Human embryonic stem cells (hES cells) have unlimited self-renewal capacity and can differentiate into most, if not all, possible cell types. This unique property makes them valuable not only for investigation of early developmental processes, but also for regenerative medicine. Mesoderm-derived cardiac cells and hematopoietic cells both have the potential for various therapeutic applications. However, efficient induction of hES cell differentiation into mesoderm remains a challenge. Here, we showed that treatment of hES cells with bone morphogenetic protein-4 (BMP-4) exhibited differential effects: long-term treatment results in trophoblast and extra-embryonic endoderm differentiation, whereas short-term treatment can promote early mesoderm induction. The induction of mesoderm in hES cells occurs at a high efficiency as measured using several markers, such as Brachyury, WNT3, and MIXL1 expression. Moreover, these mesoderm progenitor cells can differentiate into cardiac and hematopoietic lineages in vitro. Further analysis showed that the mesoderm-inducing capacity of BMP-4 requires endogenous FGF and TGF-β/Nodal/activin signaling activities. Thus, our results uncover a novel role for BMP-4 in regulation of hES cell differentiation and should provide insights into the mechanism of mesoderm induction in hES cells.
Introduction
The vertebrate mesoderm gives rise to a diverse variety of tissues, including the heart, vasculature, and blood. It is formed in the primitive streak (PS) which appears first in the posterior epiblast during gastrulation. The nascent PS then elongates to the anterior part, whereas the epiblast cells ingress through the PS, migrate to appropriate location in the embryo, and become either mesoderm or definitive endoderm.1 Although the process of mesoderm formation is well understood, the underlying molecular mechanisms remain poorly defined.
Previous studies have identified several signaling pathways involved in this process.2 One is the transforming growth factor-beta (TGF-β) superfamily member, bone morphogenetic protein-4 (BMP-4). Like other TGF-β superfamily members, BMP-4 binds to and brings together the type I and type II receptors on the cell surface, which allows receptor II to phosphorylate the receptor I kinase domain. After this activation, the type I receptor phosphorylates certain members of the Smad proteins (Smad 1, 5, 8; R-Smad). Two phosphorylated R-Smads, in combination with a common mediator Smad (Smad4; Co-Smad), form a heterotrimeric complex, which then translocates into the nucleus and cooperates with other transcription factors to modulate target gene expression.3,,–6 Genetic studies in mice have shown that BMP-4 signaling is required for mesoderm formation.7,–9 Mice deficient in the BMP-4 ligand,7 in the BMP type II receptor,8 or in a BMP type I receptor Alk3,9 all fail to develop mesoderm. It has also been demonstrated that BMP-4 plays critical roles in mesoderm induction and mesoderm lineage differentiation from mouse embryonic stem cells (mES cells).10,,,,,,,,–19
The key role of BMP-4 in mouse embryos and mES cells has led us to investigate its function in human embryonic stem cells (hES cells), the differentiation of which can, at least in part, mimic the human embryogenesis.20,21 Xu et al22 showed that long-term BMP-4 treatment (up to 7 days) induces hES cell differentiation into trophoblast. Later, Pera et al23 demonstrated that activation of the same signaling pathway by BMP-2 in hES cells results in the formation of extra-embryonic endoderm.
Surprisingly, we found that short-term BMP-4 treatment (24 hours) initiated mesoderm induction at a high efficiency in hES cells. In addition, these mesoderm progenitor cells were able to differentiate into various mesoderm lineages. The differential effects of short-term and long-term BMP-4 treatments on hES cell differentiation suggest that the BMP signaling pathway might play a flexible and time-dependent role in human embryonic development and cell fate determination.
Methods
Cell culture and differentiation
The H1, H7, and H9 hES cell lines were obtained from WiCell Research Institute (Madison, WI). Cells were cultured and passaged on mitomycin C-treated mouse embryonic fibroblast (MEF) feeders in hES cell culture medium consisting of 80% Dulbecco modified Eagle medium/F12 (Invitrogen, Carlsbad, CA), 20% KnockOut Serum Replacement (Invitrogen), 2 mM L-glutamine (HyClone, Logan, UT), 0.1 mM nonessential amino acid (NEAA), 0.1 mM 2-mercaptoethanol (2-ME), 100 U/mL penicillin, 100 μg/mL streptomycin (all from Invitrogen) and 4 ng/mL recombinant human basic fibroblast growth factor (rhbFGF; R&D Systems, Minneapolis, MN). Cultures were passaged with 1 mg/mL dispase (Invitrogen) at a 1:3 ratio every 5 to 7 days. Karyotype analysis indicated that they had a normal karyotype.
For mesoderm differentiation in feeder-free cultures, hES cells were first passaged onto plastic plates coated with matrigel (BD Biosciences, Bedford, MA) and cultured in MEF-conditioned medium as previously described.24 Then the following day after passage, differentiation was carried out in serum-free medium (SFM: RPMI 1640 (HyClone) containing 1% insulin-transferrin-selenium A (Invitrogen), 0.1 mM NEAA, 2 mM L-glutamine, 0.1 mM 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin), and supplemented where indicated with various concentrations of rhactivin A (PeproTech, Rocky Hill, NJ), rhBMP-4, rhbFGF, recombinant mouse Wnt3a (rmWnt3a), 250 ng/mL rhDkk-1 (all from R&D Systems), 10 μM SU5402 (Calbiochem, San Diego, CA), 10 μM SB431542 (Tocris Bioscience, Ellisville, MO). Before initiating differentiation, the hES cells were washed once with PBS.
For cardiac or hematopoietic induction, the hES cells were first differentiated in SFM in the presence or absence of 25 ng/mL rhBMP-4 for 24 hours, then in SFM in the presence of 300 ng/mL rmNoggin (R&D Systems) or 100 ng/mL rhbFGF for 3 days, respectively. After that, the hES cells were further differentiated in serum-containing medium (SCM: RPMI 1640 supplemented with 5% fetal bovine serum (Invitrogen), 0.1 mM NEAA, 2 mM L-glutamine, 0.1 mM 2-ME, 100 U/mL penicillin, and 100 μg/mL streptomycin) for indicated days.
Immunofluorescence
Cells at indicated days were fixed for 20 minutes at room temperature with 4% wt/vol paraformaldehyde in PBS, washed several times with PBS, permeabilized with 0.1% vol/vol Triton X-100 in PBS (PBST) for 10 minutes, and blocked for 45 minutes at room temperature in PBST containing 3% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA). The cells were then incubated at 4°C for 16 to 18 hours with primary antibodies diluted in PBST/3% normal donkey serum. After several washes with PBS, cells were incubated with secondary antibody diluted in PBS containing 0.1% bovine serum albumin (BSA; Jackson ImmunoResearch Laboratories) at room temperature for 1 hour. The cells were finally stained for the nuclei with 1 μg/mL DAPI (4,6 diamidino-2-phenylindole; Roche Applied Science, Indianapolis, IN), and observed at room temperature under the Nikon ECLIPSE TE2000-U fluorescence microscope (Nikon, Japan) equipped with Plan Fluor 4 × /0.13 and 10 × /0.30 objective lenses. Images were recorded using a SPOT RT CCD microscope digital camera (Diagnostic Instruments, Sterling Heights, MI), and processed with the Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA).
The following primary antibodies were used for this study: goat antihuman brachyury antibody (4 μg/mL; R&D Systems), rabbit antihuman brachyury antibody (4 μg/mL; Abcam, Cambridge, MA), goat antihuman SOX7 antibody (2 μg/mL; R&D Systems), goat antihuman FOXA2 antibody (2 μg/mL; R&D Systems), goat polyclonal antihuman GSC antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal antihuman Oct3/4 antibody (1:100; Santa Cruz Biotechnology). And secondary antibodies used here were donkey antigoat-fluorescein isothiocyanate (FITC), donkey antigoat-rhodamine (TRITC), donkey antirabbit-FITC, donkey antirabbit-TRITC, all from Jackson ImmunoResearch Laboratories, and were used in a 1:100 dilution.
Alkaline phosphatase detection
To detect alkaline phosphatase activity, the hES cells on matrigel were washed 3 times with PBS, fixed for 20 minutes at room temperature with 4% paraformaldehyde, and then the cells were incubated with the Vector Red alkaline phosphatase substrate (Vector Laboratories, Burlingame, CA) at room temperature in the dark for 30 minutes. Later, the cells were washed once with PBS and observed under a fluorescence microscope.
RT polymerase chain reaction (PCR) and real-time quantitative PCR
Total RNA from the cells was prepared using TriZol Reagent (Invitrogen). Residual genomic DNA was removed using RNase-free DNase I (New England Biolabs, Ipswich, MA). Then 1 μg RNA was reverse transcribed in a 20-μL reaction using the reverse transcription system (Promega, Madison, WI) according to the manufacturer's instructions. PCR was performed using EX Taq polymerase (Takara Biotechnology, Dalian, China), and 1 μL cDNA was used for each reaction. PCR conditions are as follows: 5 minutes at 94°C (hot start), 25 to 40 cycles (various numbers for different genes) of 94°C for 30 seconds, annealing temperature for 30 seconds, 72°C for 45 seconds. And a 5-minute extension at 72°C was done in the end. PCR products were analyzed on 2% agarose gel. The primer and PCR condition for each gene were shown in Table 1.
Primers used for RT PCR
| Gene name . | Forward primer 5′→3′ . | Reverse primer 5′→3′ . | Product length, bp . | Annealing temperature, °C . |
|---|---|---|---|---|
| BMP-4 | gactacatgcgggatctttacc | cccatagtttggctgcttctc | 459 | 55 |
| BMPR1b | gacctacaccctacactgcc | actttcccatccaaacttcc | 355 | 51.5 |
| BMPR1a | gcaattgctcatcgagacc | cgaagctgtagatgtcagcc | 232 | 55 |
| BMPR2 | aatgcagccataagcgagg | tggtactctggtacggattcc | 188 | 55 |
| Brachyury | gcgggaaagagcctgcagta | ttccccgttcacgtacttcc | 300 | 55 |
| Wnt3 | ggccatgaacaagcacaacaa | tgccgtgggaggtgacatt | 368 | 58 |
| Mixl1 | ggtaccccgacatccactt | tggaaggatttcccactctg | 120 | 55 |
| αMHC | gtcattgctgaaaccgagaatg | gcaaagtactggatgacacgct | 423 | 57 |
| cTnT | ggcagcggaagaggatgctgaa | gaggcaccaagttgggcatgaacga | 152 | 57 |
| GATA4 | gacgggtcactatctgtgcaac | agacatcgcactgactgagaac | 475 | 60 |
| MEF2 | agattacgaggattatggatgaacgt | acctgcacttggaggtcgatgtg | 563 | 56 |
| Nkx2.5 | cttcaagccagaggcctacg | ccgcctctgtcttcttcagc | 233 | 55 |
| SCL | tctctcggcagcgggttcttt | ccaggcggaggatctcattctt | 259 | 58 |
| CD34 | cctaagtgacatcaaggcagaa | gcaaggagcagggagcata | 201 | 55 |
| γ-Globin | cgcttctggaacgtctgaggttat | ccaggagcttgaagttctcaggat | 370 | 59 |
| ϵ-Globin | cactagcctgtggagcaagatgaa | aatcaccatcacgttacccaggag | 304 | 59 |
| GAPDH | aatcccatcaccatcttcc | catcacgccacagtttcc | 382 | 55 |
| Gene name . | Forward primer 5′→3′ . | Reverse primer 5′→3′ . | Product length, bp . | Annealing temperature, °C . |
|---|---|---|---|---|
| BMP-4 | gactacatgcgggatctttacc | cccatagtttggctgcttctc | 459 | 55 |
| BMPR1b | gacctacaccctacactgcc | actttcccatccaaacttcc | 355 | 51.5 |
| BMPR1a | gcaattgctcatcgagacc | cgaagctgtagatgtcagcc | 232 | 55 |
| BMPR2 | aatgcagccataagcgagg | tggtactctggtacggattcc | 188 | 55 |
| Brachyury | gcgggaaagagcctgcagta | ttccccgttcacgtacttcc | 300 | 55 |
| Wnt3 | ggccatgaacaagcacaacaa | tgccgtgggaggtgacatt | 368 | 58 |
| Mixl1 | ggtaccccgacatccactt | tggaaggatttcccactctg | 120 | 55 |
| αMHC | gtcattgctgaaaccgagaatg | gcaaagtactggatgacacgct | 423 | 57 |
| cTnT | ggcagcggaagaggatgctgaa | gaggcaccaagttgggcatgaacga | 152 | 57 |
| GATA4 | gacgggtcactatctgtgcaac | agacatcgcactgactgagaac | 475 | 60 |
| MEF2 | agattacgaggattatggatgaacgt | acctgcacttggaggtcgatgtg | 563 | 56 |
| Nkx2.5 | cttcaagccagaggcctacg | ccgcctctgtcttcttcagc | 233 | 55 |
| SCL | tctctcggcagcgggttcttt | ccaggcggaggatctcattctt | 259 | 58 |
| CD34 | cctaagtgacatcaaggcagaa | gcaaggagcagggagcata | 201 | 55 |
| γ-Globin | cgcttctggaacgtctgaggttat | ccaggagcttgaagttctcaggat | 370 | 59 |
| ϵ-Globin | cactagcctgtggagcaagatgaa | aatcaccatcacgttacccaggag | 304 | 59 |
| GAPDH | aatcccatcaccatcttcc | catcacgccacagtttcc | 382 | 55 |
Quantitative PCR analysis was performed using the ABI PRISM 7300 Sequence Detection System (Applied Biosystems, Foster City, CA). The PCR amplification reaction mix consisted of 12.5 μL of SYBR Green PCR Master Mix (Applied Biosystems), 0.8 μL of 10 mM forward and reverse primers, 0.5 μL of template cDNA and 10.4 μL of distilled water in a total volume of 25 μL. Cycling was performed using the default conditions of the ABI 7300 SDS software 1.3.1: 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Primer sequences are provided on request. The comparative threshold cycle (Ct) method was used to analyze data, with gene expression levels calibrated to that of the housekeeping gene GAPDH. PCR was performed in duplicate for each sample, and 3 independent experiments were carried out. The means and standard deviations were calculated and reported using data from one representative experiment.
Fluorescence-activated cell sorting
Cells were dissociated with 0.25% trypsin-ethylenediaminetetraacetic acid (Invitrogen) into a single cell suspension. Then the cells were resuspended in 0.5% BSA/PBS for 20 minutes at room temperature to block possible nonspecific antibody staining. After that, cells were stained with either monoclonal antihuman KDR-phycoerythrin (PE; R&D systems) or PE-conjugated mouse antihuman CD34 monoclonal antibody (BD Biosciences) at 5 μL/2 × 105 cells for 30 minutes on ice. After this staining, cells were washed twice in 4 mL PBS to remove unbound antibody reagent and then resuspended in 200 μL PBS. Cells were analyzed on a FACS Calibur (BD Biosciences), and data were analyzed using the CellQuest software (BD Biosciences). Three independent experiments were carried out.
Cell sorting was performed on a MoFlo high-performance cell sorter (Dako, Fort Collins, CO), and both CD34-positive (CD34+) and CD34-negative (CD34−) cells were collected.
Colony-forming unit (CFU) assay
The cells differentiated for 10 days were harvested and sorted for CD34+ and CD34− cell populations. Hematopoietic colonies were demonstrated by growing these 2 kinds of cells in Methocult GF + media H4435 (StemCell Technologies, Vancouver, BC) containing 1% methylcellulose, 30% fetal bovine serum, 1% BSA, 0.1 mM 2-ME, 2 mM l-glutamine, 50 ng/mL rh stem cell factor, 20 ng/mL rh GM-CSF, 20 ng/mL rh IL-3, 20 ng/mL rh IL-6, 20 ng/mL rh G-CSF, and 3 U/mL rh erythropoietin. After incubation in a 37°C, 5% CO2 environment with more than or equal to 95% humidity for 14 to 16 days, CFUs were counted according to their colony morphology. Three independent experiments were carried out.
Statistical analysis
For flow cytometry and CFU assays, the mean (SD) of 3 independent experiments was reported. Student t test was used for statistical evaluation, and P less than .05 was considered as statistically significant.
Results
Differential effects of short-term and long-term BMP-4 treatments on hES cell differentiation
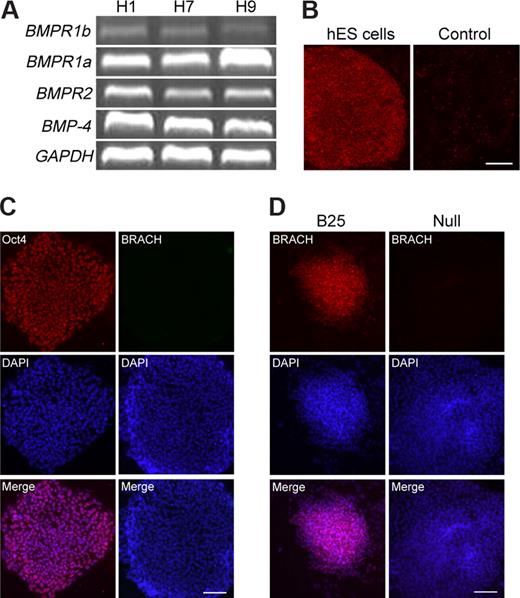
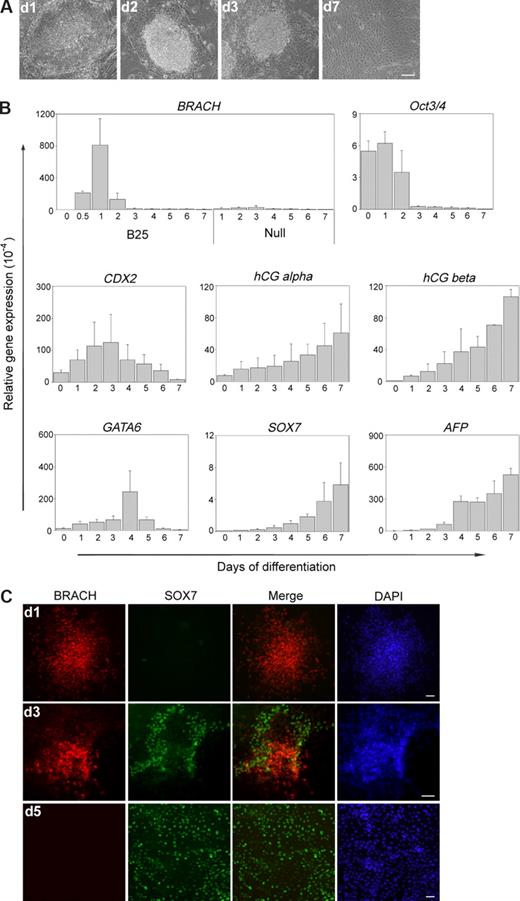
TGF-β/Nodal/activin, FGF, Wnt, and BMP are all potent mesoderm inducers in vertebrates,2 and previous reports have demonstrated that hES cells also express components of these 4 signaling transduction pathways25,–27 (Figure 1A); however, whether these mesoderm inducers function in hES cells remains unclear. Here, we tested the effects of exogenous activin A, bFGF, Wnt3a, and BMP-4 on the mesoderm initiation in hES cells, as determined by the expression analysis of the pan-mesoderm marker brachyury. Before these experiments, we first examined the hES cells for alkaline phosphatase activity, Oct3/4, and brachyury expression and confirmed that they remain in an undifferentiated state (Figure 1B,C). The cells were then examined for their response to various concentrations of these factors individually in SFM. We found that, among these factors, BMP-4 was the most efficient in inducing brachyury-positive cells, although activin A and Wnt3a also exhibited some brachyury-inducing activities (data not shown). BMP-4 treatment at a concentration of 25 ng/mL for 24 hours resulted in a high level expression of brachyury (Figure 1D), and under this condition, hES cells formed more compact aggregates at the center of the colony than untreated hES cells. The morphology of these brachyury-positive colonies was similar to that of Wnt3a-induced brachyury-positive colonies (data not shown). However, we observed that prolonged BMP-4 treatment for more than 2 days led to additional morphologic changes (Figure 2A) characterized by flattened and enlarged cells, which appeared first on the edge of a colony, then extended to the center. These changes suggested that the hES cells might have adopted further differentiated fates into specific lineages. We then monitored this process by quantitative PCR (Q-PCR) for up to 7 days, in the continuous presence of exogenous BMP-4. As shown in Figure 2B, on day 1 of differentiation, we detected the highest brachyury expression level, which, however, decreased dramatically around day 2, and was undetectable on day 7. In contrast, the expression analysis of the early trophoectoderm marker CDX2 and early extra-embryonic endoderm marker GATA6 showed that their expression was very low on day 1, started to increase on day 2, and reached their peak level on day 3 and 4, respectively. Correspondingly, the expression of trophoblast markers hCG alpha and beta or the late extra-embryonic endoderm markers SOX7 and AFP were all undetectable on day 1 and gradually increased until reaching their peak level on day 7. The sequential activation of early and late trophoectoderm and extra-embryonic endoderm genes from day 2 to day 7 strongly indicated the occurrence of extra-embryonic differentiation. As a control, hES cells differentiated in SFM in the absence of BMP-4 exhibited little or undetectable brachyury expression from day 1 to day 7. Immunofluorescence analysis of brachyury and SOX7 also confirmed our results (Figure 2C). Thus, our data showed that BMP-4 treatment exhibits time-dependent effects on hES cell differentiation: short-term BMP-4 treatment for 24 hours induces brachyury-positive cells efficiently, whereas long-term BMP-4 treatment up to 7 days leads to trophoblast and extra-embryonic endoderm differentiation. The extra-embryonic induction effects of long-term BMP treatment we observed are consistent with previous reports by Xu et al22 and Pera et al.23
Short-term BMP-4 treatment of hES cells efficiently induces brachyury expression.(A) Undifferentiated H1, H7, and H9 hES cells express BMP-4 ligand, its receptors BMPR1a, BMPR1b, and BMPR2. (B) hES cells grown on matrigel retained alkaline phosphatase activity (hES cells), whereas the differentiated hES cells did not (Control). (C) hES cells grown on matrigel expressed the pluriopotency marker Oct3/4, but did not express brachyury. (D) As measured by immunocytochemistry, hES cells differentiated in the presence of 25 ng/mL BMP-4 for 24 hours (B25) showed a high level of brachyury expression. However, when the hES cells were differentiated without BMP-4 treatment (Null), no brachyury expression could be detected. BRACH indicates brachyury. Scale bars represent 200 μm (B) and 100 μm (panels C,D).
Short-term BMP-4 treatment of hES cells efficiently induces brachyury expression.(A) Undifferentiated H1, H7, and H9 hES cells express BMP-4 ligand, its receptors BMPR1a, BMPR1b, and BMPR2. (B) hES cells grown on matrigel retained alkaline phosphatase activity (hES cells), whereas the differentiated hES cells did not (Control). (C) hES cells grown on matrigel expressed the pluriopotency marker Oct3/4, but did not express brachyury. (D) As measured by immunocytochemistry, hES cells differentiated in the presence of 25 ng/mL BMP-4 for 24 hours (B25) showed a high level of brachyury expression. However, when the hES cells were differentiated without BMP-4 treatment (Null), no brachyury expression could be detected. BRACH indicates brachyury. Scale bars represent 200 μm (B) and 100 μm (panels C,D).
Differential effects of short- and long-term BMP-4 treatments on hES cell differentiation. (A) Morphology of the differentiated hES cells at days 1, 2, 3, and 7 of BMP-4 treatment. (B) As determined by Q-PCR, the pan-mesoderm gene brachyury was highly expressed at 24 hours of hES cell differentiation by BMP-4 treatment (B25). However, the extra-embryonic lineage genes, GATA6, SOX7, AFP for extra-embryonic endoderm, and CDX2, hCG alpha, hCG beta for extra-embryonic ectoderm, were induced by long-term, but not short-term, BMP-4 treatment. And hES cells differentiated in SFM in the absence of BMP-4 (Null) showed little or undetectable brachyury expression. Numbers on the x-axis indicate days of differentiation; numbers on the y-axis indicate relative gene expression level normalized to that of GAPDH. Error bars indicate standard deviations. (C) Immunofluorescent labeling of differentiating hES cells demonstrated decreased expression of brachyury and increased expression of SOX7 with continuous BMP-4 treatment. BRACH indicates brachyury. Scale bars represent 50 μm.
Differential effects of short- and long-term BMP-4 treatments on hES cell differentiation. (A) Morphology of the differentiated hES cells at days 1, 2, 3, and 7 of BMP-4 treatment. (B) As determined by Q-PCR, the pan-mesoderm gene brachyury was highly expressed at 24 hours of hES cell differentiation by BMP-4 treatment (B25). However, the extra-embryonic lineage genes, GATA6, SOX7, AFP for extra-embryonic endoderm, and CDX2, hCG alpha, hCG beta for extra-embryonic ectoderm, were induced by long-term, but not short-term, BMP-4 treatment. And hES cells differentiated in SFM in the absence of BMP-4 (Null) showed little or undetectable brachyury expression. Numbers on the x-axis indicate days of differentiation; numbers on the y-axis indicate relative gene expression level normalized to that of GAPDH. Error bars indicate standard deviations. (C) Immunofluorescent labeling of differentiating hES cells demonstrated decreased expression of brachyury and increased expression of SOX7 with continuous BMP-4 treatment. BRACH indicates brachyury. Scale bars represent 50 μm.
We further analyzed the expression of endoderm and ectoderm markers in these BMP-4-treated hES cells over the period of 7 days. We detected little or no expression of the endoderm-associated genes CER, FOXA2, and the ectoderm-associated genes SOX1 and ZIC1 (data not shown). This result implied that little endoderm or ectoderm differentiation occurred under this condition. Interestingly, we observed that, on day 1 of the differentiation process, the expression level of the pluripotency marker Oct3/4 (POU5f1) was moderately higher than that of undifferentiated hES cells (Figure 2B). With prolonged BMP-4 treatment, the Oct3/4 expression level decreased from day 2, and at day 7 we did not detect any Oct3/4 expression. The decrease in Oct3/4 expression is consistent with the loss of pluripotency in these BMP-4-treated cells. The unexpected elevated expression of Oct3/4 during early hES cell differentiation we observed is similar to previous reports in mES cells,28 and this result suggests that Oct3/4 might be involved in BMP-4 initiated differentiation in hES cells.
Short-term BMP-4 treatment induces mesoderm progenitors in hES cells
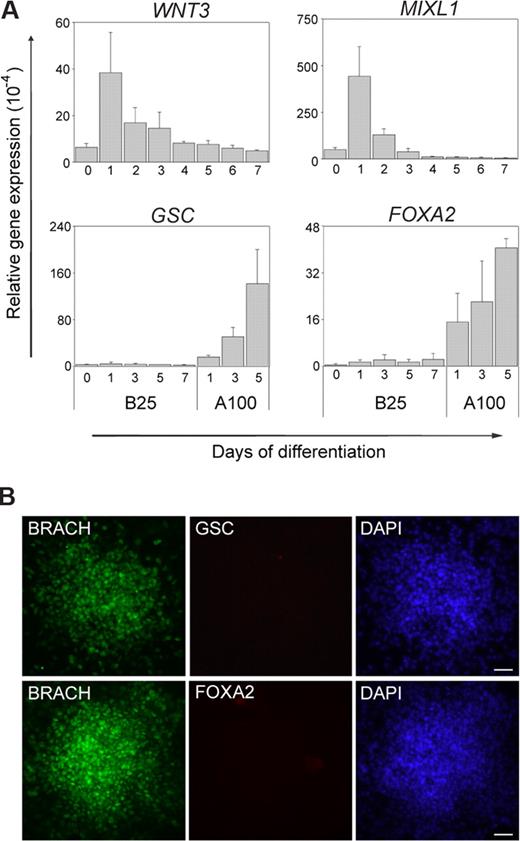
Previous reports, as well as experiments carried out in our laboratory, demonstrated that long-term BMP treatment of hES cells results in differentiation into trophoblast or extra-embryonic endoderm. Therefore, we focused our study on the brachyury-inducing effect of short-term BMP-4 treatment. Because brachyury is expressed not only in the early mesoderm, but also in the primitive streak/mesendoderm during development,29,30 it is essential to measure the expression of several other markers to characterize the brachyury-positive cells. We selected WNT3, MIXL1, GSC, and FOXA2 as additional markers. WNT3 and MIXL1 are expressed in the primitive streak/mesendoderm and early mesoderm,31,32 whereas GSC and FOXA2 are markers of the primitive streak/mesendoderm and endoderm.33,–35 We monitored the expression profiles for these 4 genes during the 7 days of differentiation. As shown in Figure 3A, on BMP-4 treatment, WNT3 and MIXL1 expression increased rapidly and reached peak level by day 1. Then their expression began to decrease, until they were undetectable on day 7. Both WNT3 and MIXL1 showed a very similar expression profile to brachyury. However, during the entire process, we detected little or no expression of GSC and FOXA2 (Figure 3A). Thus, the expression analysis of WNT3 and MIXL1, GSC, and FOXA2 strongly indicated that the BMP-4-induced brachyury-positive cell populations are possibly mesoderm progenitors instead of being at the primitive streak/mesendoderm stage.
Short-term BMP-4 treatment induces mesoderm progenitors in hES cells. (A) Q-PCR analyzes profiles of several other genes during hES cell differentiation in the presence of BMP-4 (B25). WNT3 and MIXL1, expressed in the mesendoderm and mesoderm, both had similar expression pattern to brachyury. However, GSC and FOXA2, expressed in the mesendoderm and endoderm, were not induced during this process. In this study, hES cell differentiation by 100 ng/mL activin A treatment (A100) was used as a positive control for mesendoderm and endoderm gene expression.56 Numbers on the x-axis indicate days of differentiation; numbers on the y-axis indicate relative gene expression level normalized to that of GAPDH. Error bars indicate standard deviations. (B) Immunocytochemistry results showed that, at day 1 of differentiation, when brachyury displayed high level expression, no GSC or FOXA2 expression could be detected. Scale bars represent 50 μm.
Short-term BMP-4 treatment induces mesoderm progenitors in hES cells. (A) Q-PCR analyzes profiles of several other genes during hES cell differentiation in the presence of BMP-4 (B25). WNT3 and MIXL1, expressed in the mesendoderm and mesoderm, both had similar expression pattern to brachyury. However, GSC and FOXA2, expressed in the mesendoderm and endoderm, were not induced during this process. In this study, hES cell differentiation by 100 ng/mL activin A treatment (A100) was used as a positive control for mesendoderm and endoderm gene expression.56 Numbers on the x-axis indicate days of differentiation; numbers on the y-axis indicate relative gene expression level normalized to that of GAPDH. Error bars indicate standard deviations. (B) Immunocytochemistry results showed that, at day 1 of differentiation, when brachyury displayed high level expression, no GSC or FOXA2 expression could be detected. Scale bars represent 50 μm.
In addition to Q-PCR assay, we performed immunocytochemistry to test the expression of these markers at the single-cell level in BMP-4-treated cultures. As shown in Figure 3B, on day 1 of differentiation, when brachyury expression is the highest, no expression of GSC or FOXA2 is observed. In addition, during the 7 days of differentiation, we could detect little or no expression for both GSC and FOXA2 protein (data not shown), providing further evidence that the brachyury-positive populations are mesoderm progenitors.
hES cells-derived mesoderm progenitors further differentiate in vitro
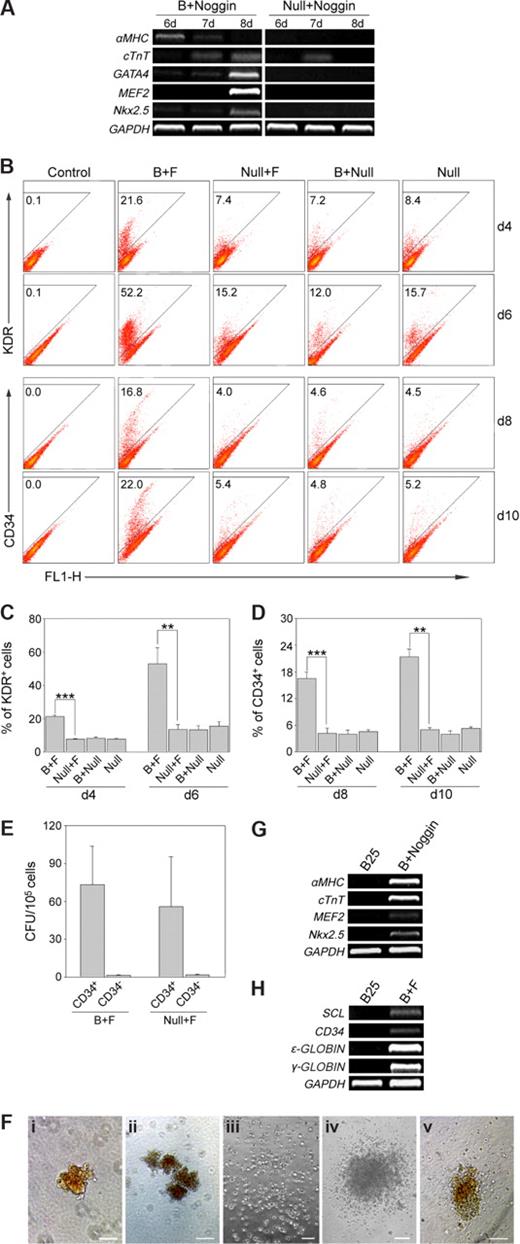
To test the developmental potential of hES cells-derived mesoderm progenitors to differentiate into mature mesoderm lineages, such as cardiac and hematopoietic cells, we further induced their differentiation in vitro. Because long-term BMP-4 treatment leads to trophoblast and extra-embryonic endoderm and BMP is able to induce its own expression through a positive feedback mechanism,23 it is essential to inhibit the long-term effect of BMP-4 after short-term treatment for mesoderm differentiation. Here we selected Noggin and bFGF because both of them could antagonize BMP-4 activity in hES cells,36 and they were able to induce cardiac and hematopoietic differentiation from ES cells, respectively.37,,–40 For cardiac differentiation, hES cells were differentiated in SFM in the presence or absence of BMP-4 for 24 hours, then in SFM in the presence of Noggin for 3 days. After that, the hES cells were further differentiated in SCM and cultures were collected at days 6, 7, and 8 for gene expression analysis. The cardiac-associated genes αMHC, cTnT, GATA4, MEF2, and Nkx2.5 were readily detectable in the BMP-4-treated cultures (Figure 4A). However, we could detect little or no expression of these genes in cultures without BMP-4 treatment.
hES cells-derived mesoderm progenitors further differentiate in vitro. (A) hES cells were differentiated with BMP-4 (B + Noggin) or without BMP-4 (Null + Noggin) for 24 hours; then cells were washed and transferred to SFM containing Noggin. Three days after that, SFM was replaced by SCM without Noggin. Then cells were collected at indicated days and analyzed for cardiac gene expression by RT-PCR. (B) hES cells were differentiated with BMP-4 (B) or without BMP-4 (Null) for 24 hours; then cells were washed and transferred to SFM supplemented with bFGF (F) or without bFGF (Null). Three days after that, SFM was replaced by SCM without bFGF. The cultures were then analyzed for hematopoietic differentiation by flow cytometry analysis of KDR and CD34 at days 4 and 6, and days 8 and 10, respectively. Numbers indicate percentage of KDR+ or CD34+ cells in each culture. (C,D) Statistical analysis of KDR+ cells at days 4 and 6 (C) and CD34+ cells at days 8 and 10 (D) under different differentiation conditions. Values are mean and SD from 3 independent experiments (**P < .01, ***P < .001). (E) Hematopoietic progenitor capacity of CD34+ and CD34− subsets from differentiated hES cells with or without BMP-4 treatment. Values are mean and SD from 3 independent experiments. (F) Examples of different CFU colonies, including (i) CFU-erythroid (CFU-E), (ii) burst-forming unit-erythroid (BFU-E), (iii) CFU-myeloid (CFU-M), (iv) CFU-granulocyte, macrophage (CFU-GM), and (v) CFU-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM). (G-H) hES cells were differentiated in the presence of 25 ng/mL BMP-4 for 7 days (B25), then analyzed for cardiac gene (G) and hematopoietic gene (H) expression. And hES cells differentiated for 7 days as described in panels A and B (B + Noggin, B + F) were used as positive controls for cardiac and hematopoietic gene expression. Control indicates isotype control. Scale bars represent 50 μm (Fi), 100 μm (Fii,iii,v), and 200 μm (Fiv).
hES cells-derived mesoderm progenitors further differentiate in vitro. (A) hES cells were differentiated with BMP-4 (B + Noggin) or without BMP-4 (Null + Noggin) for 24 hours; then cells were washed and transferred to SFM containing Noggin. Three days after that, SFM was replaced by SCM without Noggin. Then cells were collected at indicated days and analyzed for cardiac gene expression by RT-PCR. (B) hES cells were differentiated with BMP-4 (B) or without BMP-4 (Null) for 24 hours; then cells were washed and transferred to SFM supplemented with bFGF (F) or without bFGF (Null). Three days after that, SFM was replaced by SCM without bFGF. The cultures were then analyzed for hematopoietic differentiation by flow cytometry analysis of KDR and CD34 at days 4 and 6, and days 8 and 10, respectively. Numbers indicate percentage of KDR+ or CD34+ cells in each culture. (C,D) Statistical analysis of KDR+ cells at days 4 and 6 (C) and CD34+ cells at days 8 and 10 (D) under different differentiation conditions. Values are mean and SD from 3 independent experiments (**P < .01, ***P < .001). (E) Hematopoietic progenitor capacity of CD34+ and CD34− subsets from differentiated hES cells with or without BMP-4 treatment. Values are mean and SD from 3 independent experiments. (F) Examples of different CFU colonies, including (i) CFU-erythroid (CFU-E), (ii) burst-forming unit-erythroid (BFU-E), (iii) CFU-myeloid (CFU-M), (iv) CFU-granulocyte, macrophage (CFU-GM), and (v) CFU-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM). (G-H) hES cells were differentiated in the presence of 25 ng/mL BMP-4 for 7 days (B25), then analyzed for cardiac gene (G) and hematopoietic gene (H) expression. And hES cells differentiated for 7 days as described in panels A and B (B + Noggin, B + F) were used as positive controls for cardiac and hematopoietic gene expression. Control indicates isotype control. Scale bars represent 50 μm (Fi), 100 μm (Fii,iii,v), and 200 μm (Fiv).
The hematopoietic differentiation experiment was carried out in a manner similar to that of the cardiac differentiation; however, Noggin was replaced by bFGF, and cultures were collected at days 4, 6, 8, and 10 for flow cytometry analysis. As shown in Figure 4B-D, there was approximately 3-fold higher percentage of KDR+ cells at days 4 and 6, or approximately 4-fold higher percentage of CD34+ cells at days 8 and 10 in BMP-4-treated cultures than in cultures not treated by BMP-4. These results suggested that neither BMP-4 nor bFGF alone can promote the generation of KDR+ or CD34+ cells, and sequential treatment of hES cells with BMP-4 and bFGF is required for the efficient generation of KDR+ or CD34+ cells (Figure 4B-D). To further demonstrate the hematopoietic potential of the BMP-4-induced mesoderm progenitors, we performed CFU assay. The hES cells differentiated for 10 days were first sorted out by flow cytometry for CD34+ and CD34− populations, both of which were then analyzed for their capacity to generate hematopoietic colonies. As shown in Figure 4E, the CD34+ subset contained approximately 20-fold more clonogenic hematopoietic progenitors than the CD34− subset. This result, together with that described in Figure 4D, indicates that the BMP-4-induced mesoderm progenitors could give rise to functional hematopoietic cells in vitro. And the clonogenic cells were capable of normal hematopoietic maturation (Figure 4F).
To further confirm our results, hES cells differentiated in the continuous presence of BMP-4 for 7 days were also analyzed for cardiac and hematopoietic gene expression. As shown in Figure 4G,H, none of the cardiac genes αMHC, cTnT, MEF2, Nkx2.5, or the hematopoietic genes SCL, CD34, ϵ-GLOBIN, γ-GLOBIN was detected, demonstrating that long-term BMP-4 treatment was not capable of inducing mature mesoderm lineages. This result also indicated the necessity of blocking BMP activity for inducing mesoderm lineages from short-term BMP-4-treated hES cells.
FGF and TGF-β/Nodal/activin signaling pathways are involved in BMP-4 initiated mesoderm induction
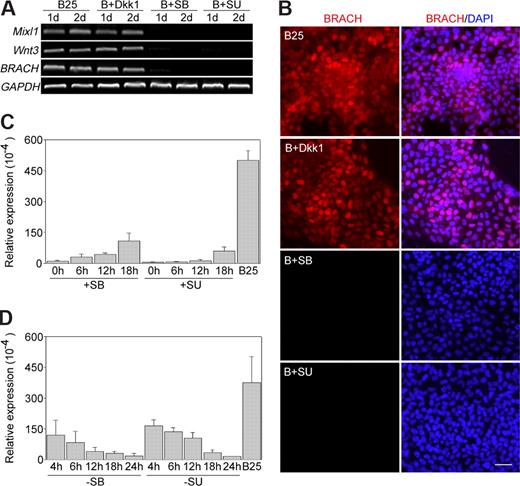
FGF, TGF-β/Nodal/activin, and Wnt signaling pathways have been demonstrated to be important for mesoderm induction in vertebrates. To investigate the possible involvement of other signaling pathways in BMP-4 induced mesoderm initiation, we first confirmed that bFGF, activin A, TGF-beta, and Wnt3a are expressed in our cultured undifferentiated hES cells (data not shown), and then we inhibited FGF and TGF-β/Nodal/activin signaling in the cultures by addition of the small molecular inhibitors SU5402 and SB431542, respectively. Furthermore, the Wnt signaling was blocked by addition of Dkk1, a specific Wnt inhibitor. We found that during differentiation of hES cells in SFM supplemented with BMP-4, addition of SU5402 or SB431542 for 1 or 2 days completely blocked the expression of brachyury, WNT3, and MIXL1 (Figure 5A,B). However, addition of Dkk-1 did not affect mesoderm induction by BMP-4 compared with the BMP-4-treated control (Figure 5A,B), even at the concentration of 500 ng/mL, which is much higher than that of previously reported to completely block Wnt signaling activity (data not shown). These results demonstrated that both FGF and TGF-β/Nodal/activin signaling pathways are involved in BMP-4 initiated mesoderm induction, whereas Wnt signaling might act upstream of or in parallel with the BMP-4 signaling in mesoderm induction from hES cells.
FGF and TGF-β/Nodal/activin signaling pathways are involved in BMP-4-initiated mesoderm induction. hES cells were differentiated in SFM in the presence of BMP-4 alone (B25), or BMP-4 and Dkk1 (B + Dkk1), or BMP-4 and SB431542 (B + SB), or BMP-4 and SU5402 (B + SU) for 2 days. Cultures were collected for (A) expression analysis of brachyury, WNT3, and MIXL1 by RT-PCR, or (B) brachyury expression at day 1 of differentiation by immunocytochemistry. (C-D) The inhibitor SB431542 (SB) or SU5402 (SU) was added at different time points of BMP-4 treatment (C), or cells were treated with BMP-4 in the presence of the inhibitor for different periods of time, washed, and then exposed to BMP-4 without inhibitors (D). Then cells were collected at 24 hours of differentiation and analyzed for brachyury expression by Q-PCR. hES cells treated by BMP-4 alone for 24 hours (B25) were used as a positive control. Numbers on the x-axis indicate time points when the inhibitors were added (C) or removed (D); numbers on the y-axis indicate relative gene expression level normalized to that of GAPDH. Error bars indicate SD. BRACH indicates brachyury. Scale bar represents 50 μm.
FGF and TGF-β/Nodal/activin signaling pathways are involved in BMP-4-initiated mesoderm induction. hES cells were differentiated in SFM in the presence of BMP-4 alone (B25), or BMP-4 and Dkk1 (B + Dkk1), or BMP-4 and SB431542 (B + SB), or BMP-4 and SU5402 (B + SU) for 2 days. Cultures were collected for (A) expression analysis of brachyury, WNT3, and MIXL1 by RT-PCR, or (B) brachyury expression at day 1 of differentiation by immunocytochemistry. (C-D) The inhibitor SB431542 (SB) or SU5402 (SU) was added at different time points of BMP-4 treatment (C), or cells were treated with BMP-4 in the presence of the inhibitor for different periods of time, washed, and then exposed to BMP-4 without inhibitors (D). Then cells were collected at 24 hours of differentiation and analyzed for brachyury expression by Q-PCR. hES cells treated by BMP-4 alone for 24 hours (B25) were used as a positive control. Numbers on the x-axis indicate time points when the inhibitors were added (C) or removed (D); numbers on the y-axis indicate relative gene expression level normalized to that of GAPDH. Error bars indicate SD. BRACH indicates brachyury. Scale bar represents 50 μm.
To further determine whether FGF or TGF-β/Nodal/activin signaling was required concurrently or subsequently to BMP-4 in the induction of mesoderm, the inhibitor SU5402 or SB431542 was added at 0, 6, 12, and 18 hours of BMP-4 treatment; or cells were treated with BMP-4 in the presence of the inhibitor for the first 4, 6, 12, and 18 hours, then washed, and further exposed to BMP-4 without the inhibitors. Cells were collected at 24 hours of differentiation and assayed for brachyury expression. As shown in Figure 5C-D, whenever the inhibitors were added, there was a reduction in brachyury expression compared with that of BMP-4-treated control (without the inhibitors). These results consistently suggested that FGF and TGF-β/Nodal/activin signaling pathways are both concurrently required for this differentiation process.
Discussion
hES cells can differentiate into definitive endoderm, mesoderm, and ectoderm in a manner similar to the embryogenesis process. Although several lineages differentiated from hES cells have been reported,22–23,41,,,,,–47 and some mesoderm inducers have been identified in mES cells,10,15,48 the mesoderm inducers in hES cells have not been well characterized. In this study, we analyzed the effects of BMP-4 treatment during hES cell differentiation and found that BMP-4 plays a time-dependent role in this process, with short-term BMP-4 treatment efficiently inducing mesoderm progenitors (Figure 1D), and the progenitor cells could further differentiate into cardiac and functional hematopoietic cells in vitro (Figure 4A-F). Importantly, BMP-4 was identified as a potent mesoderm inducer in hES cells, which might be of significance in future developmental studies and therapeutic applications.
It is very interesting that BMP-4 may play 2 differential roles during hES cell differentiation in the conditions used in our study: long-term treatment of hES cells by BMP-4 leads to extra-embryonic endoderm and trophoblast differentiation (Figure 2B,C), whereas short-term treatment can efficiently induce mesoderm progenitors (Figure 3A,B). To our knowledge, there are no reports that describe the transition from brachyury-positive cells to extra-embryonic endoderm or trophoblast. Interestingly, there are several reports demonstrating that certain genes necessary for trophoblast formation are also involved in the regulation of mesoderm development.49,50 And some markers for the extra-embryonic tissues can also be detected in the mesoderm.7,51 In addition, although previous experiments have demonstrated that activation of the BMP-2/BMP-4 signaling pathway in hES cells leads to differentiation into extra-embryonic endoderm or trophoblast, no direct evidences for involvement of BMPs in the analogous in vivo processes have been demonstrated in any mammals. However, in mouse embryos, BMP-4 signaling is required for mesoderm formation.7,–9 BMP-4 can induce mES cells to differentiate into mesoderm in vitro12,15,17 ; however, unlike hES cells, it is, to date, unfeasible to induce mES cells to differentiate into trophoblast without genetic modification.52 The extremely different responses of mES and hES cells to BMP-4 treatment in vitro indicate intrinsic differences between these 2 stem cell types. To further address this question, it is necessary to establish a brachyury reporter hES cell line and analyze the role of BMP-4 signaling in regulating human mesoderm, extra-embryonic endoderm, and trophoblast differentiation.
Because brachyury is also a primitive streak/mesendoderm marker, it is essential to measure the gene expression of several other markers to confirm that the brachyury-positive cells are mesoderm progenitors. In this study, we selected GSC and FOXA2, which are expressed in the primitive streak and endoderm, and WNT3 and MIXL1, which are expressed in the primitive streak and mesoderm. During the 7 days of BMP-4 treatment, brachyury, WNT3, and MIXL1 exhibited similar expression patterns: expression initially increased, reached a peak level, and then declined to undetectable level (Figures 2B, 3A). However, expression of GSC and FOXA2 was not induced during this process (Figure 3A). Immunofluorescence results indicated that the brachyury-positive populations exist mainly in the compact part of a hES colony (Figure 1D). On the other hand, GSC and FOXA2 are expressed in, if there are any, scattered cells, and there are few cells coexpressing brachyury and GSC or brachyury and FOXA2 (Figure 3B and data not shown). Therefore, the brachyury-positive cells are mostly mesoderm progenitors but not at the primitive streak stage.
In this study, we also investigated the functions of other signaling pathways, including FGF, TGF-β/Nodal/activin, and Wnt, during the BMP-4 induced differentiation of hES cells to mesoderm. We chose to investigate these other pathways because previous reports suggested the involvement of these pathways in mesoderm differentiation in other vertebrates. For example, studies by Cornell and Kimelman53 and LaBonne and Whiteman54 demonstrated that activin A can induce mesoderm in Xenopus. However, this mesoderm-inducing capacity by activin A requires endogenous FGF signaling. When dominant inhibitory mutants of FGF receptors are present, the mesoderm-associated genes are not induced, even in the presence of activin A. Studies by Conlon et al55 showed that disruption of TGF-β/Nodal/activin signaling pathway prevents the formation of primitive streak and the mesoderm. Recently, 2 groups38,48 using the mES cells as a model demonstrated that the Wnt signaling pathway is required for the development of primitive streak and mesoderm. Blocking this pathway either by RNAi48 or specific inhibitors38,48 prevents the associated gene expression and mature mesoderm lineage differentiation. In our experiments, we found that both endogenous FGF and TGF-β/Nodal/activin are required for BMP-4 induced differentiation into mesoderm, whereas Wnt signaling is not (Figure 5A,B). The involvement of FGF and TGF-β/Nodal/activin in BMP-4 induced mesoderm differentiation suggests the complexity of signaling pathways cross-talking in mesoderm initiation from hES cells and also underscores the necessity for a rigorous characterization of this process.
We have shown that Wnt signaling is not necessary for BMP-4-induced mesoderm initiation (Figure 5A,B); however, in mES cells, BMP-4 was unable to overcome the primitive streak-inhibition activity of saturating concentrations of Dkk1.38 These distinct observations underscore a novel difference between these 2 stem cell types, although they are both pluripotent stem cells derived from the inner cell mass of mammalian blastocysts and share certain features.
Collectively, we report 2 differential roles for BMP-4 in hES cell differentiation. Furthermore, we have developed a serum- and feeder-free system to efficiently induce mesoderm progenitors from hES cells by short-term BMP-4 treatment, which will be of great value for future therapeutic applications. However, determining the optimal methods for efficiently inducing these mesoderm cells to differentiate into mature lineages will remain a significant challenge.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hui Zhang, Dr Xinyun Huang, and Dr Matt Stremlau for critical reading of this manuscript; Haisheng Zhou, Yaxin Lv, Xiuxia Qu, Donghui Zhang, Yanxia Liu, Han Qin, Jun Cai, Yang Zhao, and other colleagues in our laboratory for technical assistance and advice during experiments, and for useful help during preparation of this manuscript; and Yizhe Zhang for performing real-time PCR.
This work was supported by National Basic Research Program of China (2007CB947901), National Nature Science Foundation of China for Creative Research Groups (30421004), the Beijing Nature Science Foundation Grant (5051003), Bill & Melinda Gates Foundation Grant (37871), and the 111 project (H.D.).
Authorship
Contribution: P.Z. designed research, performed research, analyzed data, and wrote the paper; J.L. designed research, performed research, analyzed data, and wrote the paper; Z.T. performed research; C.W. performed research; T.L. performed research; L.C. performed research; J.Y. performed research; W.J. performed research; X.S. performed research; L.D. performed research; M.D. designed research and wrote the paper; H.D. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Hongkui Deng, Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education, College of Life Sciences, Peking University, Beijing 100871, China; e-mail: hongkui_deng@pku.edu.cn.
References
Author notes
P.Z. and J.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal