To define the role of the α2β1 integrin in pathologic angiogenesis, we investigated tumor-associated growth and angiogenesis in wild-type and α2-null mice. Our findings reveal that the α2β1 integrin plays an important role in angiogenesis via regulation of VEGFR1 expression. When challenged with B16F10 melanoma cells, mice lacking α2β1 integrin ex-pression exhibit increased tumor angiogenesis associated with up-regulated VEGFR1 expression. In contrast, there was no α2β1 integrin-dependent difference in the angiogenic response to Lewis lung carcinoma (LLC) cells. Interestingly, whereas B16F10 cells secrete high levels of placental growth factor (PLGF), LLC cells produce high levels of VEGF, but low levels of PLGF. The α2β1 integrin-dependent difference in angiogenesis was restored to LLC cells by expression of PLGF, strongly suggesting that the angiogenic phenotype and tumor growth in the α2-null host is dependent on specific interactions between the tumor cell and the genetically defined integrin repertoire of the host microenvironment. Thus integrin α2-null mice represent an example of genetic alterations of “the soil” determining response to the “seed.”
Introduction
Tumor initiation and progression involve complex interactions between tumor cells and their microenvironment.1 Integrins are expressed on both tumor cells and cells of the microenvironment where they modulate tumor initiation, progression, and angiogenesis.2,,–5 Several integrins, including the αvβ3, αvβ5, α4β1, and α5β1 have been implicated in angiogenesis.6,,–9
The α1β1 and α2β1 integrins, the 2 major collagen receptors, have also been implicated in the pathobiology of tumor angiogenesis.10,,,,,,–17 Genetic deletion of the α1β1 integrin supported the concept that the α1β1 integrin was proangiogenic.16,17 The α1-deficient mice demonstrate decreased tumor growth and angiogenesis, a finding consistent with the α1β1 integrin serving a proangiogenic function. Recent wound healing studies in wild-type and α2β1 integrin–deficient mice demonstrated that deletion of the α2β1 integrin resulted in increased neoangiogenesis, suggesting that the α2β1 integrin plays a negative role in regulating neoangiogenesis within the wounded microenvironment.18,19
In this present study we investigated the molecular mechanisms whereby loss of the α2β1 integrin leads to increased neovascularization, particularly in the context of tumor-associated angiogenesis. We show that the α2β1 integrin plays an unexpected role in regulating tumor neoangiogenesis in vivo. Expression of the α2β1 integrin is up-regulated on endothelium within the tumor microenvironment. Unlike α1-null mice, α2-null mice exhibit increased tumor angiogenesis and consequent increased tumor growth when challenged with B16F10 melanoma cells. Increased expression of vascular endothelial cell growth factor receptor 1 (VEGFR-1) on α2-null endothelial cells within the tumor microenvironment is in part responsible for the increased angiogenesis. In contrast to this finding, no difference in tumor growth or angiogenesis was observed between wild-type and α2-null mice bearing Lewis lung carcinoma (LLC) cells. The differential response of the α2-null microenvironment to different tumors was in part a consequence of increased secretion of placental growth factor (PLGF) by B16F10 tumors, but not LLC tumors. Thus, tumor angiogenesis and tumor growth in the α2-null host is dependent on cross-talk between the genetically defined host microenvironment and the tumor cells, an example of genetic alterations of “the soil” determining the response to the “seed.”
Methods
Animals
The α2 integrin subunit-deficient mice, originally generated on a C57Bl/6 × 129/SvJ background were backcrossed 8 times to the C57/BL6 background using a microsatellite marker-assisted selection (“speed congenics”) approach with the assistance of the Rheumatic Diseases Core Center at Washington University.20 The mice used in these experiments were 99% genetically C57BL/6 based on markers spaced an average of about 10 cM to 20 cM across the mouse genome. Animals were housed in pathogen-free conditions at Vanderbilt University Medical Center in compliance with institutional animal care and use committee regulations. All animals were appropriately age- and sex-matched.
Tumor studies and in vivo angiogenesis
B16F10 cells, a gift from Dr Wayne Yokoyama (Washington University School of Medicine) and LLC cells, a gift from Dr Raymond Dubois (Vanderbilt University School of Medicine), were injected into the flanks of wild-type and α2-null mice (106 cells in 50 μL phosphate-buffered saline [PBS]). Tumor volume was determined at different time points following injection using the equation: volume = a × (b)2 × 0.52, where a is the longest dimension and b is the shortest. For reconstitution, B16F10 melanoma cells (106 cells) were mixed with wild-type or α2-null primary pulmonary endothelial cells (2 × 105 cells), generated as described and injected subcutaneously into the flanks of wild-type mice. After 8 days from injection, the tumors were retrieved and a portion of each tumor was fixed in 10% formalin and snap-frozen in Tissue-Tek OCT Compound (Sakura Finetek USA, Torrance, CA).
LLC cells were transfected with pcDNA3.1-mPLGF (a gift of Dr Laura Benjamin, Harvard Medical School, Boston, MA) and selected in G418 at 700 μg/mL for one week. Enzyme-linked immunosorbent assays (ELISAs) to quantitate the level of PLGF were performed on conditioned media from transfectants and control cells using the Quantikine Immunoassay kit (R&D Systems, Minneapolis, MN). B16F10 melanoma cells were transduced with mouse PLGF shRNA lentivirus or a GFP-control lentivirus (Sigma-Aldrich, St Louis, MO) and selected for one week in 1 μg/mL puromycin. The levels of PLGF in conditioned media were 94 pg/mL for the control GFP cells and 26 pg/mL for PLGF shRNA cells.
Matrigel plug assays were performed as described by Passaniti et al21 using growth factor–reduced Matrigel (200 μL; BD Biosciences, Franklin Lakes, NJ), 60 U/mL heparin (Sigma-Aldrich), and either vascular endothelial growth factor A (VEGF-A), PLGF (250 ng/mL), or VEGF-C (500 ng/mL) from R&D Systems. After 8 days, the gels were retrieved and the number of vessels in 10 high-power fields was determined.
Histology, immunohistochemistry, and immunofluorescence analyses
Tumor morphology was evaluated on paraffin-embedded, hematoxylin and eosin–stained sections. The area of tumor necrosis was determined on stained sections photographed at ×10. Necrotic areas were measured quantitatively in 9 low-power fields for each tumor using the Metamorph imaging system (Molecular Devices, Sunnyvale, CA). Immunohistochemical identification of CD31+ vessels was performed on 7 μm frozen sections stained with antimouse CD31 (BD Pharmingen, San Diego, CA) and a biotin-conjugated secondary antibody and diaminobenzidine substrate.22 Sections, with labels coded to blind the observer, were imaged with a Nikon Eclipse 80i microscope and digital camera (Pixera, Los Gatos, CA), and the images were processed by using Scion Image (Frederick, MD) software. CD31+ structures in each section were automatically counted and their areas were measured. Differences in tumor vascularity (number of and area occupied by CD31+ structures per tumor microscopic field) were determined for each section. Data were transferred to a spreadsheet and then sorted by genotype.
Immunofluorescence analyses were conducted on 7 μm frozen sections with the primary antibodies, antimouse CD31, anti-α1 integrin subunit (Clone Hα31/8), anti-α2 integrin subunit (Clone HMα2) from BD Pharmingen, anti-VEGFR1 (Flt-1), anti-VEGFR2 (Flk-1) from R&D Systems, anti–α-SMA from DAKO (Carpinteria, CA) or anti-Ki67 from Abcam (Cambridge, MA), secondary antibodies Alexa 594– or Alexa 488–conjugated IgG and DAPI, all from Molecular Probes (Eugene, OR). Quantification of the VEGFR1 immunofluorescence signal was carried out using the Scion Image system. VEGFR1+ structures in 5 high-power fields/tumor with 5 animals in each group were elucidated and their area and intensity of staining was quantitated. The total intensity was expressed as the area × density.
Ultrasound data acquisition
A VisualSonics 770 high-resolution imaging system (Visual Sonics, Toronto, ON) equipped with a 30-MHz transducer was used for these experiments. After anesthetization, scout images were obtained to determine the extent of the tumor region via 3D B-mode imaging. The transducer holder allows for the steady acquisition of a 512 × 512 acquisition matrix over a 12-mm to 15-mm field of view (depending on tumor size) and a 20-mm image width. During 3D acquisition, the holder gently and automatically slides over the length of the tumor and acquires one image at each of 90 to 170 (depending on tumor size) contiguous slices that are each 100 μm thick. Once the 3D B-mode acquisition was optimized to cover the whole length of the tumor, 3D power Doppler images were acquired with the same field of view and imaging dimensions. The scan speed and wall filters were held constant at 2.0 mm/s and 2.5 mm/s, respectively, for all studies. In power Doppler images, regions with blood flow are assigned a color level in arbitrary units from no power Doppler signal to maximum power Doppler signal. For each slice in the 3D power Doppler image stack, the fractional area displaying a power Doppler signal was calculated. By dividing the summed number of voxels displaying a power Doppler signal by the total tumor area, the percent vascularity was calculated.
Endothelial cell isolation and aortic ring assay
Recovery of primary pulmonary endothelial cells was carried out essentially as described.16,17,23 Briefly, the lung vasculature was perfused with PBS, 2.5 mM EDTA, followed by 0.25% trypsin, 2.5 mM EDTA via the right ventricle. Lungs were removed and incubated at 37°C for 20 minutes. The visceral pleura was subsequently trimmed and the perfusion was repeated. Primary endothelial cells were recovered and grown on tissue culture plastic in microvascular endothelial cell medium-2 (EGM-2-MV) containing 5% FCS (Clonetics). Primary endothelial cells greater than 90% pure by immunostaining with anti-VEGFR2 and anti-CD31 at passages 2 to 4 were used for experiments.
Tumor endothelial cells were isolated from tumor tissue by collagenase digestion and washing. Dead cells and CD45+ cells were depleted using Dead Cell Removal Kit or MACS mouse CD45 microbeads (Miltenyi Biotec, Auburn, CA), respectively. Tumor endothelial cells were sorted by FACS using PE-conjugated antimouse Flk-1 (VEGFR2) antibody (BD Biosciences).
Aortic ring assays were performed as described by Nicosia and Ottinetti.24 Thoracic aortas, isolated from 8- to 12-week-old wild-type or α2-null mice were embedded between 2 layers of 50 μL growth factor–reduced Matrigel supplemented with 60 U/mL of heparin. Vessel sprouting stimulated by the addition of 40 ng/mL VEGF or PLGF was quantitated from day 4 to day 8.
For proliferation assays, 5 × 103 primary endothelial cells in EGM-2-MV were plated on 96-well plates coated with either 10 μg/mL collagen I, 10 μg/mL collagen IV, 10 μg/mL fibronectin, or on tissue culture plastic. After 3 days, cells were pulsed for an additional 24 hours with 3H-thymidine (1 μCi/well; 3.7 × 104 Bq). In the inhibition experiment, complete medium was changed to serum-free medium 12 hours after plating. Neutralizing antibodies anti–flt-1 or anti–flk-1 (10 μg/mL; both from R&D Systems) were added and replaced fresh every other day. After 3 days, cells were pulsed for an additional 24 hours with 3H-thymidine. Proliferation assays using the inhibitory anti-α2β1 integrin antibody (BD Pharmingen) were performed using the CellTiter 96 Aqueous Nonradioactive Cell Proliferation Assay Kit (Promega, Madison, WI). The inhibitory anti-α2β1 integrin antibody (clone Ha1/29; 5 μg/mL, 10 μg/mL, and 20 μg/mL) or isotype control IgG was added to 2 × 104 cells in a 96-well plate at 24 hours. After 48 hours, 20 μL combined 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(40-sulfophenyl)-2H-tetrazolium plenazine methosulfate (MTS/PMS) substrate was added into each well and the absorbance at 492 nm was recorded after a 2-hour incubation at 37°C in the incubator.
Immunoblot analysis and ELISAs
Equal amounts of protein lysate from endothelial cells were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with either primary anti-VEGFR1 (Flt-1) or anti-VEGFR2 (Flk-1) antibody from R&D Systems at 4°C followed by secondary horseradish peroxidase–conjugated sheep antimouse or antirabbit antibody (GE Healthcare, Little Chalfont, United Kingdom). Enhanced chemiluminescence system (GE Healthcare) was used for visualization.
ELISAs were performed on conditioned media from LLC or B16F10 melanoma cells after 48 hours. The levels of VEGF and PLGF were quantitated by ELISA using the Quantikine Immunoassay kit from R&D Systems, according to the manufacturer's protocol.
Real-time quantitative reverse transcriptase–polymerase chain reaction
Total RNA was isolated from endothelial cells using the Trizol Reagent (Invitrogen, Carlsbad, CA) and further purified with the RNeasy RNA extraction kit (Qiagen, Valencia, CA). The mRNAs were reverse-transcribed into cDNAs using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time amplifications were performed in the Bio-Rad i-Cycler system with the α1 integrin subunit forward and reverse primers, respectively; 5′-CACCTTTCAAACTGAGCCCGCCA-3′ and 5′-GCTGCCCAGCGATGTAGAGCACAT-3′. The mRNA level for the α1 integrin subunit was calculated using the relative standard curve. Samples were normalized using GAPDH mRNA.
Lentiviral α2 plasmid construction and transduction of endothelial cells
pLenti-α2 was generated using the pLenti6/V5 Directional TOPO cloning kit (Invitrogen). The human α2 integrin subunit was amplified by polymerase chain reaction (PCR) from human α2 integrin subunit cDNA, as previously described.25,26 Lentivirus α2 integrin subunit was produced using the ViraPower Lentiviral Expression System (Invitrogen). Concentrated α2-lentivirus or GFP control virus was added to α2-null endothelial cells cultured in endothelial cell growth media (EGM-2-MV with 5% fetal calf serum [FCS]) and incubated at 37°C. After 72 hours, transfection efficiency was determined by fluorescence activated cell sorting (FACS) analysis using PE antihuman α2 (CD49b) antibody (BD Biosciences).
Statistical analysis
All experiments were repeated 3 or 4 times. Statistical analysis was performed using either analysis of variance (ANOVA) or the unpaired Student t test, and P < .05 was considered statistically significant. All calculations and graphs were performed using GraphPad Prism version 4 (GraphPad Software, San Diego, CA).
Results
Tumor angiogenesis is enhanced in α2β1 integrin–deficient mice
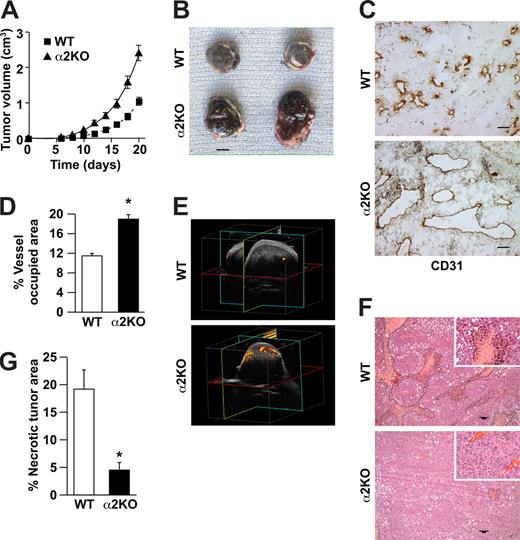
To determine whether expression of the α2β1 integrin by cells of the host microenvironment plays a role in tumor growth, wild-type and α2-null mice (on a pure C57/BL6 background) were injected subcutaneously with syngeneic B16F10 melanoma cells that do not express the integrin (data not shown). As shown in Figure 1A, tumor growth was more rapid in α2-null recipients than in wild-type mice, resulting in larger tumors at all time points from day 6 to day 21. At 21 days after injection, tumor morphology was evaluated. The tumors in the α2-null mice were larger (Figure 1B). Tumors in both wild-type and α2-null mice were composed of sheets of tumor cells with minimal inflammation, as determined by staining with anti-CD45 antibody (data not shown). Tumor vessels, identified by immunohistochemical staining with anti-CD31 antibody, were larger and occupied a significantly greater percentage of total tumor area in the α2-null than in the wild-type mice (Figure 1C,D).
Enhanced growth of syngeneic, B16F10 melanoma in α2β1 integrin–deficient mice. (A) Tumor volume of B16F10 melanoma cells in α2-null mice (KO) and their wild-type (WT) littermates as a function of time. The data are presented as the mean plus or minus the standard error of the mean (SEM) with 18 animals of each genotype (P < .001, statistical analysis by ANOVA). (B) Photograph of tumors resected after 21 days (scale bar = 5 mm). (C) Representative photomicrograph of anti-CD31 antibody staining of tumor sections (scale bar = 50 μm; 20×/0.75 NA objective). (D) Total area occupied by CD31+ structures representing tumor vascular area as a percentage of total tumor area. Tumors from α2-null hosts show increased vascularity. Data are presented as the mean plus or minus SEM (9 tumors per genotype from 3 separate experiments; *P < .001). (E) The amplitude of blood flow within the tumor determined by power Doppler. There was a marked increase in blood flow to the tumor (as seen in yellow) in the α2-null mice in comparison to wild-type mice at 14 days after tumor injection. Total blood flow to the tumor was 7.68% (± 1.24%) in α2-null mice and 2.5% (± 0.43%) in the wild-type controls. A representative image from 3 separate experiments with 2 mice in each experiment is shown. (F) Representative low power images of hematoxylin and eosin–stained sections highlight tumor necrosis (necrosis outlined in black; scale bar = 100 μm; 10×/0.45 NA objective). (G) Tumor necrosis in wild-type and α2-null mice was morphologically quantitated. Necrotic area is presented as a percentage of the total tumor area from 5 low-power fields from 11 tumors per genotype. Data are presented as mean plus or minus SEM (*P = .002).
Enhanced growth of syngeneic, B16F10 melanoma in α2β1 integrin–deficient mice. (A) Tumor volume of B16F10 melanoma cells in α2-null mice (KO) and their wild-type (WT) littermates as a function of time. The data are presented as the mean plus or minus the standard error of the mean (SEM) with 18 animals of each genotype (P < .001, statistical analysis by ANOVA). (B) Photograph of tumors resected after 21 days (scale bar = 5 mm). (C) Representative photomicrograph of anti-CD31 antibody staining of tumor sections (scale bar = 50 μm; 20×/0.75 NA objective). (D) Total area occupied by CD31+ structures representing tumor vascular area as a percentage of total tumor area. Tumors from α2-null hosts show increased vascularity. Data are presented as the mean plus or minus SEM (9 tumors per genotype from 3 separate experiments; *P < .001). (E) The amplitude of blood flow within the tumor determined by power Doppler. There was a marked increase in blood flow to the tumor (as seen in yellow) in the α2-null mice in comparison to wild-type mice at 14 days after tumor injection. Total blood flow to the tumor was 7.68% (± 1.24%) in α2-null mice and 2.5% (± 0.43%) in the wild-type controls. A representative image from 3 separate experiments with 2 mice in each experiment is shown. (F) Representative low power images of hematoxylin and eosin–stained sections highlight tumor necrosis (necrosis outlined in black; scale bar = 100 μm; 10×/0.45 NA objective). (G) Tumor necrosis in wild-type and α2-null mice was morphologically quantitated. Necrotic area is presented as a percentage of the total tumor area from 5 low-power fields from 11 tumors per genotype. Data are presented as mean plus or minus SEM (*P = .002).
We hypothesized that increased vascular area would permit increased blood flow to the tumor vascular bed. Therefore, vascular perfusion within the tumor was determined by Power Doppler. As shown in Figure 1E, there was a significant increase in blood flow to tumors in the α2-null mice (7.7% ± 1.2%) compared with wild-type mice (2.5% ± 0.4%) at 14 days after tumor injection. As expected from the dramatic differences in tumor blood flow, the area of necrotic tumor was significantly decreased in the α2β1 integrin–deficient mice when compared with their littermate controls (Figure 1F,G).
α2β1 integrin expression by endothelial cells and endothelial cell proliferation
As shown in Figure 2A, the α2β1 integrin was expressed at high levels by CD31+ endothelial cells in the wild-type tumor microenvironment. As expected, there was no expression in tumors growing in the α2-null hosts. There was no discernable expression of the α2β1 integrin by the smooth muscle cells/pericytes in the wild-type hosts. Since the tumor vasculature did not express the lymphatic marker LYVE-1, the vessels within tumors were not of lymphatic origin. In contrast to vessels of the tumor microenvironment, expression of the α2β1 integrin was not detectable on quiescent, capillary endothelial cells in many other sites including the skin, heart, kidney, liver, and lung (Figure 2A and data not shown).
α2β1 integrin expression and endothelial cell proliferation in vivo and in vitro. (A) Immunofluorescence analysis demonstrated colocalization of α2β1 integrin expression (red) and CD31 (green) on the vessels within the tumors, but not quiescent vessels in the skin of wild-type mice. Nuclei are stained with DAPI (blue; 20×/0.75 NA objective). (B) Immunofluorescence analysis of proliferation (anti-Ki67 [red]), endothelial cells (anti-CD31 [green]) and nuclei (DAPI [blue]). Ki67-positive, α2-null tumor endothelial cell nuclei or Ki67-negative, wild-type tumor endothelial cell nuclei are indicated by arrows; 20×/0.75 NA objective. (C) The percentage of the Ki67 and CD31 double-positive cells in nonnecrotic tumor tissue of wild-type or α2-null mice was quantitated by counting the number of CD31+ cells that were Ki67 positive or negative in 10 high-power fields. The data are presented as mean plus or minus SEM (*P < .001). Nine mice of each genotype were included in the analysis. (D) Proliferation of primary pulmonary microvascular endothelial cells from wild-type and α2-null animals on matrices of either type I collagen, type IV collagen, fibronectin (10 μg/mL of each), or tissue-culture plastic was determined. Bars and errors indicate the mean plus or minus SEM (3 separate experiments performed in quadruplicate; *P < .01 for each pair). (E) Expression of the α1β1 integrin on tumor endothelial cells was not significantly up-regulated on tumor endothelial cells from tumors in the α2-null mice. Quantitative RT-PCR measurement of α1 integrin subunit mRNA by primary tumor endothelial cells isolated by flow cytometric sorting from tumors implanted into wild-type and α2-null mice harboring tumors for 3 weeks is shown. Data indicate mean plus or minus SEM of 2 pairs of animals (P = .14).
