 6 | 7 | 6 | — |
| Secondary malignancy | 3 | 1 | 3 | 2 | 0 | — | |
| Relapse | 129 | 48 | 71 | 50 | 58 | 45 | |
| Site of relapse | 0 | 0 | 0 | 0 | 0 | 0 | .171 |
| Bone marrow isolated | 105 | 81 | 55 | 76 | 50 | 86 | |
| BM combined | 10 | 8 | 5 | 7 | 5 | 9 | .565 |
| + CNS | 1 | 10 | 1 | 20 | 0 | 0 | — |
| + Testis | 7 | 70 | 3 | 60 | 4 | 80 | — |
| + Other | 2 | 20 | 1 | 20 | 1 | 20 | — |
| Isolated extramedullary | 14 | 11 | 11 | 16 | 3 | 5 | .258 |
| CNS | 9 | 64 | 6 | 45 | 3 | 100 | — |
| Testis | 5 | 36 | 2 | 55 | 0 | 0 | — |
| Complete continuous remission | 97 | 36 | 50 | 36 | 47 | 37 | — |
| . | Total . | ID-MTX . | HD-MTX . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Total | 269 | (100) | 141 | (52) | 128 | (48) | .275 |
| Induction death | 8 | 3 | 4 | 3 | 4 | 3 | — |
| Nonresponse | 15 | 6 | 5 | 4 | 10 | 8 | |
| Complete remission achieved | 246 | 91 | 132 | 94 | 114 | 89 | .197 |
| Lost to follow-up | 2 | 1 | 0 | 0 | 2 | 2 | — |
| Therapy-related death | 15 | 6 | 8 | 6 | 7 | 6 | — |
| Secondary malignancy | 3 | 1 | 3 | 2 | 0 | — | |
| Relapse | 129 | 48 | 71 | 50 | 58 | 45 | |
| Site of relapse | 0 | 0 | 0 | 0 | 0 | 0 | .171 |
| Bone marrow isolated | 105 | 81 | 55 | 76 | 50 | 86 | |
| BM combined | 10 | 8 | 5 | 7 | 5 | 9 | .565 |
| + CNS | 1 | 10 | 1 | 20 | 0 | 0 | — |
| + Testis | 7 | 70 | 3 | 60 | 4 | 80 | — |
| + Other | 2 | 20 | 1 | 20 | 1 | 20 | — |
| Isolated extramedullary | 14 | 11 | 11 | 16 | 3 | 5 | .258 |
| CNS | 9 | 64 | 6 | 45 | 3 | 100 | — |
| Testis | 5 | 36 | 2 | 55 | 0 | 0 | — |
| Complete continuous remission | 97 | 36 | 50 | 36 | 47 | 37 | — |
BM indicates bone marrow; CNS, central nervous system; and —, not applicable.
Kaplan-Meier event-free survival (EFS) estimates of randomized children receiving intermediate-dose (1 g/m2, solid line, n = 141; censored = 50; pEFS = 0.36 ± 0.04) or high-dose (5 g/m2, dashed line, n = 128; censored = 49; pEFS = 0.38 ± 0.04) methotrexate (P = .919).
Kaplan-Meier event-free survival (EFS) estimates of randomized children receiving intermediate-dose (1 g/m2, solid line, n = 141; censored = 50; pEFS = 0.36 ± 0.04) or high-dose (5 g/m2, dashed line, n = 128; censored = 49; pEFS = 0.38 ± 0.04) methotrexate (P = .919).
Kaplan-Meier overall survival (OS) estimates at 10 years of randomized children receiving intermediate-dose (1 g/m2, solid line, n = 141; censored = 63; pOS = 0.47 ± 0.04) or high-dose (5 g/m2, dashed line, n = 128; censored = 55; pOS = 0.43 ± 0.04) methotrexate (P = .633).
Kaplan-Meier overall survival (OS) estimates at 10 years of randomized children receiving intermediate-dose (1 g/m2, solid line, n = 141; censored = 63; pOS = 0.47 ± 0.04) or high-dose (5 g/m2, dashed line, n = 128; censored = 55; pOS = 0.43 ± 0.04) methotrexate (P = .633).
The dose of MTX had no influence on results after SCT (P = .942) with comparable rates of treatment-related deaths (ID: n = 4; HD: n = 3) or subsequent relapses (ID: n = 15; HD: n = 12).
Subgroup analyses did not reveal a prognostic impact of the MTX dose in the following categories: sex (male, P = .892; female, P = .744), time point of relapse (very early, P = .194; early, P = .957; late, P = .433), and site of relapse (BM isolated, P = .90; BM combined, P = .593; isolated extramedullary, P = .765). Furthermore, the cumulative dose of intravenous MTX during frontline therapy had no impact on the efficacy of MTX at different dosages at relapse: EFS was not statistically different in 10 patients having received less than 20 g/m2 intravenous MTX (P = .688), or in 210 patients having received 20 g/m2 as scheduled in the standard protocol M of the ALL-BFM studies for most patients (P = .665) or in 26 patients having received more than 20 g/m2 intravenous MTX (P = .566). In 23 patients, the data on the cumulative intravenous MTX dosage at frontline therapy were not available.
Multivariate Cox regression analysis including SCT as time-dependent covariate revealed time point (P < .001; results in univariate analysis: very early: n = 10, pEFS = .40 ± .16; early: n = 112, pEFS = .21 ± .04; late: n = 147, pEFS = .48 ± .04; P < .001) and site of relapse (P < .001; results in univariate analysis: isolated BM: n = 156, pEFS = .29 ± .04; combined BM: n = 68, pEFS = .46 ± .06; isolated extramedullary: n = 45, pEFS = .50 ± .08; P = .010) as the only significant independent predictors for event-free survival, while sex (P = .666), age at relapse (P = .714), immunophenotype (missing values and 1 patient with biphenotypic ALL excluded; P = .761), peripheral blast count (P = .102), MTX dosage (P = .487), and SCT (P = .116) did not show an independent prognostic relevance.
Discussion
We report on a randomized trial ALL-REZ BFM 90 comparing the efficacy of MTX administered at a dose of 5 g/m2 over 24 hours followed by 3 scheduled doses of folinic acid versus MTX at a dose of 1 g/m2 over 36 hours followed by 2 doses of folinic acid in the treatment of childhood relapsed precursor B-cell ALL. Very high-risk patients with very early bone marrow relapse and those with T-lineage ALL have been excluded and treated with individual protocols. MTX was part of alternating multidrug chemotherapy courses. We did not find a difference in event-free and overall survival between the randomized groups, or in site or time patterns of subsequent relapses.
The antimetabolic effect of MTX and MTX-polyglutamates (MTX-PGs) is based mainly on the inhibition of dihydrofolic acid reductase (DHFR), which reduces dihydrofolates to tetrahydrofolates as essential carriers of one-carbon groups in the synthesis of nucleotides and thymidilates. In addition, MTX-PGs inhibit 2 enzymes involved in the de novo purine synthesis, 5′phosphoribosylgycinamid transformylase and aminoimidazole carboxamide transformylase.37,38 Active transmembrane transport of MTX is regulated by the reduced folate carrier (RFC) and to a minor extent also by a folate receptor. At high serum concentrations, passive diffusion of the drug becomes an additional pathway for intracellular uptake of MTX.39 Hepatic and intracellular polyglutamination are important for the antileukemic activity since polyglutamates with more than 3 glutamyl residues have a higher affinity to the target enzymes and are retained intracellularly as active metabolites for prolonged periods of time.40 Polyglutamination is catalyzed by folylpolyglutamate synthetase (FPGS); polyglutamates are cleaved by folylpolyglutamate hydrolase (FPGH).41
Several mechanisms of resistance to MTX have been described: impaired transmembrane transport due to decreased RFC activity,42 elevated levels of DHFR and amplification of the DHFR gene,43,44 impaired polyglutamination,45 and increased activity of FGPH.46
For relapsed lymphoblastic leukemias, a 3-fold higher resistance to MTX measured in the in situ thymidilate synthetase inhibition assay compared with initial ALL has been reported.47 In vitro studies on a series of antileukemic drugs revealed a 0.8- to 1.9-fold higher resistance of relapse ALL samples compared with primary ALL samples to l-asparaginase, anthracyclines, and thiopurines and a comparable resistance to Vinca alkaloids, cytarabine, ifosfamide, and epipodophyllotoxins. Only resistance to glucocorticoids was more than 24-fold higher in relapse compared with primary ALL samples.48 Hence, at relapse, sensitivity to MTX is substantially more impaired than to most other antileukemic drugs.
Most likely, the resistance of relapse ALL samples toward MTX can be explained by an increased activity of DHFR44 as a consequence of gene amplification.43 In individual patients, lack of cytotoxicity was found despite complete inhibition of thymidylate synthetase. Therefore, an alternative pathway circumventing the thymidylate synthetase has been postulated as an additional mechanism of resistance.49
Several factors of the treatment design may influence the antileukemic efficacy of MTX: the dosage, the duration of administration, and the intensity of folinic acid rescue.50
Higher doses of MTX are administered using shorter infusion times, since the duration of exposure to toxic serum levels seems to be the major factor determining the toxicity.51,52 In 1993, the ALL-REZ BFM group had already reported on a randomized comparison between MTX at a dose of 1 g/m2 administered over 24 hours followed by 2 doses of folinic acid and 12 g/m2 infused over 4 hours with 12 doses of folinic acid.29 The median serum concentration during MTX infusion was more than 700 μmol/L in the high-dose arm and 7.2 μmol/L in the intermediate-dose arm. Concentrations greater than 1 μmol/L were maintained for 36 hours with HD-MTX 12 g/m2 and 45 hours with ID-MTX 1 g/m2. EFS and survival were not significantly different, but the toxicity was even higher in the ID arm due to the substantially longer infusion duration with longer lasting serum concentrations higher than 1 μmol/L and probably also to the fact that the rescue regimens were different.
Several studies have shown that ID-MTX at a dose of 1 g/m2 leads to higher intracellular concentrations of MTX and MTX-PGs and subsequently to a better EFS than low-dose regimens.21 However, at higher doses, the intracellular uptake of MTX is not proportional to the serum concentration due to a limited capacity of the active mechanisms via RFC and folate reductase (FR) and a limited passive infusion.53 In contrast, in the CNS the MTX level is correlated with the mean serum concentration of the drug.54 Hence, at steady-state serum concentrations of even more than 20 μmol/L the antileukemic effect of MTX is related rather to the higher levels in sanctuary sites such as the CNS and the testes than to an increased intracellular accumulation of MTX and MTX-PGs.53,55 This may be the explanation as to why the arm with the 5-fold higher MTX dose did not lead to fewer subsequent BM relapses. Although the rate of subsequent isolated extramedullary relapses was higher in the ID-MTX group (16%) compared with the HD-MTX group (5%), which would fit the hypothesis of a better protection in extracompartimental sites with higher serum levels, this difference was not significant. Thus, our study design provided obviously sufficient protection to extracompartments even with ID-MTX.22
Infusion duration may be equally important for the antileukemic efficacy as the dosage. It is of note that the higher resistance toward MTX in relapsed ALL samples could not be overcome by prolongation of in vitro exposure to the drug.48,56 Therefore, an interesting question would be whether 1 g/m2 MTX administered over only 24 hours would be sufficient to exploit the antileukemic potential of MTX in this stadium of the disease. Until now, no prospective studies have been conducted investigating the importance of infusion duration of HD-MTX. The herein reported trial does not allow to assess the isolated effect of the infusion duration because of the different doses of MTX. However, this question is being addressed in the ongoing trial Total XV performed at the St Jude Children's Research Hospital, by giving 1 g/m2 MTX upfront randomly at a 4- versus 24-hour infusion.57
Toxicity of HD-MTX is usually limited to a moderate myelosuppression, reversible mucositis, and moderate hepatic or renal dysfunction. It has been substantially reduced via hydration, urinary alkalization, and monitoring of renal function and methotrexate serum concentrations.58 In case of impaired elimination, an escalation of the leucovorin rescue, or enzymatic cleavage of the drug by carboxypeptidase in selected cases, has succeeded in almost eliminating fatal toxicities.59,60 Transient neurotoxicity has been reported after diverse doses and application ways of MTX, although there are rare cases of severe encephalopathy occurring after HD-MTX.30,61,–63 Thus, the toxicity profile of HD-MTX or ID-MTX proves favorable in the context of intensive multidrug chemotherapy with myelosuppression as the prominent and dose-limiting side effect. Toxicity data on MTX therapy at several dosages had been collected in prior ALL-REZ BFM trials and have been published.29 Therefore, a detailed documentation of these data was not performed at the trial ALL-REZ BFM 90.
The long-term results of the trial reported here demonstrate that more than 35% of patients with relapsed precursor B-cell ALL (excluding very early bone marrow relapses) can be salvaged. This is in line with results reported by other collaborative trials.6,12,16,20,64 As some patients with a subsequent relapse can achieve even a long-lasting complete third remission, the overall survival rates actually exceed 45%. However, since very high-risk patients (very early BM relapse, any T-lineage relapse) were excluded from the randomization, the results are not representative for an unselected population of children with ALL relapse. The dynamics of the Kaplan-Meier plot reveal a cascade of subsequent relapses with the latest relapse occurring 7.3 years after diagnosis. The rate of second malignancies is negligible in our patient cohort; 2 of them occurred after allergenic SCT. Nevertheless, still more than half of the patients suffer a subsequent relapse. It is the most important objective of ongoing trials to predict more precisely which individual patient is at high risk for treatment failure and would therefore need an intensification of postremission therapy by allogeneic SCT, and which patients can be cured with chemotherapy/radiotherapy alone.65,66 Although SCT as time-dependent covariate was not a significant prognostic factor for EFS in multivariate analysis, this trial does not allow to assess conclusively the role of allogeneic SCT in relapsed ALL due to its rather uncontrolled use.
Although most successful protocols for treatment of ALL have incorporated ID-MTX or HD-MTX, some studies show excellent results even for high-risk patients with intensive chemotherapy not including high-dose MTX.67 The chosen study design does not permit to assess the overall impact of MTX in patients with relapsed ALL because it has been used in combination with intensive multidrug chemotherapy. However, since the results of this trial could not prove an advantage of HD-MTX over ID-MTX, but also could not disprove the role of ID-MTX in childhood relapsed ALL at all, we decided to use MTX at a dose of 1 g/m2 over 36-hour infusion as standard treatment in all subsequent ALL-REZ BFM trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to all nurses, physicians, technologists, and other support staff members involved in the BFM relapse trials. Special thanks to Marianne Löwke and Constanze Sukopp for laboratory assistance, and to Sabine Brühmüller, Andrea Kretschmann, and Steffanie Schober for preparing the data of this study. The city locations and principal investigators of the participating medical centers are listed in Document S1.
This work was supported by Deutsche Krebshilfe, Bonn, Germany; the Deutsche Kinderkrebsstiftung; and the BMBF (Federal Ministry of Education and Research, Germany).
Authorship
Contribution: A.S. wrote the paper and performed part of the data analysis; R.H. designed the statistical part of the trial and performed the data analysis; C.B. wrote part of the paper and reviewed it; R.F. designed the clinical part of the trial; G.J.-S. warranted data exchange with the COALL frontline trial and reviewed the paper; A.R. warranted the data exchange with the ALL-BFM trial and reviewed the transplantation-related data; G.M. reviewed the data of the Austrian patients; K.S. was national coordinator of the Danish patients and reviewed the paper; R.R. was responsible for reference immunophenotyping and data transfer/analysis of corresponding results; T.K. was coordinator of stem-cell transplantation and reviewed the corresponding data; J.R. contributed to the clinical design of the trial and critically reviewed the paper; G.H. was responsible for the trial design and conduction of the trial as chair of the study group and in part wrote/reviewed the paper.
A complete list of the city locations and principal investigators of the participating medical centers of the ALL-REZ BFM Study Group is provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arend von Stackelberg, Klinik für Pädiatrie m. S. Onkologie und Hämatologie, Charité Campus Virchow-Klinikum, Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany; e-mail: arend.stackelberg@charite.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal