We examined the prognostic impact of cytogenetics on the outcome of 200 acute lymphoblastic leukemia (ALL) patients 15 to 65 years of age enrolled in Southwest Oncology Group (SWOG)–9400 study. Evaluable cytogenetics or fluorescence in situ hybridization studies were available in 140 (70%) patients. Four karyotype categories (normal [n = 31, 22%], t(9;22)/BCR/ABL1 [n = 36, 26%], other unfavorable [−7, +8, or 11q23 rearrangement, n = 19, 13%], and miscellaneous [n = 54, 39%]) and the biologically and clinically relevant ALL ploidy subgroups were prospectively defined. Overall survival (OS) decreased significantly with increasing age (P = .009) and varied with karyotype category (P < .001). OS was worst for t(9;22)/BCR/ABL1 followed by other unfavorable karyotypes, with hazard ratios (HR) of 3.45 (95% confidence interval [CI], 1.88-6.31) and 2.14 (95% CI, 1.04-4.04), respectively, compared with normal diploid group. OS of the miscellaneous group was similar to that of the normal diploid group (HR = 0.82; 95% CI, 0.44-1.53). Relapse-free survival (RFS) was not significantly associated with age (P = .30) but was heterogeneous among karyotype categories (P < .001) primarily because of poor RFS in t(9;22)/BCR/ABL1 (HR = 3.49; 95% CI, 1.80-6.75) compared with the normal diploid group. After accounting for the variation among karyotype groups, age was not a significant prognostic factor for OS or RFS, highlighting cytogenetics as the most important prognostic factor in adult ALL. This trial was registered at www.ClinicalTrials.gov as #NCT00002665.
Introduction
Although the outcome of patients with acute lymphoblastic leukemia (ALL) has improved over the last 4 decades, the cure rate of adult ALL is still only about 40%, approximately only half that of childhood ALL.1,–3 Biologic differences in leukemogenesis between adult and childhood ALL are the most likely explanation for this discrepancy.1,4
Childhood and adult ALL differ markedly in the prevalences of various cytogenetic abnormalities. For example, Philadelphia chromosome (Ph)-positive ALL, a high-risk cytogenetic subset, accounts for one fourth of adult ALL cases but occurs in less than 5% of children. Similarly ETV6/RUNX1 (TEL-AML1) fusion and hyperdiploidy, both good risk genetic features, together comprise approximately 50% of childhood ALL, but only approximately 10% of adult ALL.1,4 While the impact of cytogenetic factors including specific translocations and DNA ploidy is well defined in childhood ALL, the prognostic significance of karyotype in adult ALL is much less clear, in part because the disease is less frequent. It is also not known whether the adverse prognostic impact of increasing age in adult ALL is entirely related to cytogenetic and molecular factors.
The Southwest Oncology Group (SWOG)–9400 study was a large phase 2 trial conducted to assess the effectiveness of remission induction and postconsolidation therapies in adult ALL patients. In this paper, we report the results of this trial with particular emphasis on the prognostic significance of cytogenetics in adult ALL.
Methods
Patients
SWOG-9400 was a multicenter trial of a combination chemotherapy regimen for induction and consolidation followed by allogeneic bone marrow transplantation (BMT) or maintenance chemotherapy, depending on the patient's age and availability of an human leukocyte antigen-identical sibling marrow donor. Eligible patients were between 15 and 65 years of age and had untreated ALL with French-American-British classification (FAB) L1 or L2 morphology, ECOG performance status 0 to 3, and adequate hepatic, renal, and cardiac function. During the first 2 years of the study, patients with FAB-L3 morphology were also eligible, but these are excluded from this analysis. The study was approved by the Institutional Review Boards of the participating institutions, and all patients provided written informed consent in accordance with the Declaration of Helsinki and federal and institutional guidelines.
Treatment
The chemotherapy regimen is summarized in Table 1. Thirteen patients were initially randomized to receive the induction regimen plus PIXY 321, a recombinant fusion protein composed of human granulocyte-macrophage colony-stimulating factor and interleukin-3, with the aim of shortening the duration of thrombocytopenia after induction. However, this randomization was stopped in April 1996 by the drug sponsor. The cytogenetic profile and treatment outcomes of these 13 patients did not differ significantly from those of the remaining patients (results not shown); therefore, they have been included in the following analyses.
Chemotherapy regimen of study SWOG-9400
| Agent . | Dose and route . | Days . | Notes . |
|---|---|---|---|
| Induction part 1 | |||
| Daunorubicin | 60 mg/m2 IV | 1, 2, 3 | |
| Vincristine | 1.4 mg/m2 PO | 1, 8, 15, 22 | Maximum 2 mg per administration |
| Prednisone | 60 mg/m2 per day IV | 1-42 | Full dose to day 28, taper to day 42 |
| PEG-L-asparaginase | 2000 units/m2 IM | 15 | Until protocol amendment of September 1, 1999 |
| L-Asparaginase | 10 000 units/d IV or IM | 15-24 | After protocol amendment of September 1, 1999 |
| Induction part 2 (patients with persistent leukemia on day 21) | |||
| Daunorubicin | 60 mg/m2 IV | 22, 23 | |
| Vincristine | 1.4 mg/m2 PO | 29, 36 | Maximum 2 mg per administration |
| Prednisone | 60 mg/m2 per day IV | Through day 42 | |
| PEG-L-asparaginase | 2000 units/m2 IM | Day 38 | Until protocol amendment of September 1, 1999, only |
| Consolidation | |||
| Cyclophosphamide | 650 mg/m2 IV | 1, 15, 29 | |
| Ara-C | 75 mg/m2 per day IV push | 2-5, 9-12, 16-19, 23-26 | |
| 6-Mercaptopurine | 60 mg/m2 PO | 1-28 | |
| Methotrexate | 10 mg/m2 IT or intraventricular | 2, 9, 16, 23 | |
| Maintenance course 1 | |||
| 6-Mercaptopurine | 60 mg/m2 per day PO | 1-63 | |
| Methotrexate | 20 mg/m2 per week PO | 1-63 | |
| Maintenance course 2 | |||
| Vincristine | 1.5 mg/m2 IV | 1, 8, 15, 22 | Maximum 2 mg per administration |
| Adriamycin | 25 mg/m2 IV | 1, 8, 15, 22 | |
| Dexamethasone | 10 mg/m2 PO | 1-28 | |
| Maintenance course 3 | |||
| Cyclophosphamide | 650 mg/m2 IV | 1 | |
| Thioguanine | 60 mg/m2 PO | 1-14 | |
| Ara-C | 75 mg/m2 IV push | 3-6, 10-13 | |
| Maintenance course 4 | |||
| 6-Mercaptopurine | 60 mg/m2 per day PO | Daily for 2 y | |
| Methotrexate | 20 mg/m2 per week PO | Weekly for 2 y | |
| Agent . | Dose and route . | Days . | Notes . |
|---|---|---|---|
| Induction part 1 | |||
| Daunorubicin | 60 mg/m2 IV | 1, 2, 3 | |
| Vincristine | 1.4 mg/m2 PO | 1, 8, 15, 22 | Maximum 2 mg per administration |
| Prednisone | 60 mg/m2 per day IV | 1-42 | Full dose to day 28, taper to day 42 |
| PEG-L-asparaginase | 2000 units/m2 IM | 15 | Until protocol amendment of September 1, 1999 |
| L-Asparaginase | 10 000 units/d IV or IM | 15-24 | After protocol amendment of September 1, 1999 |
| Induction part 2 (patients with persistent leukemia on day 21) | |||
| Daunorubicin | 60 mg/m2 IV | 22, 23 | |
| Vincristine | 1.4 mg/m2 PO | 29, 36 | Maximum 2 mg per administration |
| Prednisone | 60 mg/m2 per day IV | Through day 42 | |
| PEG-L-asparaginase | 2000 units/m2 IM | Day 38 | Until protocol amendment of September 1, 1999, only |
| Consolidation | |||
| Cyclophosphamide | 650 mg/m2 IV | 1, 15, 29 | |
| Ara-C | 75 mg/m2 per day IV push | 2-5, 9-12, 16-19, 23-26 | |
| 6-Mercaptopurine | 60 mg/m2 PO | 1-28 | |
| Methotrexate | 10 mg/m2 IT or intraventricular | 2, 9, 16, 23 | |
| Maintenance course 1 | |||
| 6-Mercaptopurine | 60 mg/m2 per day PO | 1-63 | |
| Methotrexate | 20 mg/m2 per week PO | 1-63 | |
| Maintenance course 2 | |||
| Vincristine | 1.5 mg/m2 IV | 1, 8, 15, 22 | Maximum 2 mg per administration |
| Adriamycin | 25 mg/m2 IV | 1, 8, 15, 22 | |
| Dexamethasone | 10 mg/m2 PO | 1-28 | |
| Maintenance course 3 | |||
| Cyclophosphamide | 650 mg/m2 IV | 1 | |
| Thioguanine | 60 mg/m2 PO | 1-14 | |
| Ara-C | 75 mg/m2 IV push | 3-6, 10-13 | |
| Maintenance course 4 | |||
| 6-Mercaptopurine | 60 mg/m2 per day PO | Daily for 2 y | |
| Methotrexate | 20 mg/m2 per week PO | Weekly for 2 y | |
IV indicates intravenously; PO, orally; IM, intramuscularly; and IT, intrathecally.
After completing consolidation chemotherapy, patients younger than 51 years with performance status 0 or 1 and suitable human leukocyte antigen-matched sibling donors were offered allogeneic BMT. Allogeneic BMT was performed by conditioning with fractionated total body radiation (1320 cGy) and etoposide (60 mg/kg) followed by infusion of donor marrow. Patients received graft-versus-host disease prophylaxis with cyclosporine, methotrexate, and methylprednisolone. Patients who were not candidates for or refused allogeneic BMT received maintenance chemotherapy (Table 1).
Conventional cytogenetics and fluorescence in situ hybridization analyses
Cytogenetic studies on pretreatment bone marrow or unstimulated blood samples were performed using standard G-banding with trypsin-Giemsa or trypsin-Wright staining in SWOG-approved cytogenetics laboratories. Karyotypes were interpreted using International System for Cytogenetic Nomenclature criteria (1995).5 Karyotypes were considered normal diploid if no clonal abnormalities were detected in a minimum of 20 metaphases examined and if at least 2 cell processing methods were used. Each karyotype was independently reviewed by at least 3 members of the SWOG Cytogenetics Committee. Pretreatment samples from 60 patients were also evaluated by MLL and BCR/ABL1 fluorescence in situ hybridization (FISH) (Abbott Molecular, Des Plaines, IL) studies to access the status of these 2 “high-risk” aberrations in 23 samples considered insufficient for adequate evaluation by conventional cytogenetics studies, 27 karyotypically normal presentation samples considered negative for t(9;22) or t(4;11), and 10 control samples (6 t(9;22) positive and 4 known negative cases). The FISH studies were performed at the City of Hope using standard methodology without prior knowledge of the conventional cytogenetics results. The probe sets used were validated, and their corresponding sensitivity and specificity were determined as described.6 Because the presentation leukemia samples were frozen before FISH studies, at least 20% of the interphase nuclei had to show either a BCR/ABL1-positive or MLL-positive FISH signal pattern to be considered positive for the gene rearrangement.
Four karyotype subgroups (normal, t(9;22)/BCR/ABL1-positive, other unfavorable, and miscellaneous) were defined at the initiation of the study to classify the combined results of conventional cytogenetic and FISH analyses, as previously defined.7 The “other unfavorable” group included −7, +8, and 11q23/MLL gene rearrangements. All other clonal cytogenetic abnormalities were classified as miscellaneous. Patients were also classified according to biologically and clinically relevant ploidy subgroups of low hypodiploidy (30-39)/triploidy (60-78)/high hyperdiploidy (51-65) and tetraploidy. At study completion, the karyotypically aberrant cases were also reclassified according to the reported MRC UKALLXII/ECOG E2993 ALL cytogenetic subgroups.8
Definitions of treatment outcomes
Complete remission (CR) was defined as less than 5% morphologically identified blasts in a bone marrow with cellularity more than 20% and normal maturation, provided the patient had a peripheral blood neutrophil count more than 1500/μL, platelet count more than 100 000/μL, and no evidence of extramedullary disease. Resistant disease was defined by the presence of persistent leukemia in the marrow and/or blood of patients who survived at least 7 days after induction therapy. Overall survival (OS) was measured from the date of entry onto the study until death from any cause, with observation censored at the date of last contact for patients last known to be alive. For patients who achieved CR, relapse-free survival (RFS) was measured from the date of CR until relapse of ALL or death from any cause, with observation censored at the date of last contact for patients last known to be alive without report of relapse. For patients registered for maintenance therapy or allogeneic BMT, postconsolidation survival and disease-free survival (DFS) were measured from the date of that registration until the respective end point for OS or RFS. Toxicities were classified by type and grade according to the National Cancer Institute's Common Toxicity Criteria version 2.X.
Statistical methods
The original goals of study SWOG-9400 included testing whether the remission induction regimen is sufficiently effective in terms of CR rate to warrant further investigation and to evaluate the efficacy and toxicity of postconsolidation allogeneic BMT and maintenance chemotherapy. A study size of 50 patients was required to meet the first objective; however, additional patients were enrolled to provide sufficient information for the latter objectives. Demographic data, clinical data at presentation, and outcome and toxicity data were collected with quality control review according to standard procedures of the SWOG. In addition to standard descriptive statistical analyses, logistic and proportional hazards (PH) regression models were used to investigate the effects of cytogenetic, demographic, and clinical variables on CR rate, OS, RFS, and DFS. Multivariate versions of these regression models were used to assess the independent effects of potential prognostic factors, such as age, karyotype category, or performance status. Distributions of OS, RFS, and DFS were estimated by the method of Kaplan and Meier.9 Because this was an exploratory analysis, statistical significance of associations was measured by 2-tailed P values, and P less than .05 was considered a general guideline for statistical significance, but not a strict criterion. Confidence intervals (CIs) were calculated at the 95% confidence level. All analyses, including maximum likelihood estimation of logistic and proportional hazards regression models, were performed using SAS, version 9 (SAS Institute, Cary, NC). Results are based on data available June 22, 2007. Detailed results for the univariate and selected multivariate regression models for CR rate, OS, RFS, and DFS are provided in Tables S1Table S2 (PDF, 40.6 KB)Table S3 (PDF, 31 KB)–S4 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Patients
A total of 218 patients entered study SWOG-9400 from August 1995 through May 2000. Eleven of the 218 had FAB-L3 ALL, and are excluded from this analysis. Five of the remaining 207 were ineligible because of diagnoses other than ALL (CML in blast crisis and Waldenstrom macroglobulinemia), inadequate liver function, chronic liver disease (hepatitis C), and absence of proper Institutional Review Board approval. Two additional patients were excluded from the analysis: one who refused to continue protocol treatment after one day and another who received no protocol treatment because a positive hepatitis C titer was observed after a 3-week delay for infection control.
Demographic and clinical characteristics of the 200 included patients are summarized in Table 2. Patients ranged in age from 16 to 65 years (median, 32 years), and all but 18 had performance status 0 or 1. White blood cell (WBC) counts ranged from 600 to 396 600 cells/mm3 (median, 15 800), and 15 patients (7.5%) had WBC counts exceeding 100 000/mm3. Conventional banded cytogenetic studies of pretreatment blood and/or bone marrow were available for 131 (65.5%) of the 200 patients, demonstrating t(9;22) for 28 cases (21%; 95% CI, 15%-29%) and t(4;11) for 5 cases (4%; 95% CI, 1%-9%). Banded studies were not available for the remaining 69 cases, primarily because of failure to submit adequate specimens to approved labs (N = 15), absence of mitotic figures (N = 21), and fewer than 20 cells for a study with no clonal abnormalities (N = 27). FISH studies for t(9;22) and/or t(4;11) were available for 60 patients, including 23 patients who had inadequate GTG-banded studies. Seven of these 23 patients showed a BCR/ABL1 gene arrangement, and 2 of the remaining 16 patients showed an MLL gene rearrangement.
Characteristics of 200 adult patients with non-L3 ALL, by karyotype category
| Characteristic . | Normal diploid, N = 31 . | t(9;22)/BCR/ABL1, N = 36 . | Other unfavorable, N = 19 . | Miscellaneous, N = 54 . | Nonevaluable, N = 60 . | All patients, N = 200 . |
|---|---|---|---|---|---|---|
| Sex, no. (%) | ||||||
| Female | 12 (39) | 19 (53) | 8 (42) | 17 (31) | 23 (38) | 79 (40) |
| Male | 19 (61) | 17 (47) | 11 (58) | 37 (69) | 37 (62) | 121 (61) |
| Race, no. (%) | ||||||
| Black | 2 (6) | 3 (8) | 2 (11) | 5 (9) | 2 (3) | 14 (7) |
| Hispanic | 10 (32) | 7 (19) | 1 (5) | 16 (30) | 16 (27) | 50 (25) |
| White, non-Hispanic | 18 (58) | 24 (67) | 14 (74) | 30 (56) | 36 (60) | 122 (61) |
| Other | 1 (3) | 2 (6) | 2 (11) | 3 (6) | 6 (10) | 14 (7) |
| Performance status, no. (%) | ||||||
| 0 to 1 | 29 (94) | 33 (92) | 18 (95) | 50 (93) | 52 (87) | 182 (91) |
| 2 to 3 | 2 (6) | 3 (8) | 1 (5) | 4 (7) | 8 (13) | 18 (9) |
| FAB classification (local diagnosis), no. (%) | ||||||
| L1 | 14 (45) | 9 (25) | 5 (26) | 20 (37) | 29 (48) | 77 (39) |
| L2 | 16 (52) | 24 (67) | 12 (63) | 29 (54) | 26 (43) | 107 (54) |
| Other, NOS | 1 (3) | 3 (8) | 2 (11) | 5 (9) | 5 (8) | 16 (8) |
| Median, age, y (range) | 25 (18-57) | 47 (17-64) | 31 (18-55) | 29 (17-63) | 38 (16-65) | 32 (16-65) |
| Median, WBCs, 1000/mm3, (range) | 11.5 (0.9-150.0) | 38.9 (1.4-141.3) | 22.5 (5.2-163.0) | 14.4 (0.6-396.6) | 5.2 (1.1-197.5) | 15.8 (0.6-396.6) |
| Peripheral blasts, % | 59 (0-91) | 64 (0-95) | 64 (20-97) | 49 (0-98) | 24 (0-92) | 52 (0-98) |
| Median peripheral blasts, 1000/mm3, (range) | 5.6 (0.0-136.5) | 29.3 (0.0-125.3) | 12.9 (1.6-124.9) | 7.5 (0.0-141.9) | 1.2 (0.0-173.8) | 8.1 (0.0-173.8) |
| Median hemoglobin, g/dL, (range) | 9.4 (4.7-18.2) | 9.9 (5.5-13.4) | 10.1 (7.0-14.1) | 9.4 (4.6-16.5) | 9.2 (4.2-18.2) | 9.7 (4.2-18.2) |
| Median platelets, 1000/mm3, (range) | 70 (16-675) | 36 (3-400) | 47 (10-171) | 48 (5-324) | 47 (3-371) | 48 (3-675) |
| Marrow blasts, % | 90 (32-99) | 88 (48-99) | 78 (38-95) | 89 (2-99) | 92 (17-99) | 90 (2-99) |
| Characteristic . | Normal diploid, N = 31 . | t(9;22)/BCR/ABL1, N = 36 . | Other unfavorable, N = 19 . | Miscellaneous, N = 54 . | Nonevaluable, N = 60 . | All patients, N = 200 . |
|---|---|---|---|---|---|---|
| Sex, no. (%) | ||||||
| Female | 12 (39) | 19 (53) | 8 (42) | 17 (31) | 23 (38) | 79 (40) |
| Male | 19 (61) | 17 (47) | 11 (58) | 37 (69) | 37 (62) | 121 (61) |
| Race, no. (%) | ||||||
| Black | 2 (6) | 3 (8) | 2 (11) | 5 (9) | 2 (3) | 14 (7) |
| Hispanic | 10 (32) | 7 (19) | 1 (5) | 16 (30) | 16 (27) | 50 (25) |
| White, non-Hispanic | 18 (58) | 24 (67) | 14 (74) | 30 (56) | 36 (60) | 122 (61) |
| Other | 1 (3) | 2 (6) | 2 (11) | 3 (6) | 6 (10) | 14 (7) |
| Performance status, no. (%) | ||||||
| 0 to 1 | 29 (94) | 33 (92) | 18 (95) | 50 (93) | 52 (87) | 182 (91) |
| 2 to 3 | 2 (6) | 3 (8) | 1 (5) | 4 (7) | 8 (13) | 18 (9) |
| FAB classification (local diagnosis), no. (%) | ||||||
| L1 | 14 (45) | 9 (25) | 5 (26) | 20 (37) | 29 (48) | 77 (39) |
| L2 | 16 (52) | 24 (67) | 12 (63) | 29 (54) | 26 (43) | 107 (54) |
| Other, NOS | 1 (3) | 3 (8) | 2 (11) | 5 (9) | 5 (8) | 16 (8) |
| Median, age, y (range) | 25 (18-57) | 47 (17-64) | 31 (18-55) | 29 (17-63) | 38 (16-65) | 32 (16-65) |
| Median, WBCs, 1000/mm3, (range) | 11.5 (0.9-150.0) | 38.9 (1.4-141.3) | 22.5 (5.2-163.0) | 14.4 (0.6-396.6) | 5.2 (1.1-197.5) | 15.8 (0.6-396.6) |
| Peripheral blasts, % | 59 (0-91) | 64 (0-95) | 64 (20-97) | 49 (0-98) | 24 (0-92) | 52 (0-98) |
| Median peripheral blasts, 1000/mm3, (range) | 5.6 (0.0-136.5) | 29.3 (0.0-125.3) | 12.9 (1.6-124.9) | 7.5 (0.0-141.9) | 1.2 (0.0-173.8) | 8.1 (0.0-173.8) |
| Median hemoglobin, g/dL, (range) | 9.4 (4.7-18.2) | 9.9 (5.5-13.4) | 10.1 (7.0-14.1) | 9.4 (4.6-16.5) | 9.2 (4.2-18.2) | 9.7 (4.2-18.2) |
| Median platelets, 1000/mm3, (range) | 70 (16-675) | 36 (3-400) | 47 (10-171) | 48 (5-324) | 47 (3-371) | 48 (3-675) |
| Marrow blasts, % | 90 (32-99) | 88 (48-99) | 78 (38-95) | 89 (2-99) | 92 (17-99) | 90 (2-99) |
NOS indicates not otherwise specified; and WBC, white blood cells.
Overall, 140 patients (70%) were evaluable for cytogenetics: 131 with banded studies and 9 others with the unfavorable t(9;22) or t(4;11) translocations by FISH only. These 140 included 31 (22%) with normal karyotypes based on conventional banded studies, 36 (26%) with t(9;22) by banded study and/or FISH. Another 19 (14%) patients had unfavorable karyotypes based on the presence of t(4;11) by banded study (N = 5) or FISH (N = 2, one with a confirmed t(4;11) at follow-up), trisomy 8 (N = 9), monosomy 7 (N = 4), or del7(q22) (N = 1); 2 of these 19 had +8 with t(4;11) and monosomy 7, respectively. The remaining 54 (38%) patients had other cytogenetic abnormalities and were categorized as miscellaneous. Recurrent abnormalities in the miscellaneous group included del(6q)/−6 (N = 12), trisomy 21 (N = 10), del(9p)/−9 (N = 8), trisomy X (N = 5), and trisomy 19 (N = 3). In addition, 7 patients in the miscellaneous group had translocations involving chromosome 14 at bands q11.2 (N = 5) or q32 (N = 2).
The t(1;19) was observed in 7 (5%) of the 131 patients with conventional banded studies: 3 with unfavorable cytogenetics resulting from the presence of +8, and 4 in the miscellaneous group [2 with del(6q), one with t(2;22;9), and one with t(1;19) as the sole abnormality].
As shown in Table 2, age varied significantly among the 4 evaluable karyotype categories (P < .001), with patients having t(9;22)/BCR/ABL1 tending to be older (median age, 47 years) than the others. Among the 140 cytogenetically evaluable patients, the peripheral blast percentage did not vary significantly among the 4 cytogenetic groups (P = .27). However, WBC (P = .007) and peripheral blast counts (P = .002) varied significantly among the 4 groups, tending to be higher in those with t(9;22)/BCR/ABL1 (median WBC 38 900/mm3 and peripheral blast count 29 300/mm3). Platelet counts varied among the 4 evaluable groups (P = .007), tending to be highest for those with normal karyotypes and lowest for those with t(9;22)/BCR/ABL1.
Treatment and outcomes
Among the 200 included patients, 159 (80%; 95% CI, 73%-85%) achieved CR and 21 (11%; 95% CI, 7%-16%) had resistant disease. 2 other patients died before response could be assessed, and response was not adequately assessed for the remaining 18 patients. Of the 159 CRs, 133 were achieved with only the initial induction, whereas 25 required part 2 of induction (treatment data were incomplete for one patient).
Among the 200 included patients, 140 have died and the other 60 were last known to be alive 5 months to 11 years after entering the study (median, 8.0 years). The estimated median OS is 22 months (95% CI, 17-31 months), and the estimated probability of surviving 5 years is 33% (95% CI, 27%-40%).
Among the 159 who achieved CR, 91 have relapsed and another 24 died without report of relapse. The estimated median RFS is 15 months (95% CI, 12-23 months), and the estimated probability of surviving 5 years without report of relapse is 29% (95% CI, 22%-36%).
Seven of the 159 patients who achieved CR did not receive protocol consolidation therapy because of early relapse (N = 2), refusal (N = 2), or other reasons (N = 3). Forty-five of the remaining 152 were not registered for protocol postconsolidation therapy, most often resulting from relapse or death (N = 11), persistent anemia or other medical reasons (N = 9), refusal (N = 4), preference for other treatment (N = 3), or prior toxicities (N = 2). The remaining 107 patients were registered for postconsolidation therapy. Four of these were found to be ineligible for that registration: one was not in CR, one was registered after starting postconsolidation therapy, and 2 had not completed prior courses of protocol therapy. Thus, 103 patients were registered and fully eligible for postconsolidation therapy on protocol: 84 for maintenance chemotherapy and 19 for allogeneic BMT.
One of the 84 maintenance patients did not receive protocol maintenance therapy and is excluded from analyses of DFS. Seventy (84%) of the remaining 83 maintenance patients received all possible protocol maintenance therapy: 32 completed all 4 courses, and the rest either relapsed (N = 35) or died (N = 3) before completing all 4 courses. Two of the remaining 13 were removed from maintenance for refusal, 3 for toxicity, and 8 others for other or unspecified reasons.
Of the 83 maintenance chemotherapy patients, 47 have relapsed and another 4 have died without report of relapse, with an estimated median DFS of 29 months (95% CI, 15-54 months). The estimated probability of DFS 5 years after registration for maintenance chemotherapy is 39% (95% CI, 28%-50%).
All 19 patients registered for allogeneic BMT received that treatment and were evaluable. Four of the 19 have relapsed and another 9 have died without report of relapse, for an estimated median DFS of 11 months (95% CI, 3-64 months). The estimated probability of DFS 5 years after registration for BMT is 37% (95% CI, 15%-59%).
Further analyses of complete remission
In univariate logistic regression analyses of CR, only age was a clearly significant prognostic factor (P = .001 treating age as a continuous variable; Table 3). The CR rate decreased from 87% for patients of age 16 to 29 years to 63% for those of age 50 to 65 years. There was a corresponding increasing risk of resistant disease with increasing age (P = .004) from 3% to 19%. The CR rate tended to decrease with increasing peripheral blast percentage (P = .033) or absolute count (P = .025) but did not decrease significantly with increasing WBC (P = .17), although the 14 patients with WBC more than 100 000/mm3 had a CR rate of only 57%. The CR rates of patients with (79%) or without (82%) evaluable cytogenetics were almost identical (P = .62).
Treatment outcomes of 200 adult non-L3 ALL patients by age
| Age, y . | No. . | Complete remission . | Resistant disease . | Overall survival at 5 y* . | Relapse-free survival at 5 y† . | Disease-free survival at 5 y‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | 95% CI, % . | % . | 95% CI, % . | Estimate . | 95% CI, % . | Estimate . | 95% CI, % . | Estimate . | 95% CI, % . | ||
| 15 to 29 | 86 | 87 | 78-93 | 3 | 1-10 | 39 | 27-49 | 32 | 21-43 | 48 | 31-65 |
| 30 to 49 | 71 | 80 | 69-89 | 14 | 7-24 | 32 | 21-43 | 29 | 17-41 | 40 | 23-58 |
| 50 to 65 | 43 | 63 | 47-77 | 19 | 8-33 | 23 | 11-36 | 22 | 7-38 | 25 | 4-46 |
| Age, y . | No. . | Complete remission . | Resistant disease . | Overall survival at 5 y* . | Relapse-free survival at 5 y† . | Disease-free survival at 5 y‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | 95% CI, % . | % . | 95% CI, % . | Estimate . | 95% CI, % . | Estimate . | 95% CI, % . | Estimate . | 95% CI, % . | ||
| 15 to 29 | 86 | 87 | 78-93 | 3 | 1-10 | 39 | 27-49 | 32 | 21-43 | 48 | 31-65 |
| 30 to 49 | 71 | 80 | 69-89 | 14 | 7-24 | 32 | 21-43 | 29 | 17-41 | 40 | 23-58 |
| 50 to 65 | 43 | 63 | 47-77 | 19 | 8-33 | 23 | 11-36 | 22 | 7-38 | 25 | 4-46 |
Five years after study entry.
Five years after complete remission.
Five years after start of maintenance chemotherapy (maintenance patients only).
Among the 140 patients with evaluable cytogenetics, the CR rate did not vary significantly according to karyotype category (P = .21), although the 36 patients with t(9;22)/BCR/ABL1 had a somewhat lower CR rate (67%) than the remaining patients with evaluable karyotypes (86 of 104, 83%, P = .051; Table 4). There was a corresponding higher risk of resistant disease in patients with t(9;22)/BCR/ABL1, 19%, compared with the remaining patients with evaluable karyotypes (8 of 104, 8%, P = .053). The lower CR rate of patients with t(9;22)/BCR/ABL1 was largely explained by their older age: after adjusting for the effect of age in multivariate logistic regression (P = .053), the effect of t(9;22)/BCR/ABL1 on CR rate was not statistically significant (P = .36).
Treatment outcomes of 200 adult non-L3 ALL patients, by karyotype and ploidy categories
| Category . | No. of patients . | Complete remission . | Resistant disease . | Overall survival at 5 y* . | Relapse-free survival at 5 y† . | Disease-free survival at 5 y‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | 95% CI, % . | % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | ||
| Normal diploid | 31 | 87 | 70-96 | 10 | 2-26 | 50 | 32-68 | 43 | 24-62 | 64 | 39-89 |
| t(9;22)/BCR/ABL1 | 36 | 67 | 49-81 | 19 | 8-36 | 8 | 2-22 | 0 | 0-14 | 0 | 0-34 |
| Other unfavorable§ | 19 | 79 | 54-94 | 16 | 3-40 | 26 | 9-51 | 33 | 9-57 | 50 | 15-85 |
| Miscellaneous | 54 | 81 | 69-91 | 4 | 0-13 | 52 | 38-65 | 41 | 26-56 | 45 | 23-66 |
| P‖¶ | .21 | .091 | <.001 | <.001 | .006 | ||||||
| Not evaluable | 60 | 82 | 70-90 | 10 | 4-21 | 26 | 15-37 | 24 | 12-36 | 30 | 13-48 |
| Normal diploid | 31 | 87 | 70-96 | 10 | 2-26 | 50 | 32-68 | 43 | 24-62 | 64 | 39-89 |
| Hypodiploid | 11 | 73 | 39-94 | 27 | 6-61 | 24 | 0-51 | 38 | 4-71 | —** | — |
| Pseudodiploid | 45 | 78 | 63-89 | 4 | 1-15 | 42 | 27-56 | 39 | 23-55 | 58 | 34-82 |
| Hyperdiploid | 38 | 71 | 54-85 | 13 | 4-28 | 33 | 17-48 | 26 | 9-42 | 24 | 3-44 |
| P‖# | .42 | .18 | .38 | .82 | .10 | ||||||
| Other | 6 | 83 | 36-100 | 17 | 0-64 | 17 | 0-64 | 0 | 0-52 | —** | — |
| Not evaluable | 69 | 83 | 72-91 | 10 | 4-20 | 24 | 14-34 | 20 | 10-31 | 26 | 11-42 |
| Category . | No. of patients . | Complete remission . | Resistant disease . | Overall survival at 5 y* . | Relapse-free survival at 5 y† . | Disease-free survival at 5 y‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | 95% CI, % . | % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | ||
| Normal diploid | 31 | 87 | 70-96 | 10 | 2-26 | 50 | 32-68 | 43 | 24-62 | 64 | 39-89 |
| t(9;22)/BCR/ABL1 | 36 | 67 | 49-81 | 19 | 8-36 | 8 | 2-22 | 0 | 0-14 | 0 | 0-34 |
| Other unfavorable§ | 19 | 79 | 54-94 | 16 | 3-40 | 26 | 9-51 | 33 | 9-57 | 50 | 15-85 |
| Miscellaneous | 54 | 81 | 69-91 | 4 | 0-13 | 52 | 38-65 | 41 | 26-56 | 45 | 23-66 |
| P‖¶ | .21 | .091 | <.001 | <.001 | .006 | ||||||
| Not evaluable | 60 | 82 | 70-90 | 10 | 4-21 | 26 | 15-37 | 24 | 12-36 | 30 | 13-48 |
| Normal diploid | 31 | 87 | 70-96 | 10 | 2-26 | 50 | 32-68 | 43 | 24-62 | 64 | 39-89 |
| Hypodiploid | 11 | 73 | 39-94 | 27 | 6-61 | 24 | 0-51 | 38 | 4-71 | —** | — |
| Pseudodiploid | 45 | 78 | 63-89 | 4 | 1-15 | 42 | 27-56 | 39 | 23-55 | 58 | 34-82 |
| Hyperdiploid | 38 | 71 | 54-85 | 13 | 4-28 | 33 | 17-48 | 26 | 9-42 | 24 | 3-44 |
| P‖# | .42 | .18 | .38 | .82 | .10 | ||||||
| Other | 6 | 83 | 36-100 | 17 | 0-64 | 17 | 0-64 | 0 | 0-52 | —** | — |
| Not evaluable | 69 | 83 | 72-91 | 10 | 4-20 | 24 | 14-34 | 20 | 10-31 | 26 | 11-42 |
Est. indicates estimated.
Five years after study entry.
Five years after complete remission.
Five years after start of maintenance chemotherapy (maintenance patients only).
Other unfavorable is defined by the presence of −7, +8, and 11q23/MLL gene rearrangements.
P values based on logistic (complete remission, resistant disease) or proportional hazards (overall survival, relapse-free survival, disease-free survival) regression analysis.
P value for heterogeneity of outcomes among four categories with evaluable karyotype.
P value for heterogeneity of outcomes among four ploidy categories (normal, hypodiploid, pseudodiploid, and hyperdiploid).
DFS was not estimated for hypodiploid (N = 2) and other (N = 0) ploidy categories.
Further analyses of overall survival
Univariate PH regression analyses identified age as a statistically significant prognostic factor for overall survival (OS, P = .008 treating age as a continuous variable, Table 3). In addition, OS decreased significantly with increasing absolute peripheral blast count (P = .002) or peripheral blast percentage (P = .045), although not with WBC (P = .091). Patients with performance status 2 or 3 had somewhat poorer OS compared with those with PS 0 or 1 (hazard ratio [HR] = 1.60; 95% CI, 0.93-2.73, P = .11). OS was somewhat higher for the 140 patients with evaluable cytogenetics, although this difference was not statistically significant (HR = 0.78; 95% CI, 0.55-1.11, P = .18) and could not be attributed to confounding effects of age, blast count or percentage, or PS (results not shown).
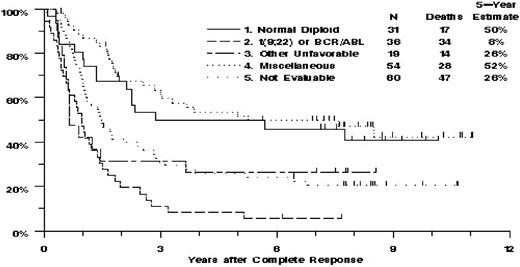
Among the 140 patients with evaluable cytogenetics, there was highly significant heterogeneity of OS among the 4 karyotype categories (P < .001, Table 4; Figure 1). This was largely because of the poor OS of the patients with t(9;22)/BCR/ABL1 or with other unfavorable abnormalities, for whom the mortality hazard ratios, compared with the normal diploid group, were 3.36 (95% CI, 1.86-6.09) and 2.03 (95% CI, 1.00-4.13), respectively. OS of the miscellaneous group was similar to that of the normal diploid group, with hazard ratio 0.83 (95% CI, 0.46-1.52).
Estimated overall survival of 200 adult patients with non-L3 ALL, by karyotype category.
Estimated overall survival of 200 adult patients with non-L3 ALL, by karyotype category.
In multivariate PH regression analysis, the heterogeneity of OS among the 4 karyotype categories remained statistically significant (P < .001), whereas age (P = .65), WBC (P = .34), absolute blast count (P = .18), and peripheral blast percentage (P = .27) did not. Performance status 2 or 3 did retain a large detrimental effect on OS (HR = 1.99; 95% CI, 0.90-4.41, P = .089). Additional analyses of OS were performed for the 104 cytogenetically evaluated patients without t(9;22)/BCR/ABL1. After accounting for heterogeneity of OS among the normal diploid, other unfavorable, and miscellaneous categories (P = .041), OS was not significantly related to age (P = .55), WBC count (P = .15), peripheral blast percentage (P = .31), or PS 2 or 3 (P = .68) but did tend to decrease with increasing absolute blast count (P = .020).
Further analyses of relapse-free survival
Univariate PH regression analyses identified peripheral blast percentage (P = .039) and peripheral blast count (P = .036) as prognostic factors for RFS. Age (P = .32), WBC (P = .56), and performance status (P = .57) were not significantly associated with RFS. RFS was somewhat higher for patients with evaluable cytogenetics, although the difference was not statistically significant (HR = 0.86; 95% CI, 0.58-1.27, P = .45).
Among the 110 patients with evaluable banded studies or FISH who achieved CR, there was highly significant heterogeneity of RFS among the 4 karyotype categories (P < .001; Table 4). This was primarily because of the poor RFS of the patients with t(9;22)/BCR/ABL1, for whom the HR, compared with the normal diploid group, was 3.24 (95% CI, 1.70-6.15). All 24 of these patients relapsed (N = 21) or died (N = 3) within 3 years after achieving CR. RFS of the 15 patients with other unfavorable abnormalities was not significantly different from that of the normal diploid patients (HR = 1.22; 95% CI, 0.56-2.64, P = .62). RFS of the miscellaneous group was also similar to that of the normal diploid group, with HR = 0.72 (95% CI, 0.39-1.32).
In multivariate PH regression analyses that adjusted for the heterogeneity of RFS among the 4 karyotype categories, no other factors were significantly prognostic for RFS, including age (P = .57), performance status (P = .74), WBC (P = .80), peripheral blast percentage (P = .45), and peripheral blast count (P = .34).
Further analyses of disease-free survival with maintenance chemotherapy
Univariate PH regression analyses identified several marginally significant prognostic factors for improved DFS among the 83 maintenance chemotherapy patients: younger age (P = .074), female sex (P = .039), lower peripheral blast percentage (P = .031), higher absolute neutrophil count (P = .034), and higher hemoglobin (P = .041). Performance status (P = .55), WBC count (P = .43), and absolute peripheral blast count (P = .11) were not significant prognostic factors for DFS. DFS was somewhat higher for patients with evaluable cytogenetics, although the difference was not statistically significant (HR = 0.77; 95% CI, 0.44-1.36, P = .38).
Among the 55 of the 83 maintenance patients who had evaluable banded or FISH studies, DFS varied significantly among the 4 karyotype categories (P = .001; Table 4). This was primarily because of the poor DFS of the 9 patients with t(9;22)/BCR/ABL1, for whom the HR, compared with the normal diploid group, was 5.84 (95% CI, 2.02-16.9). All 9 of these patients relapsed (N = 8) or died (N = 1) within 21 months after starting maintenance therapy. DFS of the other unfavorable (P = .70) and miscellaneous (P = .52) groups was not significantly different from that of the normal diploid group; however, because of the small numbers of patients, this cannot be viewed as definitive evidence of equivalent outcomes.
In multivariate PH regression analyses adjusted for the heterogeneity of DFS among karyotype categories, no other factors were significantly prognostic for DFS, including age (P = .89), gender (P = .84), peripheral blast percentage (P = .25), absolute neutrophil count (P = .17), hemoglobin (P = .26), and platelet count (P = .41).
Further analyses of cytogenetic subgroups based on proposed MRC UKALLXII/ECOG E2993 adult ALL classification
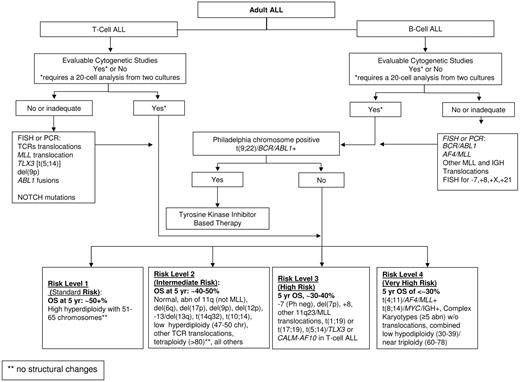
An attempt was made to reclassify the 140 patients with evaluable cytogenetics using the karyotype categories recently proposed for adult ALL based on the results of studies MRC UKALLXII/ECOG E2993 (Figure 3). Given the small number of patients in each of the approximately 20 proposed ALL subgroups, it was necessary to combine subgroups into a smaller number of risk categories. This was accomplished by combining groups with similar OS at 5 years as reported by Moorman et al.8 Patients with t(9;22) or BCR/ABL1 (N = 36) were identified as a distinct risk group because of their very poor prognosis and because they are now treated with tyrosine kinase inhibitors. Four risk groups were defined for the remaining patients. The very high risk group included t(4;11) (N = 6), complex defined as more than or equal to 5 abnormalities without known translocations (N = 12), or low hypodiploidy (N = 1). The high risk group included other MLL translocations (N = 2), monosomy 7 with less than 5 abnormalities (N = 1), t(1;19) (N = 7), or del(7p) (N = 2). Intermediate risk included normal diploid (N = 31), low hyperdiploidy (N = 6), del(9p) (N = 3), or any karyotypic changes not identified with a different risk group (N = 32). Standard risk was defined by high hyperdiploidy (N = 1). The single patient in the standard risk group who had resistant disease and died 107 days after entering the study was combined with the intermediate risk group for further analyses. Despite the limited numbers of patients in the present study, there was significant heterogeneity of treatment outcomes among the standard/intermediate risk, high risk, very high risk, and t(9;22)/BCR/ABL1 patients (P = .036 for CR, P < .001 for OS and RFS, P = .009 for DFS; Table 5,Figure 2). For each endpoint, however, this heterogeneity was largely the result of poor outcomes in very high risk and t(9;22)/BCR/ABL1 groups, which compared with all other patients combined, had significantly lower CR rate (67% vs 86%, P = .011), the highest percentage of patients with resistant disease (18% vs 6%, P = .018), and the poorest OS (13% vs 52% at 5 years, HR = 2.99; 95% CI, 1.97-4.53, P < .001) and RFS (11% vs 42% at 5 years, HR = 2.72; 95% CI, 1. 71-4.33, P < .001). It was noted that the patients with t(9;22)/BCR/ABL1 had poorer OS (P = .054; 8% vs 22% at 5 years) and RFS (P = .014; 0% vs 31% at 5 years) than the patients in the very high risk group. With respect to ploidy analysis in adult ALL, this study had too few patients (10 total) in the 3 biologically and clinically relevant ALL ploidy subgroups of low hypodiploidy (30-39 chromosomes)/triploidy (60-78 chromosomes, 2 patients), high hyperploidy (51-65 chromosomes, 8 patients), and or tetraploidy (no patients), precluding an informative analysis of the ploidy data.
Estimated overall survival by MRC UKALLXII/ECOG E2993 karyotypic categories.
Proposed cytogenetic and molecular genetic prognostic risk grouping for adult acute lymphoblastic leukemia.
Proposed cytogenetic and molecular genetic prognostic risk grouping for adult acute lymphoblastic leukemia.
Treatment outcomes of 200 adult non-L3 ALL patients, by modified karyotypic risk categories
| Risk category . | No. of patients . | Complete remission . | Resistant disease . | Overall survival at 5 y* . | Relapse-free survival at 5 y† . | Disease-free survival at 5 y‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | 95% CI, % . | % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | ||
| Standard | 1 | 0 | — | 100 | — | — | — | — | — | — | — |
| Intermediate | 72 | 85 | 73-92 | 6 | 2-14 | 52 | 40-64 | 40 | 27-53 | 53 | 34-71 |
| High | 12 | 100 | 74-100 | 0 | 0-26 | 47 | 17-76 | 47 | 17-76 | 47 | 10-83 |
| Very high | 19 | 68 | 43-87 | 16 | 3-40 | 22 | 3-42 | 22 | 9-61 | 43 | 10-82 |
| t(9;22)/BCR/ABL1 | 36 | 67 | 49-81 | 19 | 8-36 | 8 | 2-22 | 0 | 0-14 | 0 | 0-34 |
| P§ | .036 | .11 | <.001 | <.001 | .009 | ||||||
| Not evaluable | 60 | 82 | 70-90 | 10 | 4-21 | 26 | 15-37 | 24 | 12-36 | 30 | 13-48 |
| Risk category . | No. of patients . | Complete remission . | Resistant disease . | Overall survival at 5 y* . | Relapse-free survival at 5 y† . | Disease-free survival at 5 y‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | 95% CI, % . | % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | Est. % . | 95% CI, % . | ||
| Standard | 1 | 0 | — | 100 | — | — | — | — | — | — | — |
| Intermediate | 72 | 85 | 73-92 | 6 | 2-14 | 52 | 40-64 | 40 | 27-53 | 53 | 34-71 |
| High | 12 | 100 | 74-100 | 0 | 0-26 | 47 | 17-76 | 47 | 17-76 | 47 | 10-83 |
| Very high | 19 | 68 | 43-87 | 16 | 3-40 | 22 | 3-42 | 22 | 9-61 | 43 | 10-82 |
| t(9;22)/BCR/ABL1 | 36 | 67 | 49-81 | 19 | 8-36 | 8 | 2-22 | 0 | 0-14 | 0 | 0-34 |
| P§ | .036 | .11 | <.001 | <.001 | .009 | ||||||
| Not evaluable | 60 | 82 | 70-90 | 10 | 4-21 | 26 | 15-37 | 24 | 12-36 | 30 | 13-48 |
Est. indicates estimated; and —, the single patient in the standard risk category had resistant disease and died on day 107.
Five years after study entry.
Five years after complete remission.
Five years after start of maintenance chemotherapy (maintenance patients only).
P values for heterogeneity of outcomes among four categories (standard/intermediate, high, very high risk, and t(9;22)/BCR/ABL1) based on Pearson χ 2 test (complete remission, resistant disease) or proportional hazards (overall survival, relapse-free survival, disease-free survival) regression analysis.
Discussion
Our results demonstrate that cytogenetics is the most important prognostic factor in adult ALL. The 4 karyotype categories used in this study (t(9;22)/BCR/ABL1, other unfavorable (UNF), miscellaneous, and normal diploid) were able to separate patients into distinct subsets with significant differences in outcome. The normal diploid group had the best OS and RFS followed by miscellaneous, UNF, and t(9;22)/BCR/ABL1 groups in descending order. Age, gender, and WBC count were not independently predictive of OS or RFS in multivariate analysis. Although only 70% of the patients in this study had evaluable cytogenetics, their outcomes did not differ significantly from those of patients without evaluable cytogenetics, suggesting that the results reported here are not likely to be highly unrepresentative of the entire cohort.
The importance of cytogenetics, as the single most important prognostic factor in adult ALL, has been reported previously by the CALGB and more recently by the GIMEMA and MRC UKALLXII/ECOG study groups.7,8,,,–12 The CALGB examined the impact of cytogenetics on the prognosis of 256 adult patients with non-FAB-L3 ALL treated from 1984 to 1994 on one of 6 protocols in whom adequate cytogenetic studies were available.7 The frequency of clonal aberrations observed in the CALGB study (69%) is similar to SWOG-9400 (70%) and within the range reported by others (66%-85%).8,10,–12 Likewise, the incidence of the miscellaneous subgroup was similar in the CALGB and SWOG studies (27% vs 28%). In the CALGB study, karyotype remained an independent prognostic factor even when more intensified chemotherapy regimens were introduced in 1988. However, unlike our study where both OS and DFS were markedly inferior in the UNF category compared with normal and miscellaneous groups, in the CALGB study, karyotype retained prognostic significance for DFS but not OS when multivariate analysis was performed. The differences in categorization (eg, the t(9;22) subgroup was included in the unfavorable CALGB group but classified as a separate entity in the SWOG analysis), age differences (32 years [SWOG] vs 46 years [CALGB]), or the difference in use of allogeneic HCT between these 2 studies may explain this discrepancy.
The GIMEMA categorized the cytogenetic data of 325 patients treated homogenously on their protocol 0496 into 6 groups.12 These groups were: normal, t(9;22)/BCR/ABL1, t(4;11)/MLL/AF4, t(1;19)TCF3/PBX1, 9p/CDKN2A-CDKN2B deletions, 6q deletions, miscellaneous, and hyperdiploid. Among these, patients with normal karyotype and those with isolated 9p/CDKN2A-CDKN2B deletions had a relatively favorable (standard) prognosis, whereas those with 6q deletions, miscellaneous, and hyperdiploid karyotype had an intermediate prognosis, and patients with t(9;22)/BCR/ABL1, t(4;11)/MLL/AF4, t(1;19)/TCF3/PBX1 constituted the unfavorable prognosis group. Based on these results, these authors were able to classify patients as standard, intermediate, and high risk groups with significantly different DFS at 2 years. However, at 5 years their standard and intermediate groups had similar outcomes.
In the largest adult ALL cytogenetic analysis reported to date, Moorman et al analyzed cytogenetic data for the 1522 patients enrolled on the MRC UKALLXII/ECOG E2993 trial.8 In this trial, cytogenetic analysis was attempted in 1366 (90%) patients; however, only 73% (1003 cases) were considered evaluable by central review with 796 patients showing clonal aberrations. Because of the relatively large number of samples available, the clinical outcome of approximately 20 specific cytogenetic subgroups were presented. These authors found that patients with t(9;22), t(4;11), t(8;14), complex karyotype (defined as ≥ 5 abnormalities) or low hypodiploidy/near triploidy had a poor outcome in comparison to more favorable cytogenetic subgroups of high hyperdiploidy or del(9p). In multivariate analysis, only t(8;14), complex karyotypes, and low hypodiploidy/near triploidy retained significance as prognostic factors independent of age, sex, and WBC count among t(9;22)-negative patients. For comparison, the SWOG cytogenetic data were reclassified using the MRC UKALLXII/ECOG E2993. Despite the small number of cases in each subgroup, the SWOG-9400 data provide an incomplete validation of the proposed adult ALL prognostic subgroups. It is obvious that the patients at very high risk due to abnormalities other than t(9;22)/BCR/ABL1, who had only 22% 5-year OS, compared with approximately 50% for the standard/intermediate- and high-risk groups, urgently require novel therapeutic strategies.
Based on the results of this SWOG study and the larger trials mentioned in the previous 3 paragraphs, age and WBC count do not appear to be independent prognostic factors. In our trial, patients with UNF cytogenetics, and with t(9;22)/BCR/ABL1 in particular, tended to be older and have higher WBC counts (Table 2). Studies have shown progressively worse outcome for ALL with each advancing decade after infancy.13,14 Some differences have been described between blasts of childhood and adult ALL with respect to sensitivity to steroids and methotrexate metabolism.15,16 Even among patients with the same cytogenetic abnormality, for example, the t(9;22)/BCR/ABL1, there is a suggestion that older patients have worse outcomes.17 Also, older patients are more likely to have treatment-related complications with intense chemotherapy or BMT. In our study, when the effect of cytogenetics on OS was accounted for, the effect of age was not significant (P = .81). This suggests that the worsening prognosis with advancing age in adult ALL is a manifestation of the age-related increase in unfavorable cytogenetics, at least until age 65, which was the upper limit of enrollment in our trial. In contrast, poor performance status, which may reflect the patient's general health and ability to withstand the effects of both treatment and ALL, retained an independent association with poor OS after accounting for the effect of cytogenetics (P = .058).
In addition to t(9;22)/BCR/ABL1-positive adult ALL, the presence of −7,+8, and t(4;11) also showed inferior OS and RFS compared with the normal and miscellaneous subgroups. Our data agree with the unfavorable prognostic categorization of −7 and +8 in adult ALL as originally proposed by CALGB.7 This finding, however, was not confirmed in the larger MRC UKALLXII/ECOG 2993 trial discussed, which reported that patients with −7 and +8 fare as well as the other t(9;22) or Ph chromosome–negative ALL patients.8 Differences in sample size and treatment protocol between the studies may account for this discrepancy. Regardless, further study of these specific numerical aberrations, with and without structural aberrations, remains an important objective in adult ALL.
Because of the rarity of adult ALL, the genetic risk for current therapeutic protocols has been simply categorized into intermediate (normal and miscellaneous karyotypes) and unfavorable (t(9;22)/BCR/ABL1 and t(4;11)). The prognostic relevance of nonrandom cytogenetic aberrations usually referred to as miscellaneous clonal karyotypic aberrations in adult ALL remains to be further refined. In the large UKALLXII/ ECOG 2993 trial, 16 specific cytogenetic abnormalities failed to show any significance with disease outcome, justifying the grouping of many of these nonrandom aberrations in the miscellaneous risk stratification group. In the current study, too few patients had any specific miscellaneous abnormality for definitive analysis. It must be noted that “cryptic” cytogenetic changes may be present in T-cell and B-cell ALL with normal karyotype.18,,–21 In the case of T-cell ALL, where approximately 50% of cases show normal karyotypes, microdeletions or “cryptic” cytogenetic aberrations have been detected by FISH or other molecular methods (polymerase chain reaction, arrays, mutation analyses) in almost every case.18 The detection and characterization of these genetic aberrations are beginning to increase our understanding of the leukomogenesis and emphasizes the need for standardizing the methods for detecting cytogenetic and molecular genetic aberrations in future clinical trials for ALL.
Numerical chromosomal abnormalities alone are less common in adult ALL, possibly reflecting a fundamental difference in the pathogenesis between childhood and adult ALL.1 Ploidy is a well-established prognostic factor in childhood ALL.22 Children with hyperdiploidy (defined as > 50 chromosomes) have an especially good prognosis, a finding attributed to the increased accumulation of methotrexate polyglutamates in these leukemic blasts23 as well as the increased tendency of these leukemic blasts to undergo apoptosis.24 A similar favorable effect of hyperdiploidy (51-65 chromosomes) was seen in some studies of adult patients10, as was an unfavorable effect in hypodiploidy10,11 or low hypodiploidy/near-triploidy in adult ALL.8,25 The number of adult patients who have been reported in these previously defined biologic and clinically relevant ploidy subgroups is very limited. In the SWOG-9400 cohort, only 10 patients fell within these ploidy subgroups, precluding a robust analysis of these ploidy subgroups with outcome. This apparent ploidy frequency discrepancy between adults and pediatric patients clearly reflects a key biologic difference that requires further characterization, especially in young adult and adolescent patients with ALL.
A standardized prognostic cytogenetic/molecular classification system is needed to compare future ALL clinical trials. Based on the estimated overall survival data published in 5 adult ALL clinical trials (CALGB, GIMEMA, ECOG/MRC, Group Francais/LALA studies and the current study)7,8,10,12 with more weight given to the recent and largest adult ALL trial with 5 years of follow data (ECOG/MRC),8 at least 5 practical cytogenetic risk subgroups are recurring and have led us to propose a standardized, working cytogenetic/molecular classification scheme as shown in Figure 3. As proposed, the classification system is expected to evolve as the clinical significance of rare cytogenetic abnormalities and other molecular changes become evident.
In conclusion, cytogenetics is the single most important factor that predicts risk of treatment failure in adult ALL, and a more detailed risk stratification based on cytogenetic and molecular abnormalities similar to that described for acute myeloid leukemia26 and most recently in pediatric ALL27 is becoming feasible. The cytogenetic stratification used in our study is practical and its prognostic significance has been validated with more than 5 years of follow-up in this study and the CALGB and MRC/ECOG trials.7,8 Because the outcome of adult ALL remains poor, new therapies that target specific cytogenetic or molecular abnormalities, in combination with conventional or high dose chemotherapy appear to be the logic next step. This approach is already being tested in clinical trials of imatinib and other tyrosine kinase inhibitors for Ph+ ALL and Notch pathway inhibitors for T- ALL. Particular cytogenetic subsets of ALL have been shown to have complex yet characteristic gene expression profiles.28,–30 Knowledge of genetic abnormalities obtained by cytogenetics in conjunction with microarray analysis would be fundamental to guide targeted therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Holly Gundacker and Amy Edwards for their expertise in cytogenetic data collection and validation.
This work was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA 32102, CA-38926, CA-33572, CA-30206, CA-46368, CA-35431, CA-46441, CA-46282, CA-20319, CA-42777, CA-52654, CA-14028, CA-45450, CA-63844, CA-04919, CA-45377, CA-67575, CA-35090, CA-35176, CA-63845, CA-35119, CA-35192, CA-74647, CA-58882, CA-35178, and CA-35261.
National Institutes of Health
Authorship
Contribution: V.P. and M.L.S. analyzed the data and wrote the manuscript; K.J.K. performed statistical analysis; S.J.F. and F.R.A. conceived and designed the clinical trial and provided intellectual input; and all authors reviewed and revised the final manuscript.
A full list of Southwest Oncology Group institutions that participated in trial SWOG-9400 appears as Table S5.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vinod Pullarkat, Division of Hematology and Hematopoietic Cell Transplantation, City of Hope Medical Center, 1500 East Duarte Road, Duarte, CA 91010; e-mail: vpullarkat@coh.org.
References
Author notes
V.P. and M.L.S. contributed equally to this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal