Among dendritic cell (DC) subsets, CD8α+ DCs and plasmacytoid DCs (pDCs) produce high levels of IL12 and type I interferons (IFNs), respectively, and confer early innate immunity. Development of CD8α+ DCs and pDCs requires the interferon regulatory factor 8 (IRF8). Recently, a spontaneous point mutation was identified in the Irf8/Icsbp gene in the BXH2 mouse, which exhibits an immunodeficient phenotype similar to the IRF8 knockout (KO) mouse. We show that this mutation, designated IRF8R294C, abolishes the development of CD8α+ DCs without impairing pDC development, and eliminates production of IL12p40, while retaining that of type I IFNs. Electrophoretic mobility shift and chromatin immunoprecipitation assays indicated that IRF8R294C failed to interact with partner transcription factors and did not bind certain promoters that require partner interactions. Together, this work indicates that IRF8-partner interactions play different roles in CD8α+ DCs and pDCs, revealing a mechanistic separation that underlies development of these DC subsets.
Introduction
Dendritic cells (DCs) are composed of multiple subsets that collectively provide early innate immunity, leading to subsequent adaptive immunity.1 Plasmacytoid DCs (pDCs) and CD8α+ DCs produce signature cytokines, type I IFNs, and IL12 and help to establish antiviral antimicrobial states in the body. Other functions, including antigen presentation, are carried out more efficiently by CD4+ DCs and CD4−CD8α− DCs. The origin of DC subsets and the mechanism underlying their development are not fully understood.2,3 Recent studies show that IRF4 and IRF8, transcription factors of the IRF family, play critical roles in DC subset development.4,,,,–9 Whereas the development of CD8α+ DCs and pDCs requires IRF8, that of the remaining subsets is dependent on IRF4. Other IRF members are also involved in modulating DC development and function.10,–12
BXH2 is a recombinant inbred mouse strain displaying a phenotype similar to IRF8 KO mice.13 They have splenomegaly with increased granulocytes and develop spontaneous myeloid leukemia. BXH2 mice are susceptible to M bovis (BCG), and are defective in IL12p40 production.13 Recently, Turcotte et al14 showed that BXH2 mice carry a point mutation in the Irf8/Icsbp gene that changes arginine (R) to cysteine (C) in position 294 (IRF8R294C). The mutation is within the IRF association domain (IAD) important for the interaction of IRF8 with partner proteins.12,15 Here we investigated the effects of the IRF8R294C mutation on DC development.
Methods
BXH2 mice obtained from National Cancer Institute were crossed with C57BL/6J (B6) female mice for 3 generations to eliminate maternal transmission of ecotropic murine leukemia viruses (MuLVs). Progeny mice were crossed to generate mice homozygous for IRF8R294C and wild-type IRF8 (IRF8WT), genotyped as in Figure S2, and were used for experiments at 8 to 12 weeks of age.
Magnetic bead separation of splenic DCs, flow cytometry, and the generation of bone marrow–derived DCs (BMDCs) in fms-like tyrosine kinase ligand (Flt3L) were described.6,9 DCs were stimulated with indicated Toll-like receptor (TLR) ligands, and induction of IL12p40 and IFNα was measured as described.6,7
Electrophoretic mobility shift assays (EMSAs) were performed using in vitro–transcribed and – translated IRF8WT, IRF8R294C, and IRF8R289E cloned in pcDNA as described.6 Chromatin immunoprecipitation (ChIP) analysis was performed for BMDCs (see Figure S4 for details).9,16 For gene transfer experiments, IRF8R294C in the pMSCV-puro retroviral vector was transduced into BM progenitor cells from IRF8 KO mice, and tested by flow cytometry and for cytokine expression on day 8 or day 9.6,9
Results and discussion
Selective loss of CD8α+ DCs in BXH2 mice
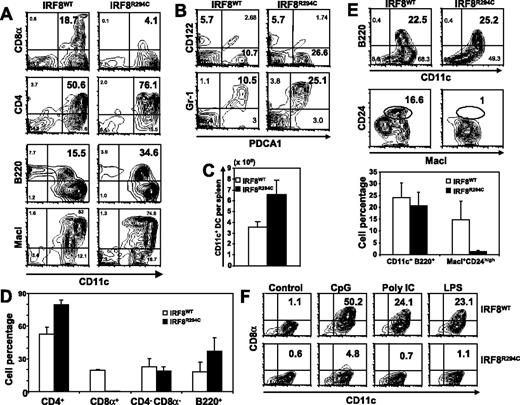
Maternal transmission of MuLV facilitates leukemia progression in BXH2 mice initiated by the IRF8 mutation, suggesting that the phenotypes resulting from the IRF8 mutation may be modulated by MuLV.13,14 Since our aim was to study the effect of the mutation, independent of MuLV production, we generated mice carrying IRF8R294C without virus by crossing BXH2 mice with B6 females preventing vertical transmission of the virus. Figure 1A-D depicts flow cytometry analysis of DC subsets in IRF8WT and IRF8R294C spleens. The most striking feature of IRF8R294C DC subsets was the almost total absence of CD8α+ DCs detected in total spleen and CD11chigh populations (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, B220+ pDCs expressing PDCA1 and Gr-1 were present in somewhat greater numbers in IRF8R294C spleens. Similarly, CD4+ DCs were increased in the mutants, both in number and frequency. On the other hand, levels of CD4−CD8α− DCs and the recently identified IFN-producing natural killer DCs (CD11cint, B220+ CD122+, PDCA1−, Gr-1−)17 were similar in IRF8WT and IRF8R294C spleens. The total number of splenic CD11c+ DCs was higher in IRF8R294C mice than in IRF8WT mice (Figure 1C). BXH2 mice producing MuLV showed a very similar phenotype (Figure S2). Together, the representation of DC subsets in IRF8R294C mice is markedly different from that of IRF8 KO mice, which lack both pDCs and CD8α+ DCs.4,7,9 These data indicate that CD8α+ DC lineage development is selectively obliterated by the IRF8R294C mutation. The increase in pDCs and CD4+ DCs in the mutant mice may suggest that their developmental pathways are influenced by that of CD8α+ DCs.
Analysis of DC subsets in IRF8R294C mice. (A) CD11c+ cells in spleen were analyzed for expression of the indicated DC subset markers by flow cytometry. (B) CD11c+ gated cells were tested for the pDC and IKDC markers, CD122 and PDCA1. (C) Total CD11c+ DCs per spleen. Values represent the average of 3 mice (± SD). (D) The percentages of 4 DC subsets in IRF8WT and IRF8R294C spleens. Values represent the average of 3 spleens (± SD). (E) BMDCs from IRF8WT and IRF8R294C mice grown in Flt3L were tested for B220 and CD24 as markers for pDCs and CD8α+ DCs, respectively. (F) BMDCs from IRF8WT and IRF8R294C mice were stimulated with CpG oligomer DNA D19 (1 μg/mL), LPS (100 ng/mL), or Poly IC (100 μg/mL) for 24 hours, and expression of CD8α was tested by flow cytometry. Numbers on plots are percentages of total cells.
Analysis of DC subsets in IRF8R294C mice. (A) CD11c+ cells in spleen were analyzed for expression of the indicated DC subset markers by flow cytometry. (B) CD11c+ gated cells were tested for the pDC and IKDC markers, CD122 and PDCA1. (C) Total CD11c+ DCs per spleen. Values represent the average of 3 mice (± SD). (D) The percentages of 4 DC subsets in IRF8WT and IRF8R294C spleens. Values represent the average of 3 spleens (± SD). (E) BMDCs from IRF8WT and IRF8R294C mice grown in Flt3L were tested for B220 and CD24 as markers for pDCs and CD8α+ DCs, respectively. (F) BMDCs from IRF8WT and IRF8R294C mice were stimulated with CpG oligomer DNA D19 (1 μg/mL), LPS (100 ng/mL), or Poly IC (100 μg/mL) for 24 hours, and expression of CD8α was tested by flow cytometry. Numbers on plots are percentages of total cells.
Flt3L supports the generation of CD24high DCs in vitro, which correspond to splenic CD8α+ DCs, although CD8α itself is not expressed in DCs cultured in vitro.18 In line with data for splenic DCs, CD24high cells were present in IRF8WT cultures, but were absent in IRF8R294C cultures (Figure 1E). B220+ pDCs, present in IRF8R294C spleens, were also generated from mutant BM cells at levels similar to those of IRF8WT mice. Following TLR stimulation by CpG, LPS, and Poly IC, IRF8WT BMDCs expressed CD8α, as reported,6 while IRF8R294C BMDCs remained negative for this marker (Figure 1F), further supporting the inability of the mutant to generate CD8α+ DCs.
Selective loss of cytokine production in IRF8R294C DCs
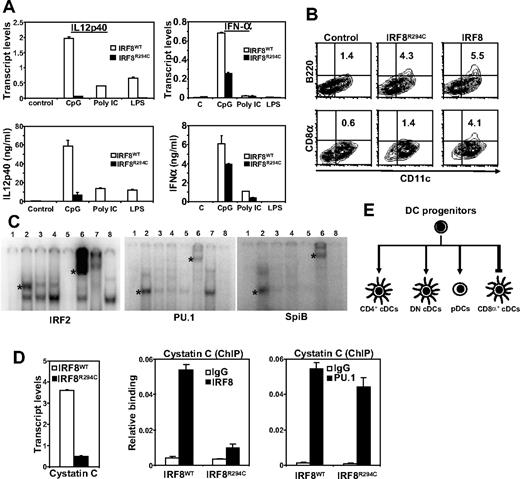
TLR ligands stimulate IL12p40 expression in CD8α+ DCs, often at higher levels than in other DCs.9,18,19 In contrast, type I IFNs are expressed at higher levels in pDCs than in other DCs.20 As seen in Figure 2A, IL12p40 transcripts and protein were essentially undetectable in IRF8R294C BMDCs following TLR stimulation, while high levels of IL12p40 were induced in IRF8WT DCs by the same stimulation. In contrast to their failure to induce IL12p40, IRF8R294C DCs expressed IFNα transcripts and protein in response to TLR stimulation. Thus, the IRF8R294C mutation selectively abolished cytokine induction predominant in CD8α+ DCs, without affecting that in pDCs. These results reinforce the concept that the IRF8R294C mutation selectively affects the development of CD8α+ DCs. The differential effect of the mutation on cytokine induction may explain why BXH2 mice are susceptible to certain pathogens but not others.13
Impaired cytokine induction and partner interactions in IRF8R294C DCs. (A) BMDCs from IRF8WT and IRF8R294C mice were stimulated with indicated TLR ligands. IL12p40 and IFNα transcripts (measured 6 hours after stimulation) and proteins (measured 24 hours after stimulation) were measured by quantitative polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Error bars represent SD. (B) BM cells from IRF8 KO mice were transduced with pMSCV vectors for IRF8WT or IRF8R294C, and expression of B220 and CD8α (24 hours after CpG stimulation) was detected by flow cytometry. (C) EMSA analysis: in vitro–transcribed and – translated proteins from control pcDNA, IRF8WT, IRF8R294C, and IRF8R289E vectors (lanes 1–4) were mixed with the indicated in vitro–transcribed and – translated partner proteins and 32p-labeled ISRE (for IRF2) and EICE (for PU.1 and SpiB). Asterisks indicates IRF8-partner complexes. Specificity of mobility shifts was verified by adding excess unlabeled probes (lane 5), which removed the shifted band or by adding antibodies for IRF8 (lane 6) or partner proteins (lane 7), which “supershifted” the band mobility. Lane 8 contained partner proteins without IRF8, which produced no shifted band. (D) Cystatin C transcript expression was tested for IRF8WT and IRF8R294C BMDCs (left) by quantitative reverse-transcription (RT)–PCR. ChIP analysis was performed for the Csc3 promoter for binding of IRF8 or PU.1 in above DCs. Normal rabbit IgG was used as a control. Data represent the average of 3 determinations (± SD). (E) Diagram of IRF8R294C-directed DC development. The mutation abolishes the development of CD8α+ DCs, without affecting that of pDCs. The mutation results in increased CD4+ DCs and pDCs. The impaired ability to interact with partner proteins may partly account for the differential effect of this mutation on DC subset development.
Impaired cytokine induction and partner interactions in IRF8R294C DCs. (A) BMDCs from IRF8WT and IRF8R294C mice were stimulated with indicated TLR ligands. IL12p40 and IFNα transcripts (measured 6 hours after stimulation) and proteins (measured 24 hours after stimulation) were measured by quantitative polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Error bars represent SD. (B) BM cells from IRF8 KO mice were transduced with pMSCV vectors for IRF8WT or IRF8R294C, and expression of B220 and CD8α (24 hours after CpG stimulation) was detected by flow cytometry. (C) EMSA analysis: in vitro–transcribed and – translated proteins from control pcDNA, IRF8WT, IRF8R294C, and IRF8R289E vectors (lanes 1–4) were mixed with the indicated in vitro–transcribed and – translated partner proteins and 32p-labeled ISRE (for IRF2) and EICE (for PU.1 and SpiB). Asterisks indicates IRF8-partner complexes. Specificity of mobility shifts was verified by adding excess unlabeled probes (lane 5), which removed the shifted band or by adding antibodies for IRF8 (lane 6) or partner proteins (lane 7), which “supershifted” the band mobility. Lane 8 contained partner proteins without IRF8, which produced no shifted band. (D) Cystatin C transcript expression was tested for IRF8WT and IRF8R294C BMDCs (left) by quantitative reverse-transcription (RT)–PCR. ChIP analysis was performed for the Csc3 promoter for binding of IRF8 or PU.1 in above DCs. Normal rabbit IgG was used as a control. Data represent the average of 3 determinations (± SD). (E) Diagram of IRF8R294C-directed DC development. The mutation abolishes the development of CD8α+ DCs, without affecting that of pDCs. The mutation results in increased CD4+ DCs and pDCs. The impaired ability to interact with partner proteins may partly account for the differential effect of this mutation on DC subset development.
A differential effect of the IRF8R294C mutation on DC subset development was corroborated by gene transfer experiments in IRF8 KO BM progenitors. In Figure 2B, introduction of expression vectors for IRF8WT but not for IRF8R294C led to the generation of CD8α+ DCs, although both IRF8WT and IRF8R294C generated B220+ DCs at comparable levels. Similarly, IL12p40 induction was rescued only by IRF8WT, while IFNα induction was rescued by both IRF8WT and IRF8R294C (Figure S3).
Loss of partner protein interactions in the IRF8R294C mutant
IRF8 binds to certain DNA motifs such as ISRE and Ets/IRF composite elements by interacting with partner proteins that include IRF members and Ets family proteins.12,15 Although IRF8 can regulate gene expression through other mechanisms, the interaction with partners is thought to be a critical requirement for macrophage development.16 In EMSA analyses shown in Figure 2C, IRF8WT interacted with IRF2, PU.1, and SpiB21 to bind the probes. In contrast, IRF8R294C and the engineered IAD mutant, IRF8R289E, did not interact with the partners and failed to bind to these elements.6 Thus, the R294C mutation disabled partner-dependent DNA-binding activity of IRF8 in vitro. To ascertain this defect in vivo, we performed ChIP analysis first for the Csc3 (cystatin C) promoter. Cystatin C is expressed highly in CD8α+ DCs and its expression depends on the IRF8-PU.1 interaction.16,18 In agreement, cystatin C expression was high in IRF8WT DCs, but was greatly diminished in IRF8R294C DCs (Figure 2D). ChIP analysis showed that whereas IRF8WT bound to the Csc3 promoter well, IRF8R294C showed meager binding despite that PU.1 binding was comparable in both of the samples. We also noted that IRF8R294C bound to the IFNa4 promoter, albeit less efficiently than IRF8WT, in agreement with the expression of IFNa4 in IRF8R294C DCs (Figure S4). Further, IRF8R294C failed to bind to the IL12p40 promoter, again in agreement with the failure of the mutant to support cytokine expression (Figure S4). Together, our data indicate that IRF8 by interacting with partners may regulate many genes specifically required for the development and functional elicitation of CD8α+ DCs.22,23 Based on the fact that the IRF8R294C mutation did not affect pDC development, an alternative mechanism(s) of IRF8 action is likely to operate in directing pDC development and their function.12
In summary, analyses of BXH2 mice revealed that IRF8 uses distinct mechanisms to direct the development of CD8α+ DCs and pDCs (Figure 2E). These mice will serve as a useful model to further study the role of IRF8 in DC subset gene expression, and to investigate the mechanisms of its action in directing DC and lymphocyte development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. Dasenbrock, M. Smith, and B. Oldfield for genotyping and technical assistance; T. Kuwata for breeding suggestions; and B. Levi for discussions.
This work was supported by the Intramural Research Programs of the NIH, NICHD, and NIAID.
National Institutes of Health
Authorship
Contribution: P.T., T.T., H.C.M., and K.O. designed experiments; P.T. carried out the experiments; all authors contributed to data analysis and discussions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keiko Ozato, Laboratory of Molecular Growth Regulation, NICHD, National Institutes of Health, Bldg 6, Rm 2A01, 6 Center Dr, Bethesda, MD, 20892-2753; e-mail: ozatok@nih.gov.