α2β1 integrin expression and endothelial cell proliferation in vivo and in vitro. (A) Immunofluorescence analysis demonstrated colocalization of α2β1 integrin expression (red) and CD31 (green) on the vessels within the tumors, but not quiescent vessels in the skin of wild-type mice. Nuclei are stained with DAPI (blue; 20×/0.75 NA objective). (B) Immunofluorescence analysis of proliferation (anti-Ki67 [red]), endothelial cells (anti-CD31 [green]) and nuclei (DAPI [blue]). Ki67-positive, α2-null tumor endothelial cell nuclei or Ki67-negative, wild-type tumor endothelial cell nuclei are indicated by arrows; 20×/0.75 NA objective. (C) The percentage of the Ki67 and CD31 double-positive cells in nonnecrotic tumor tissue of wild-type or α2-null mice was quantitated by counting the number of CD31+ cells that were Ki67 positive or negative in 10 high-power fields. The data are presented as mean plus or minus SEM (*P < .001). Nine mice of each genotype were included in the analysis. (D) Proliferation of primary pulmonary microvascular endothelial cells from wild-type and α2-null animals on matrices of either type I collagen, type IV collagen, fibronectin (10 μg/mL of each), or tissue-culture plastic was determined. Bars and errors indicate the mean plus or minus SEM (3 separate experiments performed in quadruplicate; *P < .01 for each pair). (E) Expression of the α1β1 integrin on tumor endothelial cells was not significantly up-regulated on tumor endothelial cells from tumors in the α2-null mice. Quantitative RT-PCR measurement of α1 integrin subunit mRNA by primary tumor endothelial cells isolated by flow cytometric sorting from tumors implanted into wild-type and α2-null mice harboring tumors for 3 weeks is shown. Data indicate mean plus or minus SEM of 2 pairs of animals (P = .14).
The presence of increased vasculature in tumors growing in α2-deficient hosts suggested an increase in endothelial cell proliferation. Therefore, the proliferative activity of tumor endothelial cells within nonnecrotic regions of tumor was assessed by costaining tumor sections with anti-Ki67 and anti-CD31 antibodies. Endothelial cell nuclei were defined by DAPI staining of CD31+ cells. The α2-deficient endothelial cells demonstrated significantly increased coexpression of CD31 and Ki67 and therefore proliferation in vivo (Figure 2B,C). To examine further the functional role of α2β1 integrin on endothelial cell proliferation, primary pulmonary microvascular endothelial cells were isolated from wild-type and α2β1 integrin–deficient mice. Endothelial cells were plated on type I or type IV collagen (both α2β1 integrin-dependent ligands), fibronectin (an α2β1 integrin-independent ligand), or tissue culture plastic, and cell proliferation evaluated. α2-null endothelial cells showed a 4- to 5-fold higher proliferative index when compared with their wild-type counterparts on all matrices (Figure 2D).
The matrix-independent enhanced proliferation suggested that α2-null endothelial cells were intrinsically more proliferative. Since the α1β1 integrin provides a positive proliferative signal to endothelial cells,16,17 compensatory overexpression of the α1β1 integrin collagen receptor could account for the enhanced proliferative activity. Expression of α1 integrin subunit mRNA by primary tumor endothelial cells isolated by flow cytometric sorting from tumors 21 days after injection into wild-type or α2-null mice, as well as from primary pulmonary endothelial cells, was determined by quantitative reverse transcription (qRT)-PCR. As shown in Figure 2E and Figure S1A (available on the Blood website; see the Supplemental Materials link at the top of the online article), no significant difference in the level of the α1 integrin subunit mRNA was observed. Expression of the α1β1 integrin on tumor vessels was also evaluated by immunofluorescence analysis. Consistent with the qRT-PCR data there were no detectable differences in the α1β1 integrin protein expression in vivo (Figure S1B). Thus enhanced expression of the α1β1 integrin by the α2-null endothelial cells cannot account for the increased proliferative activity.
Endothelial cell–dependent modulation of tumor angiogenesis
To define the direct contribution of α2β1 integrin expression by endothelial cells to angiogenesis, B16F10 melanoma cells, mixed at a ratio of 5:1 with wild-type or α2-null primary pulmonary endothelial cells, were injected into the flanks of wild-type mice. Tumor growth was significantly more rapid in wild-type mice receiving tumor cells mixed with α2-null endothelial cells than wild-type mice receiving tumor cells with wild-type endothelial cells (P = .009, statistical analysis by ANOVA; Figure 3A). In addition, the tumor vessels derived from α2-null endothelial cells were larger and resembled the vessels seen in the tumors grown in the α2-null animals. In contrast, the vessels derived from wild-type endothelial cells were small and tortuous and resembled the tumor vessels in the wild-type host (Figure 3B). These findings indicate that the enhanced vascular morphogenesis in tumors in mice lacking α2β1 integrin expression are, at least in part, a consequence of the absence of the integrin on the endothelial cells. As shown in Figure 3C, many of the vessels in the wild-type host receiving α2-null endothelial cells were a mixture of wild-type cells (α2β1 integrin-positive) and transplanted α2-null endothelial cells (α2β1 integrin-negative). Although wild-type endothelial cells were also incorporated into the tumor vessels, the presence of the transplanted α2-null endothelial cells was sufficient to enhance tumor growth and tumor morphogenesis.
The vascular phenotype of α2-null mice is dependent on α2-null endothelial cells within the wild-type tumor microenvironment. (A) Tumor volume stimulated from B16F10 melanoma cells mixed at a ratio of 5:1 with wild-type or α2-null primary pulmonary endothelial cells in wild-type mice as a function of time is shown. The data are presented as the mean plus or minus SEM of 5 animals per endothelial cell genotype repeated 3 times (P = .009, statistical analysis by ANOVA). Therefore, tumors intermixed with α2-null endothelial cells grew faster at all time points than those intermixed with wild-type endothelial cells. (B) Immunofluorescence analysis of CD31+ tumor vessels (green) within the tumors of wild-type mice receiving α2-null endothelial cells (KO-ECs) or wild-type endothelial cells (WT-ECs). Vessels formed by α2-null endothelial cells in wild-type mice were larger than vessels formed by wild-type endothelial cells in wild-type mice (20×/0.75 NA objective). (C) Immunofluorescence analysis of tumor vessels in wild-type mice receiving α2-null endothelial cells. Tumor vessels were highlighted with anti-CD31 (green) and those cells that expressed the α2β1 integrin were stained with the anti-α2β1 integrin antibody (red). The tumor vessels were a mixture of wild-type (CD31+ and α2β1 integrin–positive [yellow]) and α2-null endothelial cells (CD31+ and α2β1 integrin–negative [green]; 20×/0.75 NA objective).
The vascular phenotype of α2-null mice is dependent on α2-null endothelial cells within the wild-type tumor microenvironment. (A) Tumor volume stimulated from B16F10 melanoma cells mixed at a ratio of 5:1 with wild-type or α2-null primary pulmonary endothelial cells in wild-type mice as a function of time is shown. The data are presented as the mean plus or minus SEM of 5 animals per endothelial cell genotype repeated 3 times (P = .009, statistical analysis by ANOVA). Therefore, tumors intermixed with α2-null endothelial cells grew faster at all time points than those intermixed with wild-type endothelial cells. (B) Immunofluorescence analysis of CD31+ tumor vessels (green) within the tumors of wild-type mice receiving α2-null endothelial cells (KO-ECs) or wild-type endothelial cells (WT-ECs). Vessels formed by α2-null endothelial cells in wild-type mice were larger than vessels formed by wild-type endothelial cells in wild-type mice (20×/0.75 NA objective). (C) Immunofluorescence analysis of tumor vessels in wild-type mice receiving α2-null endothelial cells. Tumor vessels were highlighted with anti-CD31 (green) and those cells that expressed the α2β1 integrin were stained with the anti-α2β1 integrin antibody (red). The tumor vessels were a mixture of wild-type (CD31+ and α2β1 integrin–positive [yellow]) and α2-null endothelial cells (CD31+ and α2β1 integrin–negative [green]; 20×/0.75 NA objective).
Regulation of VEGFR1 expression by the α2β1 integrin
Cross-talk between integrins and growth factor receptors has been well described. In mice deficient in the αv or β3 integrin subunits, endothelial cells up-regulate the expression of VEGFR2. Increased expression of angiogenic growth factor receptors such as VEGFR1 and/or VEGFR2 might also account for increased endothelial cell proliferation in the α2-null mouse. Immunoblot analysis revealed that VEGFR2 was expressed at similar levels by wild-type and α2-null endothelial cells (Figure 4A). In contrast, VEGFR1 was expressed at significantly higher levels by α2-null endothelial cells compared with wild-type cells in vitro (P < .001, statistical analysis by t test; Figure 4A). Tumor vessels in the α2-null mouse also expressed higher levels of VEGFR1, but not VEGFR2, compared with their wild-type counterparts (Figure 4B,C). If increased levels of VEGFR1 expression by the α2-null primary endothelial cells contributed to the intrinsic proliferative potential, then inhibition of VEGFR1 should abrogate the proliferative advantage. As shown in Figure 4D, incubation with an anti-VEGFR1 inhibitory antibody significantly reduced proliferation of both the wild-type and α2-null endothelial cells, but the effect was greater in the α2-null endothelial cells. These findings suggest that, both in vitro and in vivo, the increased tumor angiogenesis and endothelial cell proliferation in the α2-null animals are in part due to increased expression of VEGFR1.
VEGFR1 but not VEGFR2 expression is up-regulated on α2-null endothelial cells in vitro and in vivo. (A) The levels of VEGFR1 and VEGFR2 expression by primary pulmonary microvascular endothelial cells were evaluated by immunoblot analysis. β-actin was used as loading control. Representative images are shown. Quantitative data are presented as the mean plus or minus SEM (4 immunoblots per genotype pair from 4 endothelial cell preparations; P < .001, statistical analysis by t test). (B) Immunofluorescence analysis of VEGFR1 (red) or VEGFR2 (green) expression on tumor endothelial cells in B16F10 melanoma. Nuclei were stained with DAPI (blue; 20×/0.75 NA objective). VEGFR1 intensity was quantitated and the data presented as mean plus or minus SEM (5 tumors per genotype repeated in 2 separate experiments; *P < .001). (C) Immunofluorescence analysis colocalized CD31 (red) and VEGFR1 (green) on endothelial cells in the tumors of the α2-null mouse (20×/0.75 NA objective). (D) Inhibition of VEGFR1 reduced endothelial cell proliferation in vitro. Proliferation of primary pulmonary microvascular endothelial cells from wild-type and α2-null animals was inhibited by anti-VEGFR1–neutralizing antibodies (10 μg/mL), as designated. Absolute counts per minute (cpm) incorporated after 72 hours is shown. Bars and errors indicate the mean and SEM (3 experiments were performed in quadruplicate; *P < .01 for both wild-type and α2-null endothelial cells). (E) Rescue of α2-null endothelial cells in vitro. Primary α2-null endothelial cells were transfected with lentiviral vectors expressing either the full-length human α2 integrin subunit cDNA or GFP-control vector. Endothelial cell expression of the human α2 integrin subunit was analyzed by flow cytometric analysis using PE-conjugated antihuman α2 integrin subunit antibody. Histograms show the expression of the α2β1 integrin by cells transfected with the α2 integrin subunit cDNA, but not by control transfectants. (F) Re-expression of human α2β1 integrin resulted in decreased expression of VEGFR1 to levels similar to wild-type endothelial cells. Expression of VEGFR1 by α2-null primary pulmonary endothelial cells transfected with the α2 integrin cDNA was compared with α2-null or wild-type primary pulmonary endothelial cells transfected with GFP by immunoblot analysis.
VEGFR1 but not VEGFR2 expression is up-regulated on α2-null endothelial cells in vitro and in vivo. (A) The levels of VEGFR1 and VEGFR2 expression by primary pulmonary microvascular endothelial cells were evaluated by immunoblot analysis. β-actin was used as loading control. Representative images are shown. Quantitative data are presented as the mean plus or minus SEM (4 immunoblots per genotype pair from 4 endothelial cell preparations; P < .001, statistical analysis by t test). (B) Immunofluorescence analysis of VEGFR1 (red) or VEGFR2 (green) expression on tumor endothelial cells in B16F10 melanoma. Nuclei were stained with DAPI (blue; 20×/0.75 NA objective). VEGFR1 intensity was quantitated and the data presented as mean plus or minus SEM (5 tumors per genotype repeated in 2 separate experiments; *P < .001). (C) Immunofluorescence analysis colocalized CD31 (red) and VEGFR1 (green) on endothelial cells in the tumors of the α2-null mouse (20×/0.75 NA objective). (D) Inhibition of VEGFR1 reduced endothelial cell proliferation in vitro. Proliferation of primary pulmonary microvascular endothelial cells from wild-type and α2-null animals was inhibited by anti-VEGFR1–neutralizing antibodies (10 μg/mL), as designated. Absolute counts per minute (cpm) incorporated after 72 hours is shown. Bars and errors indicate the mean and SEM (3 experiments were performed in quadruplicate; *P < .01 for both wild-type and α2-null endothelial cells). (E) Rescue of α2-null endothelial cells in vitro. Primary α2-null endothelial cells were transfected with lentiviral vectors expressing either the full-length human α2 integrin subunit cDNA or GFP-control vector. Endothelial cell expression of the human α2 integrin subunit was analyzed by flow cytometric analysis using PE-conjugated antihuman α2 integrin subunit antibody. Histograms show the expression of the α2β1 integrin by cells transfected with the α2 integrin subunit cDNA, but not by control transfectants. (F) Re-expression of human α2β1 integrin resulted in decreased expression of VEGFR1 to levels similar to wild-type endothelial cells. Expression of VEGFR1 by α2-null primary pulmonary endothelial cells transfected with the α2 integrin cDNA was compared with α2-null or wild-type primary pulmonary endothelial cells transfected with GFP by immunoblot analysis.
To determine whether expression of the α2β1 integrin regulates the expression of VEGFR1, α2-null primary pulmonary endothelial cells were transfected with a lentiviral vector encoding either the full-length human α2 integrin subunit or green fluorescent protein (GFP) as control. Wild-type endothelial cells were also transfected with the control GFP vector. As expected and shown in Figure 4E, α2 integrin-transfectants, but not control GFP-transfectants, expressed the human α2 integrin subunit on the cell surface, as evaluated by FACS analysis. Furthermore, expression of the human α2 integrin subunit in α2-null cells resulted in markedly decreased expression of VEGFR1 to levels comparable to primary wild-type endothelial cells (Figure 4F). Expression of GFP did not alter the level of VEGFR1 in either α2-null or wild-type cells. These findings demonstrate direct regulation of the VEGFR1 expression by the α2β1 integrin.
Differential growth factor secretion modulates angiogenesis in a manner dependent on host genetic background
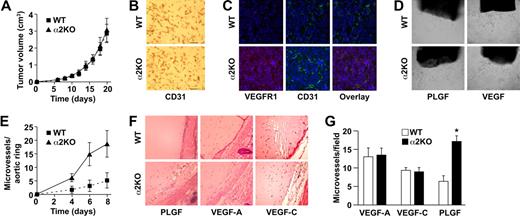
Since increased neoangiogenesis in the α2-null mouse was unexpected, we sought to determine whether these findings were restricted to the B16 melanoma model or whether they were more generally applicable to other tumor types. Somewhat surprisingly, when we examined neoangiogenesis in response to syngeneic LLC cells, tumor growth was similar in both wild-type and α2-null mice (Figure 5A). The CD31+ vasculature in both genotypes was composed of small, tortuous vessels. There was no difference in vessel area (12.55% for wild type and 13.75% for α2-null) or vessel morphology (Figure 5B). The vessels in LLC-derived tumors in α2-null mice expressed increased VEGFR1 (Figure 5C), although the increase was less than that observed in B16F10 tumors. These rather unexpected results indicated that the impact of host expression of the α2β1 integrin in the tumor microenvironment was dependent on tumor type. The observed angiogenic phenotype appeared to be dependent on cross-talk between tumor cells and the microenvironment.
α2β1 integrin–deficient tumor angiogenesis is PLGF dependent. (A) Tumor volume of LLC cells injected into α2-null mice (KO) and their wild-type (WT) littermates as a function of time. The data are presented as the mean plus or minus SEM with 5 animals of each genotype (P = .99, statistical analysis by ANOVA). (B) Immunohistochemical analysis of CD31+ vessels in LLC tumor sections. No difference in vascularity was observed (scale bar = 50 μm; 20×/0.75 NA objective). (C) Immunofluorescence analysis of VEGFR1 (red) expression on tumor endothelial cells in LLC tumors from wild-type and α2-null mice. Nuclei were stained with DAPI (blue; 20×/0.75 NA objective). (D) Light microscopic images of representative wild-type and α2-null aortic ring microvessels embedded in growth factor–reduced Matrigel supplemented with either PLGF or VEGF (10×/0.75 NA objective). (E) Quantitation of the number of vascular sprouts in the presence of PLGF. PLGF stimulated a significantly increased number of sprouts from the α2-null, but not wild-type aortic rings. Fifteen aortic rings of each genotype are included in the analysis (P = .002, statistical analysis by ANOVA). (F) Light microscopic images of neoangiogenesis in growth factor–reduced Matrigel supplemented with either PLGF, VEGF-A, or VEGF-C after 8 days (20×/0.75 NA objective). (G) Quantitation of the number of microvessels infiltrating into the Matrigel plug in response to either PLGF, VEGF-A, or VEGF-C (3 animals of each genotype and each experimental condition; *P < .001, statistical analysis by t test).
α2β1 integrin–deficient tumor angiogenesis is PLGF dependent. (A) Tumor volume of LLC cells injected into α2-null mice (KO) and their wild-type (WT) littermates as a function of time. The data are presented as the mean plus or minus SEM with 5 animals of each genotype (P = .99, statistical analysis by ANOVA). (B) Immunohistochemical analysis of CD31+ vessels in LLC tumor sections. No difference in vascularity was observed (scale bar = 50 μm; 20×/0.75 NA objective). (C) Immunofluorescence analysis of VEGFR1 (red) expression on tumor endothelial cells in LLC tumors from wild-type and α2-null mice. Nuclei were stained with DAPI (blue; 20×/0.75 NA objective). (D) Light microscopic images of representative wild-type and α2-null aortic ring microvessels embedded in growth factor–reduced Matrigel supplemented with either PLGF or VEGF (10×/0.75 NA objective). (E) Quantitation of the number of vascular sprouts in the presence of PLGF. PLGF stimulated a significantly increased number of sprouts from the α2-null, but not wild-type aortic rings. Fifteen aortic rings of each genotype are included in the analysis (P = .002, statistical analysis by ANOVA). (F) Light microscopic images of neoangiogenesis in growth factor–reduced Matrigel supplemented with either PLGF, VEGF-A, or VEGF-C after 8 days (20×/0.75 NA objective). (G) Quantitation of the number of microvessels infiltrating into the Matrigel plug in response to either PLGF, VEGF-A, or VEGF-C (3 animals of each genotype and each experimental condition; *P < .001, statistical analysis by t test).
These observations raised the possibility that the tumors secreted different angiogenic growth factors and that these factors mediated the cross-talk. Conditioned media derived from LLC cells contained 1160 pg/mL VEGF and 0.78 pg/mL PLGF. In contrast, media from B16F10 melanoma cells contained 168 pg/mL VEGF and 108 pg/mL PLGF (140-fold increase, P = .008). The difference in the growth factor milieu of B16F10 melanoma and LLC suggested that the different angiogenic responses in α2-null mice might be due to a differential response to growth factors. Therefore, the differential response of wild-type and α2-null endothelial cells to PLGF and VEGF was evaluated ex vivo and in vivo. In the ex vivo aortic ring assay, microvessel sprouts per aortic ring stimulated by either PLGF or VEGF were quantitated. PLGF stimulated increased numbers of vascular sprouts by the α2-null aortic rings when compared with wild-type rings (Figure 5D,E). VEGF stimulated slightly more vascular sprouts in the α2-null than in the wild-type aortic rings; however, the difference was not statistically significant (data not shown). The finding of increased responsiveness to PLGF is consistent with the increased VEGFR1 expression by α2-null endothelium.
To further evaluate the impact of VEGF or PLGF on neoangiogenesis in vivo, Matrigel plugs containing either PLGF, VEGF-A, or VEGF-C were implanted into wild-type or α2-null mice. PLGF stimulated robust angiogenesis with dilated blood vessels in the α2-null animals (Figure 5F). PLGF stimulated limited angiogenesis with only a small number of microvessels in the wild-type mouse (Figure 5F,G). The response to VEGF-A and VEGF-C was similar in both wild-type and α2-null mice (Figure 5F,G), demonstrating that the α2-null microenvironment augments neoangiogenesis in response specifically to PLGF.
To determine if the observed difference in tumor angiogenesis was dependent on secretion of PLGF, LLC cells were transfected with a PLGF-expression vector or control vector.27 Conditioned media from PLGF or control transfectants contained 1843 pg/mL or 4.4 pg/mL PLGF, respectively. PLGF transfectants grew faster in α2-null mice than in controls (Figure 6A). In both wild-type and α2-null mice, LLC-PLGF–stimulated tumor vessels occupied a greater percentage of tumor area and were larger than LLC-stimulated vessels (Figure 6B-D). Furthermore, LLC-PLGF–stimulated vessels in the α2-null microenvironment occupied a greater area and were larger than LLC-PLGF–stimulated vessels in the wild type microenvironment (Figure 6B-D). Thus, the observed increased angiogenesis and endothelial cell proliferation in the α2-null tumor microenvironment depend, in part, on increased expression of VEGFR1 by the α2-null endothelial cells and secretion of PLGF by the tumor cells.
Augmented angiogenesis in the α2β1 integrin–deficient animals is mediated by PLGF. (A) Tumor growth of LLC-PLGF or vector-only (control) transfectants in α2-null mice (KO) and their wild-type (WT) littermates as a function of time over 14 days. Tumors expressing PLGF grew significantly faster in α2-null mice than in wild-type mice. The data are presented as the mean plus or minus SEM with 5 animals of each genotype (P = .007, statistical analysis by ANOVA). (B) Immunohistochemical analysis with anti-CD31 staining of LLC-control and LLC-PLGF tumor sections in wild-type (WT) and α2-null (KO) animals showed an increased vascular area and increased size of the LLC-PLGF tumor vessels in α2-null mice (scale bar = 50 μm; 20×/0.75 NA objective). (C, D) In both wild-type and α2-null mice, LLC-PLGF–stimulated tumor vessels occupied a greater percentage of tumor area (***P = .002 for WT and **P < .001 for KO) and were larger (***P = .006 for WT and **P < .001 for KO) than LLC-stimulated vessels. Total area of CD31+ vessels as a percentage of total tumor area (*P = .003) and average vessel size (*P < .001) were increased in α2-deficient hosts bearing LLC-expressing PLGF tumors in comparison to wild-type hosts bearing LLC-expressing PLGF tumors. Data are presented as the mean plus or minus SEM (5 animals for each tumor/genotype combination). (E) Tumor growth of B16 melanoma-shPLGF or GFP-only (control) cells in wild-type (WT) and α2-null (KO) animals as a function of time over 14 days. The data are presented as the mean plus or minus SEM with 5 animals of each genotype (P = .91, statistical analysis by ANOVA). (F) In both wild-type and α2-null mice, B16 melanoma-shPLGF–stimulated tumor vessels occupied a similar percentage of tumor area in wild-type and α2-null mice. Data are presented as the mean plus or minus SEM (5 animals for each tumor/genotype combination; P = .521).
Augmented angiogenesis in the α2β1 integrin–deficient animals is mediated by PLGF. (A) Tumor growth of LLC-PLGF or vector-only (control) transfectants in α2-null mice (KO) and their wild-type (WT) littermates as a function of time over 14 days. Tumors expressing PLGF grew significantly faster in α2-null mice than in wild-type mice. The data are presented as the mean plus or minus SEM with 5 animals of each genotype (P = .007, statistical analysis by ANOVA). (B) Immunohistochemical analysis with anti-CD31 staining of LLC-control and LLC-PLGF tumor sections in wild-type (WT) and α2-null (KO) animals showed an increased vascular area and increased size of the LLC-PLGF tumor vessels in α2-null mice (scale bar = 50 μm; 20×/0.75 NA objective). (C, D) In both wild-type and α2-null mice, LLC-PLGF–stimulated tumor vessels occupied a greater percentage of tumor area (***P = .002 for WT and **P < .001 for KO) and were larger (***P = .006 for WT and **P < .001 for KO) than LLC-stimulated vessels. Total area of CD31+ vessels as a percentage of total tumor area (*P = .003) and average vessel size (*P < .001) were increased in α2-deficient hosts bearing LLC-expressing PLGF tumors in comparison to wild-type hosts bearing LLC-expressing PLGF tumors. Data are presented as the mean plus or minus SEM (5 animals for each tumor/genotype combination). (E) Tumor growth of B16 melanoma-shPLGF or GFP-only (control) cells in wild-type (WT) and α2-null (KO) animals as a function of time over 14 days. The data are presented as the mean plus or minus SEM with 5 animals of each genotype (P = .91, statistical analysis by ANOVA). (F) In both wild-type and α2-null mice, B16 melanoma-shPLGF–stimulated tumor vessels occupied a similar percentage of tumor area in wild-type and α2-null mice. Data are presented as the mean plus or minus SEM (5 animals for each tumor/genotype combination; P = .521).
To further document the role of PLGF in mediating augmented tumor angiogenesis in α2-null hosts, we returned to the B16 melanoma model. Populations of nonclonal B16 melanoma cells secreting either high or low concentrations of PLGF were generated using either a PLGF-directed or a control short hairpin (sh) RNA–expressing virus. Conditioned media from shPLGF cells contained 26 pg/mL PLGF, whereas media from control (sh)RNA cells contained 94 pg/mL. Wild-type or α2-null mice were injected subcutaneously with B16 melanoma-shPLGF cells. In contrast to results obtained with the parental B16 melanoma cells shown in Figure 1A, growth of B16 melanoma-shPLGF was similar in wild-type and α2-null animals (Figure 6E). After 14 days the animals were killed and tumor vessel morphology was evaluated. The area occupied by CD31+ vessels relative to total B16 shPLGF tumor area was similar in the α2-null and wild-type mice (Figure 6F). Therefore, the augmented neoangiogenesis observed in the α2-null animals harboring wild-type B16 melanoma cells was in part a consequence of high PLGF levels.
Discussion
To define the role of the α2β1 integrin in pathologic angiogenesis, we investigated tumor growth and tumor angiogenesis in wild-type and α2-null mice. Our findings reveal that the α2β1 integrin plays an important regulatory role in the angiogenic response via regulation of VEGFR1 expression. When challenged with B16F10 melanoma cells, mice lacking α2β1 integrin expression exhibit increased tumor growth and tumor angiogenesis in association with increased VEGFR1 expression in a PLGF-rich environment. In contrast, there was no α2β1 integrin–dependent difference in the angiogenic response to LLC cells, which produce a PLGF-poor, VEGF-rich microenvironment. The α2β1 integrin–dependent difference in tumor growth and tumor angiogenesis was restored to LLC cells by expression of PLGF. Thus, the angiogenic response of the α2-null mouse varies with tumor type and is dependent on specific angiogenic growth factor secretion. The molecular basis of enhanced tumor angiogenesis in the α2-null host is dependent on interactions between the host microenvironment and specific tumor type, an example of genetic alterations of “the soil” determining the response to the “seed.”
We also demonstrate that the α2β1 integrin is not expressed at detectable levels on quiescent murine microvascular endothelial cells. In contrast, expression of the integrin is markedly up-regulated within the tumor microenvironment. These in vivo findings are consistent with in vitro data.10 Unstimulated microvascular endothelial cells failed to express the α2β1 integrin, but integrin expression was increased in response to VEGF. VEGF secreted by the tumor cells may alone, or in combination with other angiogenic factors, up-regulate α2β1 integrin expression in vivo. In turn, the angiogenic response is modulated by α2β1 integrin expression.
The ability of the α2β1 integrin-null mouse to produce normal or increased vessels within the tumor microenvironment was initially surprising in light of the previously published data using inhibitory antibodies, endorepellin, or angiocidin. Blocking antibodies directed against the α1β1 or α2β1 integrin, alone or in combination, inhibited VEGF-induced angiogenesis.10,–12 Fragments of perlecan (endorepellin) and thrombospondin (angiocidin) that demonstrate antiangiogenic activity also serve as novel ligands of the α2β1 integrin and prevent integrin interactions with type I collagen. Both endorepellin and angiocidin inhibit angiogenesis in vitro and in vivo and inhibit tumor growth in vivo.28,–30 Together these observations were interpreted to suggest that the α2β1 integrin was required for neoangiogenesis. Our studies suggest that the role of the α2β1 integrin in pathologic angiogenesis is more complex than heretofore appreciated.
Our data suggest that a shift in the angiogenic signal to increased PLGF-mediated stimulation of VEGFR1 relative to VEGF-mediated stimulation of VEGFR1 and VEGFR2 may in part explain the differences in vascular morphology observed between α2-null mice implanted with PLGF-rich tumors (B16F10 melanoma and PLGF-transfected LLC) and VEGF-rich tumors (LLC). We observed up-regulation of VEGFR1 upon deletion of the α2β1 integrin from cells within the tumor microenvironment. Re-expression of the α2β1 integrin in α2-null endothelial cells not only restored integrin expression but also resulted in decreased expression of VEGFR1. When up-regulation of the α2β1 integrin in the tumor microenvironment is prevented by genetic deletion, then VEGFR1 is up-regulated.
The apparently discordant results obtained by deletion of the α2β1 integrin and those obtained by inhibition of the α2β1 integrin with antibodies and matrix fragments are reminiscent of the conflicting results reported for the αvβ3 and αvβ5 integrins in tumor angiogenesis using genetic deletion and antibody inhibition.2,4,,–7,31,–33 Similarities between the roles of the α2β1 integrin and the αvβ3 integrin were not appreciated before this report. First, both α2β1 and αvβ3 integrin expression is significantly up-regulated by tumor vessels. Second, genetic ablation of the α2 subunit, like ablation of the β3 or β5 integrin subunits, does not cause significant vascular abnormalities during development. Third, although antibody inhibition of either the α2β1 integrin or the αvβ3 integrin impairs angiogenesis, genetic ablation of either integrin results in enhanced tumor growth and tumor angiogenesis. Finally, genetic ablation of the α2 integrin subunit results in up-regulation of VEGFR1; genetic ablation of the β3 integrin results in up-regulation of VEGFR2.
We propose several alternative, but not mutually exclusive, explanations for the α2β1 integrin paradox. First, we suggest that a balance between the contributions of α2β1 integrin and the α1β1 integrin maintains the angiostatic set point. In the resting state, endothelial cells express extremely low levels of the α1β1 and α2β1 integrins. However, under circumstances of pathologic angiogenesis, such as the tumor microenvironment or wound healing, expression of both the α1β1 and the α2β1 integrins is up-regulated. The 2 integrins are not redundant, but may have distinct roles in angiogenesis. The α1β1 integrin provides pro-proliferative signals that may be counterbalanced by signals from the α2β1 integrin. Part of the balance via integrin cross-talk involved down-regulation of VEGFR1 as a consequence of α2β1 integrin expression. Whereas, genetic deletion eliminates the cross-talk, inhibitory antibodies and matrix fragments inhibit adhesive activity but not regulatory cross-talk.
Alternatively, integrin-mediated death (IMD), which occurs when cells expressing a specific integrin are present in an inappropriate matrix that does not allow for integrin ligation, may account for the discordance. IMD has been suggested to be the mechanism by which inhibition of the αv integrins blocked angiogenesis and by which expression of α3β1 integrin prevents metastasis of neuroblastoma.34,,–37 In this context, alternative ligands for the α2β1 integrin such as angiostatin, endorepellin, inhibitory antibodies, or other features unique to the matrix of the tumor microenvironment might contribute to IMD.
Finally, the absence of α2β1 integrin expression may lead to embryonic compensation and up-regulated expression of VEGFR1. Since the α2-null animals are viable, it was assumed that the α2β1 integrin was not involved in developmental angiogenesis. Perhaps VEGFR1 and the α2β1 integrin mediate similar roles at early stages of development and up-regulated VEGFR1 compensates for the lack of the integrin. Under physiologic conditions in the α2-null mouse, low levels of VEGFR1 are able to compensate for the absence of the α2β1 integrin. In contrast, under pathologic conditions, the absence of the α2β1 integrin leads to up-regulated VEGFR1 expression and increased tumor angiogenesis.
In summary, the angiogenic phenotype observed in the absence of the α2β1 integrin is profoundly dependent on growth factors present in the microenvironment and up-regulated expression of VEGFR1 that occurs in the absence of the integrin. Our results point to the critical importance of the interplay between the tumor cell–derived growth factors and integrin-mediated determinants on cellular responsiveness of the microenvironment. We would also suggest that cross-talk between the α2β1 integrin and the α1β1 integrin dampens the proangiogenic effect of the α1β1 integrin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Samuel A. Santoro for advice and encouragement in many helpful discussions, and Brent Weedman and Jean McClure for excellent photographic and secretarial assistance. We acknowledge the assistance of the speed congenics facility supported by the Rheumatic Diseases Core Center at Washington University (no. P30-AR48335).
This work was supported by grants CA115984, CA098027, and CA70275 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: Z.Z. designed and performed the research and analyzed the data; N.R. carried out the lentiviral experiments; T.Y. conducted the imaging studies; Y.Q. developed the sorting technique to purify primary tumor endothelial cells; L.F. and Z.L. provided technical assistance; A.P. assisted in the design of the experiments, data analysis, and writing the paper; M.Z. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary M. Zutter, Department of Pathology, C-3321A, Vanderbilt University School of Medicine, 1161 21st Ave S, Nashville, TN 37232-2561; e-mail: mary.zutter@vanderbilt.edu.

![Figure 2. α2β1 integrin expression and endothelial cell proliferation in vivo and in vitro. (A) Immunofluorescence analysis demonstrated colocalization of α2β1 integrin expression (red) and CD31 (green) on the vessels within the tumors, but not quiescent vessels in the skin of wild-type mice. Nuclei are stained with DAPI (blue; 20×/0.75 NA objective). (B) Immunofluorescence analysis of proliferation (anti-Ki67 [red]), endothelial cells (anti-CD31 [green]) and nuclei (DAPI [blue]). Ki67-positive, α2-null tumor endothelial cell nuclei or Ki67-negative, wild-type tumor endothelial cell nuclei are indicated by arrows; 20×/0.75 NA objective. (C) The percentage of the Ki67 and CD31 double-positive cells in nonnecrotic tumor tissue of wild-type or α2-null mice was quantitated by counting the number of CD31+ cells that were Ki67 positive or negative in 10 high-power fields. The data are presented as mean plus or minus SEM (*P < .001). Nine mice of each genotype were included in the analysis. (D) Proliferation of primary pulmonary microvascular endothelial cells from wild-type and α2-null animals on matrices of either type I collagen, type IV collagen, fibronectin (10 μg/mL of each), or tissue-culture plastic was determined. Bars and errors indicate the mean plus or minus SEM (3 separate experiments performed in quadruplicate; *P < .01 for each pair). (E) Expression of the α1β1 integrin on tumor endothelial cells was not significantly up-regulated on tumor endothelial cells from tumors in the α2-null mice. Quantitative RT-PCR measurement of α1 integrin subunit mRNA by primary tumor endothelial cells isolated by flow cytometric sorting from tumors implanted into wild-type and α2-null mice harboring tumors for 3 weeks is shown. Data indicate mean plus or minus SEM of 2 pairs of animals (P = .14).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-06-094680/3/m_zh80030814670002.jpeg?Expires=1765898310&Signature=JTfucidY8DFEzkeDIk2v-IWijCdv61Y~6bcosC1HKufJrtNKQGXP16ggXxw20zR8mdA3v9Tdhdb4sxr3rn9CQ6dAHly-H19W34BIkB2ciPLoWMPl2ybHpCG8gwtT4jDyNINtUn3v~Pa72WejWjpT8HHf2o6ehr1apH4AZUFXYifldFeTzhdNS7jvFGxZLIBGVFiqbZ4RQaL0g6GeyTOYauvqNfQ4QKM50u~LFW~LaO9THjpUdRzwesP5iRqohrKJPRIYTEknnKsWr3p4JMxFeIUhdJiuw04le-H0tWDErNQ-41Gbd4rTw7ptpgrfpK7pv2ad-yTfnaQkXvhgHC1AMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. The vascular phenotype of α2-null mice is dependent on α2-null endothelial cells within the wild-type tumor microenvironment. (A) Tumor volume stimulated from B16F10 melanoma cells mixed at a ratio of 5:1 with wild-type or α2-null primary pulmonary endothelial cells in wild-type mice as a function of time is shown. The data are presented as the mean plus or minus SEM of 5 animals per endothelial cell genotype repeated 3 times (P = .009, statistical analysis by ANOVA). Therefore, tumors intermixed with α2-null endothelial cells grew faster at all time points than those intermixed with wild-type endothelial cells. (B) Immunofluorescence analysis of CD31+ tumor vessels (green) within the tumors of wild-type mice receiving α2-null endothelial cells (KO-ECs) or wild-type endothelial cells (WT-ECs). Vessels formed by α2-null endothelial cells in wild-type mice were larger than vessels formed by wild-type endothelial cells in wild-type mice (20×/0.75 NA objective). (C) Immunofluorescence analysis of tumor vessels in wild-type mice receiving α2-null endothelial cells. Tumor vessels were highlighted with anti-CD31 (green) and those cells that expressed the α2β1 integrin were stained with the anti-α2β1 integrin antibody (red). The tumor vessels were a mixture of wild-type (CD31+ and α2β1 integrin–positive [yellow]) and α2-null endothelial cells (CD31+ and α2β1 integrin–negative [green]; 20×/0.75 NA objective).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-06-094680/3/m_zh80030814670003.jpeg?Expires=1765898310&Signature=HMnSbHPXWy8tiGREisyP~ULfmJ0~G7Jkt2q0TFBG6Jcn3~iF8~TSfqAZxogJ63dvRGNM3k3lFWr-4b0yncCsLuy5BBwU8YbxDYO5jDiGiWRmldYqsJ9SRBfcHjntmrPe2RXdwDASuMJTRSsekCm~MqnWLBaAOCc0FlbF~ioWKKwav0LH9GF8ySGhHXpyDSK7FKFS4l4PD4v1iZOeH9KjxxZcnKIYIhzT2KPE7mCsiu0DnpTAHsJxIRA5mTC1XND5qaH~H-DK3aohZlLmu4iCnL3XkOlktJqNXZmZ1Zrg8FeIaXiDXp4H6JJ05hf2JCfYBHcy53cHfgWoSVxKb95Bvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal