Healing of skin wounds is delayed in hemophilia B (HB) mice. HB mice do not bleed excessively at wounding, yet rebleed hours to days later. Tissue factor (TF) expression is up-regulated by inflammatory cytokines and has been linked to angiogenesis. We hypothesized that impaired thrombin generation in HB leads to impaired TF expression following injury. Punch biopsies were placed on wild-type (WT) and HB mice. Tissues from wound sites were immunostained for TF. Blood vessels are normally surrounded by a coat of pericytes expressing TF. Surprisingly, within a day after wounding TF disappeared from around nearby vessels; returning after 8 days in WT and 10 days in HB mice. The granulation tissue filling the wound during healing also lacked TF around angiogenic vessels. Thus, perivascular TF expression is down-regulated during wound healing. This may prevent thrombosis of neovessels during angiogenesis but renders hemophiliacs vulnerable to hemorrhage during healing.
Introduction
Wound healing consists of (1) hemostasis, (2) inflammation, (3) cellular proliferation, and (4) remodeling. During hemostasis platelets provide an initial hemostatic plug and support the burst of thrombin production that forms a fibrin clot. Platelets also release a variety of growth factors.1,2 Thrombin has cytokine-like properties that promote influx of neutrophils3 and monocytes,4 which in turn release additional cytokines.5 Fibrin forms a scaffold for tissue repair,6 and localizes growth factors to the wound site.7 Thus, adequate hemostatic function is necessary for appropriate tissue repair
Our group has demonstrated that healing of skin wounds is delayed and histologically abnormal in hemophilia B (HB) mice.8 HB mice also exhibit bleeding near the wound site for an extended period of time, even after the surface wound has closed.
The major initiator of coagulation, tissue factor (TF), is expressed around blood vessels, to create a “hemostatic envelope.”9,10 TF expression by several cell types is increased by inflammatory mediators in vitro.11,–13 Thrombin triggers secretion of inflammatory mediators and also synergizes with interleukin-1 and tumor necrosis factor to induce endothelial TF.14 TF messenger RNA in cutaneous tissue is up-regulated immediately following wounding in a mouse model.15 TF expression has also been closely linked to angiogenesis in malignancy.16,17 Therefore, we expected that TF would be highly expressed near a wound site — especially during angiogenesis.
We hypothesized that the failure of thrombin generation in hemophilia would reduce expression of inflammatory mediators, resulting in reduced TF expression after wounding. The current study tests this hypothesis by comparing TF expression after wounding in WT and HB mice.
Methods
Adult WT (C57BL/6) and factor IX-knockout (HB) mice were used in these studies.18 These studies were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of North Carolina as in our previous studies.8
A 3 mm biopsy punch wound was placed on the back of each mouse. The epithelial defect healed by 10 days in WT and 12 days in HB mice, as we previously reported.8 Tissue harvesting, processing, and staining were carried out as described.8 TF was detected using a rabbit polyclonal antibody against the extracellular domain of mouse TF (gift of Dr Mirella Ezban, Novo Nordisk, Bagsvaerd, Denmark, used at 1:1000 dilution). This antibody inhibited FX activation by lipopolysaccharide (LPS)–treated mouse mononuclear cells and recognized a single protein band in Western blots of sodium dodecyl sulfate (SDS)–denatured lysates of these cells. Pericytes were distinguished from endothelial cells by staining for desmin with a rabbit polyclonal antibody (Abcam, Cambridge, MA; 1:100 dilution). Binding of both rabbit primary antibodies was visualized with a Dako LASB2 kit (DakoCytomation, Carpinteria, CA). Image acquisition and processing were carried out as proviously reported.8
TF expression around vessels within 2 high-power (40×) fields of the wound bed was counted for tissues from 3 or 4 mice per time point, and expressed as the percentage of vessels with at least half of their circumference surrounded by TF staining.
Results and discussion
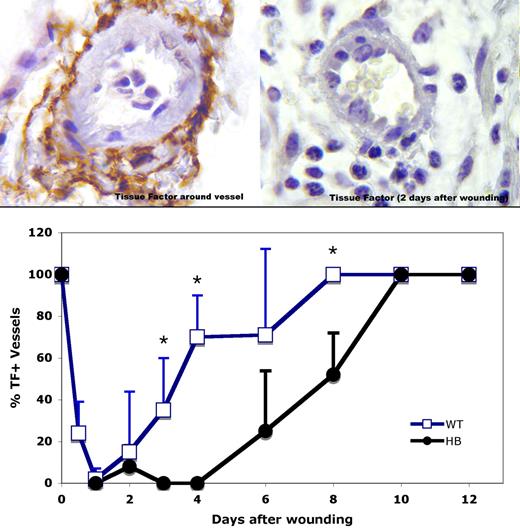
Immunostaining of unwounded skin from WT and HB mice showed strong TF staining in the squamous epithelium and around dermal vessels (left panel, Figure 1) as previously reported in human tissues.9,10,19 To our surprise, TF was absent from around vessels near wounds in both WT and HB mice by 1 day after wounding. A section from 2 days after wounding is shown in the right panel of Figure 1. Over time TF expression returned around dermal vessels, as is plotted in the lower panel of Figure 1. By day 8, TF again surrounded all vessels near the wound bed in WT mice. However, reexpression of TF was delayed in the HB mice, with full expression occurring only after 10 days.
TF immunostaining around dermal vessels near sites of wounding. (Top panels) TF antigen is indicated by the brown color. Representative vessel profiles from WT animals are shown. Normal TF distribution around a dermal vessel is shown in the upper left, and 2 days after wound placement in the upper right. Original magnification 1000×. (Bottom panel) TF expression around vessels within 2 high-power (40×) fields of the wound bed was scored for 3 or 4 mice per time point, and means with SD are plotted. The SD values are 0 for days 0, 8, 10, and 12 for WT mice and 0, 10, and 12 for HB mice because all vessels were surrounded with a TF coat at these times. *P < .05 vs HB.
TF immunostaining around dermal vessels near sites of wounding. (Top panels) TF antigen is indicated by the brown color. Representative vessel profiles from WT animals are shown. Normal TF distribution around a dermal vessel is shown in the upper left, and 2 days after wound placement in the upper right. Original magnification 1000×. (Bottom panel) TF expression around vessels within 2 high-power (40×) fields of the wound bed was scored for 3 or 4 mice per time point, and means with SD are plotted. The SD values are 0 for days 0, 8, 10, and 12 for WT mice and 0, 10, and 12 for HB mice because all vessels were surrounded with a TF coat at these times. *P < .05 vs HB.
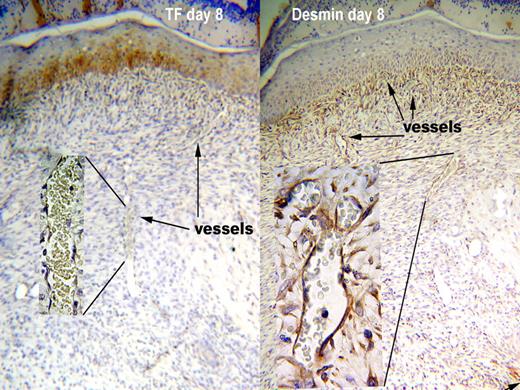
The defect produced by a punch biopsy is filled by a highly vascular stroma called “granulation tissue.” The vessels within the granulation tissue also did not express TF in either WT or HB mice during healing. In contrast to the reappearance of TF around dermal vessels, the newly formed vessels in the granulation tissue lacked TF through the 20 days of the experiment. The lack of TF at the site of a biopsy wound is illustrated in the left panel of Figure 2 from an 8 day old wound. The inset shows a high-power view of a vessel within the granulation tissue demonstrating its lack of TF staining. Strong TF staining (brown color) can be seen in the overlying squamous epithelium. Squamous epithelium stained for TF throughout the healing process, serving as an internal positive control.
TF is absent from around vessels in granulation tissue even though pericytes are present. Antigen is indicated by the brown color. Representative sections from WT at day 8 after wounding are shown. TF was not present around vessels in the granulation tissue (left panel), since the intensity of staining was no greater than negative control sections stained with nonimmune rabbit IgG. The squamous epithelium above the granulation tissue does stain for TF. Vessels in the granulation tissue were surrounded by pericytes, as indicated by a layer of desmin staining around the vessels (right panel). The pattern of staining was consistent in 15 skin samples for TF and 23 for desmin, and was the same for WT and HB mice. Original magnification 100× and 1000× for insets.
TF is absent from around vessels in granulation tissue even though pericytes are present. Antigen is indicated by the brown color. Representative sections from WT at day 8 after wounding are shown. TF was not present around vessels in the granulation tissue (left panel), since the intensity of staining was no greater than negative control sections stained with nonimmune rabbit IgG. The squamous epithelium above the granulation tissue does stain for TF. Vessels in the granulation tissue were surrounded by pericytes, as indicated by a layer of desmin staining around the vessels (right panel). The pattern of staining was consistent in 15 skin samples for TF and 23 for desmin, and was the same for WT and HB mice. Original magnification 100× and 1000× for insets.
TF around vessels is primarily expressed by pericytes; myofibroblast-like cells that surround the endothelium of capillaries and the muscular layer of arterioles and venules. Therefore, we explored whether the pericytes themselves disappeared from around vessels after wounding. We used the expression of desmin, an intermediate filament found in cells of muscle origin, to identify pericytes.20 We found that dermal vessels retain their surrounding pericytes after wounding, even while TF is absent (data not shown).
Ensheathment by pericytes stabilizes small vessels. Pericytes also have a role in the process of angiogenesis.20,21 Pericytes have been visualized at the tip of growing capillary sprouts, apparently serving as guiding structures.20 We initially thought that the lack of TF around neovessels in the granulation tissue might reflect a lack of pericyte ensheathment. However, the developing blood vessels in granulation tissue were surrounded by pericytes throughout the healing process (Figure 2 right panel).
At this point we can only speculate on the mechanism of TF disappearance around preexisting vessels. We favor the hypothesis that TF loss might occur by proteolytic shedding. TF is not homologous to coagulation proteins, but is a member of the cytokine receptor superfamily. Soluble forms of many cytokine receptors are formed by regulated proteolytic release from the cell surface.22 Growth hormone receptor, for example, is structurally related to TF and is shed from cell surfaces by cleavage near the transmembrane domain in response to cell-signaling events.23 Such regulated shedding could account for the rapid and complete loss of TF antigen from pericytes.
TF antigen could also be removed from pericyte surfaces by endocytosis. While we expect TF antigen would still be detectable within pericytes under these conditions, it might be degraded and the fragments no longer recognized by our antibody. It is also possible that a dramatic conformational change in the TF molecule could reduce its reactivity with our antibody reagent. This is unlikely, because the polyclonal antibody we used was raised to the entire extracellular domain of TF and even recognizes denatured and reduced TF on Western blots.
Mature vessels that provide sites for angiogenic sprouting exhibit vasodilatation, increased permeability and detachment of supporting cells.24 Endothelial sprouts migrate into the healing wound, acquire lumens and fuse with existing the vasculature.24 We propose that a lack of TF around angiogenic vessels during wound healing may reflect a mechanism to prevent thrombosis of newly formed, delicate and leaky vessels. This leads to an increased risk of bleeding from granulation tissue that is not a significant liability in normal individuals. However, in hemophilia the absence of TF near wound sites could lead to repeated hemorrhage in and near the wound site, even after the surface defect has been healed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Angela Lenkowski for her excellent technical assistance in immunostaining and Jacqueline Holman for her excellent technical assistance in the animal experiments.
This work was supported in part by a research grant from Novo Nordisk (D.M.M.), the Donald B. Hackel Pathology Fellowship at Duke University School of Medicine (A.G.M.), the Howard Hughes Undergraduate Research Program (K.Y.), and the US Department of Veterans Affairs (M.H.).
Authorship
Contribution: A.G.M. and K.Y. carried out immunostaining and interpreted results; H.R.R. interpreted data; D.M.M. designed research and interpreted data; and M.H. designed research, reviewed histology slides, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Maureane Hoffman, Laboratory Service (113), Durham Veterans Affairs Medical Center, 508 Fulton St, Durham, NC 27705; e-mail: maureane@med.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal