Hepcidin, a master regulator of iron homeostasis, is produced in small amounts by inflammatory monocytes/macrophages. Chronic immune activation leads to iron retention within monocytes/macrophages and the development of anemia of chronic disease (ACD). We questioned whether monocyte-derived hepcidin exerts autocrine regulation toward cellular iron metabolism. Monocyte hepcidin mRNA expression was significantly induced within 3 hours after stimulation with LPS or IL-6, and hepcidin mRNA expression was significantly higher in monocytes of ACD patients than in controls. In ACD patients, monocyte hepcidin mRNA levels were significantly correlated to serum IL-6 concentrations, and increased monocyte hepcidin mRNA levels were associated with decreased expression of the iron exporter ferroportin and iron retention in these cells. Transient transfection experiments using a ferroportin/EmGFP fusion protein construct demonstrated that LPS inducible hepcidin expression in THP-1 monocytes resulted in internalization and degradation of ferroportin. Transfection of monocytes with siRNA directed against hepcidin almost fully reversed this lipopolysaccharide-mediated effect. Using ferroportin mutation constructs, we found that ferroportin is mainly targeted by hepcidin when expressed on the cell surface. Our results suggest that ferroportin expression in inflammatory monocytes is negatively affected by autocrine formation of hepcidin, thus contributing to iron sequestration within monocytes as found in ACD.
Introduction
A dysregulated iron homeostasis is a cornerstone of acute and chronic inflammatory processes involving cell-mediated immunity and frequently leads to the development of anemia, termed as anemia of chronic disease (ACD), or anemia of inflammation.1,2 ACD is a multifactorial disease, where immune mechanisms play key pathogenetic roles. These include cytokine-mediated suppression of erythropoiesis,3,4 a blunted erythropoietin response,5,–7 and an increased iron accumulation in and a defective iron recycling from the reticuloendothelial system.8,,,,–13 The liver-derived acute phase protein hepcidin, which is induced by cytokines and iron, plays a key role in this concert.14,15 It causes anemia when overexpressed,16,17 whereas hepcidin mutations lead to hepatic iron overload,18,19 which can be referred to its regulatory effects on cellular iron efflux. This is exerted after binding of hepcidin to the only known cellular iron exporter ferroportin,20,–22 leading to ferroportin internalization and blockade of duodenal iron absorption and macrophage iron recycling.23 Because hepcidin expression is induced by inflammatory stimuli, including interleukin-6 (IL-6) or lipopolysaccharide (LPS),24,,,,–29 an increased expression of this acute phase protein has been found to be associated with macrophage iron retention in ACD patients.30,31 In addition, hepcidin-independent inhibition of ferroportin mRNA expression by inflammatory cytokines also contributes to macrophage iron retention under inflammatory conditions.32,33
Interestingly, tissues other than the liver can also synthesize hepcidin, including the kidney, the right heart atrium, and the spinal cord.14,34 Moreover, significant hepcidin expression has been found in spleen, alveolar, and bone marrow-derived murine macrophages.14,35,–37 Although the basal expression is relatively low in comparison to liver hepcidin expression, LPS, group A Streptococcus strains and Pseudomonas aeruginosa can induce a 20- to 80-fold increase of hepcidin expression in these cells by a TLR-4–dependent pathway.35,36
Based on these observations and being aware of a strong correlation between pro-hepcidin serum levels, macrophage ferroportin expression, and iron sequestration within the reticuloendothelial system,30 we now investigated whether monocyte-derived hepcidin may effectively modulate ferroportin expression and thus monocyte iron homeostasis under inflammatory conditions in an autocrine fashion.
Methods
The study was approved by the ethics board of the Medical University, Innsbruck, Austria.
Patients
A total of 25 patients were included in the study. Informed consent for obtaining additional blood samples for scientific purposes during routine blood drawing was obtained before the procedure from each subject in accordance with the Declaration of Helsinki. Patients were considered to suffer from ACD when (1) they had a chronic infection or autoimmune disease, (2) when they were anemic with a hemoglobin of less than 13 g/dL for men and less than 12 g/dL for women, and (3) when they had low transferrin saturation (TfS < 16%) but normal or increased serum ferritin concentrations (> 100 ng/mL).1 Among the 12 patients with ACD, 5 had recurrent pneumonia, 3 had rheumatoid arthritis, 1 had large cell vasculitis, and 3 had chronic osteomyelitis. Although some patients received antibiotics at enrollment, none of the patients with newly diagnosed autoimmune disorders had been treated with immunosuppressive drugs before study enrollment. We also studied a group of age-matched controls (n = 13) with no signs of anemia, normal serum iron status, and no signs of inflammation (normal serum concentrations of C-reactive protein < 0.7 mg/dL). We did not include patients with malignancies because radio- and/or chemotherapeutic regimens as well as bone marrow infiltration by the tumor alter the pathophysiology of the anemia compared with subjects with ACD on the basis of an autoimmune or infectious disease.1,38
None of our patients was under treatment with iron, recombinant human erythropoietin, and/or received blood transfusion before study entry.
The baseline characteristics of the different patient groups are shown in Table 1.
Patients' baseline characteristics
| . | Control . | ACD . |
|---|---|---|
| N | 13 | 12 |
| Age (yr) | 55.0 ± 17.0 | 60.0 ± 23.0 |
| Sex, female/male | 5/8 | 5/7 |
| Hb, g/dL | 14.9 ± 1.1 | 11.4 ± 1.5* |
| MCH, pg | 31.2 ± 1.9 | 29.3 ± 2.1† |
| MCV, fl | 92.6 ± 4.0 | 88.5 ± 6.1 |
| CRP, mg/dL | 0.36 ± 0.4 | 12.0 ± 7.2* |
| Fe, μmol/L | 21.6 ± 9.9 | 5.1 ± 1.5* |
| Ferritin, μg/L | 91.8 ± 93.3 | 446.5 ± 388.5* |
| Transferrin, mg/dL | 292.8 ± 38.5 | 173.5 ± 50.38.5* |
| TfS, % | 28.9 ± 11.3 | 12.1 ± 3.4* |
| Folic acid, μg/L | 10.4 ± 3.7 | 7.5 ± 5.7 |
| Cobalamine, ng/L | 495.0 ± 204.0 | 588.0 ± 567.0 |
| IL-6, pg/mL | 1.3 ± 0.8 | 41.8 ± 32.5* |
| . | Control . | ACD . |
|---|---|---|
| N | 13 | 12 |
| Age (yr) | 55.0 ± 17.0 | 60.0 ± 23.0 |
| Sex, female/male | 5/8 | 5/7 |
| Hb, g/dL | 14.9 ± 1.1 | 11.4 ± 1.5* |
| MCH, pg | 31.2 ± 1.9 | 29.3 ± 2.1† |
| MCV, fl | 92.6 ± 4.0 | 88.5 ± 6.1 |
| CRP, mg/dL | 0.36 ± 0.4 | 12.0 ± 7.2* |
| Fe, μmol/L | 21.6 ± 9.9 | 5.1 ± 1.5* |
| Ferritin, μg/L | 91.8 ± 93.3 | 446.5 ± 388.5* |
| Transferrin, mg/dL | 292.8 ± 38.5 | 173.5 ± 50.38.5* |
| TfS, % | 28.9 ± 11.3 | 12.1 ± 3.4* |
| Folic acid, μg/L | 10.4 ± 3.7 | 7.5 ± 5.7 |
| Cobalamine, ng/L | 495.0 ± 204.0 | 588.0 ± 567.0 |
| IL-6, pg/mL | 1.3 ± 0.8 | 41.8 ± 32.5* |
Data are mean ± SD in each group.
ACD indicates anemia of chronic disease; Hb, hemoglobin; MCH, mean cellular hemoglobin; MCV, mean cellular volume; CRP, C-reactive protein; Fe, serum iron concentration; TfS, transferrin saturation; IL-6, interleukin 6.
P < .001, control vs ACD (Student t test).
P < .05, control vs ACD (Student t test).
Blood samples were drawn on a routine basis and laboratory parameters (eg, alanine aminotransferase, aspartate aminotransferase, hemoglobin, blood cell count, and serum iron parameters) were examined by routinely used automated laboratory tests, ferritin concentration by an immunoassay, and transferrin concentration by a turbidimetric method. Serum specimens were drawn during this routine examination and stored at −70°C until cytokine assays were performed. Determination of serum IL-6 concentrations was carried out using a commercially available enzyme-linked immunosorbent assay kit obtained from R&D (Quantikine HS ELISA Kit; Minneapolis, MN). The detection limit was 0.04 pg/mL.
Monocyte isolation and cell culture
Peripheral blood mononuclear cells were freshly isolated from whole blood by Ficoll-Paque separation (Pharmacia, Uppsala, Sweden) as previously described.39 For monocyte isolation by plastic adherence, peripheral blood mononuclear cells were resuspended with RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 2 mM l-glutamine, 10% (v/v) heat inactivated fetal calf serum (PAA Laboratories, Pasching, Austria), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Biochrom). Cells were seeded into a 100-mm dish (BD Biosciences, Franklin Lakes, NJ) and allowed to adhere in a 5% CO2 incubator at 37% for 45 minutes. Nonadherent cells were removed, and the adherent cells were washed carefully, at least twice, with prewarmed phosphate-buffered saline (PBS; Biochrom) before being harvested. The purity of the resulting cells suspension was randomly tested by fluorescent-activated cell sorting analysis and yielded more than 96% monocytes.
THP-1 cells, a human monocytic cell line, were cultured in RPMI 1640 medium with the same supplements as used for monocytes. Cells were seeded in 6-well plates at passage 5 to 15 at a density of 1.5 × 106 cells per well in 3 mL of medium. After allowing them to rest for 12 hours, cells were stimulated with 1 μg/mL LPS, 10 ng/mL IL-6, or 50 μM of ferric chloride for 3, 6, 12, and 24 hours, respectively.
Determination of hepcidin mRNA in human monocytes by TaqMan RT-PCR
Total RNA was extracted from monocytes and THP-1 cells using a guanidinium-isothiocyanate-phenol-chloroform-based procedure as previously described.33 Reverse transcription was performed with 1 μg of total RNA, 10 ng/μL random hexamer primers (Roche Diagnostics, Mannheim, Germany), dNTPs (Roche Diagnostics), 500 μM each, and 200 U M-MLV reverse transcriptase (Invitrogen, Vienna, Austria) in 1 times reverse transcription buffer for 50 minutes at 37°C. TaqMan reverse-transcribed polymerase chain reaction (RT-PCR) primers and probes were designed, and quantification of targets genes by RT-PCR was carried out exactly as described.40 The following hepcidin primers and TaqMan probe were used: forward primer 5′-TTTCCCACAACAGACGGGAC-3′, reverse primer 5′-AGCTGGCCCTGGCTCC-3′, probe 5′-FAM-CAGAGCTGCAACCCCAGGACAGAGC-BHQ-3′, purchased from Microsynth (Balgach, Switzerland). For quantification of the human housekeeping gene beta-actin, the PerkinElmer Life and Analytical Sciences (Waltham, MA) predeveloped assay kit was used.
Western blotting
Membrane protein extracts were prepared from THP-1 and human monocytes as described40 and run on a 10% SDS-polyacrylamide gel. Proteins were transferred onto a nylon membrane (Hybond-P; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) and after blocking incubated either with a rabbit anti-GFP-antibody (2 μg/mL, Invitrogen), a mouse monoclonalbiotin conjugated anti-c-Myc-antibody (Santa Cruz Biotechnology, Heidelberg, Germany), a rabbit antihuman ferritin-antibody (2μg/mL, DakoCytomation, Vienna, Austria), a rabbit antihuman ferroportin antibody (1:250; kindly provided by Andrew McKie), or a mouse antihuman β-actin antibody (2 μg/mL; Sigma-Aldrich, St Louis, MO), which was used as a loading control.41
Immunofluorescence
THP-1 cells were harvested and cytospun at 320g for 5 minutes. For fixation, cells were washed twice with ice-cold PBS and treated with a 4% paraformaldehyde PBS solution for 20 minutes on ice. Afterward, cells were washed twice with PBS again and treated with methanol/acetone (1:1) for 6 minutes. Fixed cells were rinsed 2 times with PBS and incubated with blocking solution (PBS supplemented with 10 mg/mL bovine serum albumin) for 1 hour. THP-1 cells were transfected with ferroportin tagged with fluorescent EmGFP to visualize ferroportin and PKH26 RED Fluorescence Cell Linker (Sigma-Aldrich) was used to visualize the membrane localizing the cell boarder. For microscopic analysis, the cells were covered into a mounting medium to prevent fading on glass slides and observed under an inverted confocal microscope from Carl Zeiss (Jena, Germany). The base frame is an Axiovert 100M with a highly integrated LSM 510 scanning head. Acquisition was done with the Carl Zeiss LSM imaging software SP2 version 2.81. The following objectives were used: Plan-Apochromat objective, ×63 oil, numerical aperture of 1.4, and EC Plan Neofluar ×40, numerical aperture of 0.75. Laser lines at 488 nm and 543 nm were used for excitation. The emission filter for GFP was 505 to 530 nm and for PKH26 RED LP 585 nm.
Purification of native human hepcidin
For the purification of hepcidin, human urine was centrifuged at 3000g for 30 minutes and filtered through a 0.45-μm filter, before it was diluted with PBS. Hepcidin purification was essentially carried out as previously described14 with a minor modification of the first purification step. In the original protocol, CM Sepharose was used, which was substituted for copper charged chelating Sepharose (GE Healthcare) in our modification.
After loading the diluted urine sample, the chelating resin was eluted with 0.1% aqueous trifluoroacetic acid (TFA) and the elution fraction was collected. A 1-μL aliquot of the elution fraction was subsequently analyzed for hepcidin by MALDI-TOF MS (Ultraflex MALDI TOF-TOF; Bruker Daltonics, Bremen, Germany) using an equal volume of saturated α-cyano-4-hydroxy-cinnamic acid in 50% acetonitrile/0.1% TFA as matrix. After spotting onto a stainless steel target and drying in air, fractions were analyzed on a Bruker Ultraflex mass spectrometer operated in reflectron mode. All spectra were recorded by summarizing 400 laser shots, using a 337-nm nitrogen laser with a pulse of 50 Hz. The instrument was calibrated externally using the peptide calibration standard II, purchased from Bruker. The Flex Analysis version 2.4 software packages provided by the manufacturer were used for data processing.
Subsequently, hepcidin was purified by RP HPLC, where the lyophilized and redissolved eluate from the first purification step was further separated on a semipreparative 10 × 250-mm C18 column (218TP510; Grace Vydac, Hesperia, CA) using a gradient from 0.1% TFA in water to 0.1% TFA in acetonitrile. The chromatography was carried out on a Shimadzu High Performance Liquid Chromatography LC-10Avp instrument equipped with an autosampler, gradient pumps, a UV-VIS photodiode array detector, and a fraction collector (Shimadzu, Duisburg, Germany). Data were recorded and handled using LabSolutions software provided by the manufacturer.
To analyze for the presence of hepcidin isoforms, 0.5-mL fractions were collected and 1 μL of each fraction was analyzed by MALDI-TOF MS as described above. Fractions containing hepcidin were pooled and relyophilized, being finally redissolved in distilled water at a concentration of 1 mg/mL.
Plasmid construction and transient transfection
Human wild-type pro-hepcidin was amplified from HepG2 cDNA using 5′-cagggcaggtaggttctagc-3′ as forward and 5′-acagacggcacgatggcact-3′as reverse primer. The PCR product was cloned into the PCR2.1 vector using the TA cloning Kit (Invitrogen). For subcloning into pcDNA3, a eukaryotic expression vector, both plasmids were digested with HindIII/XbaI restriction enzymes.
For generation of an EmGFP N-terminally tagged ferroportin expression clone, we used the Gateway recombination system (Invitrogen). As entry vector, pENTR 221 containing the ferroportin open reading frame was obtained from Invitrogen. LR recombination reaction was performed for transfer of ferroportin open reading sequence into a Vivid Colors pcDNA 6.2/EmGFP-DEST vector to create an EmGFP N-terminally tagged ferroportin expression clone. As control vector, we used Vivid Colors pcDNA 6.2 EmGFP/CAT (chloramphenicol acetyltransferase) plasmid provided by Invitrogen.
pcDNA3.1/c-Myc expression vectors containing the gene encoding ferroportin, and the N144H and A77D ferroportin mutants were designed as described elsewhere.42
Transfection of THP-1 cells was performed using the Amaxa (Cologne, Germany) system after a routine protocol.43 In brief, THP-1 cells were seeded in 12-well plates at a density of 106 cells per well in 2-mL medium 6 hours before transfection. Cells were centrifuged at 90g for 10 minutes and thereafter resuspended in Cell Line Nucleofector Solution V (Amaxa) to a final concentration of 106 cells/100 μL; 100 μL of cell suspension was mixed with 5 μg of plasmid DNA and transferred to an Amaxa certified cuvette. For transfection, we used the program V-01. Transfection efficiency was between 70% and 80% as checked by immunofluorescence.
Transient hepcidin knockdown by siRNA
Target-specific and control siRNA double-stranded molecules were purchased from Dharmacon RNA Technologies (Lafayette, CO); 3 μg siRNA was nucleofected in 106 cells because this was the optimal amount for protein knockdown. Efficiency of nucleofection was measured by flow cytometry after nucleofecting a TAMRA-labeled siRNA into the same cells under the same conditions. To exclude unspecific effects, each experiment was controlled by the additional nucleofection of a scrambled siRNA. This is defined as reference.
Transfection of THP-1 cells was performed using the Amaxa system after a routine protocol; 100 μL of cell suspension, which was prepared as for transient transfection, was mixed with 3 μg of siRNA and transferred to an Amaxa-certified cuvette. For transfection, we used the program V-01. Transfection efficiency was between 70% and 80% as checked by immunofluorescence. The effect of siRNA knockdown on hepcidin mRNA levels was quantified by real-time PCR.
Intracellular iron determination by graphite furnace atomic absorption spectrometry
Graphite furnace atomic absorption spectrometry was used for quantitative iron determination and carried out with a Unicam Model Solaar, 939 QZ, atomic absorption spectrometer, equipped with a Zeeman-effect background corrector, an FS90 furnace autosampler, and a longitudinally heated atomizer with extended lifetime graphite tubes (Thermo Electron, Waltham, MA). Slit 0.2 and wavelength λ 271.9 nm were used as spectrometer parameters. A Thermo hollow cathode iron lamp (15 mA maximum operating current) was run at 100% maximum current. The calibration solutions (25.0 μg/L to 1.5 μg/L) were prepared by adequate dilution of a 1000 mg/L Titrisol (Merck, Darmstadt, Germany) stock solution with 0.1% Ultrapure nitric acid (Merck), 0.2% Triton X-100, and high-purity water (Milli-Q system, Millipore, Billerica, MA). Samples were suspended by ultrasonication for 40 seconds in 0.1% Ultrapure nitric acid and 0.2% Triton X-100. A pyrolysis temperature of 1000°C and an atomization temperature of 2000°C were used. Argon was used as inert gas at a constant flow of 1 L/min throughout the heating program, except during the atomization step, when the gas flow was interrupted. The accuracy of the procedure was assessed through the analysis of lyophilized control samples (Recipe Chemicals and Instruments, Munich, Germany), which were reconstituted according to the manufacturer's instruction with Milli-Q water and further diluted with 0.1% Ultrapure nitric acid and 0.2% Triton X-100.
Data analysis
Statistical analysis was carried out using SPSS statistics package. Calculations for statistical differences between the various groups were carried out by Student t test or by nonparametric Kruskal-Wallis test. Associations among the various parameters in the different groups were calculated using Spearman's rank correlation technique and Bonferroni correction for multiple tests.
Results
Hepcidin mRNA in primary monocytes and THP-1 cells is induced by LPS and IL-6 but not by iron
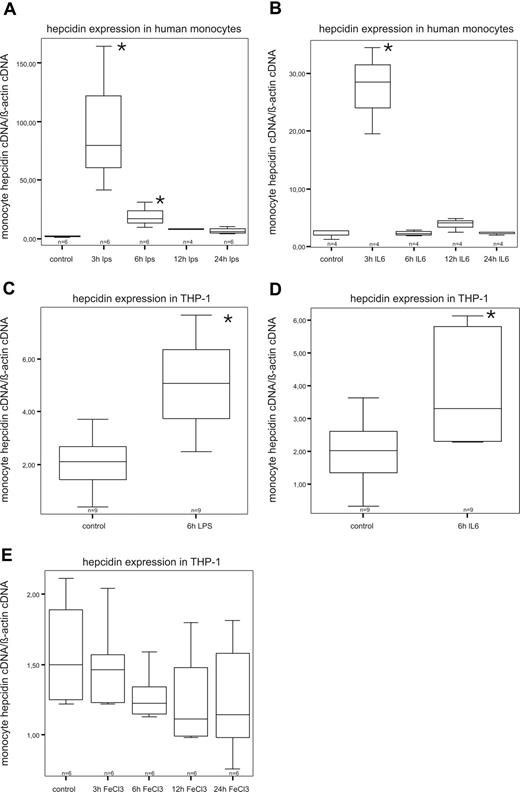
Basal levels of hepcidin mRNA could be detected in both freshly isolated human monocytes and THP-1 cells (Figure 1). In time course experiments, we found that hepcidin mRNA expression in human monocytes is most prominently induced after 3 hours of LPS stimulation. Thereafter, hepcidin mRNA expression declines and returns to baseline levels (Figure 1A). Interestingly, IL-6 had a similar kinetic effect (Figure 1B). The same also holds true for THP-1 cells (Figure 1C,D). However, hepcidin induction by LPS and IL-6 in THP-1 cells was less pronounced than in primary monocytes and reached maximum levels 6 hours after stimulation (Figure 1C,D). Interestingly, supplementation of THP-1 cells with ferric chloride had no significant regulatory effect on hepcidin mRNA expression in monocytes (Figure 1E).
Expression and regulation of hepcidin mRNA in primary monocytes and THP-1 cells by LPS, IL-6, and iron. THP-1 cells (C-E) were stimulated for 3, 6, 12, and 24 hours with 1μg/mL LPS (C), 10 ng/mL IL-6 for (D), or 50μM ferric chloride (E). Freshly isolated human monocytes were stimulated for 6 hours with 1 μg/mL LPS (A) or 10 ng/mL IL-6 (B). After that, cells were subjected to RNA preparation, followed by reverse transcription and quantitative TaqMan PCR. Specific values of target genes were normalized to those of beta-actin. Data are depicted as lower quartile, median and upper quartile (boxes), and minimum/maximum ranges (whiskers; *P < .05, control cells vs stimulated cells by Student t test).
Expression and regulation of hepcidin mRNA in primary monocytes and THP-1 cells by LPS, IL-6, and iron. THP-1 cells (C-E) were stimulated for 3, 6, 12, and 24 hours with 1μg/mL LPS (C), 10 ng/mL IL-6 for (D), or 50μM ferric chloride (E). Freshly isolated human monocytes were stimulated for 6 hours with 1 μg/mL LPS (A) or 10 ng/mL IL-6 (B). After that, cells were subjected to RNA preparation, followed by reverse transcription and quantitative TaqMan PCR. Specific values of target genes were normalized to those of beta-actin. Data are depicted as lower quartile, median and upper quartile (boxes), and minimum/maximum ranges (whiskers; *P < .05, control cells vs stimulated cells by Student t test).
Monocyte hepcidin expression in ACD patients and association with cellular iron retention
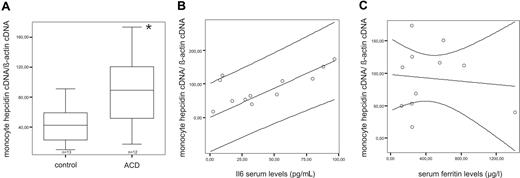
To study monocyte hepcidin expression in vivo and its possible association with monocyte iron retention during inflammation, we investigated primary monocytes of 12 ACD patients and 13 age-matched controls. Hepcidin mRNA expression was detected in monocytes of both groups and was significantly higher in ACD subjects than in controls (P < .05; Figure 2A). Accordingly, as ACD patients suffered from inflammatory diseases, we found a strong positive correlation (R = −0.892, P = .001) between monocyte hepcidin mRNA expression and serum IL-6 levels (Figure 2B), as a marker of inflammation.
Monocyte hepcidin mRNA expression and correlation to serum IL-6 and ferritin levels in ACD patients. (A) Freshly isolated blood monocytes from control (n = 13) and ACD (n = 12) patients were subjected to RNA preparation, followed by reverse transcription and quantitative TaqMan PCR. Specific values of target genes were normalized to those of beta-actin. Data are depicted as lower quartile, median and upper quartile (boxes), and minimum/maximum ranges (whiskers; *P < .05, monocytes from control patients vs monocytes from ACD patients by Student t test). (B) Correlation between monocyte hepcidin mRNA and IL-6 (R = −0.892, P = .001) in ACD patients as determined by Spearman Rank correlation technique. The regression line and the 95% confidence interval are plotted. (C) Correlation between monocyte hepcidin mRNA and serum ferritin (R = −0.027, P = .937) in ACD patients as determined by Spearman Rank correlation technique. The regression line and the 95% confidence interval are plotted.
Monocyte hepcidin mRNA expression and correlation to serum IL-6 and ferritin levels in ACD patients. (A) Freshly isolated blood monocytes from control (n = 13) and ACD (n = 12) patients were subjected to RNA preparation, followed by reverse transcription and quantitative TaqMan PCR. Specific values of target genes were normalized to those of beta-actin. Data are depicted as lower quartile, median and upper quartile (boxes), and minimum/maximum ranges (whiskers; *P < .05, monocytes from control patients vs monocytes from ACD patients by Student t test). (B) Correlation between monocyte hepcidin mRNA and IL-6 (R = −0.892, P = .001) in ACD patients as determined by Spearman Rank correlation technique. The regression line and the 95% confidence interval are plotted. (C) Correlation between monocyte hepcidin mRNA and serum ferritin (R = −0.027, P = .937) in ACD patients as determined by Spearman Rank correlation technique. The regression line and the 95% confidence interval are plotted.
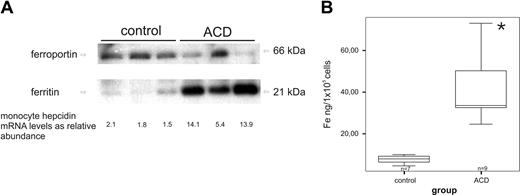
In primary monocytes from ACD patients, we observed decreased ferroportin protein levels and increased ferritin levels, a marker of cellular iron retention, compared with controls (Figure 3A). The decrease in ferroportin expression was paralleled by a corresponding increase in monocyte hepcidin mRNA expression (Figure 3A).
Association of monocyte hepcidin mRNA levels with monocyte ferroportin/ferritin expression and intracellular iron levels. (A) Freshly isolated blood monocytes were subjected to protein and RNA preparation for subsequent Western blotting and TaqMan PCR for determination of protein levels of ferroportin, ferritin, and β-actin as well as hepcidin mRNA levels. For Western blotting, one of 4 representative experiments is shown. (B) Freshly isolated blood monocytes were used for intracellular iron measurement by atom absorption technique as described in “Intracellular iron determination by graphite furnace atomic absorption spectrometry.” Data are depicted as lower quartile, median and upper quartile (boxes), and minimum/maximum ranges (whiskers; *P < .05, iron content in monocytes of control vs ACD patients by Student t test).
Association of monocyte hepcidin mRNA levels with monocyte ferroportin/ferritin expression and intracellular iron levels. (A) Freshly isolated blood monocytes were subjected to protein and RNA preparation for subsequent Western blotting and TaqMan PCR for determination of protein levels of ferroportin, ferritin, and β-actin as well as hepcidin mRNA levels. For Western blotting, one of 4 representative experiments is shown. (B) Freshly isolated blood monocytes were used for intracellular iron measurement by atom absorption technique as described in “Intracellular iron determination by graphite furnace atomic absorption spectrometry.” Data are depicted as lower quartile, median and upper quartile (boxes), and minimum/maximum ranges (whiskers; *P < .05, iron content in monocytes of control vs ACD patients by Student t test).
In parallel to the increased ferritin levels, we detected enhanced intracellular iron concentrations in monocytes of ACD patients compared with control subjects as measured by atom absorption (Figure 3B).
Monocyte-derived hepcidin causes ferroportin degradation in vitro
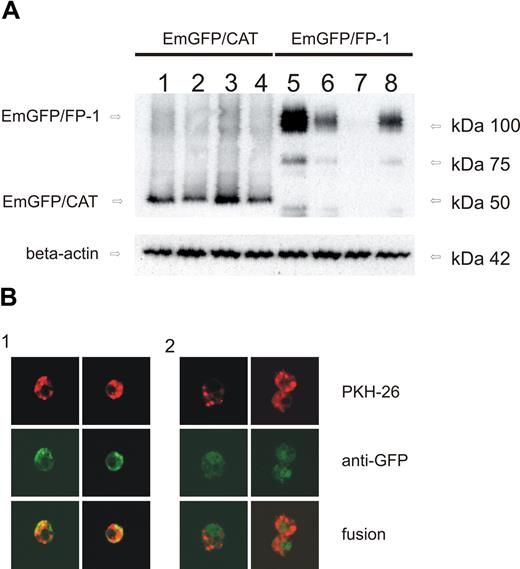
To specifically study the effects of monocyte hepcidin on ferroportin expression, an N-terminal EmGFP/ferroportin fusion protein was overexpressed in THP-1 cells as described in “Plasmid construction and transient transfection.” The EmGFP/ferroportin fusion protein was well expressed in transiently transfected THP-1 cells as estimated by Western blot analysis (Figure 4A). Exogenous addition of purified human hepcidin to the cells resulted in a dramatic reduction in EmGFP/ferroportin protein expression (Figure 4A lane 7).
Monocyte-derived hepcidin causes ferroportin degradation in vitro. (A) Western blot of THP-1 cells transfected with EmGFP/CAT expression vector (lanes 1–4; as control) or EmGFP/ferroportin expression vector (lanes 5–8). Cells were either left untreated (control, lanes 1 and 5), cotransfected with a hepcidin expression vector for another 12 hours (lanes 2 and 6), treated with natural human hepcidin (1 μg/mL) for 3 hours (lanes 3 and 7), or stimulated with LPS 1 μg/mL for 6 hours (lanes 4 and 8). Monocytes were subjected to cell membrane extract preparation and subsequent Western blotting for quantification of EmGFP protein expression. Western blotting for β-actin was used as an internal control. One of 4 representative experiments is shown. (B) Immunofluorescence of THP-1 cells: Cells were transfected with ferroportin/EmGFP expression vector and left untreated (lane 1) or stimulated with LPS (1 μg/mL) for 6 hours (lane 2). PKH-26, a red fluorescence cell linker, was used for cell membrane staining. Ferroportin/EmGFP fusion protein showed green fluorescence. Colocalization of both results in a yellow staining. The size of the images is 360 × 360 μm displayed in original resolution of 1024 × 1024 pixel colored in 8 bit using the ×630 magnification.
Monocyte-derived hepcidin causes ferroportin degradation in vitro. (A) Western blot of THP-1 cells transfected with EmGFP/CAT expression vector (lanes 1–4; as control) or EmGFP/ferroportin expression vector (lanes 5–8). Cells were either left untreated (control, lanes 1 and 5), cotransfected with a hepcidin expression vector for another 12 hours (lanes 2 and 6), treated with natural human hepcidin (1 μg/mL) for 3 hours (lanes 3 and 7), or stimulated with LPS 1 μg/mL for 6 hours (lanes 4 and 8). Monocytes were subjected to cell membrane extract preparation and subsequent Western blotting for quantification of EmGFP protein expression. Western blotting for β-actin was used as an internal control. One of 4 representative experiments is shown. (B) Immunofluorescence of THP-1 cells: Cells were transfected with ferroportin/EmGFP expression vector and left untreated (lane 1) or stimulated with LPS (1 μg/mL) for 6 hours (lane 2). PKH-26, a red fluorescence cell linker, was used for cell membrane staining. Ferroportin/EmGFP fusion protein showed green fluorescence. Colocalization of both results in a yellow staining. The size of the images is 360 × 360 μm displayed in original resolution of 1024 × 1024 pixel colored in 8 bit using the ×630 magnification.
A similar effect was also seen when THP-1 cells expressing the EmGFP/ferroportin fusion protein were transiently transfected with a hepcidin expression plasmid. Twelve hours after transfection, we found EmGFP/ferroportin levels to be significantly reduced (Figure 4A lane 6), compared with controls (Figure 4A lane 5) or cells transfected with the parent plasmid not containing hepcidin cDNA (not shown).
Finally, we stimulated EmGFP/ferroportin protein expressing cells with LPS for 6 hours because this resulted in maximum hepcidin mRNA expression in these cells (Figure 1). Again, this resulted in reduced EmGFP/ferroportin protein surface expression compared with controls (Figure 4A lane 8).
To further study the effect of monocyte-derived hepcidin on ferroportin surface expression, a double staining of cells was used. Therefore, PKH-26, a red fluorescence dye, was used for membrane staining. As EmGFP/ferroportin shows green fluorescence, colocalization of both was shown by yellow fluorescence (Figure 4Bi). Stimulation of cells with LPS (1 μg/mL) reduced yellow fluorescence at the cell surface, indicating internalization of ferroportin (Figure 4Bii).
Hepcidin knockdown inhibits ferroportin degradation in vitro
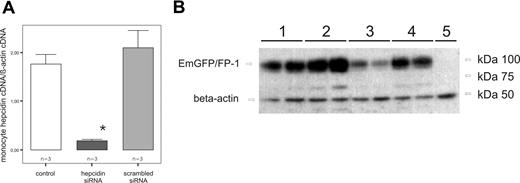
To examine whether the reduced ferroportin surface expression after LPS stimulation is indeed mediated by an increased endogenous formation of hepcidin mRNA in THP-1 cells, we performed hepcidin knockdown experiments using a hepcidin siRNA approach. After nucleofection of THP-1 cells with the specific siRNA, we found highly significant reduction of hepcidin mRNA levels (P = .001) compared with control cells and cells transfected with the scrambled siRNA, respectively (Figure 5A).
Hepcidin knockdown inhibits ferroportin degradation in vitro. (A) Determination of relative abundance of hepcidin expression in THP-1 cells. Cells were left untreated, as a control, or nucleofected with 3 μg of hepcidin siRNA, or, to exclude unspecific effects, nucleofected with a scrambled siRNA. At 36 hours after nucleofection, the mRNA degradation was monitored by real-time PCR. Data are shown as mean plus or minus SD for relative abundances (*P < .001, THP-1 cells nucleofected with 3 μg of hepcidin siRNA vs THP-1 cells transfected with a scrambled siRNA by Student t test). (B) Western blot of THP-1 cells transfected with EmGFP/FP-1 expression vector (lanes 1–4) or mock transfected (lane 5) as a control. Cells were left untreated (lane 1), cotransfected with hepcidin siRNA (lane 2), treated with LPS (1 μg/mL) for 6 hours (lane 3), or cotransfected with hepcidin siRNA and stimulated with LPS (1 μg/mL) for 6 hours (lane 4). Monocytes were subjected to cell membrane extract preparation and subsequent Western blotting for quantification of EmGFP protein expression. Western blotting for β-actin was used as an internal control. One of 4 representative experiments is shown.
Hepcidin knockdown inhibits ferroportin degradation in vitro. (A) Determination of relative abundance of hepcidin expression in THP-1 cells. Cells were left untreated, as a control, or nucleofected with 3 μg of hepcidin siRNA, or, to exclude unspecific effects, nucleofected with a scrambled siRNA. At 36 hours after nucleofection, the mRNA degradation was monitored by real-time PCR. Data are shown as mean plus or minus SD for relative abundances (*P < .001, THP-1 cells nucleofected with 3 μg of hepcidin siRNA vs THP-1 cells transfected with a scrambled siRNA by Student t test). (B) Western blot of THP-1 cells transfected with EmGFP/FP-1 expression vector (lanes 1–4) or mock transfected (lane 5) as a control. Cells were left untreated (lane 1), cotransfected with hepcidin siRNA (lane 2), treated with LPS (1 μg/mL) for 6 hours (lane 3), or cotransfected with hepcidin siRNA and stimulated with LPS (1 μg/mL) for 6 hours (lane 4). Monocytes were subjected to cell membrane extract preparation and subsequent Western blotting for quantification of EmGFP protein expression. Western blotting for β-actin was used as an internal control. One of 4 representative experiments is shown.
We then could demonstrate that transfection of EmGFP/ferroportin expressing THP-1 cells with a hepcidin siRNA already results in a higher EmGFP/ferroportin expression compared with cells transfected with the scrambled siRNA. Moreover, the hepcidin specific siRNA almost fully prevented the LPS-induced reduction in EmGFP/ferroportin expression (Figure 5B).
Effects of hepcidin challenge on the expression of ferroportin mutation variants
To specifically study whether hepcidin affects ferroportin only on the cell surface or also intracellularly, we transiently transfected THP-1 cells with 2 different ferroportin mutants. Whereas the A77D variant is not expressed on the cell surface and does not show any response to extracellular hepcidin,44 the N144H mutant is found on the cell surface but only partially internalizes in response to hepcidin exposure.45
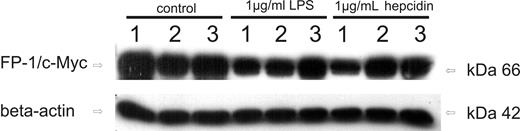
As can be seen in Figure 6 (left panel), wild-type ferroportin as well as the 2 mutant ferroportin variants are expressed in transiently transfected THP-1 cells and can be detected by Western blot. The lower basal expression of the A77D mutant after transfection is in accordance with previous observations.42 When treating cells with LPS for 6 hours, wild-type ferroportin expression was clearly reduced compared with baseline levels. Whereas a small but in comparison to wild-type ferroportin much less pronounced reduction in the expression of A77D ferroportin was observed compared with baseline levels, no change could be detected for the N144H mutant on LPS treatment. Accordingly, addition of purified human hepcidin (1 μg/mL) to the culture medium reduces wild-type ferroportin expression, whereas almost no effect is observed toward the 2 ferroportin mutation variants.
Effects of hepcidin challenge on the expression of ferroportin mutation variants. Western blots of THP-1 cells transfected with ferroportin/c-Myc expression vector (lanes 1, 4, and 7), A77D ferroportin mutant/c-Myc expression vector (lanes 2, 5, and 8), and N144H ferroportin mutant/c-Myc expression vector (lanes 3, 6, and 9). Cells were either left untreated (lanes 1–3), treated with LPS (1 μg/mL) for 6 hours (lanes 4–6) or exposed to native human hepcidin (1 μg/mL) for 3 hours (lanes 7–9). Monocytes were subjected to protein extract preparation and subsequent Western blotting for quantification of c-Myc protein expression. Western blotting for β-actin was used as an internal control. One of 3 representative experiments is shown.
Effects of hepcidin challenge on the expression of ferroportin mutation variants. Western blots of THP-1 cells transfected with ferroportin/c-Myc expression vector (lanes 1, 4, and 7), A77D ferroportin mutant/c-Myc expression vector (lanes 2, 5, and 8), and N144H ferroportin mutant/c-Myc expression vector (lanes 3, 6, and 9). Cells were either left untreated (lanes 1–3), treated with LPS (1 μg/mL) for 6 hours (lanes 4–6) or exposed to native human hepcidin (1 μg/mL) for 3 hours (lanes 7–9). Monocytes were subjected to protein extract preparation and subsequent Western blotting for quantification of c-Myc protein expression. Western blotting for β-actin was used as an internal control. One of 3 representative experiments is shown.
Discussion
Herein we examined the endogenous formation of hepcidin in human monocytic cells and primary monocytes from patients with ACD and investigated its impact on monocyte iron homeostasis.
We found that both freshly isolated human monocytes and THP-1 cells, a monocytic cell line, express significant levels of hepcidin mRNA, which is in accordance with data obtained in mice.35,–37 LPS as well as IL-6 significantly induced hepcidin formation in both cell types, but the inducibility of hepcidin by these inflammatory stimuli was more pronounced in primary monocytes compared with THP-1 cells. These results are at least in part in contrast to macrophage results as published by Nguyen et al37 and Liu et al,35 as they found no induction of hepcidin mRNA by IL-6. This may be referred to the well-known differences in immune response pattern and metabolic pathways between monocytes and macrophages occurring during differentiation and cell maturation. Interestingly, after a single stimulus of LPS and/or IL-6, the induction of hepcidin mRNA in human monocytes was very rapid, showing a maximum expression already 3 hours after stimulation. Thereafter, hepcidin mRNA levels returned to baseline within a few hours, indicating either low stability of hepcidin mRNA and/or the presence of potent negative regulators of hepcidin gene expression within monocytes.
Accordingly, hepcidin mRNA expression was increased in monocytes of ACD patients, and its relationship to inflammation was supported by a strong correlation with IL-6 levels, which could not be found in control patients. This is in accordance with data obtained from mice, indicating that hepcidin formation by cells of the reticuloendothelial system is primarily regulated by inflammatory stimuli35,–37 rather than by iron status.37
So far it is not clear whether the same signals regulate hepcidin formation in the liver and/or myeloid cells. In murine macrophages, hepcidin is induced by a TLR-4–dependent pathway,36 whereas in human monocytes, as shown here, it can also be induced via IL-6 induction, which is TLR-4 independent and involves STAT-3-dependent activation.46,–48 Moreover, the induction kinetics of hepcidin appears to differ between monocytes/macrophages and hepatocytes. Whereas in vivo LPS stimulation results in a 2-phase kinetic of liver hepcidin formation, showing a rapid induction after a few hours, a subsequent decline thereafter, and a second peak after more than 24 hours,49 hepcidin formation by monocytes is short and self-limited as shown herein. These observations may be traced back to variations in the hepcidin promoter region or different signaling pathways between these tissues.
The functionality of endogenous hepcidin on monocyte iron homeostasis was confirmed by experiments using an EmGFP/ferroportin fusion protein expressed in THP-1 cells. In accordance with Nemeth et al,23 we found that overexpression of endogenous hepcidin or exogenous administration of human hepcidin significantly reduced ferroportin expression (Figure 4A) and the localization of EmGFP/ferroportin at the cell surface as indicated by immunofluorescence (Figure 4B).
The functional importance of monocyte-derived hepcidin for ferroportin expression was further confirmed by our results, demonstrating that siRNA directed against hepcidin impairs the LPS-mediated reduction of ferroportin levels.
It needs to be considered that the hepcidin mRNA content of monocytes is approximately 1000 times lower than that of hepatocytes.36 However, whereas liver-derived hepcidin is the master regulator of iron homeostasis in the circulation, we propose that hepcidin formation by activated monocytes/macrophages results in a biologically significant accumulation of this peptide in the inflammatory environment, which then affects iron homeostasis of monocytes/tissue macrophages in an autocrine and paracrine fashion. This may be of specific importance at inflammatory sites with poor perfusion, such as the interstitium, where circulating hepcidin is not sufficiently abundant.
To study whether endogenously formed hepcidin acts as a secreted peptide on monocyte ferroportin or directly within the cell by affecting the posttranslational processing and trafficing of ferroportin to the outer membrane, we performed transient transfection experiments using 2 ferroportin mutation variants. Thereby, we found that neither LPS nor exogenous addition of purified hepcidin significantly affected the expression of the N144H variant, which is expressed on the cell surface but only partially internalizes on hepcidin treatment. When using the cytoplasmatic ferroportin mutant A77D, which is not exposed on the cell surface, we found no change in its expression on addition of hepcidin to the culture medium, whereas a small reduction was observed on LPS treatment. These results indicate that hepcidin produced by monocytes targets membrane bound ferroportin primarily as a secreted peptide in an autocrine way; however, hepcidin also affects, to an albeit much lesser extent, ferroportin expression within the cell. These latter data are in accordance with a recent study50 indicating 2 pathways of hepcidin trafficking within macrophages.
Formation of hepcidin by monocytes may contribute to the development of iron retention seen in ACD. This is indicative from our observations of increased hepcidin formation by monocytes of ACD patients together with the association between increased hepcidin mRNA, monocyte ferritin levels, and monocyte iron concentrations on the one side and reduced monocyte ferroportin expression. As cytokines and LPS have also been shown to down-regulate the mRNA expression of ferroportin, thus acting upstream of the hepcidin-ferroportin interaction,32,33 we hypothesize that cytokines and hepcidin collaborate in modulating monocyte iron homeostasis in the following way.
In the case of an infectious challenge, monocytes/macrophages are aimed to restrict iron to invading pathogens to limit their growth.51,52 As monocytes/macrophages express ferroportin at their cell membrane to maintain iron recirculation, the rapid induction of hepcidin by cytokines and LPS would result in immediate blockage of iron export by ferroportin because of hepcidin-mediated degradation of the protein, thus resulting in iron restriction within macrophages/monocytes. This would fit to the rapid iron sequestration in macrophages seen within hours after LPS stimulation.33
Subsequently, LPS and cytokines, such as interferon-γ inhibit ferroportin mRNA expression to keep iron export low and to withhold iron from pathogens. These series of events ultimately lead to iron accumulation in monocytes/macrophages, hypoferremia, and an iron-restricted erythropoiesis contributing to the development of ACD.
Thus, the formation of hepcidin by monocyte/macrophage and its autocrine effects on iron homeostasis of these cells would be part of the innate immune defense to reduce the availability of the essential nutrient iron from pathogens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr A. McKie (Kings College, London, United Kingdom) for his kind gift of ferroportin antibody and Dr Lisa Schimanski (Institute for Molecular Medicine, Oxford, United Kingdom) for generously providing the ferroportin mutation plasmids.
This work was supported by the Austrian Research Funds FWF (P-19 664; G.W.), the TWF (0404/237; I.T.), and Research Funds from the OENB (P-12 558; I.T.).
Authorship
Contribution: I.T. and G.W. designed the research; I.T., M.T., M.S., S.M., M.N., H.Z., R.B.-W., H.N., H.T., and G.W. performed research and examination of patients; I.T., M.T., and G.W. controlled and analyzed data; I.T. and G.W. wrote the paper; I.T., M.T., M.S., S.M., M.N., H.R., H.Z., R.B.-W., H.N., H.T., and G.W. checked the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Günter Weiss, Medical University, Departmentof General Internal Medicine, Clinical Immunology and Infectious Diseases, Anichstr 35, A-6020 Innsbruck, Austria; e-mail: guenter.weiss@i-med.ac.at.
References
Author notes
I.T. and M.T. contributed equally to this study.