Interleukin (IL)-27, one of the most recently discovered IL-6 family cytokines, activates both the signal transducer and activator of transcription (STAT)1 and STAT3, and plays multiple roles in pro- and anti-inflammatory immune responses. IL-27 acts on various types of cells including T, B, and macrophage through the common signal-transducing receptor gp130 and its specific receptor WSX-1, but the effect of IL-27 on hematopoietic stem cells (HSCs) remains unknown. Here, we show that IL-27 together with stem cell factor (SCF) directly acts on HSCs and supports their early differentiation in vitro and in vivo. CD34−/lowc-Kit+Sca-1+lineage marker− (CD34−KSL) cells, a population highly enriched in mouse HSCs, were found to express both IL-27 receptor subunits. In vitro cultures of CD34−KSL cells with IL-27 and SCF resulted in an expansion of progenitors including short-term repopulating cells, while some of their long-term repopulating activity also was maintained. To examine its in vivo effect, transgenic mice expressing IL-27 were generated. These mice exhibited enhanced myelopoiesis and impaired B lymphopoiesis in the bone marrow with extramedullary hematopoiesis in the spleen. Moreover, IL-27 similarly acted on human CD34+ cells. These results suggest that IL-27 is one of the limited cytokines that play a role in HSC regulation.
Introduction
Hematopoietic stem cells (HSCs) are defined as cells that have the ability to self-renew to maintain the HSC pool and differentiate into all myeloid and lymphoid cell lineages of the blood.1 These HSC functions, including self-renewal and multilineage differentiation, are precisely regulated through tight interactions with stromal cells, cytokines, and the extracellular matrix (ECM) in bone marrow (BM) niche. Loss of function studies demonstrated important roles of multiple stimulatory cytokines, such as c-Kit ligand (or stem cell factor, [SCF]) and thrombopoietin (TPO), in maintenance of the HSC pool.2,3
The interleukin (IL)-6 family cytokines, including IL-6, IL-11, oncostatin M (OSM), and leukemia inhibitory factor (LIF), exhibit both functional redundancy and pleiotropy in regulating proliferation, differentiation, and functional maturation of cells belonging to multiple hematopoietic lineages.4,–6 This functional redundancy of all the IL-6 family cytokines results from the use of the common signal-transducing receptor β subunit glycoprotein (gp)130 as one of their receptor subunits and signal transducer and activator of transcription (STAT)3. IL-11 was identified as a potent cooperating factor with SCF and Flt3-ligand in stimulating the expansion of murine HSCs in vitro.7,,–10 A fusion protein composed of IL-6 joined to the sIL-6Rα by a flexible linker, named HyperIL-6,11 enables cells that do not express IL-6Rα, including HSCs, to become IL-6 responsive, and was shown to substitute for IL-11 in stimulating BM stem cell self-renewal divisions in short-term serum-free cultures containing SCF and Flt3-ligand.12 The importance of IL-6 family cytokines in regulating hematopoiesis in vivo was further demonstrated in mice by genetic modification of individual genes encoding members of this family or their receptor subunits. For instance, mice null for the gp130 display severely impaired fetal hematopoiesis,13 and mice lacking IL-6, LIF, or the LIF receptor (LIFR) β subunit exhibit hematopoietic defects characterized by a reduction in the number of primitive hematopoietic progenitor cells.14,–16 Moreover, continuous activation of gp130 within the hematopoietic compartment in mice overexpressing either LIF or both IL-6 and sIL-6Rα was shown to result in pathological consequences such as hyperproliferative abnormalities including splenomegaly and thrombocytosis.17,18
IL-27 is one of the most recently discovered IL-6 family cytokines that consists of an IL-12 p40-related protein, Epstein-Barr virus–induced gene 3 (EBI3), and an IL-12 p35-related protein, p28.19 The WSX-1/T-cell cytokine receptor (TCCR), which is homologous to the IL-12Rβ2 subunit, and the common signal-transducing receptor, gp130, constitute a functional signal-transducing receptor for IL-27.19,20 IL-27 activates both STAT1 and STAT3 in naive CD4+ T cells21,,–24 and enhances proliferation in naive but not memory CD4+ T cells. IL-27 plays multiple roles in proinflammatory and anti-inflammatory immune responses including induction of helper T (Th)1 differentiation and suppression of inflammatory cytokine production, respectively.25,26 In addition to CD4+ T cells, IL-27 acts on various types of cells including CD8+ T, B, natural killer (NK)T, and macrophage. The effect of IL-27 on HSCs, however, is not known.
In the present study, we found that mouse HSCs express both IL-27R subunits, gp130 and WSX-1, and that in combination with SCF, IL-27 directly acts on HSCs and induces their differentiation. In addition, we observed enhanced myelopoiesis and impaired B-cell development with splenomegaly in the transgenic (Tg) mice expressing IL-27 under the control of a liver-specific promoter. Interestingly, the number of CD34-KSL cells remarkably increased in these Tg mice, but they did not show any HSC activity. These mice died earlier than control littermates, presumably due to dysregulated hematopoiesis. This is the first report on the effect of IL-27 on HSCs.
Methods
Reagents
Recombinant mouse and human IL-27 were prepared as described before.27 Anti–TCCR/WSX-1 was purchased from Abcam (Cambridge, United Kingdom).
Purification of mouse CD34−KSL cells
CD34−KSL cells were purified from BM cells as described.28,29 In brief, low-density cells were isolated on Ficoll-Paque PLUS (Amersham Bioscience, Uppsala, Sweden). The cells were stained with an antibody mixture consisting of biotinylated anti–Gr-1, anti–Mac-1, anit-B220, anti-CD4, anti-CD8, and anti–Ter-119 antibodies (BD Biosciences, San Jose, CA). The cells were subsequently labeled with MACS goat antirat immunoglobulin (Ig)G microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Lineage-positive cells were then depleted by using the Midi-MACS system (Miltenyi Biotec). The cells were further stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD34, phycoerythrin (PE)–conjugated anti–Sca-1, and allophycocyanin (APC)–conjugated anti–c-Kit antibodies (BD Biosciences). Biotinylated antibodies were detected with streptavidin-APC-Cy7 (Molecular Probes, Carlsbad, CA). Four-color analysis and sorting were performed on a MoFlo Cell Sorter (DakoCytomation, Glostrup, Denmark).
RT-PCR
Hematopoietic lineage cDNA panels were prepared as previously reported.30 Briefly, purified hematopoietic cells prepared from bone marrow, spleen, and thymus were sorted into Trizol-LS (Invitrogen, Carlsbad, CA). Total RNA samples were reverse transcribed with ThermoScript reverse-transcription polymerase chain reaction (RT-PCR) system and oligo-dT primer (Invitrogen). Resultant cDNA samples were applied for conventional semiquantitative RT-PCR. PCR primers used are as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense primer, 5′-ATTGTCAGCAATGCATCCTGC-3′; GADPH antisense primer, 5′-TCATACTTGGCAGGTTTCTCC-3′; WSX-1 sense primer, 5′-ACCCAAATGAAGCCAGACAC-3′; WSX-1 antisense primer, 5′-CACACAAGGTCTTGGGTCCT-3′; gp130 sense primer, 5′-AGTCTGGGTGGAAGCAGAGA-3′; gp130 antisense primer, 5′-CTTGGTGGTCTGGATGGTCT-3′.
Immunofluorescent staining
Single-cell immunofluorescent staining was performed as described.31 CD34−KSL cells were directly sorted into droplets of medium on poly-L-lysine–coated glass slides, followed by fixation with 2% paraformaldehyde and blocking in 10% goat serum for 1 hour at room temperature. Cells were incubated with rabbit antimouse TCCR/WSX-1 polyclonal antibody or control rabbit IgG for 12 hours at 4°C. The cells were then washed and incubated with Alexa-488–conjugated goat antirabbit IgG (Molecular Probes) for 30 minutes at room temperature. After staining with 4, 6-diamidino-2-phenylindole (DAPI), cells were analyzed and micrographs were acquired and processed with a Leica TCS SP2 AOBS confocal microscope system fitted with a 40′ NA oil objective (Leica Microsystems, Wetzlar, Germany Images were processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Culture of mouse CD34−KSL cells
Purified mouse CD34−KSL cells (single cell/well or 40 cells/well) were cultured in serum-free S-clone SF-O3 medium (Sanko-Junyaku, Tokyo, Japan) supplemented with 0.5% bovine serum albumin (BSA) and mouse SCF (10 ng/mL) (PeproTech, Rocky Hill, NJ) in the presence of IL-27 (10 ng/mL). CD34−KSL cells also were cultured under myeloid conditions containing 10% fetal calf serum (FCS), mouse SCF (10 ng/mL), human TPO (10 ng/mL), mouse IL-3 (10 ng/mL), and mouse EPO (1 U/mL; all from PeproTech) in the presence of IL-27 (10 ng/mL). The cells were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. At several time points, numbers of cells per well were counted under an inverted microscope or by the standard trypan blue exclusion method.
In vivo analysis of CD34−KSL cells stimulated by IL-27 and SCF
CD34−KSL cells (40 cells/well) were stimulated with SCF (50 ng/mL) and IL-27 (10 ng/mL) for 7 days in serum-free culture. Cultured cells were transplanted with competitor cells (4 × 105). Freshly isolated CD34−KSL cells (40 cells) also were transplanted with competitor cells (4 × 105) as controls. At 3, 7, and 12 weeks after transplantation, recipients (n = 3 for each group) were killed and peripheral blood, spleen, and thymus were analyzed. Donor- and competitor-derived cells were recognized by Ly5 congenic mouse system. Peripheral blood cells and spleen cells were stained with FITC-conjugated anti-Ly5.1, a mixture of PE-conjugated anti-CD4 and anti-CD8, PE-Cy7–conjugated anti-B220, and biotinylated anti–Gr-1. Thymus cells were stained with FITC-conjugated anti-Ly5.1, PE-conjugated anti-CD4, APC conjugated anti-CD8, PE-Cy7–conjugated anti-B220, and biotinylated anti–Gr-1. The biotinylated antibody was detected with streptavidin-Texas red. Cells were analyzed on a FACSVantage (Becton Dickinson, Franklin Lakes, NJ).
Colony-formation assay
Colony-formation assay was performed as described.32 Briefly, BM cells (2 × 104 cells/mL) were seeded in complete αMEM medium with methylcellulose containing SCF, TPO, IL-3, and EPO, or SCF, IL-7, and Flt3 ligand (all from PeproTech) for 7 days. Colonies were recovered, cytospun onto slide glass, and subjected to May-Gruenwald-Giemsa staining for morphologic examination.
Transplantation assay
HSCs in BM cells were quantitatively and qualitatively analyzed by both competitive and noncompetitive transplantation assay. For competitive transplantation, BM cells (106) from IL-27 Tg mice were transplanted into a lethally irradiated (9.5 Gy) wild-type recipient mouse with BM cells (106) from wild-type mice as competitor cells. For noncompetitive transplantation, BM cells (2 × 106) from WT or IL-27 Tg mice were transplanted into a lethally irradiated wild-type or IL-27 Tg recipient mouse. Donor-, competitor-, and recipient-derived cells were recognized by Ly5 congenic mouse system. Twenty weeks after transplantation, peripheral blood cells of the recipients were stained with FITC-conjugated anti-Ly5.2(104) and biotinylated anti-Ly5.1 (A4) (BD Biosciences). The cells were simultaneously stained with PE-Cy7–conjugated anti-B220 antibody and a mixture of APC-conjugated anti–Mac-1 and anti–Gr-1 antibodies and a mixture of PE-conjugated anti-CD4 and -CD8 antibodies (BD Biosciences). The biotinylated antibody was detected with streptavidin-Texas red. Cells were analyzed on a FACSVantage (Becton Dickinson). Percentage chimerism was calculated as (percentage of donor cells) × 100/(percentage of donor cells + percentage of competitor cells).
Generation of IL-27 Tg mice
The cDNA for 3×FLAG-tagged single-chain mouse IL-27, in which EBI3 flexibly links to p28 with the (Gly4Ser)3 linker, in p3xFLAG-CMV-9 (Sigma Chemical, St Louis, MO) vector33 was amplified using standard PCR methods and inserted into the pLG1-SAP vector34 that contains the liver-specific human serum amyloid P component (SAP) promoter and the rabbit β-globin gene. Two independent IL-27 Tg mouse lines (no. 1 and no. 6) were generated by pronuclear microinjection of the cDNA into fertilized eggs obtained from C57BL/6 mice. All animal experiments were performed in accordance with our institutional guidelines.
Culture of human CD34+ cells
Human CD34+ cells purified from umbilical cord blood were obtained from the Cell Bank, RIKEN BioResource Center (Ibaraki, Japan). Purified human CD34+ cells (2 × 104 cells/mL) were cultured in serum-free X-VIVO 10 medium (StemCell Technologies, Vancouver, BC) with a human cytokine cocktail consisting of SCF (50 ng/mL), Flt3 ligand (20 ng/mL), and TPO (10 ng/mL), and with and without IL-6 (50 ng/mL) and soluble (s)IL-6Rα (50 ng/mL) (all from PeproTech). The cells were incubated in flat-bottomed 24-well plates in triplicate for 7, 9, and 14 days and harvested. Then, the cell number was counted and assayed for expression of lineage markers by flow cytometry (FACSCalibur; Becton Dickinson). All studies using human samples were performed in accordance with our institutional guidelines. Approval was obtained from Tokyo Medical University's Institutional Review Board for these studies.
Results
CD34−KSL HSCs express IL-27R subunits, gp130 and WSX-1, and IL-27 induces their proliferation in synergy with SCF
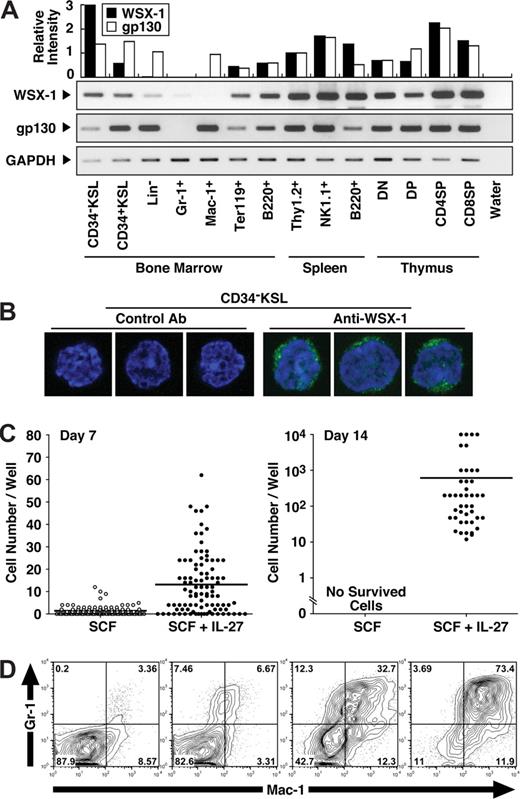
To explore the effect of IL-27 on HSCs, CD34−KSL cells, which are highly enriched in HSCs,28 were purified as described in “Purification of mouse CD-KSL cells.” We first examined whether CD34−KSL cells express IL-27R subunits, gp130 and WSX-1, by semiquantitative RT-PCR using cDNAs prepared from various purified hematopoietic cells (Figure 1A). The expression of both gp130 and WSX-1 was detected at mRNA level in CD34−KSL cells. The cell surface expression of WSX-1 on CD34−KSL cells at single cell level also was detected by immunofluorescence staining with anti–WSX-1 but not control antibody (Figure 1B). To further examine the effect of IL-27 on HSCs in vitro, we next cultured purified CD34−KSL cells (single cell/well) with IL-27 in the presence of SCF in serum-free medium supplemented with 0.5% BSA (Figure 1C). CD34−KSL cells only slightly increased in number but never survived until day 14 in the presence of SCF or IL-27 alone (Figure 1C and data not shown). In marked contrast, almost all CD34−KSL cells responded to the stimulation with both IL-27 and SCF at day 7, and around 50% of these cells formed colonies by day 14. Cells composing day 14 colonies showed morphologically immature myeloid cells such as myelocyte- and metamyelocyte-like cells (Figure S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article). Very few neutrophils and macrophages were identified among colonies. Surface phenotypes of colony cells were examined for individual colonies (Figure 1D). Some cells expressed Gr-1 and Mac-1 antigens. Proportions of these lineage marker–positive cells markedly varied among individual colonies. These results suggest that HSCs express IL-27R subunits, gp130 and WSX-1, and that IL-27 induces their proliferation and differentiation in synergy with SCF.
CD34−KSL HSCs express IL-27R subunits, gp130 and WSX-1, and IL-27 induces their proliferation in synergy with SCF. (A) Semiquantitative RT-PCR for analyzing WSX-1 and gp130 mRNA expression was carried out using cDNAs prepared from cells including mouse BM CD34−c-Kit+Sca-1+ lineage marker− HSCs (CD34−KSL), CD34+KSL progenitors, lineage marker− cells (Lin-), Gr-1+ neutrophils, Mac-1+ monocytes/macrophages, TER119+ erythroblasts, and B220+ B cells; spleen Thy-1.2+ T cells, NK1.1+ natural killer cells, and B220+ B cells; and thymic CD4−CD8− T cells (DN), CD4+CD8+ T cells (DP), CD4+CD8− T cells (CD4SP), and CD4−CD8+ T cells (CD8SP). The intensity of each band was densitometrically measured, and relative intensity was calculated after normalization by the intensity for GAPDH. (B) CD34−KSL cells were stained with anti–WSX-1 or control antibody followed by incubation with Alexa-488–conjugated antimouse IgG and DAPI. Three representative cells were shown. At least 50 cells were analyzed with a confocal microscope, and similar results were obtained. (C) CD34−KSL cells (single cell/well) were cultured in serum-free medium supplemented with 0.5% BSA and SCF (10 ng/mL) in the presence of IL-27 (10 ng/mL). At several time points, the number of cells per well was counted under an inverted microscope or standard trypan blue exclusion method. Each dot shows number of cells in individual wells. Horizontal lines are median values. (D) Individual day-14 colonies were stained with anti–Gr-1 and anti–Mac-1 and analyzed by flow cytometry. Numbers represent the percentage of cells in each area.
CD34−KSL HSCs express IL-27R subunits, gp130 and WSX-1, and IL-27 induces their proliferation in synergy with SCF. (A) Semiquantitative RT-PCR for analyzing WSX-1 and gp130 mRNA expression was carried out using cDNAs prepared from cells including mouse BM CD34−c-Kit+Sca-1+ lineage marker− HSCs (CD34−KSL), CD34+KSL progenitors, lineage marker− cells (Lin-), Gr-1+ neutrophils, Mac-1+ monocytes/macrophages, TER119+ erythroblasts, and B220+ B cells; spleen Thy-1.2+ T cells, NK1.1+ natural killer cells, and B220+ B cells; and thymic CD4−CD8− T cells (DN), CD4+CD8+ T cells (DP), CD4+CD8− T cells (CD4SP), and CD4−CD8+ T cells (CD8SP). The intensity of each band was densitometrically measured, and relative intensity was calculated after normalization by the intensity for GAPDH. (B) CD34−KSL cells were stained with anti–WSX-1 or control antibody followed by incubation with Alexa-488–conjugated antimouse IgG and DAPI. Three representative cells were shown. At least 50 cells were analyzed with a confocal microscope, and similar results were obtained. (C) CD34−KSL cells (single cell/well) were cultured in serum-free medium supplemented with 0.5% BSA and SCF (10 ng/mL) in the presence of IL-27 (10 ng/mL). At several time points, the number of cells per well was counted under an inverted microscope or standard trypan blue exclusion method. Each dot shows number of cells in individual wells. Horizontal lines are median values. (D) Individual day-14 colonies were stained with anti–Gr-1 and anti–Mac-1 and analyzed by flow cytometry. Numbers represent the percentage of cells in each area.
IL-27 enhances the differentiation of CD34−KSL HSCs
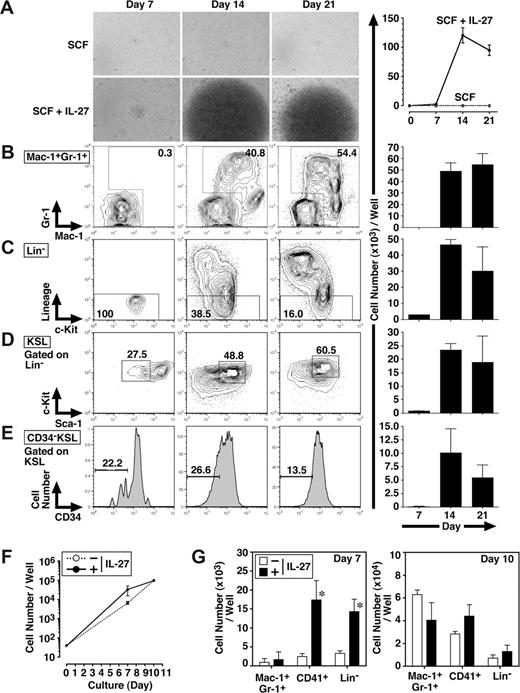
To further examine whether IL-27 affects the self-renewal and/or differentiation of CD34−KSL cells, FACS analyses were performed 7, 14, and 21 days after the stimulation of CD34−KSL cells (40 cells/well) with IL-27 and SCF (Figure 2A-E). In response to IL-27 and SCF, CD34−KSL cells expanded and also differentiated to Lin+ populations. Consistent with the result in Figure 1D, the number of Mac-1+Gr-1+ cells was mainly increased together with that of Mac-1+Gr-1− cells. In Lin− populations, the number of CD34−KSL cells also was increased.
IL-27 enhances the differentiation of CD34−KSL HSCs. Mouse CD34−KSL cells (40 cells/well) were cultured in serum-free medium supplemented with 0.5% BSA and SCF (10 ng/mL) in the presence or absence of IL-27 (10 ng/mL) in triplicate. On days 7, 14, and 21, cell growth was monitored using a light microscopy (A left panels), and number of cells per well was counted and images were acquired under an inverted microscope (Leica DB IRBE) or standard trypan blue exclusion method (A-E right panels). Expanded cells also were stained with various combinations of antibodies and analyzed by flow cytometry (B-E left panels). Numbers represent the percentage of cells in each box or area. Data are shown as the mean (± SD). Mouse CD34−KSL cells (40 cells/well) were cultured under myeloid conditions in medium supplemented with 10% FCS, SCF (10 ng/mL), TPO (10 ng/mL), IL-3 (10 ng/mL), and EPO (1 U/mL) in the presence or absence of IL-27 (10 ng/mL) in triplicate. On days 7 and 10, number of cells per well was counted under an inverted microscope (F). Cells also were stained with various combinations of antibodies and analyzed by flow cytometry (G). Data are shown as the mean (± SD). * indicates P less than .05, compared with controls.
IL-27 enhances the differentiation of CD34−KSL HSCs. Mouse CD34−KSL cells (40 cells/well) were cultured in serum-free medium supplemented with 0.5% BSA and SCF (10 ng/mL) in the presence or absence of IL-27 (10 ng/mL) in triplicate. On days 7, 14, and 21, cell growth was monitored using a light microscopy (A left panels), and number of cells per well was counted and images were acquired under an inverted microscope (Leica DB IRBE) or standard trypan blue exclusion method (A-E right panels). Expanded cells also were stained with various combinations of antibodies and analyzed by flow cytometry (B-E left panels). Numbers represent the percentage of cells in each box or area. Data are shown as the mean (± SD). Mouse CD34−KSL cells (40 cells/well) were cultured under myeloid conditions in medium supplemented with 10% FCS, SCF (10 ng/mL), TPO (10 ng/mL), IL-3 (10 ng/mL), and EPO (1 U/mL) in the presence or absence of IL-27 (10 ng/mL) in triplicate. On days 7 and 10, number of cells per well was counted under an inverted microscope (F). Cells also were stained with various combinations of antibodies and analyzed by flow cytometry (G). Data are shown as the mean (± SD). * indicates P less than .05, compared with controls.
We also examined the effect of IL-27 on the differentiation of CD34−KSL cells under myeloid differentiation conditions containing FCS, SCF, TPO, IL-3, and EPO. The addition of IL-27 did not change the total number of cells significantly (Figure 2F). In contrast, IL-27 significantly increased the numbers of CD41+ cells and Lin− cells but not that of Mac-1+Gr-1+ cells on day 7, whereas these increases were caught up with by the number of cells stimulated without IL-27 until day 10 (Figure 2G). Thus, IL-27 enhances the differentiation of CD34−KSL cells.
IL-27 and SCF generate short-term repopulating cells from CD34−KSL cells
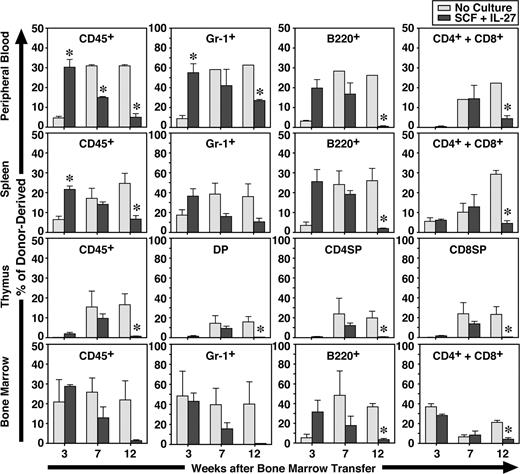
To further examine the function of CD34−KSL cells after in vitro stimulation with IL-27 and SCF for 7 days, the expanded cells were transplanted with competitor cells. Freshly isolated CD34−KSL cells without culture also were similarly transplanted with competitor cells as controls. At 3, 7, and 12 weeks after transplantation, recipients were killed and peripheral blood, spleen, thymus, and BM were analyzed (Figure 3). Donor- and competitor-derived cells were recognized by the Ly5 congenic mouse system. At 3 weeks, the percentages of CD45+ and Gr-1+ cells derived from CD34−KSL cells cultured with IL-27 and SCF in peripheral blood and spleen were significantly greater than those derived from freshly isolated CD34−KSL cells. At 7 weeks these differences became comparable, and at 12 weeks the percentages of CD45+, Gr-1+, B220+, CD4+, CD8+, and CD4+CD8+ cells derived from cultured CD34−KSL cells were significantly decreased in peripheral blood, spleen, and thymus. Similar tendency was observed in BM. These results suggest that short-term repopulating cells were generated in vitro from HSCs by IL-27 and SCF. Of note is that a significant level of repopulation in the myeloid lineage remained detectable at 12 weeks, although it had gradually decreased over time. Since B- and T-lymphoid lineages were poorly repopulated at 12 weeks, these cells could be myeloid lineage–dominant long-term repopulating cells as previously observed in aged mice.29 Nevertheless, IL-27 can maintain long-term repopulating activity of HSCs to some extent in vitro for a week.
IL-27 and SCF generate short-term repopulating cells from CD34−KSL cells. CD34−KSL cells (40 cells) were stimulated with SCF (50 ng/mL) and IL-27 (10 ng/mL) for 7 days in serum-free culture. Cultured cells were transplanted with competitor cells (SCF + IL-27). Freshly isolated CD34−KSL cells (40 cells) also were transplanted with competitor cells as controls (No Culture). At 3, 7, and 12 weeks after transplantation, recipients (n = 3 for each group) were killed and peripheral blood, spleen, thymus, and BM were analyzed. Data are shown as the mean (± SEM). * indicates P < .05 compared with controls.
IL-27 and SCF generate short-term repopulating cells from CD34−KSL cells. CD34−KSL cells (40 cells) were stimulated with SCF (50 ng/mL) and IL-27 (10 ng/mL) for 7 days in serum-free culture. Cultured cells were transplanted with competitor cells (SCF + IL-27). Freshly isolated CD34−KSL cells (40 cells) also were transplanted with competitor cells as controls (No Culture). At 3, 7, and 12 weeks after transplantation, recipients (n = 3 for each group) were killed and peripheral blood, spleen, thymus, and BM were analyzed. Data are shown as the mean (± SEM). * indicates P < .05 compared with controls.
IL-27 Tg mice exhibit an increased number of megakaryocytes and splenomegaly due to extramedullary hematopoiesis
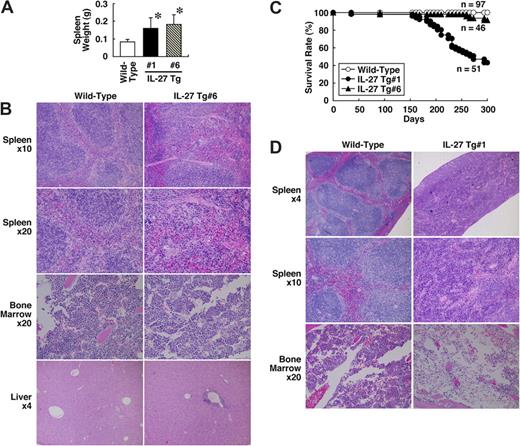
Next, to investigate the in vivo effect of IL-27 on development of hematopoeitic cells, we established 2 IL-27 Tg mouse lines, no. 1 and no. 6, in which the expression of FLAG-tagged IL-27 is regulated by a liver-specific promoter.34 Serum levels of the IL-27 protein in these Tg mice at 6 to 8 weeks of age were determined by specific ELISA using anti-FLAG and biotin–anti-IL-27p28. They were 0.821 (± 0.627) ng/mL (line no. 1, n = 8) and 0.514 (± 0.306) ng/mL (line no. 6, n = 12). Both of these Tg mice developed splenomegaly with a mean 2-fold increase in spleen weight at 8 to 12 weeks of age (Figure 4A). Histological analysis revealed that the number of megakaryocytes appears to increase in spleen and BM of IL-27 Tg no. 6 mice compared with wild-type mice (Figure 4B). The extramedullary hematopoiesis also was observed in spleen of IL-27 Tg no. 6 mice. In addition, an infiltration of small numbers of mononuclear cells was occasionally seen in the liver. However, hematological examination of peripheral blood revealed no significant difference in the number of leukocytes, erythrocytes, and platelets (data not shown). Similar results were obtained using IL-27 Tg no. 1 mice (data not shown). Thus, IL-27 Tg mice exhibit an increased number of megakaryocytes and splenomegaly due to extramedullary hematopoiesis.
IL-27 Tg mice exhibit an increased number of megakaryocytes and splenomegaly with extramedullary hematopoiesis and shortened survival. (A) Spleen weight of wild-type (n = 31), IL-27 Tg no. 1 (n = 15) and no. 6 (n = 30) mice at 8 to 12 weeks of age was determined. Data are shown as the mean (± SD). * indicates P < .05, compared with wild-type mice. (B) Spleen, BM, and liver tissues of wild-type and IL-27 Tg no. 6 mice at 8 weeks of age were fixed in 10% buffered formalin, sectioned, stained with hematoxylin and eosin, and evaluated microscopically. (C) Survival rate of wild-type (n = 97), IL-27 Tg no. 1 (n = 51) and no. 6 (n = 46) mice was determined. (D) Spleen and BM tissues of wild-type and IL-27 Tg no. 1 mice at 40 weeks of age were fixed in 10% buffered formalin, sectioned, stained with hematoxylin and eosin, and evaluated microscopically. Images were acquired with an Olympus BX50 microscope system and 4×/0.13, 10×/0.30, and 20×/0.50 NA objectives (Olympus, Tokyo, Japan).
IL-27 Tg mice exhibit an increased number of megakaryocytes and splenomegaly with extramedullary hematopoiesis and shortened survival. (A) Spleen weight of wild-type (n = 31), IL-27 Tg no. 1 (n = 15) and no. 6 (n = 30) mice at 8 to 12 weeks of age was determined. Data are shown as the mean (± SD). * indicates P < .05, compared with wild-type mice. (B) Spleen, BM, and liver tissues of wild-type and IL-27 Tg no. 6 mice at 8 weeks of age were fixed in 10% buffered formalin, sectioned, stained with hematoxylin and eosin, and evaluated microscopically. (C) Survival rate of wild-type (n = 97), IL-27 Tg no. 1 (n = 51) and no. 6 (n = 46) mice was determined. (D) Spleen and BM tissues of wild-type and IL-27 Tg no. 1 mice at 40 weeks of age were fixed in 10% buffered formalin, sectioned, stained with hematoxylin and eosin, and evaluated microscopically. Images were acquired with an Olympus BX50 microscope system and 4×/0.13, 10×/0.30, and 20×/0.50 NA objectives (Olympus, Tokyo, Japan).
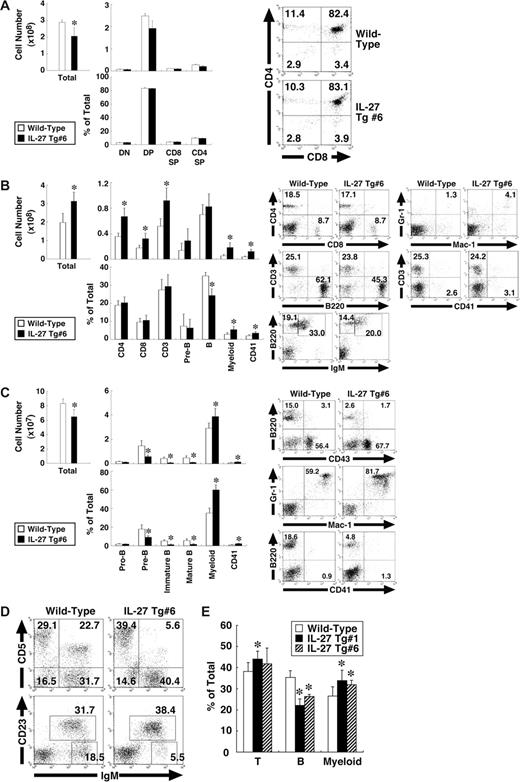
IL-27 Tg mice exhibit BM dysfunction with enhanced myelopoiesis and impaired development of B-cell lineages
We then examined which cell population was affected in the IL-27 Tg mice. Cells from the thymus, spleen, BM, peritoneal cavity, and peripheral blood of IL-27 Tg no. 6 mice were subjected to FACS analysis using various combinations of antibodies. The total cell number in the thymus of IL-27 Tg no. 6 mice was slightly less than that of wild-type mice, whereas cell numbers and percentages of each population including CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ T cells were comparable between them (Figure 5A). Correlating with the splenomegaly, the total cell number in spleen was significantly increased, approximately 1.5-fold (Figure 5B). Concomitantly, numbers of CD4+ and CD8+ T cells, myeloid (Mac-1+Gr-1+) cells and CD41+ cells but not B cells were increased. However, percentages of CD4+ and CD8+ T cells were comparable, while those of myeloid and CD41+ cells were increased, while in contrast that of B cells was decreased. In BM, not only total cell numbers but also numbers and percentages of pre-B (B220+CD43−IgM−), immature B (B220lowIgM+), and mature B (B220highIgM+) cells were decreased (Figure 5C). In contrast, numbers and percentages of myeloid cells and CD41+ cells were increased. Percentage of B1 (IgM+CD5+ or IgM+CD23−) cells in peritoneal cavity also was decreased (Figure 5D). In addition, reduced percentage of B cells and increased percentages of CD3+ T cells and myeloid cells were observed in peripheral blood lymphocytes (Figure 5E). Similar results were obtained using IL-27 Tg no. 1 mice (data not shown). Thus, IL-27 Tg mice exhibit BM dysfunction with enhanced myelopoiesis and impaired development of B-cell lineages.
IL-27 Tg mice exhibit BM dysfunction with enhanced myelopoiesis and impaired development of B-cell lineages. Cells from thymus (A), spleen (B), BM (C), peritoneal cavity (D), and peripheral blood (E) of wild-type and IL-27 Tg no. 1 and/or no. 6 mice (n = 3 each) at 8 weeks of age were stained with various combinations of antibodies and analyzed by flow cytometry. Thymus: DN (CD4−CD8− T), DP (CD4+CD8+ T), CD4SP (CD4+CD8− T), and CD8SP (CD4−CD8+ T). Spleen: CD4+ T, CD8+ T, CD3+ T, pre-B (B220+CD43−IgM−), B (B220+IgM+), myeloid (Mac-1+Gr-1+), and CD41+. BM: pro-B (B220+CD43+), pre-B, immature B (B220lowIgM+), mature B (B220highIgM+), myeloid, and CD41+. Peritoneal cavity: B1a (IgM+CD5+), B1 (IgM+CD23−), and B2 (IgM+CD23+). Data are shown as the mean (± SD). * indicate P < .05, compared with wild-type mice. Numbers represent the percentage of cells in each box or area.
IL-27 Tg mice exhibit BM dysfunction with enhanced myelopoiesis and impaired development of B-cell lineages. Cells from thymus (A), spleen (B), BM (C), peritoneal cavity (D), and peripheral blood (E) of wild-type and IL-27 Tg no. 1 and/or no. 6 mice (n = 3 each) at 8 weeks of age were stained with various combinations of antibodies and analyzed by flow cytometry. Thymus: DN (CD4−CD8− T), DP (CD4+CD8+ T), CD4SP (CD4+CD8− T), and CD8SP (CD4−CD8+ T). Spleen: CD4+ T, CD8+ T, CD3+ T, pre-B (B220+CD43−IgM−), B (B220+IgM+), myeloid (Mac-1+Gr-1+), and CD41+. BM: pro-B (B220+CD43+), pre-B, immature B (B220lowIgM+), mature B (B220highIgM+), myeloid, and CD41+. Peritoneal cavity: B1a (IgM+CD5+), B1 (IgM+CD23−), and B2 (IgM+CD23+). Data are shown as the mean (± SD). * indicate P < .05, compared with wild-type mice. Numbers represent the percentage of cells in each box or area.
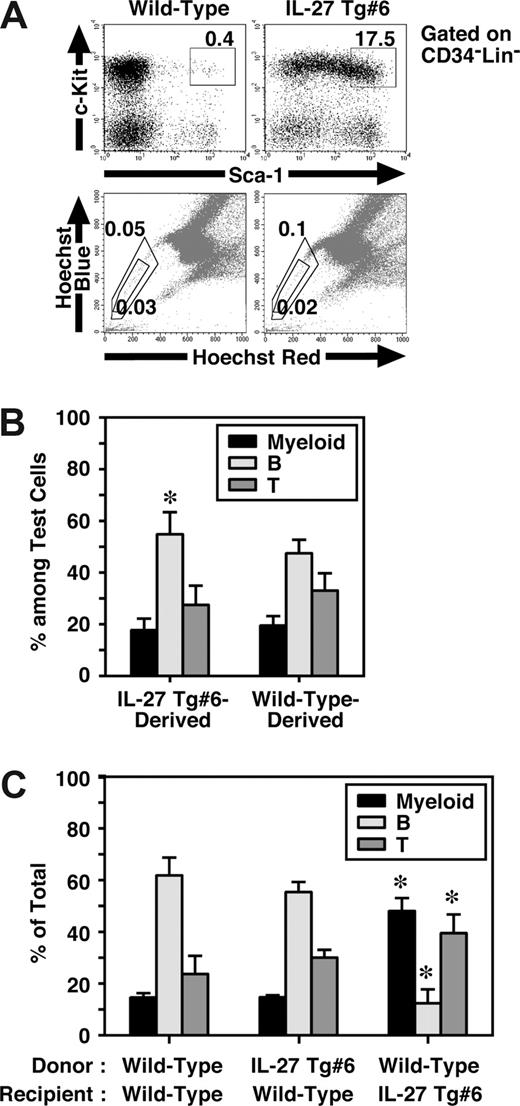
IL-27 Tg mice have an increased number of CD34−KSL cells in BM, but they failed to show any HSC activity
Mouse CD34−KSL cells are highly enriched in the HSCs,28 and most HSCs also are assumed to reside in the so-called side population (SP), which is capable of efficient Hoechst dye efflux.35 Since BM dysfunction of IL-27 Tg mice might have resulted from intrinsic abnormalities in HSCs, we next examined HSCs in IL-27 Tg mice. To our surprise, FACS analysis revealed that the percentage of CD34−KSL cells in BM of IL-27 Tg no. 6 mice increased approximately 50-fold compared with that in wild-type mice (Figure 6A). However, no significant difference was observed in the percentage of SP cells between IL-27 Tg no. 6 and wild-type mice. Similar results were obtained using IL-27 Tg no. 1 mice (data not shown). To further examine their function, we first performed a colony-formation assay using unfractionated BM cells. Consistent with the results obtained in the analysis of SP cells, almost no marked increase in the number of colonies of IL-27 Tg no. 6 mice compared with wild-type mice was observed (data not shown). HSC activity in unfractionated BM cells of IL-27 Tg mice were then analyzed in vivo by both competitive (Figure 6B) and noncompetitive transplantation assays (Figure 6C). Chimerism of white blood cells (WBCs) derived form IL-27 Tg no. 6 mice in the competitive transplantation assay at 4 months after transplantation was approximately 50% (n = 10, data not shown). The lineage distributions of IL-27 Tg no. 6 mouse– and wild-type mouse–derived cells including myeloid, B and T cells were similar, although only a slight increase in the B-cell lineage distribution of IL-27 Tg no. 6 mouse–derived cells was observed as compared with that of wild-type mouse–derived cells (Figure 6B). Moreover, lineage distribution of IL-27 Tg no. 6 mouse–derived cells in wild-type recipients was similar to that of wild-type mouse–derived cells after transplantation without competitor cells (Figure 6C). In contrast, the lineage distribution of wild-type mouse–derived cells in IL-27 Tg no. 6 recipients was different from that in wild-type recipients, but similar to the pattern seen in peripheral blood of IL-27 Tg mice (Figure 5E). These results suggest that IL-27 Tg mice have a markedly increased number of CD34−KSL cells, most of which neither engraft nor repopulate after transplantation.
IL-27 Tg mice have an increased number of CD34−KSL cells in BM, but they failed to show any HSC activity. (A) Cells from BM of wild-type and IL-27 Tg no. 1 and/or no. 6 mice (n = 3) at 8 weeks of age were stained with various combinations of antibodies or Hoechst 33342 and analyzed by flow cytometry. (B) HSC function in BM cells of IL-27 Tg mice were analyzed by transplantation assays. Chimerism of IL-27 Tg no. 6 mouse-derived WBCs in competitive repopulation assays at 4 months after transplantation (n = 10). Lineage distribution of IL-27 Tg no. 6 mouse- or wild-type mouse–derived cells was determined. * indicates P < .05, compared with wild-type mouse–derived cells. (C) Lineage distribution of IL-27 Tg no. 6 mouse- or wild-type mouse–derived cells in wild-type or Tg recipients at 3 months after transplantation without competitor cells. * indicates P < .05, compared with wild-type to wild-type transplantation. Data are shown as the mean (± SD).
IL-27 Tg mice have an increased number of CD34−KSL cells in BM, but they failed to show any HSC activity. (A) Cells from BM of wild-type and IL-27 Tg no. 1 and/or no. 6 mice (n = 3) at 8 weeks of age were stained with various combinations of antibodies or Hoechst 33342 and analyzed by flow cytometry. (B) HSC function in BM cells of IL-27 Tg mice were analyzed by transplantation assays. Chimerism of IL-27 Tg no. 6 mouse-derived WBCs in competitive repopulation assays at 4 months after transplantation (n = 10). Lineage distribution of IL-27 Tg no. 6 mouse- or wild-type mouse–derived cells was determined. * indicates P < .05, compared with wild-type mouse–derived cells. (C) Lineage distribution of IL-27 Tg no. 6 mouse- or wild-type mouse–derived cells in wild-type or Tg recipients at 3 months after transplantation without competitor cells. * indicates P < .05, compared with wild-type to wild-type transplantation. Data are shown as the mean (± SD).
IL-27 Tg mice exhibit shortened survival, presumably due to the BM dysfunctions
Finally, we have noticed that approximately half of IL-27 Tg no. 1 mice and 10% of IL-27 Tg no. 6 mice died by 10 months of age (Figure 4C), although the IL-27 Tg mice appeared normal at birth. More shortened survival rate in IL-27 Tg no. 1 mice than that in IL-27 Tg no. 6 mice could be due to more increased expression of FLAG-tagged IL-27 in the liver of IL-27 Tg no. 1 mice, which was detected by RT-PCR (data not shown). Reduced body weight and anemia sometimes preceded death. Histological analysis of IL-27 Tg no. 1 mice at 10 months of age revealed that normal splenic architecture was not seen and BM became hypocellular and developed expanded sinusoidal spaces (Figure 4D). Taken together, these results suggest that IL-27 Tg mice exhibit shortened survival, presumably due to the BM dysfunctions, including anemia.
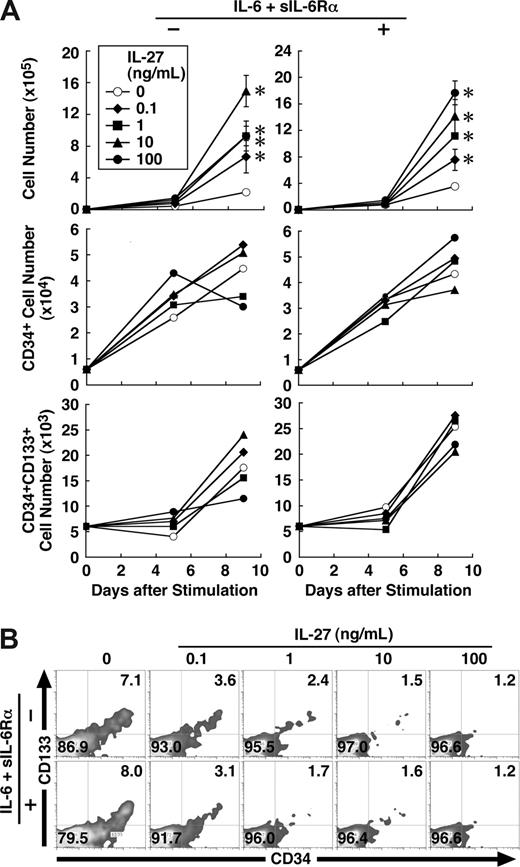
IL-27 enhances proliferation and differentiation of human CD34+ cells
Finally, we examined the effect of IL-27 on human hematopoietic stem/progenitor cells. Human CD34+ HSCs purified from umbilical cord blood were cultured in the medium containing SCF, Flt3 ligand, TPO, and IL-27 in the presence or absence of IL-6 and sIL-6Rα. IL-27 significantly augmented the proliferation of CD34+ cells and increased their cell number approximately 8 times irrespective of the presence of IL-6 and sIL-6Rα (Figure 7A). Then, we performed FACS analysis using various combinations of antibodies on days 5 and 9. However, IL-27 failed to increase the percentage of hematopoietic stem/progenitor population such as CD34+ and CD34+CD133+ cells (Figure 7A,B). Under these conditions, IL-27 rather appeared to inhibit the expression of most cell surface markers such as CD3, CD14, CD15, CD19, and CD41 (data not shown). To confirm these results, a colony-formation assay also was performed using the expanded cells with and without IL-27 for 9 days in the absence of IL-6 and sIL-6Rα. However, no increase in the number of colonies, rather a slight decrease, was observed with IL-27 (data not shown). These results suggest that IL-27 also enhances proliferation and differentiation of human hematopoietic stem/progenitor cells.
IL-27 enhances proliferation and differentiation of human CD34+ cells. (A) Human CD34+ cells (104 cells/0.5 mL/well) were cultured in medium supplemented with SCF (50 ng/mL), TPO (10 ng/mL), and Flt3 ligand (20 ng/mL) and with or without IL-6 (50 ng/mL) and sIL-6Rα (50 ng/mL) in the presence of IL-27 (0.1, 1, 10, and 100 ng/mL) in triplicate. On days 5 and 9, cells were counted and stained with various combinations of antibodies and analyzed by flow cytometry. Data in the total cell number are shown as the mean (± SD). * indicates P < .05, compared with control. Numbers represent the percentage of cells in each area. A representative result obtained day 9 from 2 independent experiments was shown (B). Similar results were obtained in more than 2 independent experiments.
IL-27 enhances proliferation and differentiation of human CD34+ cells. (A) Human CD34+ cells (104 cells/0.5 mL/well) were cultured in medium supplemented with SCF (50 ng/mL), TPO (10 ng/mL), and Flt3 ligand (20 ng/mL) and with or without IL-6 (50 ng/mL) and sIL-6Rα (50 ng/mL) in the presence of IL-27 (0.1, 1, 10, and 100 ng/mL) in triplicate. On days 5 and 9, cells were counted and stained with various combinations of antibodies and analyzed by flow cytometry. Data in the total cell number are shown as the mean (± SD). * indicates P < .05, compared with control. Numbers represent the percentage of cells in each area. A representative result obtained day 9 from 2 independent experiments was shown (B). Similar results were obtained in more than 2 independent experiments.
Discussion
Since IL-27 is one of the most recently discovered cytokines using gp130 as one of their receptor subunits and activates not only STAT3 but also STAT1 through another receptor subunit WSX-1, we explored the effect of IL-27 on HSCs in vitro and in vivo using purified mouse CD34−KSL HSCs, IL-27 Tg mice, and human CD34+ cells in the present study. CD34−KSL HSCs were revealed to express both IL-27R subunits, and IL-27 directly acted on HSCs and induced their proliferation and differentiation in synergy with SCF. IL-27 Tg mice exhibited BM dysfunction with enhanced myelopoiesis and impaired development of B-cell lineages. When CD34−KSL cells were cultured in the presence or absence of IL-27 under the B-cell differentiation conditions (100 ng/mL SCF, 10 ng/mL IL-7, 10 ng/mL Flt3 ligand, and 10 ng/mL IL-12), no significant difference in the number of B-cell lymphoid progenitors was observed (data not shown). Therefore, IL-27 may not have any direct effects on the expansion of B-cell progenitors. Since IL-27 has effects on lymphoid cells, and lymphoid cells have regulatory actions on hematopoietic progenitors and/or stem cells,36 the effects of IL-27 in vivo in IL-27 Tg mice may be at least somewhat mediated by indirect effects of IL-27 on lymphoid cells. Moreover, histological analysis on the structure of spleens in IL-27 Tg mice (Figure 4) suggests that IL-27 Tg mouse has extramedullary hematopoiesis, but appears to have normal architecture. Since tumor necrosis factor (TNF)–α and lymphotoxin-α were reported to be required for development of specialized lymphoid tissue structures such as follicular dendritic cell or germinal centers,37 we have measured the serum TNF-α level by an ELISA kit (R&D Systems). However, the TNF-α levels in both IL-27 Tg and wild-type mice were less than the lowest sensitivity of the ELISA kit (data not shown). Therefore, the phenotype of IL-27 Tg mice is unlikely to be related to TNF-α. Notably, although IL-27 Tg mice also have increased number of CD34−KSL cells in BM, these CD34−KSL cells are not functional HSCs. Thus, this is the case where phenotype and function are not always consistent. Human IL-27 also augmented the proliferation of human CD34+ hematopoietic stem/progenitor cells purified from umbilical cord blood in the medium containing SCF, Flt3 ligand, and TPO. However, IL-27 failed to maintain the percentage of hematopoietic stem/progenitor populations such as CD34+ and CD34+CD133+ cells. Overall, IL-27 may induce early stages of differentiation in HSCs in synergy with SCF and, consequently, accumulation of cells phenotypically similar but functionally dissimilar to CD34−KSL HSCs. These non-HSCs might belong to a certain subset of progenitor cells. Further studies are necessary to elucidate the function and role of these unusual CD34−KSL cells. Since a previous study using WSX-1–deficient mice reported that the absence of WSX-1 does not affect the development of hematopoietic or lymphoid systems,38 the physiological role of endogenous IL-27 appears to be negligible or compensated by other IL-6 family cytokines. However, under stressed or pathological circumstances, IL-27 may play another role.
A large body of evidence has highlighted the central role of the ETS family transcription factor PU.1 in many aspects of hematopoiesis. An instructive role of PU.1 in promoting the development of myeloid lineages such as macrophages and dendritic cells was demonstrated by enforced expression of PU.1 in hematopoietic progenitors.39 In contrast, deletion of the PU.1 gene leads to defects in myelopoiesis, including loss of monocytes and macrophages.40,41 PU.1 performs these important roles by regulating the expression of numerous genes within the myeloid and lymphoid lineages, including those encoding many of the developmentally important cytokine receptors such as macrophage colony-stimulating factor (M-CSFR), granulocyte-macrophage colony-stimulating factor (GM-CSFR)α, and IL-7Rα. Moreover, STAT3 signaling is important for cytokine-inducible expression of PU.1, which results in promotion of myeloid cell development and the concomitant induction of proteins including αMβ2 integrin (Mac-1).42 Since IL-27 can activate STAT3, IL-27 may induce PU.1 expression, which could presumably account for the effects of IL-27 on hematopoietic cells to some extent. Further studies on the molecular mechanism whereby IL-27 regulates hematopoiesis are currently under investigations.
It was previously demonstrated that IL-6/sIL-6Rα transgenic mice exhibit a reduced body weight with splenomegaly and a dramatic increase of extramedullary hematopoietic progenitor cells in liver and spleen but not in BM.18 This is in contrast with the fact that IL-27 Tg mice show BM dysfunction with splenomegaly and extramedullary hematopoiesis only in the spleen. Recently, similar BM abnormalities to those in IL-27 Tg mice were reported in mice lacking gp130 in hematopoietic and endothelial cells and also in mice lacking STAT3 in BM progenitor cells.43,44 Mice lacking gp130 in hematopoietic and endothelial cells show BM dysfunction with anemia that persisted despite compensatory extramedullary hematopoiesis. Long-term BM cultures and transplantation experiments revealed that the hematopoietic defect results from gp130 deficiency in the BM microenvironment, especially endothelial cells, rather than in the hematopoietic cells themselves.43 Furthermore, mice lacking STAT3 in BM progenitor cells display impaired B-cell development and reduced B-cell lineages, including pro-B, pre-B, immature B, and mature B cells.44 IL-7–mediated proliferation of BM cells also was significantly reduced, which correlated with a decreased number of IL-7–responsive precursors in the BM. In addition, since ectopic expression of STAT3 promotes stem -ell self-renewal and maintenance of pluripotency in the absence of LIF45 and inactivation of STAT3 function in LIF-maintained embryonic stem (ES) cells promotes spontaneous differentiation,46 STAT3 is an essential component of the LIF-dependent self-renewal pathway. On the other hand, accumulating evidence suggests that IL-27 possesses multiple functions such as pro-inflammatory and anti-inflammatory properties including induction of Th1 differentiation and suppression of proinflammatory cytokine production, respectively.25,26 Recently, it was demonstrated that IL-27 inhibits development of autoimmune encephalomyelitis (EAE) by suppressing the generation of IL-17–producing helper T (Th)17 cells induced by IL-6 and transforming growth factor (TGF)–β1.47 This suppressive effect is dependent on STAT1 and considered to be mediated by the inhibition of IL-6 function by IL-27. We previously demonstrated that IL-27 inhibits primary IL-2 production mediated by co-stimulation with CD28 through induction of signal transducer and activator of transcription (SOCS)3 in STAT1-dependent mechanism.48 These results suggest that IL-27 has an ability to induce not only positive signals such as proliferation through STAT3 activation as does IL-6, but also negative signals through STAT1 activation. Therefore, IL-27 may both positively and negatively regulate the differentiation of HSCs through activation of STAT1 and STAT3. Indeed, interferon (IFN)–γ, which activates STAT1, was reported to inhibit the in vitro cellular expansion and self renewal of human CD34+CD38− cells and promote their differentiation, which may account for the suppressive effects of IFN-γ in BM failure syndromes.49 Therefore, the balance between activation status of STAT1 and STAT3 under microenvironmental conditions may have a major impact on how cells behave in response to IL-27.
Thus, although further studies are necessary to elucidate the molecular mechanism whereby IL-27 regulates the HSC differentiation and perhaps also maintains the HSC self-renewal activity to a certain extent, IL-27 might be a unique cytokine, which has a potential to be applied for the development of new methods to regulate the early stages of HSC differentiation in vivo and ex vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Animal Research Center in Tokyo Medical University and the Foundation for Biomedical Research and Innovation for animal care and human CD34+ cells, respectively. The authors are indebted to Professor J.P. Barron (Tokyo Medical University) for his review of this manuscript.
This work was supported in part by Grant-in-Aid for Exploratory Research, High-Tech Research Center Project, and University-Industry Joint Research Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan; by The Mitsubishi Pharma Research Foundation; and by The Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Authorship
Contribution: J.M., H.E., H.N., and T.Y. designed the experiments and analyzed data; H.E. and T.Y. wrote the manuscript; J.S, M.A., J.O., and S.T. performed research and analyzed data; Y.M. and N.W. performed FACS analysis; and K.F. and M.K. performed the histologic analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takayuki Yoshimoto, Intractable Immune System Disease Research Center, Tokyo Medical University, 6-1-1 Shinjuku, Shinjuku-ku, Tokyo 160-8402, Japan; e-mail: yoshimot@tokyo-med.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal