During inflammation, E-selectin expressed on cytokine-activated endothelial cells mediates leukocyte rolling under flow. E-selectin undergoes endocytosis and may associate with lipid rafts. We asked whether distribution of E-selectin in membrane domains affects its functions. E-selectin was internalized in transfected CHO cells or cytokine-activated human umbilical vein endothelial cells (HUVECs). Confocal microscopy demonstrated colocalization of E-selectin with α-adaptin, a clathrin-associated protein. Deleting the cytoplasmic domain of E-selectin or disrupting clathrin-coated pits with hypertonic medium blocked internalization of E-selectin, reduced colocalization of E-selectin with α-adaptin, and inhibited E-selectin-mediated neutrophil rolling under flow. Unlike CHO cells, HUVECs expressed a small percentage of E-selectin in lipid rafts. Even fewer neutrophils rolled on E-selectin in HUVECs treated with hypertonic medium and with methyl-β-cyclodextrin, which disrupts lipid rafts. These data demonstrate that E-selectin clusters in both clathrin-coated pits and lipid rafts of endothelial cells but is internalized in clathrin-coated pits. Distribution in both domains markedly enhances E-selectin's ability to mediate leukocyte rolling under flow.
Introduction
Recruitment of leukocytes into secondary lymphoid organs or inflamed tissues requires that the cells first tether to and roll on vascular surfaces. Interactions of selectins with their glycoconjugate ligands mediate tethering and rolling,1 which precedes integrin-dependent deceleration, arrest, and transmigration of leukocytes across the endothelial cell layer.2 L-selectin, expressed on leukocytes, binds to ligands on other leukocytes and on endothelial cells in lymph nodes and in some regions of inflammation. P- and E-selectin, expressed on activated platelets or endothelial cells, bind to ligands on leukocytes. The leukocyte mucin P-selectin glycoprotein ligand-1 (PSGL-1) is the dominant ligand for P- and L-selectin and an important ligand for E-selectin.3,4
The manner in which selectins or their ligands distribute on cell surfaces has major influence on rolling behavior.5 For example, L-selectin and PSGL-1 are located on tips of microvilli,6,7 which enhances the initial tethering of leukocytes.8 The extended length of P-selectin helps capture flowing neutrophils and slow their rolling velocities, presumably by increasing encounters with PSGL-1.9 The dimeric structures of P-selectin and PSGL-1 allow them to form dimeric bonds that prolong tether duration and strength.10 Membrane anchorage of L-selectin and P-selectin through binding of their cytoplasmic domains to cytosolic proteins favors rolling. L-selectin anchors through interactions of its cytoplasmic domain with the cytoskeleton to effectively engage its ligands under flow.11 P-selectin uses a different mechanism to cluster on the cell surface. Thrombin or histamine mobilizes P-selectin from the membranes of Weibel-Palade bodies to the plasma membranes of endothelial cells.12,13 Sequences in the cytoplasmic domain enable P-selectin to cluster in clathrin-coated pits and then enter the cell in clathrin-coated vesicles.14 This limits inflammation by clearing P-selectin from the cell surface. Before P-selectin is internalized, however, its locally high concentration in clathrin-coated pits allows it to form clusters of bonds with PSGL-1 on rolling leukocytes; this increases the number of rolling leukocytes per unit area and favors slower and more regular rolling steps.15 As a result, P-selectin makes important contributions to leukocyte rolling during inflammation.
Much less is known about the distribution of E-selectin in membrane domains and about the potential effects of such distribution on its adhesive properties. Like P-selectin, E-selectin is usually expressed on the endothelial surface only during inflammation. Mediators, such as tumor necrosis factor-α, interleukin-1β (IL-1β), or lipopolysaccharide cause transient synthesis of E-selectin that is transported directly to the plasma membrane. In vitro, the density of E-selectin on activated endothelial cells (∼350 sites/μm2) is much higher than that of P-selectin (∼25–50 sites/μm2),16 and it is possible that inflamed endothelial cells express higher levels of E-selectin than P-selectin in vivo. On one hand, the higher densities of E-selectin might obviate a requirement for clustering in membranes to optimize its ability to mediate leukocyte rolling. On the other hand, E-selectin binds with lower affinity than P-selectin to its ligands, which might make clustering in the membrane more important for rolling.17,18 Like P-selectin, E-selectin is internalized from the endothelial cell surface to limit the inflammatory response.19,20 However, it is unclear whether internalization of E-selectin affects leukocyte rolling. Sequences in the cytoplasmic domain of E-selectin promote endocytosis in transfected cells.21,22 Whether E-selectin requires its cytoplasmic domain to mediate leukocyte rolling under flow is not known. Furthermore, whether E-selectin is internalized through clathrin-coated pits or by another mechanism has not been determined. In flow-free conditions, adhesion of leukocytes to cytokine-activated endothelial cells induces association of E-selectin with lipid raft domains, cytoskeletal components, and signaling molecules.23,,,–27 Indeed, it has been suggested that E-selectin is primarily associated with lipid rafts even before it engages leukocytes.27 If this were true, E-selectin would probably not cluster in clathrin-coated pits, as lipid rafts are not known to mix with clathrin-enriched structures.28 Here, we demonstrate that E-selectin distributes in clathrin-coated pits where it is internalized, but unlike P-selectin, it also distributes in lipid rafts of endothelial cells. Importantly, partitioning of E-selectin in both membrane domains significantly enhances its ability to mediate leukocyte rolling under flow. These data underscore the importance of the cell surface organization of E-selectin for its adhesive function as well as its clearance from the cell surface.
Methods
Blood collected from healthy volunteers was used to isolate neutrophils. The blood collection protocol was approved by the Institutional Review Board of the Oklahoma Medical Research Foundation, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Umbilical cords for isolation of endothelial cells were provided by the Pathology Department of Mercy Lab Oklahoma according to a protocol approved by the Institutional Review Board of Mercy Lab Oklahoma.
Cells, proteins, and other materials
Cultured human umbilical vein endothelial cells (HUVECs) were passaged 2 times or less.15 Stably transfected CHO cells expressing wild-type human E-selectin or a truncated E-selectin containing only the first arginine residue of the 32-residue cytoplasmic domain (tail-less E-selectin) were prepared by previously described methods.10,14 Human neutrophils were isolated as described.29 The antihuman E-selectin mAb ES1 was prepared as described.30 Goat polyclonal antibody to human E-selectin was raised and then biotinylated using a previously described protocol.14 For confocal microscopy, mAbs to α-adaptin and caveolin-1 were from BD Biosciences, (San Jose, CA). For immunoblotting, mAbs to flotillin-1, caveolin-1, moesin, and transferrin receptor were from BD Biosciences. Mouse anti–goat IgG-horseradish peroxidase (HRP) and goat anti–mouse IgG-HRP were from Pierce Chemical (Rockford, IL). Recombinant human interleukin-1β (IL-1β) was from R&D Systems (Minneapolis, MN).
Internalization assays
HUVECs were stimulated with 1 μg/mL IL-1β for 4 hours to induce expression of E-selectin. The internalization rate of E-selectin in IL-1β– stimulated HUVECs and transfected CHO cells was measured by 2 methods. The first method measured the ability of an acidic buffer to remove 125I-labeled mAb ES1, prebound to the cell surface at 4°C, after warming to 37°C for various intervals.14 The cell-bound radioactivity remaining at each time point represents the amount of internalized E-selectin and is presented as a percentage of the initial cell-bound radioactivity. In the second method, cell surface E-selectin was first biotinylated by incubation with sulfo-NHS-ss-biotin at 4°C. The internalization rate of biotinylated E-selectin was measured by the ability of sodium 2-mercaptoethanesulfonate to remove biotin from cell surface E-selectin after warming the cells to 37°C for various intervals.14 The biotin that remained bound to E-selectin, which represents the amount of internalized E-selectin, was quantified by enzyme-linked immunosorbent assay. The results are presented as percentage of the total amount of biotinylated E-selectin at time 0.
Adhesion assay under flow
Rolling adhesion of neutrophils on HUVECs or transfected CHO cells was assayed in a 35-mm dish incorporated into a parallel-plate flow chamber.15,31 Site densities of E-selectin were measured by binding of 125I-labeled mAb ES1.15 Site densities of E-selectin on transfected CHO cell clones were measured within one week of each series of experiments to confirm that cells expressing comparable densities of E-selectin were used. Consistent adhesion data were obtained with several independent clones of CHO cells expressing each form of E-selectin. Neutrophils (106/mL in Hanks balanced salt solution containing 0.5% human serum albumin) were perfused over cells at defined wall shear stresses. The number of rolling cells and their rolling behavior were analyzed by videomicroscopy (30 frames per second) using imaging software (Inovision). In some experiments, neutrophils were fixed with 1% paraformaldehyde for 10 minutes before use. The number of rolling neutrophils that accumulated on HUVECs or transfected CHO cells was analyzed when it reached a plateau 4 minutes after neutrophil perfusion was initiated. Because fixed neutrophils rolled at lower levels, we injected them at a higher concentration (2 × 106/mL) into the flow chamber. Frame-by-frame analysis was used to measure the mean rolling velocity and the variance of rolling velocity for a population of neutrophils.10 Individual unfixed rolling neutrophils were tracked for 5 seconds. Individual fixed rolling neutrophils were tracked for 15 seconds.
Disruption of clathrin-coated pits or lipid rafts
Hypertonic medium containing sucrose was used to block clathrin-mediated endocytosis of E-selectin in IL-1β-stimulated HUVECs and transfected CHO cells.14,15 Lipid rafts in IL-1β-stimulated HUVECs and transfected CHO cells were disrupted by chelating cholesterol with water-soluble methyl-β-cyclodextrin (MβCD) as described.32,–34 Briefly, the cells were washed in the isotonic medium in which they were cultured, but without serum or antibiotics. Then, the cells were incubated in the same medium containing 10 mM MβCD for 15 minutes at 37°C. Controls included treatment with 10 mM α-cyclodextrin, an inactive analog of MβCB, or treatment with MβCD followed by the respective medium containing 20% serum, which replenishes cholesterol in the membrane. Treatment with hypertonic medium or MβCD did not alter the surface density of E-selectin on IL-1β-stimulated HUVECs or transfected CHO cells.
Detergent-resistant membrane preparation
Chilled HUVECs or transfected CHO cells were lysed with 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA containing complete protease inhibitors (Roche Applied Science, Indianapolis, IN) and 1% Triton X-100. Using a cell scraper, cell lysate was collected and incubated in ice for 30 minutes. The cell lysate was homogenized using a tissue grinder and centrifuged at 960g for 15 minutes at 4°C. The supernatant was collected and centrifuged in a separation medium, OptiPrep (Sigma- Aldrich, St Louis, MO), according to the manufacturer's recommendation. Briefly, a 600-μL mixture of cell lysate and OptiPrep at a final concentration of 40% was layered at the bottom of a 2.4-mL centrifuge tube. On top of this mixture, Optiprep solutions at concentrations of 30% (600 μL), 20% (600 μL), and 5% (500 μL) were layered sequentially. After centrifugation at 100 000g for 4 hours, at 4°C, 250-μL fractions from the top to the bottom of the tube were collected. Fractions were separated by SDS-PAGE and then analyzed by Western blotting with specific primary antibodies followed by secondary antibodies conjugated with HRP. To identify the ganglioside GM1, cholera toxin B subunit directly conjugated with HRP (Sigma-Aldrich) was added. Bound proteins were revealed with the ECL detection system (GE Healthcare, Little Chalfont, United Kingdom).
Immunofluorescence confocal microscopy
IL-1β-stimulated HUVECs and transfected CHO cells were cultured on glass coverslips in a 12-well plate. The distribution of E-selectin, α-adaptin, caveolin-1, GM1, and flotillin-1 was examined by immunofluorescence confocal microscopy with previously described protocols.15 In addition, the confocal microscopy images were deconvolved using constrained iterative frame in iVision image processing software (BioVision, Mountain View, CA). The extent of colocalization of cell surface E-selectin with α-adaptin, caveolin-1, GM1, or flotillin-1 was quantified.15
Results
E-selectin is rapidly internalized in clathrin-coated pits of transfected CHO cells and cytokine-stimulated endothelial cells
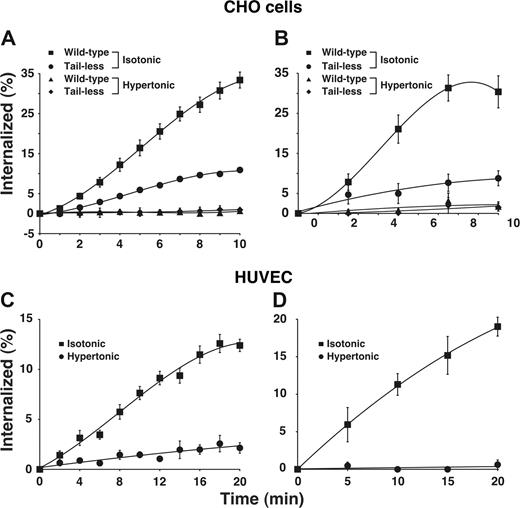
Using 2 well-characterized assays, we found that E-selectin was rapidly internalized from the surfaces of transfected CHO cells (Figure 1A,B) and IL-1β-stimulated HUVECs (Figure 1C,D), confirming previous observations.19,20 E-selectin was internalized at a similar rate in HUVECs stimulated with tumor necrosis factor-α (H.S. and R.P.M., unpublished data, September 2006). We also expressed a form of E-selectin that lacked the entire cytoplasmic domain except for a single membrane-proximal arginine. The internalization rate of this tail-less E-selectin was markedly diminished but not eliminated in transfected cells. This confirms previous reports that the cytoplasmic domain of E-selectin supports internalization21,22 but also shows that E-selectin can be endocytosed at a slow rate in the absence of the cytoplasmic domain. To determine whether E-selectin is internalized through clathrin-coated pits, we treated cells with hypertonic medium, a well-characterized method to block clathrin-mediated endocytosis by disassembling clathrin lattices.35,,,,,–41 This treatment eliminated internalization of wild-type E-selectin in both HUVECs and transfected CHO cells, and also eliminated the residual internalization of tail-less E-selectin in transfected CHO cells.
E-selectin is rapidly internalized in clathrin-coated pits of transfected CHO cells and cytokine-stimulated HUVECs. (A,B) The internalization rate of wild-type or tail-less E-selectin in transfected CHO cells was measured in isotonic medium or in hypertonic medium, which disrupts clathrin-coated pits. (C,D) The internalization rate of E-selectin in IL-1β–stimulated HUVECs was measured in isotonic or hypertonic medium. The internalization rate was measured by the ability of acidic buffer to remove surface-bound 125I-labeled anti-E-selectin mAb (A-C) or by the ability of sodium 2-mercaptoenthanesulfonate to remove biotin from cell surface E-selectin (B-D), after warming cells to 37°C for the indicated intervals. The data represent the mean plus or minus SEM from 3 to 16 experiments for each experimental condition.
E-selectin is rapidly internalized in clathrin-coated pits of transfected CHO cells and cytokine-stimulated HUVECs. (A,B) The internalization rate of wild-type or tail-less E-selectin in transfected CHO cells was measured in isotonic medium or in hypertonic medium, which disrupts clathrin-coated pits. (C,D) The internalization rate of E-selectin in IL-1β–stimulated HUVECs was measured in isotonic or hypertonic medium. The internalization rate was measured by the ability of acidic buffer to remove surface-bound 125I-labeled anti-E-selectin mAb (A-C) or by the ability of sodium 2-mercaptoenthanesulfonate to remove biotin from cell surface E-selectin (B-D), after warming cells to 37°C for the indicated intervals. The data represent the mean plus or minus SEM from 3 to 16 experiments for each experimental condition.
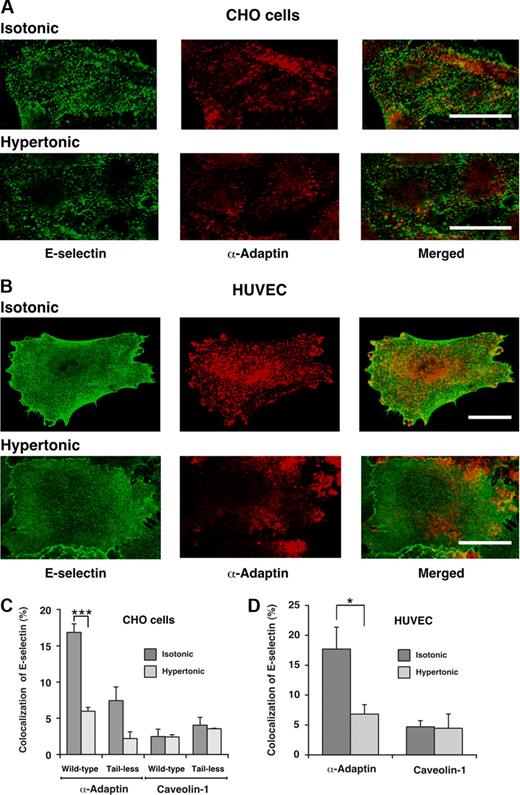
To further determine whether E-selectin enters clathrin-coated pits, we used confocal immunofluorescence microscopy to ascertain whether E-selectin colocalized with α-adaptin, a component of clathrin-coated pits.42,43 After the clathrin-coated pit buds to form a vesicle, α-adaptin dissociates as the vesicle loses its coat. Therefore, the total pool of α-adaptin is distributed between clathrin-coated pits and the cytoplasm. Green or red staining illustrates the respective distributions of E-selectin and α-adaptin. In both transfected CHO cells and HUVECs, there was partial colocalization of E-selectin with α-adaptin (Figure 2A,B). Quantification of the percentage of E-selectin pixels that colocalized with α-adaptin pixels supported the conclusions from the representative images (Figure 2C,D). Incubation of cells in hypertonic medium abrogated the observed colocalization, confirming that it represented colocalization in clathrin-coated pits. Furthermore, E-selectin did not significantly colocalize with caveolin-1, a component of caveolae44 (Figure 2C,D). These results demonstrate that E-selectin clusters in clathrin-coated pits of both HUVECs and transfected CHO cells, and are consistent with a dominant role for endocytosis of E-selectin through a clathrin-mediated pathway.
Cell-surface E-selectin colocalizes with α-adaptin, a component of clathrin-coated pits but not with caveolin-1, a component of caveolae. IL-1β-stimulated HUVECs or transfected CHO cells expressing wild-type or tail-less E-selectin were incubated in isotonic or hypertonic medium for 15 minutes, then fixed and incubated with biotinylated polyclonal antibodies to E-selectin, followed by streptavidin conjugated to Alexa 488. After permeabilization, the cells were incubated with a mAb to α-adaptin or caveolin-1, followed by donkey antimouse Ig conjugated to Cy-3. Using a confocal microscope, an optical section at several levels of each cell was examined for staining of E-selectin (green) or α-adaptin or caveolin-1 (red). Representative images of transfected CHO cells expressing wild-type E-selectin (A) or of IL-1β-stimulated HUVECs (B) revealed partial colocalization of E-selectin with α-adaptin in isotonic medium but significantly less colocalization in hypertonic medium. Bar represents 20 μm. (C,D) The degree of colocalization was quantified by measuring the percentage of green pixels (E-selectin) that colocalized with red pixels (α-adaptin or caveolin-1). The data represent the mean plus or minus SEM of at least 4 experiments, with at least 10 cells counted in each experiment (*P < .05; ***P < .001, as measured by the unpaired t test).
Cell-surface E-selectin colocalizes with α-adaptin, a component of clathrin-coated pits but not with caveolin-1, a component of caveolae. IL-1β-stimulated HUVECs or transfected CHO cells expressing wild-type or tail-less E-selectin were incubated in isotonic or hypertonic medium for 15 minutes, then fixed and incubated with biotinylated polyclonal antibodies to E-selectin, followed by streptavidin conjugated to Alexa 488. After permeabilization, the cells were incubated with a mAb to α-adaptin or caveolin-1, followed by donkey antimouse Ig conjugated to Cy-3. Using a confocal microscope, an optical section at several levels of each cell was examined for staining of E-selectin (green) or α-adaptin or caveolin-1 (red). Representative images of transfected CHO cells expressing wild-type E-selectin (A) or of IL-1β-stimulated HUVECs (B) revealed partial colocalization of E-selectin with α-adaptin in isotonic medium but significantly less colocalization in hypertonic medium. Bar represents 20 μm. (C,D) The degree of colocalization was quantified by measuring the percentage of green pixels (E-selectin) that colocalized with red pixels (α-adaptin or caveolin-1). The data represent the mean plus or minus SEM of at least 4 experiments, with at least 10 cells counted in each experiment (*P < .05; ***P < .001, as measured by the unpaired t test).
Interactions of E-selectin with clathrin-coated pits enhance leukocyte rolling under flow
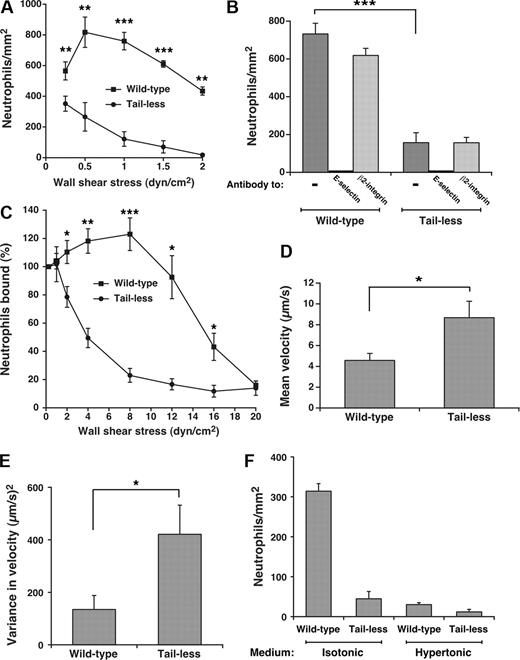
Clustering of P-selectin in clathrin-coated pits improves its ability to mediate leukocyte rolling under flow.15 To determine whether such clustering augments the adhesive function of E-selectin, we compared rolling of human neutrophils on E-selectin on transfected CHO cells or HUVECs under conditions that supported or prevented interactions of E-selectin with clathrin-coated pits. As measured by binding of radiolabeled anti-E-selectin mAb, the transfected CHO cells expressed approximately 350 E-selectin molecules/μm2, the same surface density measured on cytokine-activated HUVECs (370 ± 35 molecules/μm2, mean ± SEM, n = 9). Flow cytometry confirmed uniform expression of E-selectin on all cells, excluding clonal variation in expression (H.S. and R.P.M., unpublished data, May 2005). Significantly more neutrophils rolled on matched densities of wild-type than tail-less E-selectin on CHO cells over a range of wall shear stresses (Figure 3A). The observed rolling was strictly dependent on E-selectin as it was blocked by a mAb to E-selectin but not by a mAb to β2 integrins (Figure 3B). Compared with those rolling on wild-type E-selectin, neutrophils rolling on tailless E-selectin detached much more readily as wall shear stress increased (Figure 3C), and rolled with higher velocities and greater variance in rolling steps even at low wall shear stress where the number of cells rolling on wild-type and tailless E-selectin was similar (Figure 3D,E). As wall shear stress increased, neutrophils rolled even more rapidly and irregularly on tailless E-selectin until they detached (unpublished data). We compared neutrophil rolling on transfected CHO cells in control isotonic medium or in hypertonic medium, which disrupts clathrin lattices. Because hypertonic medium alters neutrophil shape, we used fixed neutrophils, which roll on selectins although less efficiently than unfixed cells because of the loss of membrane deformability.5,45 Far fewer fixed neutrophils rolled on wild-type E-selectin on CHO cells incubated in hypertonic than in isotonic medium (Figure 3F). The reduction in rolling neutrophils was equivalent to that observed on tailless E-selectin in either isotonic or hypertonic medium. This suggests that the cytoplasmic domain of E-selectin supports leukocyte rolling largely through interactions with clathrin-coated pits.
Interactions of the cytoplasmic domain of E-selectin with clathrin-coated pits of CHO cells enhance leukocyte rolling under flow. (A) Neutrophils were perfused over transfected CHO cells expressing wild-type or tailless E-selectin over a range of wall shear stresses. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of at least 4 experiments. (B) Neutrophils were perfused over CHO cells expressing wild-type or tailless E-selectin in the absence or presence of 10 μg/mL anti-E-selectin mAb ES1 or anti-β2 integrin mAb IB4 at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. (C) Neutrophils were perfused for 5 minutes over CHO cells expressing wild-type or tailless E-selectin at a wall shear stress of 0.25 dyne/cm2. Without stopping flow, cell-free buffer was perfused at the same wall shear stress for 30 seconds, and the number of cells still rolling was taken as 100%. The wall shear stress was then increased incrementally every 30 seconds, and the percentage of neutrophils still rolling was determined. The data represent the mean plus or minus SEM of 5 experiments. (D,E) The mean velocity and the variance of velocity of neutrophils rolling on CHO cells expressing wild-type or tailless E-selectin were measured. The wall shear stress was 1 dyne/cm2. The data represent the mean plus or minus SEM for at least 4 experiments. (F) Fixed neutrophils were perfused over CHO cells expressing wild-type or tailless E-selectin in isotonic or hypertonic medium at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of at least 3 experiments (*P < .05; **P < .01; ***P < .001, as measured by the unpaired t test).
Interactions of the cytoplasmic domain of E-selectin with clathrin-coated pits of CHO cells enhance leukocyte rolling under flow. (A) Neutrophils were perfused over transfected CHO cells expressing wild-type or tailless E-selectin over a range of wall shear stresses. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of at least 4 experiments. (B) Neutrophils were perfused over CHO cells expressing wild-type or tailless E-selectin in the absence or presence of 10 μg/mL anti-E-selectin mAb ES1 or anti-β2 integrin mAb IB4 at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. (C) Neutrophils were perfused for 5 minutes over CHO cells expressing wild-type or tailless E-selectin at a wall shear stress of 0.25 dyne/cm2. Without stopping flow, cell-free buffer was perfused at the same wall shear stress for 30 seconds, and the number of cells still rolling was taken as 100%. The wall shear stress was then increased incrementally every 30 seconds, and the percentage of neutrophils still rolling was determined. The data represent the mean plus or minus SEM of 5 experiments. (D,E) The mean velocity and the variance of velocity of neutrophils rolling on CHO cells expressing wild-type or tailless E-selectin were measured. The wall shear stress was 1 dyne/cm2. The data represent the mean plus or minus SEM for at least 4 experiments. (F) Fixed neutrophils were perfused over CHO cells expressing wild-type or tailless E-selectin in isotonic or hypertonic medium at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of at least 3 experiments (*P < .05; **P < .01; ***P < .001, as measured by the unpaired t test).
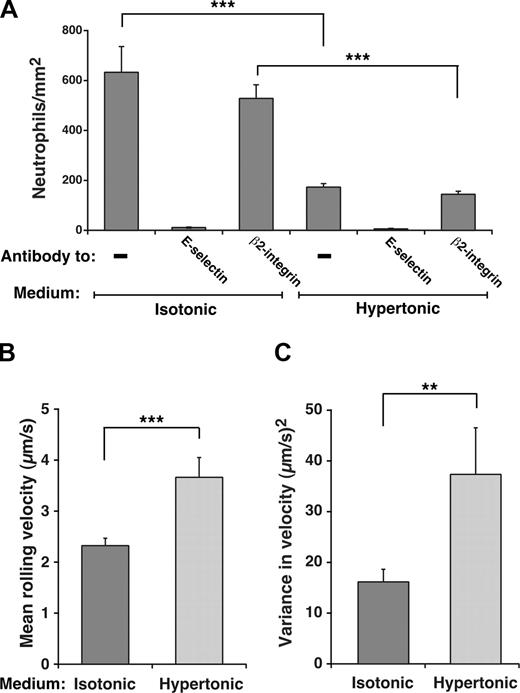
Unfixed neutrophils perfused over IL-1β-activated HUVECs normally roll on E-selectin and decelerate and arrest through β2 integrin interactions as they become activated.46 Because we used fixed neutrophils to compare rolling on HUVECs in isotonic or hypertonic medium, the contribution of β2 integrins to adhesion was eliminated, as confirmed by the elimination of rolling by anti-E-selectin mAb and the lack of effect on rolling by antiβ2 integrin mAb (Figure 4A). Furthermore, no neutrophils arrested on the HUVEC surface (H.S. and R.P.M., unpublished data, April 2006). Significantly fewer fixed neutrophils rolled on E-selectin on IL-1β-stimulated HUVECs incubated in hypertonic than in isotonic medium (Figure 4A), and cells in hypertonic medium rolled with faster velocities and greater variance in rolling steps (Figure 4B,C). However, the degree of reduction in rolling was not as great as that on transfected CHO cells, and those neutrophils still rolling in hypertonic medium exhibited slower velocities and less variance in rolling steps than those on transfected CHO cells, despite similar E-selectin densities (compare Figure 4B,C with Figure 3D,E). Nevertheless, these data demonstrate that interactions of E-selectin with clathrin-coated pits augment its adhesive function on HUVECs as well as transfected CHO cells.
Interactions of E-selectin with clathrin-coated pits of HUVECs enhance leukocyte rolling under flow. (A) Fixed neutrophils were perfused over IL-1β–stimulated HUVECs in isotonic or hypertonic medium in the absence or presence of 10 μg/mL anti-E-selectin mAb ES1 or anti-β2 integrin mAb IB4. The wall shear stress was 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. (B,C) The mean velocity and the variance of velocity of neutrophils rolling on IL-1β–stimulated HUVECs in isotonic or hypertonic medium was measured. The data represent the mean plus or minus SEM for at least 4 experiments (**P < .01; ***P < .001, as measured by the unpaired t test).
Interactions of E-selectin with clathrin-coated pits of HUVECs enhance leukocyte rolling under flow. (A) Fixed neutrophils were perfused over IL-1β–stimulated HUVECs in isotonic or hypertonic medium in the absence or presence of 10 μg/mL anti-E-selectin mAb ES1 or anti-β2 integrin mAb IB4. The wall shear stress was 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. (B,C) The mean velocity and the variance of velocity of neutrophils rolling on IL-1β–stimulated HUVECs in isotonic or hypertonic medium was measured. The data represent the mean plus or minus SEM for at least 4 experiments (**P < .01; ***P < .001, as measured by the unpaired t test).
A portion of E-selectin resides in lipid rafts of HUVECs but not of transfected CHO cells
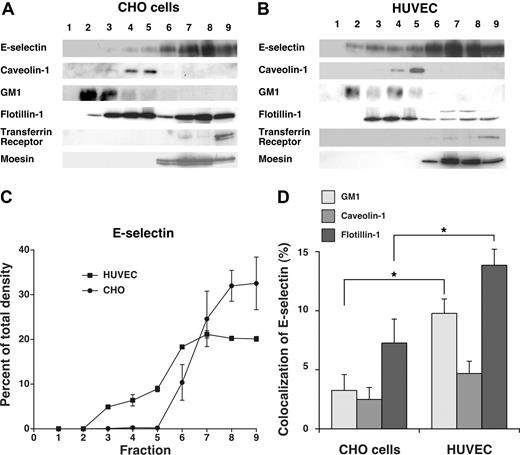
Although E-selectin was internalized through clathrin-coated pits of both HUVECs and transfected CHO cells, the residual neutrophil rolling on HUVECs in hypertonic medium raised the possibility that some E-selectin molecules on HUVEC, but not on CHO cells, were in domains that slowed movement into clathrin-coated structures. We considered that these domains might be lipid rafts in view of a report that E-selectin was largely distributed in rafts of HUVECs.27 To address this possibility, we extracted transfected CHO cells and HUVECs in cold 1% Triton X-100 and fractionated the extracts by centrifugation in a discontinuous OptiPrep gradient. The most buoyant fractions at the top of the gradient are enriched in cholesterol-rich raft microdomains that resist disruption with Triton X-100, whereas the less buoyant fractions at the bottom of the gradient contain nonraft components. We analyzed the fractions by blotting with probes to E-selectin, to caveolin-1 and GM1 (a membrane protein and a ganglioside enriched in lipid rafts), to transferrin receptor and moesin (a membrane protein and a cytosolic protein excluded from rafts), and to flotillin-1 (a membrane protein found in both raft and nonraft domains; Figure 5A,B). Although E-selectin was largely distributed in the nonraft fractions of both cell types, a small percentage of E-selectin was consistently found in lipid raft fractions of HUVECs but not of CHO cells (Figure 5C). This observation differed from a previous report that virtually all E-selectin was in lipid rafts of HUVECs.27 That report used a detergent-free procedure in which sonicated cell membranes were fractionated on a discontinuous sucrose gradient, as originally used to isolate caveolae.47 In this procedure, nonraft components might float in more buoyant fractions if they associate with raft structures.48 Detergent-based procedures can also yield variable results, as manifested by the divergent abilities of nonionic detergents to extract particular components of cholesterol-rich membrane domains.49 Caveolin-1 is found in the buoyant fractions of cells extracted with or without detergent, whereas some markers fractionate very differently depending on the extraction method.50 The clathrin-dependent endocytosis of E-selectin (Figure 1) and the colocalization of a portion of E-selectin with the clathrin-pit marker α-adaptin (Figure 2) strongly suggest that not all E-selectin is in lipid rafts. We also used confocal microscopy to compare the colocalization of E-selectin with caveolin-1, GM1, and flotillin-1 in CHO cells and HUVECs. There was significantly more colocalization of E-selectin with GM1 and flotillin-1 in HUVECs than in CHO cells (Figure 5D). In contrast, very little E-selectin colocalized with caveolin-1 in either cell type. These combined data demonstrate that a portion of E-selectin on HUVECs membranes is in noncaveolar lipid rafts that may also contain GM1 and flotillin-1.
A portion of E-selectin distributes in detergent-resistant fractions from HUVECs but not from transfected CHO cells. IL-1β–stimulated HUVECs or transfected CHO cells expressing wild-type E-selectin were lysed in cold 1% Triton X-100, and the lysate was centrifuged in a discontinuous Optiprep gradient. Fractions collected from top to bottom were resolved by SDS-PAGE and analyzed by Western blotting with mAbs to the indicated proteins, except for GM1, which was identified with cholera toxin B. (A,B) Representative Western blots of gradients from CHO cells and HUVECs. (C) The percentage of E-selectin in each fraction was quantified by the density of blotted signals using Adobe Photoshop software. The data represent the mean plus or minus SEM of at least 4 experiments. (D) Confocal microscopy was used to measure colocalization of E-selectin with GM1, caveolin-1, or flotillin-1 in IL-1β–stimulated HUVECs or transfected CHO cells expressing wild-type E-selectin, using methods, such as those in Figure 2 and detailed in “Methods.” The degree of colocalization was quantified by measuring the percentage of green pixels (E-selectin) that colocalized with red pixels (GM1, caveolin-1, or flotillin-1). The data represent the mean plus or minus SEM of at least of 3 experiments, with at least 10 cells counted in each experiment (*P < .05, as measured by the unpaired t test).
A portion of E-selectin distributes in detergent-resistant fractions from HUVECs but not from transfected CHO cells. IL-1β–stimulated HUVECs or transfected CHO cells expressing wild-type E-selectin were lysed in cold 1% Triton X-100, and the lysate was centrifuged in a discontinuous Optiprep gradient. Fractions collected from top to bottom were resolved by SDS-PAGE and analyzed by Western blotting with mAbs to the indicated proteins, except for GM1, which was identified with cholera toxin B. (A,B) Representative Western blots of gradients from CHO cells and HUVECs. (C) The percentage of E-selectin in each fraction was quantified by the density of blotted signals using Adobe Photoshop software. The data represent the mean plus or minus SEM of at least 4 experiments. (D) Confocal microscopy was used to measure colocalization of E-selectin with GM1, caveolin-1, or flotillin-1 in IL-1β–stimulated HUVECs or transfected CHO cells expressing wild-type E-selectin, using methods, such as those in Figure 2 and detailed in “Methods.” The degree of colocalization was quantified by measuring the percentage of green pixels (E-selectin) that colocalized with red pixels (GM1, caveolin-1, or flotillin-1). The data represent the mean plus or minus SEM of at least of 3 experiments, with at least 10 cells counted in each experiment (*P < .05, as measured by the unpaired t test).
Interactions of E-selectin with lipid rafts in HUVECs enhance leukocyte rolling under flow
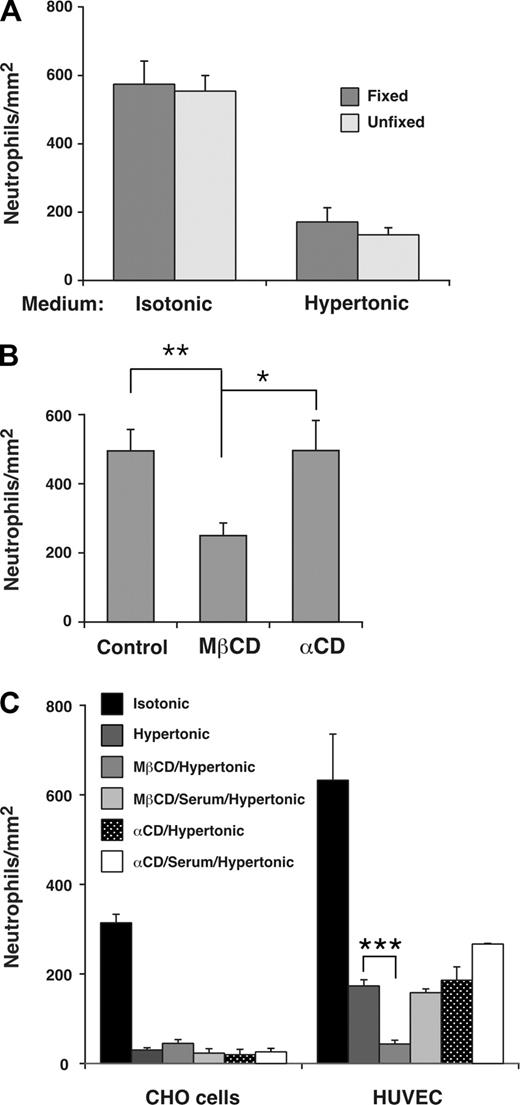
To determine whether the distribution of a subset of E-selectin in lipid rafts affected leukocyte rolling on E-selectin, we treated HUVECs with MβCD, which reversibly disrupts lipid rafts by extracting cholesterol from the cell membrane.51 The flexibility of leukocyte membranes contributes significantly to rolling efficiency, and MβCD-treated leukocytes roll less efficiently because MβCD also increases the rigidity of cell membranes by extracting cholesterol.5 To determine whether membrane flexibility of HUVECs affects rolling, we incubated HUVECs in isotonic or hypertonic medium and then fixed a portion of the HUVECs in paraformaldehyde, which increases membrane rigidity more than MβCD does.5 Neutrophils rolled on fixed or unfixed HUVECs in similar numbers (Figure 6A) and with equivalent velocities (H.S. and R.P.M., unpublished data, February 2007). This demonstrates that the deformability of HUVECs membranes is not a major contributor to leukocyte rolling under these experimental conditions, and indicates that MβCD would not affect leukocyte rolling through changes in HUVEC membrane rigidity. We next pretreated HUVECs in control isotonic medium or in isotonic medium containing MβCD or the inactive analog α-cyclodextrin. Neutrophils rolled on MβCD-treated HUVECs in significantly fewer numbers (Figure 6B) and with faster velocities (H.S. and R.P.M., unpublished data, October 2007) than on control or α-cyclodextrin-treated HUVECs.
Interactions of E-selectin with lipid rafts in HUVECs enhance leukocyte rolling under flow. (A) Fixed neutrophils were perfused over unfixed or fixed IL-1β–stimulated HUVECs in isotonic or hypertonic medium at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of 3 experiments. (B) IL-1β–stimulated HUVECs were pretreated in control isotonic medium or in medium containing MβCD or α-cyclodextrin (α-CD). Fixed neutrophils were then perfused in isotonic medium at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of 5 experiments. (C) Fixed neutrophils were perfused over transfected CHO cells expressing wild-type E-selectin or IL-1β–stimulated HUVECs in isotonic medium; hypertonic medium; hypertonic medium after pretreatment of CHO cells or HUVECs with MβCD; hypertonic medium after pretreatment with MβCD and then 20% serum; hypertonic medium after pretreatment with α-cyclodextrin; or hypertonic medium after pretreatment with α-cyclodextrin and then 20% serum. The wall shear stress was 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of at least 3 experiments (*P < .05; **P < .01; ***P < .001, as measured by the unpaired t test).
Interactions of E-selectin with lipid rafts in HUVECs enhance leukocyte rolling under flow. (A) Fixed neutrophils were perfused over unfixed or fixed IL-1β–stimulated HUVECs in isotonic or hypertonic medium at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of 3 experiments. (B) IL-1β–stimulated HUVECs were pretreated in control isotonic medium or in medium containing MβCD or α-cyclodextrin (α-CD). Fixed neutrophils were then perfused in isotonic medium at a wall shear stress of 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of 5 experiments. (C) Fixed neutrophils were perfused over transfected CHO cells expressing wild-type E-selectin or IL-1β–stimulated HUVECs in isotonic medium; hypertonic medium; hypertonic medium after pretreatment of CHO cells or HUVECs with MβCD; hypertonic medium after pretreatment with MβCD and then 20% serum; hypertonic medium after pretreatment with α-cyclodextrin; or hypertonic medium after pretreatment with α-cyclodextrin and then 20% serum. The wall shear stress was 1 dyne/cm2. The number of rolling neutrophils was measured after 4 minutes. The data represent the mean plus or minus SEM of at least 3 experiments (*P < .05; **P < .01; ***P < .001, as measured by the unpaired t test).
Because MβCD may inhibit clathrin-mediated endocytosis,32,52 we treated HUVECs with hypertonic medium as well as MβCD to eliminate the effects of MβCD on clathrin lattices. As described earlier, incubation of transfected CHO cells in hypertonic medium alone virtually eliminated their ability to support neutrophil rolling, whereas incubation of HUVECs in hypertonic medium alone significantly impaired but did not eliminate neutrophil rolling (Figure 6C). Sequential treatment of HUVECs with MβCD and hypertonic medium abrogated this residual neutrophil rolling on E-selectin (Figure 6C). The effect of MβCD required chelation of cholesterol because sequential treatment with the inactive analog α-cyclodextrin and hypertonic medium did not eliminate rolling. Furthermore, replacing MβCD with medium containing 20% serum, which restores cholesterol to cell membranes, did not eliminate the residual neutrophil rolling on E-selectin (Figure 6C). These results demonstrate that positioning a subset of E-selectin molecules in lipid rafts further enhances leukocyte rolling on HUVECs under flow.
Discussion
Our data establish that E-selectin distributes in 2 distinct membrane domains of human endothelial cells: clathrin-coated pits and lipid rafts. Although E-selectin is internalized through clathrin-coated pits, clustering in both membrane domains markedly enhances its ability to mediate leukocyte rolling under flow.
P-selectin is internalized through clathrin-coated pits at an even faster rate than E-selectin, suggesting that it has a higher affinity for these structures.14 Deleting the cytoplasmic domain of P-selectin eliminates its ability to undergo clathrin-mediated endocytosis and significantly impairs its ability to support leukocyte rolling.14,15 E-selectin lacking the cytoplasmic domain was still internalized through a clathrin-dependent pathway in transfected CHO cells, albeit at a very slow rate. This residual endocytosis might be mediated by weak associations of the extracellular or transmembrane domain of E-selectin with another membrane protein that binds through its cytoplasmic domain to the clathrin endocytic machinery. However, any remaining clustering of tailless E-selectin in clathrin-coated pits of CHO cells was insufficient to augment neutrophil rolling over that observed in hypertonic medium. Thus, the cytoplasmic domain of E-selectin, like that of P-selectin, makes a major contribution to its adhesive function under flow.
On both CHO cells and HUVECs, the residual neutrophils that rolled on E-selectin in hypertonic medium did so with much faster velocities and more irregular rolling steps than those in isotonic medium. The simplest interpretation of these results is that clustering of E-selectin in clathrin-coated pits favors clusters of bonds with E-selectin ligands on rolling neutrophils. When one bond in a cluster dissociates, it has an opportunity to reassociate because the remaining bonds in the cluster support the adhesive tether at the trailing edge of the leukocyte, where force is applied.10 This allows more time for new bonds to form at the leading edge of the cell before the last bond at the trailing edge dissociates. The result is slower and more regular rolling motions. In vivo, slow neutrophil rolling on E-selectin prolongs transit times in postcapillary venules. This allows more opportunity for endothelial cell chemokines to activate neutrophil β2 integrins to a high-affinity conformation, which causes neutrophils to arrest.53,54 Clustering of E-selectin may be essential for neutrophils to roll sufficiently slowly so that chemokines can signal. Furthermore, interactions of E-selectin with neutrophil PSGL-1 signal activation of β2 integrins to a low affinity but extended conformation that further slows rolling.55 Signaling may require crosslinking of PSGL-1, which is more likely to occur if rolling neutrophils encounter E-selectin clustered in membrane domains.
Neutrophils treated to prevent β2 integrin interactions rolled with slower velocities and more regular motions on E-selectin on IL-1β–activated HUVECs than on transfected CHO cells, even though the surface density of E-selectin was equivalent on both cell types. Although this might be due to greater clustering of E-selectin in clathrin-coated pits of HUVECs than of CHO cells, the slower internalization rate of E-selectin in HUVECs than in CHO cells (Figure 1) argues against this interpretation. Instead, E-selectin also clustered in lipid rafts of HUVECs. Importantly, disruption of lipid rafts with the cholesterol chelator MβCD virtually eliminated the residual neutrophil rolling on IL-1β–stimulated HUVECs in hypertonic medium. These combined data support a model where E-selectin must distribute in both clathrin-coated pits and lipid rafts of endothelial cells to support optimal leukocyte rolling under flow (Figure 7). Many lipid rafts are much smaller than clathrin-coated pits,48,56 as depicted in Figure 7, although cytoskeletal interactions can drive small rafts to merge into much larger structures.57 Neutrophils rolled faster on HUVECs that were treated to disrupt either clathrin-coated pits or lipid rafts, suggesting that both membrane domains contribute to the efficiency of E-selectin–mediated rolling. Each membrane pool of E-selectin might also support specific functions such as the initial tethering of flowing leukocytes, postadhesion signaling in endothelial cells, or cooperative signaling with endothelial cell-bound chemokines to activate integrins on rolling leukocytes. Selectins require force loading to stabilize their bonds with glycosylated ligands.45,58,–60 This force loading may require adequate anchorage of selectins or their ligands in the cell membrane.11 Interactions of the cytoskeleton with clathrin-coated pits61 and lipid rafts57 may contribute to anchorage and prevent forcible extraction of selectins or their ligands as leukocytes roll along the vascular surface. Disruption of membrane domains in endothelial cells might diminish the ability of the cytoskeleton to optimize force loading on E-selectin–ligand bonds.
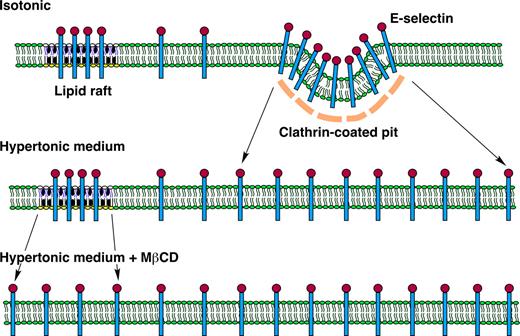
Model of E-selectin distribution in membrane domains of endothelial cells. E-selectin distributes reversibly among nonraft and raft domains in the plasma membrane of endothelial cells. At any time point, most E-selectin molecules are in nonraft domains, and some of these are clustered in clathrin-coated pits. A minor population of E-selectin clusters in lipid rafts that appear to be distinct from caveolae. The major pathway for internalization of E-selectin is through clathrin-coated pits. Hypertonic medium prevents clustering of E-selectin in clathrin-coated pits by disrupting the clathrin lattice structure. MβCD prevents clustering of E-selectin in lipid rafts by chelating cholesterol, a major component of the rafts. Disruption of both structures on endothelial cells markedly impairs the ability of E-selectin to mediate leukocyte rolling under flow, probably by hindering the ability of E-selectin to form clusters of bonds with ligands on rolling leukocytes.
Model of E-selectin distribution in membrane domains of endothelial cells. E-selectin distributes reversibly among nonraft and raft domains in the plasma membrane of endothelial cells. At any time point, most E-selectin molecules are in nonraft domains, and some of these are clustered in clathrin-coated pits. A minor population of E-selectin clusters in lipid rafts that appear to be distinct from caveolae. The major pathway for internalization of E-selectin is through clathrin-coated pits. Hypertonic medium prevents clustering of E-selectin in clathrin-coated pits by disrupting the clathrin lattice structure. MβCD prevents clustering of E-selectin in lipid rafts by chelating cholesterol, a major component of the rafts. Disruption of both structures on endothelial cells markedly impairs the ability of E-selectin to mediate leukocyte rolling under flow, probably by hindering the ability of E-selectin to form clusters of bonds with ligands on rolling leukocytes.
E-selectin appeared to reside in lipid rafts that were distinct from caveolae. Why E-selectin distributes in rafts of HUVECs but not of CHO cells is not known. Lipid rafts are extremely heterogeneous,48,56 and endothelial cells are rich in these structures.62 Perhaps endothelial cells synthesize a particular type of raft domain that has greater affinity for E-selectin than rafts in CHO cells. Indeed, it is possible that even more E-selectin partitions in rafts of endothelial cells in vivo. Although both P- and E-selectin enter clathrin-coated pits, it is noteworthy that only E-selectin was detectably associated with lipid rafts. The molecular basis for the association of E-selectin with rafts requires further study.
Proteins and lipids can be internalized through a clathrin-dependent pathway or a clathrin-independent, lipid raft-mediated pathway.63,64 Flotillin-1 participates in a clathrin-independent endocytic pathway,65 and the ganglioside GM1 is excluded from clathrin-coated pits.28 Therefore, the subset of E-selectin molecules that colocalize with these markers in lipid rafts might enter cells by a clathrin-independent mechanism. If so, they must be internalized in small quantities and at a very slow rate that we could not detect with our assays. We found that E-selectin was primarily internalized by a clathrin-dependent mechanism because endocytosis was blocked by hypertonic medium, which disrupts clathrin lattices but not lipid rafts.35,36 There are precedents for membrane proteins that distribute into lipid rafts but are internalized through clathrin-coated pits.33,66,67 Membrane proteins interact reversibly with lipid rafts or clathrin-coated pits. Depending on its relative affinity for these structures, E-selectin might dissociate from a lipid raft and diffuse in the plasma membrane until it encounters a clathrin-coated pit. It might then interact with components of the pit and be internalized, unless it dissociates before the pit buds off to form a vesicle. Both P-selectin and E-selectin are endocytosed through a constitutive clathrin-mediated pathway. However, inflammatory mediators differentially regulate the rate that P-selectin enters clathrin-coated pits.31 Signaling also regulates the rate at which some proteins in lipid rafts become internalized through clathrin-coated pits.67 Perhaps thrombotic or inflammatory mediators differentially affect the clustering and/or internalization of E-selectin through clathrin-dependent or -independent pathways. This could affect how leukocytes initially roll on E-selectin as well as how E-selectin signals integrin activation on rolling leukocytes. Leukocyte adhesion may then cause E-selectin to cluster into larger raft domains. These domains may propagate signals into endothelial cells that regulate later events in the inflammatory cascade.27
Our results, in conjunction with previous studies, underscore the importance of particular cell surface presentations of selectins or their ligands during leukocyte rolling on vascular surfaces under flow. Unlike several other adhesion receptors, P-selectin and E-selectin undergo rapid internalization from the cell surface. Leukocytes expressing α4 integrins also roll on endothelial cells through interactions with vascular cell adhesion molecule-1.68,69 There is some evidence that vascular cell adhesion molecule-1 is internalized through clathrin-coated pits.70 Perhaps adhesion molecules on endothelial cells have evolved to associate with coated pits and other specialized structures to better support the initial capture and rolling of leukocytes under flow. It is noteworthy that both P-selectin and E-selectin must cluster in membrane domains of endothelial cells to enhance their functions, even though they differ significantly in their cell-surface densities and in their affinity and specificity for ligands on leukocytes. In contrast to P-selectin, which is clustered only in clathrin-coated pits, E-selectin distributes into both clathrin-coated pits and lipid rafts, each of which augments its adhesive properties.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lisa Mayer, Cindy Carter, Courtney Kerbo, and Todd Walker for excellent technical assistance and Anna Patrick for her contributions during her summer research project as a Sir Alexander Fleming Scholar. The authors also thank the Pathology Department of Mercy Lab Oklahoma for providing umbilical cords, and Mark Coggeshall, William Rodgers, Florea Lupu, and David Schmidtke for critical reading of the manuscript. Confocal microscopy was performed in the Flow Cytometry and Confocal Microscopy Laboratory of the University of Oklahoma Health Sciences Center.
This work was supported by National Institutes of Health (grant HL 34363).
National Institutes of Health
Authorship
Contribution: H.S. designed and conducted research and wrote the first draft of the manuscript; R.P.M. designed research, analyzed and interpreted experimental data, and wrote the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rodger P. McEver, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: rodger-mcever@omrf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal