The retinoblastoma tumor suppressor protein (RB) plays important roles in the control of the cell division cycle. It is estimated that RB is dysfunctional/inactivated in up to 40% of human leukemias. The consequences of loss of RB on hematopoietic stem and progenitor cell (HSPC) function in vivo are incompletely understood. Here, we report that mice genetically deficient in Rb in all hematopoietic cells (Vav-Cre Rb knockout [KO] animals) showed altered contribution of distinct hematopoietic cell lineages to peripheral blood, bone marrow, and spleen; significantly increased extramedullary hematopoiesis in the spleen; and a 2-fold increase in the frequency of hematopoietic progenitor cells in peripheral blood. Upon competitive transplantation, HSPCs from Vav-Cre Rb KO mice contributed with an at least 4- to 6-fold less efficiency to hematopoiesis compared with control cells. HSPCs deficient in Rb presented with impaired cell-cycle exit upon stress-induced proliferation, which correlated with impaired function. In summary, Rb is critical for hematopoietic stem and progenitor cell function, localization, and differentiation.
Introduction
The retinoblastoma tumor suppressor protein (RB) plays important roles in the control of the cell division cycle, the DNA-damage cell-cycle checkpoint activation, and cell differentiation and apoptosis in a variety of cell types and tissues (as reviewed by Khidr and Chen1 ). The RB protein is responsive to mitogenic and antiproliferative signals to integrate cell-cycle control with the cellular environment. In quiescent cells, RB is hypophosphorylated and mediates the repression of many genes required for cell-cycle progression to inhibit proliferation. Mitogenic signals activate cycling-dependent kinases (CDKs)/cyclin complexes that phosphorylate RB, which alleviates transcriptional repression and thus facilitates progression through the cell cycle.2,3 RB sequesters and inactivates several proteins (including the E2F family of proteins) necessary for S-phase progression. Due to the fact that various cell cycle–inhibitory as well as cell cycle–promoting signals converge in regulating RB activity, posphorylation of RB is regarded as a “master switch” in cell-cycle progression by regulating the G1-S transition restriction point.4
Mice deficient in Rb are not viable and show defects in multiple tissues, including the hematopoietic compartment, with reduced formation of blood islands in the fetal liver and an increased proportion of immature nucleated erythroid cells.5,,–8 A role for RB in hematopoiesis is also supported by the fact that levels of RB are high during erythorid differentiation, whereas expression of RB is down-regulated during granulocyte maturation.9
It has been recently suggested that the retinoblastoma gene product might be involved in multiple aspects of stem-cell biology.10 The CDK inhibitors p21CIP1, p27KIP1, p16INK4a, p18INK4c, and p19ARF, which regulate the phosphorylation status of Rb by CDKs, have been recently implicated in modulating hematopoietic stem cell (HSC) fates.11,,,,–16 In addition, mice that were reconstituted with Rb−/− fetal liver cells containing fetal HSCs presented with progressive hematopoietic depletion in the bone marrow (BM).8 These data therefore imply a specific role for Rb in the regulation of HSCs. On the other hand, it has been recently reported that Rb is dispensable for self-renewal and multilineage differentiation of adult HSCs.17
Thus, further investigations on the consequence of loss of Rb on hematopoietic stem and progenitor cell (HSPC) function are warranted, especially as Rb is dysfunctional/inactivated in up to 40% of acute lymphoblastic leukemias (ALLs) and in up to 25% of myeloid leukemias (acute myeloid leukemia [AML] and chronic myelogenous leukemia [CML]).4 Although Rb belongs to a family of proteins called pocket proteins, mutations affecting the other pocket proteins p107 and p130 are not frequently observed in human cancers, supporting a unique role for the loss of RB in cancer development.
To circumvent the embryonic lethal phenotype of Rb−/− animals, we developed a new animal model system in which floxed Rb alleles were deleted in the hematopoietic system by Cre recombinase driven by the Vav1 promoter.18 Our analyses revealed that Rb is indispensable for the correct function of adult HSCs under stress, and that Rb regulates HSPC function, localization, and differentiation ability.
Methods
Generation of mice
Floxed Rb mice (RbloxP/loxP) on a mixed 129/FVBN background were crossed with vav1Cre+ mice. RbloxP/+, vav1Cre+/− female mice were backcrossed on RbloxP/loxP animals for generating RbloxP/loxP, vav1Cre+/− (Vav-Cre Rb knockout [KO]) and RbloxP/loxP, vav1Cre−/− (control) mice. Polymerase chain reaction (PCR) was performed on tail DNA to confirm presence of the Cre transgene and homozygosity of the RbloxP/loxP allele and on blood, muscle, brain, bone marrow, and colony-forming cells (CFCs) to test for/confirm recombined alleles as previously described.18,19 Mice were analyzed between 5 and 7 weeks of age.
Hematologic analysis and histology
Cellularity of peripheral blood (PB), BM, and spleen were determined using a Hemavet 850 (Drew Scientific, Dallas, TX). Blood smears were stained with Wright-Giemsa (EMD Chemicals, San Diego, CA). Cytospins of BM and spleen cells were generated from single-cell suspensions (Cytospin-4; Thermo Shandon, Waltham, MA) and stained according to the manufacturer's protocol (Hema 3 Manual Staining Systems; Fisher Scientific, Pittsburgh, PA). For histology, fixed, paraffin-embedded spleen sections were stained with hematoxylin-eosin.
Figure 1A was acquired with a Nikon (Melville, NY) Coolpix 3200 digital camera and further processed with iPhoto 6.06 software (Apple, Cupertino, CA). Figure 1B was acquired by the Pathology Core of Cincinnati Children's Hospital Medical Center using standard microscopy equipment. Figure 1E was acquired on a Nikon OptiPhot-2 using an Olympus (Melville, NY) 40×0.65 NA objective with a Diagnostic Instruments (Sterling Heights, MI) RT SPOT camera and SPOT 4.0.9 software. Figure 4A was acquired using an Olympus CK40 microscope with an Olympus 20×0.4 NA objective with an RTn Color SPOT camera and SPOT 4.0.9 software. All raw pictures were further processed with Adobe Photoshop Elements 2.0 Software (Adobe Systems, San Jose, CA).
Hematopoietic cell isolation and identification
BM cells were flushed from femora and tibiae and suspended in Hanks balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Atlanta Biologicals, Laurenceville, GA), and 2% penicillin (5000 U/mL)/streptomycin (5000 μg/mL; Cambrex Bio Science, East Rutherford, NJ). Spleen cell suspensions were passed through a 70-μm filter. PB was obtained by retro-orbital bleeding. Mononuclear cells were isolated by low-density centrifugation (Histopaque 1083; Sigma-Aldrich, St Louis, MO) and incubated with a cocktail of biotinylated antibodies against epitopes on differentiated leukocytes: anti-CD11b (clone M1/70), anti-B220 (clone RA3-6B2), anti-CD5 (clone 53-7.3), anti-Gr-1 (clone RB6-8C5), anti-Ter119, anti-CD8a (Clone 53-6.7; all from BD Pharmingen, San Diego, CA), anti–Sca-1 (clone D7; eBioscience, San Diego, CA), anti–C-kit (clone 2B8; BD Pharmingen), and streptavidin-APC-Cy7 (BD Pharmingen), and identified on a BD FacsCanto (BD Biosciences, San Diego, CA).
CFC assay
CFC assays were performed as previously described.20 Cultures were plated in triplicate and incubated for 7 days with 5% CO2 at 37°C. On day 7, colonies containing at least 50 cells were scored. Colonies were harvested and replated at 104 cells in 1 mL.
CAFC progenitor assay
BM transplantation and engraftment analysis
Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice aged 2 to 3 months were placed on doxycycline (625 mg/kg of diet) 3 weeks prior to transplantation and remained on doxycycline 1 week following transplantation. NOD/SCID mice were irradiated (3 or 3.5 Gy) and subsequently transplanted with either 2 × 105 or 106 BM cells via retro-orbital sinus. To determine chimerism, PB was incubated with FITC-conjugated anti–mouse H-2Kd (clone SF1-1.1), PE-conjugated anti–mouse H-2Kb (clone AF6-88.5), and APC-conjugated anti–mouse CD45 (Ly-5; clone 30-F11; all from BD Pharmingen). After red blood cell (RBC) lysis, cells were analyzed for CD45+, H-2Kd on a BD FacsCanto.
For competitive transplantations, BM cells were combined with BM cells isolated from C57BL/6 CD45.1 (BoyJ) mice (Charles River Laboratories, Wilmington, MA). BoyJ recipient mice were lethally irradiated with a dosage of 11.75 Gy (7 Gy + 4.75 Gy, 4 hours apart) and subsequently underwent transplantation. For secondary transplantations, either 2 × 106 fresh BM cells or 107 previously frozen BM cells were transplanted into irradiated BoyJ recipients. Chimerism in PB was determined by incubating cells with FITC-conjugated anti–mouse CD45.2 (104), PE-conjugated anti–mouse CD45.1 (A20), APC-conjugated anti–mouse CD45R/B220 (RA3-6B2), APC-Cy7–conjugated anti–mouse CD11b (M1/70), and APC-Cy7–conjugated anti–mouse Ly-6G/Ly-6C (Gr-1; all from BD Pharmingen). After RBC lysis, cells were analyzed on a BD FacsCanto.
Homing assay
A total of 2 × 107 whole BM cells from Vav-Cre Rb KO or control mice were intravenously injected into irradiated (11.75 Gy) BoyJ mice. BM was harvested 18 hours later, 1/12 of the total bone marrow cells were resuspended in 4 mL of CFU culture (CFU-C) medium supplemented with cytokines, and 1 mL of the cell suspension was plated in triplicate. The colonies were scored 7 days later, and homing efficiencies were calculated on the basis of the number of input CFU-Cs and the number of CFU-Cs retrieved from tibia and femur.
Analysis of apoptosis
Low-density cells (2 × 106) were immunostained as described, washed with cold phosphate-buffered saline (PBS), and stained for annexin V and 7AAD according to the manufacturer's protocol (BD Pharmingen) and analyzed on a BD FacsCanto.
Cell-cycle analysis
Some animals were injected with 5-fluorouracil (5-FU; 150 mg/kg; Sicor Pharmaceutical, Irvine, CA) intraperitoneally either 16 hours or 4 days prior to BrdU labeling. Mice were subsequently injected intraperitoneally with 200 μL, BrdU (2.5 μg/μL). BM and spleen cells were harvested 45 minutes later and immunostained as described. Anti-BrdU staining was performed using the manufacturer's protocol (APC BrdU flow kit; BD Pharmingen). Total DNA content was stained with 20 μL 7AAD solution (12.5 ng/μL), and cell-cycle status was analyzed on a BD FacsCanto. For the determination of the frequency of Lin−, Sca-1+, and c-Kit+ (L−S+K+) Mac-1+ cells in S-phase, Mac-1 was eliminated from the Lin cocktail and independently provided as an Alexa Fluor 700 conjugate, and 7AAD was omitted from the analysis. Cells were analyzed on a BD FacsCantoII.
Results
Generation of animals deficient in Rb in the hematopoietic system
Animals deficient in Rb in all hematopoietic cells (Vav-Cre Rb KO mice) were generated by crossing a conditional (floxed) allele of Rb19 onto mice expressing Cre under the control of the promoter of the Vav1 gene18 (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The Vav1-Cre transgene targets Cre recombinase expression to hematopoietic cells, and, to some extent, during embryonic development to vascular endothelial cells.18,22 Deletion of the floxed Rb allele in hematopoietic cells in adult animals was confirmed by PCR of specific hematopoietic tissues and hematopoietic progenitor cells (HPCs; Figure S1B,C). Animals deficient in Rb in hematopoietic cells were born in the expected Mendelian ratio, and homozygote animals were able to give rise to offspring up to 1 year of age (data not shown). We detected partial deletion of the floxed allele in muscle tissue as well as in the brain (Figure S1B), which might be due to the presence of hematopoietic-derived cells in these tissues. As Vav1-driven Cre expression results at least during embryonic development also in deletion of floxed genes in the endothelial lineage, we cannot exclude though that nonhematopoietic cells also are deleted for Rb in Vav-Cre Rb KO animals.
Loss of Rb results in altered relative contribution of hematopoietic cell lineages
We detected an increase in reticulocytes and nucleated red blood cells (Figure S2A) in the PB of Vav-Cre Rb KO animals, indicating a potential perturbation in erythrocyte maturation. These changes correlated with significant anemia (hemoglobin [Hb] of 121 g/L [12.1 g/dL] in Vav-Cre Rb KO animals vs 136 g/L [13.6 g/dL] in controls, and RBC count of 6.7 × 1012/L [6.7 × 106/μL] in Vav-Cre Rb KO animals vs 8.8 × 1012/L [8.8 × 106/μL] in controls; Tables 1 and 2. We further detected a significant decrease in the number of B220+ B cells (Figure S2B), indicating that loss of Rb causes perturbed frequency of various hematopoietic cell lineages.
Cell counts in PB
| Tissue/genotype . | Leukocytes, × 109/L . | RBC, × 109/L . | Hb, g/L . | Platelets, × 109/L . |
|---|---|---|---|---|
| PB/control | 7.7 ± 1.2 | 8.8 ± 0.2* | 136 ± 3.0* | 807 ± 27 |
| PB/Vav-Cre Rb KO | 8.5 ± 1.0 | 6.7 ± 0.2* | 121 ± 3.0* | 760 ± 35 |
| Tissue/genotype . | Leukocytes, × 109/L . | RBC, × 109/L . | Hb, g/L . | Platelets, × 109/L . |
|---|---|---|---|---|
| PB/control | 7.7 ± 1.2 | 8.8 ± 0.2* | 136 ± 3.0* | 807 ± 27 |
| PB/Vav-Cre Rb KO | 8.5 ± 1.0 | 6.7 ± 0.2* | 121 ± 3.0* | 760 ± 35 |
Shown are mean values plus or minus 1 SEM. n = 5 for controls and n = 6 for Vav-Cre Rb KO animals.
Significantly different between control and Vav-Cre Rb KO animals (P < .05).
Cell counts in spleen and BM
| Tissue/genotype . | Cell count, × 107 . | Red cells, × 108 . | Platelets, × 108 . |
|---|---|---|---|
| Spleen/control | 1.7 ± 0.2* | 4.9 ± 0.3* | 5.8 ± 0.5* |
| Spleen/Vav-Cre Rb KO | 6.0 ± 0.3* | 15.5 ± 3.4* | 37.2 ± 6.4* |
| BM/control | 8.3 ± 0.8 | 19.0 ± 2.4 | ND |
| BM/Vav-Cre Rb KO | 7.9 ± 0.4 | 11.7 ± 1.1 | ND |
| Tissue/genotype . | Cell count, × 107 . | Red cells, × 108 . | Platelets, × 108 . |
|---|---|---|---|
| Spleen/control | 1.7 ± 0.2* | 4.9 ± 0.3* | 5.8 ± 0.5* |
| Spleen/Vav-Cre Rb KO | 6.0 ± 0.3* | 15.5 ± 3.4* | 37.2 ± 6.4* |
| BM/control | 8.3 ± 0.8 | 19.0 ± 2.4 | ND |
| BM/Vav-Cre Rb KO | 7.9 ± 0.4 | 11.7 ± 1.1 | ND |
ND indicates not determined. Shown are mean values plus or minus 1 SEM. Total cell counts in BM are based on tibia and femur combined. n = 5 for controls and n = 6 for Vav-Cre Rb KO animals.
Significantly different between control and Vav-Cre Rb KO animals (P < .05).
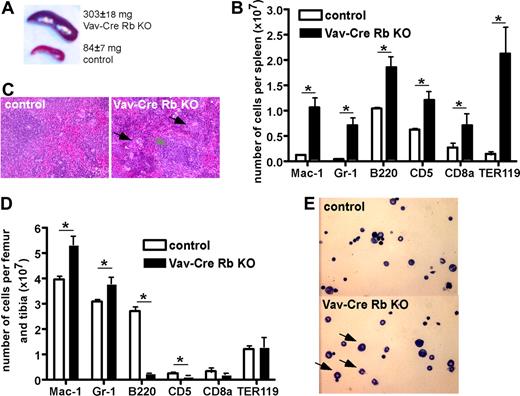
Vav-Cre Rb KO animals presented with a significant splenomegaly (mean organ weight of 303 mg vs 84 mg in control animals; Figure 1A) and significant extramedullary hematopoiesis, with the overall architecture of the spleen still intact (Figure 1B). Flow cytometric analyses revealed an increase in the number of myeloid cells (Mac-1+ and Gr-1+ cells) as well as B cells (B220+ and CD5+ cells), and a prominent increase in the number of Ter119+ erythroid cells (Figure 1C).
Altered contribution of distinct hematopoietic cell lineages in BM and spleen in Vav-Cre Rb KO animals. (A) Spleen size in control and Vav-Cre Rb KO animals (n = 7 for control, n = 10 for Vav-Cre Rb KO). (B) Hematoxylin-eosin stain of spleens from control and Vav-Cre Rb KO animals, indicating increased number of neutrophils and erythroblasts in spleen, although the overall architecture of the spleen is still preserved. (C) Flow cytometric analyses of spleens from control and Vav-Cre Rb KO animals for cell-surface markers associated with myeloid cells (Mac-1, Gr-1), B-cells (B220), T and B cells in the spleen (CD5), T cells (CD8a), and erythroid cells (Ter119; n = 5 for both control and Vav-Cre Rb KO). (D) Flow cytometric analyses of BM from control and Vav-Cre Rb KO animals for cell-surface markers associated with myeloid cells (Mac-1, Gr-1), B cells (B220), T cells (CD5, CD8a), and erythroid cells (Ter119; n = 5 for both control and Vav-Cre Rb KO). (E) Cytospins of BM from control and Vav-Cre Rb KO animals, indicating increased frequency of neutrophils in BM of Vav-Cre Rb KO animals. Shown are mean values plus or minus 1 SEM; *P < .05.
Altered contribution of distinct hematopoietic cell lineages in BM and spleen in Vav-Cre Rb KO animals. (A) Spleen size in control and Vav-Cre Rb KO animals (n = 7 for control, n = 10 for Vav-Cre Rb KO). (B) Hematoxylin-eosin stain of spleens from control and Vav-Cre Rb KO animals, indicating increased number of neutrophils and erythroblasts in spleen, although the overall architecture of the spleen is still preserved. (C) Flow cytometric analyses of spleens from control and Vav-Cre Rb KO animals for cell-surface markers associated with myeloid cells (Mac-1, Gr-1), B-cells (B220), T and B cells in the spleen (CD5), T cells (CD8a), and erythroid cells (Ter119; n = 5 for both control and Vav-Cre Rb KO). (D) Flow cytometric analyses of BM from control and Vav-Cre Rb KO animals for cell-surface markers associated with myeloid cells (Mac-1, Gr-1), B cells (B220), T cells (CD5, CD8a), and erythroid cells (Ter119; n = 5 for both control and Vav-Cre Rb KO). (E) Cytospins of BM from control and Vav-Cre Rb KO animals, indicating increased frequency of neutrophils in BM of Vav-Cre Rb KO animals. Shown are mean values plus or minus 1 SEM; *P < .05.
We detected a similar cellularity in the BM of Vav-Cre Rb KO and control animals (Tables 1 and 2). However, Vav-Cre Rb KO animals presented with an increased number of neutrophils in BM (Figure 1E). Consistent with the differential cells counts, flow cytometric analyses identified an increased number of Mac-1+ and Gr-1+ cells (Figure 1D) as well as a significant reduction in the number of B220+ B cells (Figure 1D). Interestingly, Vav-Cre Rb KO mice showed a decrease in the number of red cells in the BM compared with control littermates. As reported in Figures 1B and S2 for PB and spleen, we did not detect a significant difference in a mature T-cell compartment in BM (CD8a+ cells). The overall cellularity as well as the distribution of CD4/CD8 cell populations in the thymus were also not significantly altered in Vav-Cre Rb KO animals compared with control animals (data not shown). As osteoclasts are part of the myeloid cell family, we also determined the frequency of osteoclasts in bone in control and Vav-Cre Rb KO animals.23,24 We did not observe a significant difference in the number of osteoclasts in bone sections (data not shown).
Influence of loss of Rb on the frequency of primitive hematopoietic cells in BM and PB
We next determined the consequences of Rb deficiency on the total number of primitive hematopoietic cells in BM. The numbers of L−S−K+ HPCs and L−S+K+ HSCs in Vav-Cre Rb KO BM were almost identical to the numbers in BM of control animals (Figure 2A). Colony-forming assays confirmed a similar number of progenitor cells (CFCs) in the BM of Vav-Cre Rb KO and control animals (Figure 2B). We also analyzed the proliferative potential of the CFCs by determining the number of cells generated per colony and the self-renewal potential by replating CFCs in fresh methylcellulose medium. We detected almost identical cell numbers per colony (Figure 2C), and an almost identical ability of Vav-Cre Rb KO and control cells to form secondary colonies (Figure 2D).
Normal progenitor and stem-cell parameters in Vav-Cre Rb KO animals in BM under steady-state conditions. (A) Total number of cell populations enriched for HPCs (L−S−K+) and HSCs (L−S+K+) in BM of control and Vav-Cre Rb KO animals (n = 5 for control and n = 7 for Vav-Cre Rb KO). (B) Total number of committed progenitor cells (CFCs; n = 7 for control and n = 9 for Vav-Cre Rb KO). (C) Number of cells per CFC colony to determine the proliferative potential of the CFCs (n = 7 for control and n = 9 for Vav-Cre Rb KO). (D) Number of colonies per 1 × 104 cells from primary colonies to determine the self-renewal potential of CFCs (n = 7 for control and n = 9 for Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM.
Normal progenitor and stem-cell parameters in Vav-Cre Rb KO animals in BM under steady-state conditions. (A) Total number of cell populations enriched for HPCs (L−S−K+) and HSCs (L−S+K+) in BM of control and Vav-Cre Rb KO animals (n = 5 for control and n = 7 for Vav-Cre Rb KO). (B) Total number of committed progenitor cells (CFCs; n = 7 for control and n = 9 for Vav-Cre Rb KO). (C) Number of cells per CFC colony to determine the proliferative potential of the CFCs (n = 7 for control and n = 9 for Vav-Cre Rb KO). (D) Number of colonies per 1 × 104 cells from primary colonies to determine the self-renewal potential of CFCs (n = 7 for control and n = 9 for Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM.
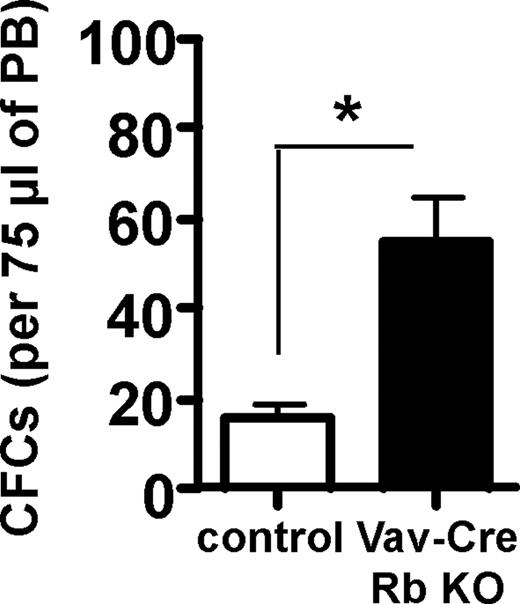
The elevated extramedullary hematopoiesis in the Vav-Cre Rb KO animals lead us also to the examination of the frequency of HSPC in PB. HSPCs normally reside in BM and are almost absent from peripheral blood. Vav-Cre Rb KO animals showed a significant increase in the frequency of CFCs in PB (Figure 3), indicating altered progenitor cell trafficking in Vav-Cre Rb KO animals.
Increased spontaneous HSPC mobilization proficiency in Vav-Cre Rb KO animals. Frequency of CFCs in 75 μL PB. n = 6 for control and n = 5 for Vav-Cre Rb KO. Shown are mean values plus or minus 1 SEM; *P < .05.
Increased spontaneous HSPC mobilization proficiency in Vav-Cre Rb KO animals. Frequency of CFCs in 75 μL PB. n = 6 for control and n = 5 for Vav-Cre Rb KO. Shown are mean values plus or minus 1 SEM; *P < .05.
Loss of Rb results in reduced function of HSPCs
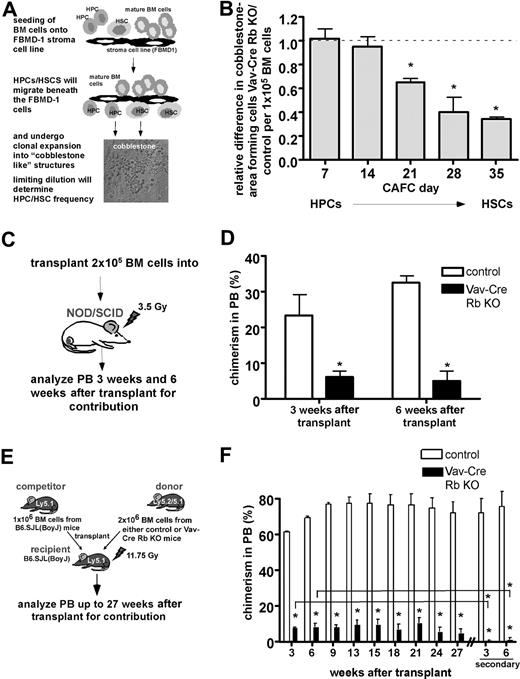
To further determine stem-cell function in Vav-Cre Rb KO animals, CAFC assays were performed (Figure 4A). The CAFC assay is a well-established limiting-dilution in vitro surrogate assay to measure progenitor and stem-cell activity.21 The frequency of CAFC day-28 and day-35 cells correlates with the frequency of long-term repopulating hematopoietic stem cells.21,25 Despite similar frequencies of HSCs in BM as determined by flow cytometry (Figure 2A), BM from Vav-Cre Rb KO animals showed a significant reduction in the frequency of CAFC day-21, day-28, and day-35 cells compared with control animals (Figure 4B). Interestingly, the relative difference in CAFC frequencies between Vav-Cre Rb and control animals increased with the stemness of the cells (Figure 4B).
Intrinsically reduced function of Vav-Cre Rb KO HSPCs outside the BM and upon transplantation. (A) Frequency/function of HPCs and HSCs analyzed by the CAFC assay. Shown is the experimental setup of the assay. (B) Frequency/function of distinct CAFC populations in Vav-Cre Rb KO animals in BM relative to control animals analyzed in parallel (n = 6 for control and Vav-Cre Rb KO). The dotted line (value of 1) indicates the level of no relative difference in CAFCs between Vav-Cre Rb KO and control animals in this figure. Starting from CAFC day 21 on, Vav-Cre Rb KO primitive hematopoietic cells are significantly reduced in their frequency/function. (C) Analysis of the progenitor cell function of Vav-Cre Rb KO cells upon transplantation of BM cells into sublethally irradiated NOD/SCID recipients. Shown is the experimental setup. (D) Determination of the contribution of control and Vav-Cre Rb KO cells to PB 3 weeks and 6 weeks after transplantation (chimerism in PB) by flow cytometric analyses (n = 4 recipients for control and n = 4 recipients for Vav-Cre Rb KO). (E) Analysis of stem- cell function by competitive repopulation assays into C57BL/6 CD45.1 animals. Shown is the experimental setup. (F) Determination of the contribution of control and Vav-Cre Rb KO cells to PB up to 27 weeks after transplantation and up to 6 weeks in secondary recipients (chimerism in PB) by flow cytometric analyses (n = 3 recipients for control and n = 3 recipients for Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM; *P < .05.
Intrinsically reduced function of Vav-Cre Rb KO HSPCs outside the BM and upon transplantation. (A) Frequency/function of HPCs and HSCs analyzed by the CAFC assay. Shown is the experimental setup of the assay. (B) Frequency/function of distinct CAFC populations in Vav-Cre Rb KO animals in BM relative to control animals analyzed in parallel (n = 6 for control and Vav-Cre Rb KO). The dotted line (value of 1) indicates the level of no relative difference in CAFCs between Vav-Cre Rb KO and control animals in this figure. Starting from CAFC day 21 on, Vav-Cre Rb KO primitive hematopoietic cells are significantly reduced in their frequency/function. (C) Analysis of the progenitor cell function of Vav-Cre Rb KO cells upon transplantation of BM cells into sublethally irradiated NOD/SCID recipients. Shown is the experimental setup. (D) Determination of the contribution of control and Vav-Cre Rb KO cells to PB 3 weeks and 6 weeks after transplantation (chimerism in PB) by flow cytometric analyses (n = 4 recipients for control and n = 4 recipients for Vav-Cre Rb KO). (E) Analysis of stem- cell function by competitive repopulation assays into C57BL/6 CD45.1 animals. Shown is the experimental setup. (F) Determination of the contribution of control and Vav-Cre Rb KO cells to PB up to 27 weeks after transplantation and up to 6 weeks in secondary recipients (chimerism in PB) by flow cytometric analyses (n = 3 recipients for control and n = 3 recipients for Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM; *P < .05.
To further characterize the function of HSCs from Vav-Cre Rb KO animals in vivo, transplantation/repopulation experiments were performed. As Vav-Cre Rb KO animals are on a mixed genetic background, we initially transplanted equal numbers of BM cells from either control or Vav-Cre Rb KO animals into sublethally irradiated immunocompromised NOD/SCID mice (Figures 4C and S3A) to avoid possible histoincompatibilities of the graft in these experiments. Recipient animals were analyzed by flow cytometry for donor contribution in PB. At both 3 and 6 weeks after transplantation, animals receiving Vav-Cre Rb KO BM displayed a significant 3- to 5-fold reduced contribution to PB compared with animals that received transplants of control BM cells (Figures 4D and S3B), indicating a reduced function of HPCs from Vav-Cre Rb KO animals.
As transplantation in NOD/SCID mice does not allow long-term follow-up on the activity of the donor cells, we also performed competitive transplantation experiments using C57BL/6-CD45.1 animals as recipients and competitors (Figure 4E). As Vav-Cre Rb and control mice are on a mixed genetic background (contribution from 129 as well as FVB), hematopoietic cells from these animals are positive for both Ly5.1 and Ly5.2. Analyzing the contribution of Vav-Cre Rb KO and control cells to PB up to 27 weeks after transplantation, Vav-Cre Rb KO cells displayed a stable but 8-fold lower contribution to chimerism in PB compared with control cells, which was a further 5-fold reduced upon secondary transplantation (Figure 4F). Additional experiments in which BM from individual littermate control and Vav-Cre Rb KO animals was competitively transplanted into NOD/SCID as well as C57BL/6-CD45.1 recipients further revealed that the observed reduction in chimerism derived from Vav-Cre Rb KO donor cells is most likely not due to histoincompatibilities of the mixed genetic background of our experimental animals (Figure S3C,D), although we can formally not completely exclude alterations of the chimersim due to histoincompatibilities between donor and recipients in our experiments. In addition, spleen cells from Vav-Cre Rb KO animals depleted for differentiated hematopoietic cells (Lin−) and thus enriched for HSPCs also presented with a reduced ability to contribute to PB upon transplantation (Figure S3E). As the frequency of phenotypically determined HSPCs was almost identical in BM in Vav-Cre Rb KO and control animals (see Figure 2A), these data suggest that HSPCs from Vav-Cre Rb KO animals are functionally impaired upon transplantation.
Homing, adhesion, cell-cycle, and apoptotic status of Rb-deficient primitive hematopoietic cells
Successful reconstitution upon transplantation depends on multiple parameters, including the ability of HSCPs to home to the BM and26 to adhere to stroma in BM,27,28 the cell-cycle status of the transplanted cells,29,30 and the rate of apoptosis. Consequently, we determined these properties in primitive hematopoietic cells of Vav-Cre Rb KO animals.
Vav-Cre Rb KO HPCs were not impaired in their ability to home to the BM (Figure 5A). Primitive hematopoietic cells from Vav-Cre Rb KO animals (CAFC day-7 to day-28 cells) showed a similar frequency of adhesion to FBMD-1 stroma cells compared with control cells (Figure 5B). The literature supports a scenario in which cells that are in the S-phase of the cell cycle are impaired in their reconstitution activity.29,30 We detected almost identical frequencies of both HSCs and HPCs in either the G0/G1, the S, or the G2/M phases of the cell division cycle in control and Vav-Cre Rb KO animals by measuring BrdU incorporation in vivo (Figure 5C). Since the transplantation results could also be explained by reduced survival of HSPCs, we determined the rate of apoptosis in HSPCs. HSPCs from Vav-Cre Rb KO animals showed no significant change in the frequency of annexin V+ apoptotic cells (Figure 5D). In summary, the reduced function of Vav-Cre Rb KO cells upon transplantation does not correlate with homing, adhesion, cell-cycle distribution, or apoptosis.
Homing, adhesion to stroma, cell-cycle status, and apoptosis in Vav-Cre Rb KO primitive hematopoietic cells do not correlate with reduced function. (A) CFCs were transplanted into lethally irradiated animals, and the percentage of CFCs homed to the BM was determined 18 hours after transplantation (n = 4 for both control and Vav-Cre Rb KO). (B) The ability of distinct CAFC populations to adhere to the FBMD-1 stroma cell line after 4 hours was determined using the CAFC adhesion assay as described in “Methods.” There is no difference in the ability to efficiently adhere to stroma cells between control and Vav-Cre Rb KO cells (n = 6 for both control and Vav-Cre Rb KO). (C) BM cells were pulse-labeled with BrdU in vivo, and the frequency of HPCs (L−S−K+ cells) and HSCs (L−S+K+) in the G0/G1, S, and G2/M phases of the cell division cycle were analyzed by flow cytometry (n = 5 for control and n = 7 for Vav-Cre Rb KO). (D) Frequency of HPCs (L−S−K+) and HSCs (L−S+K+) undergoing spontaneous apoptosis in control and Vav-Cre Rb KO animals, determined by staining for annexin V and 7AAD by flow cytometry (n = 3 for both control and Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM.
Homing, adhesion to stroma, cell-cycle status, and apoptosis in Vav-Cre Rb KO primitive hematopoietic cells do not correlate with reduced function. (A) CFCs were transplanted into lethally irradiated animals, and the percentage of CFCs homed to the BM was determined 18 hours after transplantation (n = 4 for both control and Vav-Cre Rb KO). (B) The ability of distinct CAFC populations to adhere to the FBMD-1 stroma cell line after 4 hours was determined using the CAFC adhesion assay as described in “Methods.” There is no difference in the ability to efficiently adhere to stroma cells between control and Vav-Cre Rb KO cells (n = 6 for both control and Vav-Cre Rb KO). (C) BM cells were pulse-labeled with BrdU in vivo, and the frequency of HPCs (L−S−K+ cells) and HSCs (L−S+K+) in the G0/G1, S, and G2/M phases of the cell division cycle were analyzed by flow cytometry (n = 5 for control and n = 7 for Vav-Cre Rb KO). (D) Frequency of HPCs (L−S−K+) and HSCs (L−S+K+) undergoing spontaneous apoptosis in control and Vav-Cre Rb KO animals, determined by staining for annexin V and 7AAD by flow cytometry (n = 3 for both control and Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM.
Primitive hematopoietic cells from Vav-Cre Rb KO animals are impaired in cell-cycle regulation upon replicative stress
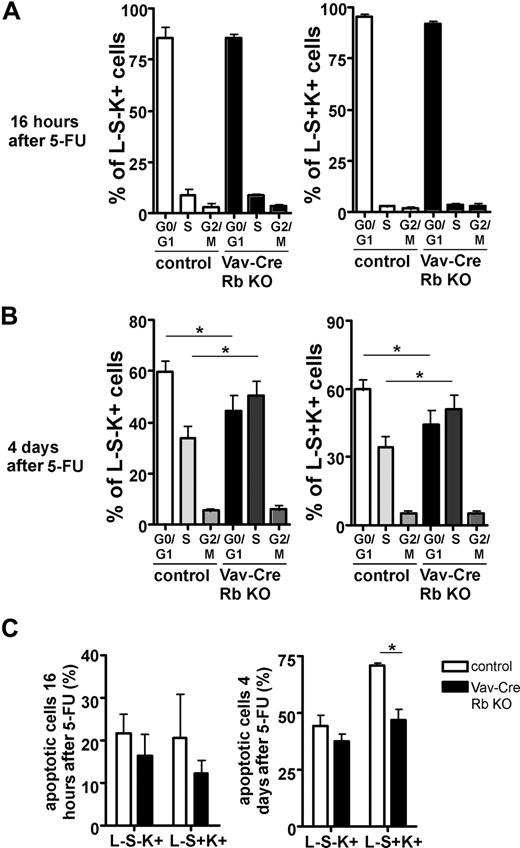
It has been previously reported that erythropoiesis in Rb−/− fetal liver cells is especially susceptible to stress.8 We thus determined the cell-cycle status in HPCs (L−S−K+) and HSCs (L−S+K+) by in vivo BrdU labeling after a 5-FU challenge to induce replicative stress.31,32 At 16 hours after 5-FU, Vav-Cre Rb KO cells showed no difference in the frequency of cells in S-phase compared with control cells (Figure 6A). At 4 days after 5-FU though, Vav-Cre Rb KO cells presented with a significant increase in cells in S-phase in both HPCs and HSCs (Figure 6B). Experiments published by Randall et al indicate that long-term repopulating HSCs transiently express Mac-1 after 5-FU treatment.32 To exclude that the increased frequency of cells in S-phase is not due to Rb−/− cell–specific changes regarding Mac-1 expression on HSCs after 5-FU, we further analyzed the percentage of BrdU+ Lin−, Sca-1+, c-Kit+, Mac-1+ cells 4 days after 5-FU. While the percentage of Mac-1+ cells in the L−S+K+ compartment was almost identical for both strains of animals (6.9% for Vav-Cre Rb animals vs 6.4% for controls), the percentage of BrdU+ L−S+K+ Mac-1+ cells in Vav-Cre Rb animals was significantly elevated over the percentage in control animals (58% ± 8% vs 11% ± 3%; n = 4, P = .001). In addition, except for a lower frequency of apoptotic L−S+K+ cells in Vav-Cre Rb KO animals at 4 days after 5-FU, the frequency of apoptotic L−S−K− and L−K+S+ cells remained similar in both groups (Figure 6C). These results imply that stress conditions result in an increased frequency of cells in S-phase in Vav-Cre Rb KO animals in comparison with controls.
Primitive hematopoietic cells from Vav-Cre Rb KO animals are impaired in cell-cycle exit upon replicative stress. BM cells were pulse-labeled with BrdU in vivo, and the frequency of HPCs (L−S−K+ cells) and HSCs (L−S+K+) in the G0/G1, S, and G2/M phases of the cell division cycle was analyzed by flow cytometry after 5-FU treatment (150 mg/kg) at (A) 16 hours (n = 3 for both control and Vav-Cre Rb KO) and (B) 4 days (n = 9 for both control and Vav-Cre Rb KO). (C) Frequency of HPCs (L−S−K+) and HSCs (L−S+K+) undergoing apoptosis in control and Vav-Cre Rb KO animals either 16 hours or 4 days after 5-FU treatment, determined by staining for annexin V and 7AAD by flow cytometry (n = at least 3 for each data point). Shown are mean values plus or minus 1 SEM; *P < .05.
Primitive hematopoietic cells from Vav-Cre Rb KO animals are impaired in cell-cycle exit upon replicative stress. BM cells were pulse-labeled with BrdU in vivo, and the frequency of HPCs (L−S−K+ cells) and HSCs (L−S+K+) in the G0/G1, S, and G2/M phases of the cell division cycle was analyzed by flow cytometry after 5-FU treatment (150 mg/kg) at (A) 16 hours (n = 3 for both control and Vav-Cre Rb KO) and (B) 4 days (n = 9 for both control and Vav-Cre Rb KO). (C) Frequency of HPCs (L−S−K+) and HSCs (L−S+K+) undergoing apoptosis in control and Vav-Cre Rb KO animals either 16 hours or 4 days after 5-FU treatment, determined by staining for annexin V and 7AAD by flow cytometry (n = at least 3 for each data point). Shown are mean values plus or minus 1 SEM; *P < .05.
Hematopoiesis in spleen in Vav-Cre Rb KO animals is characterized by increased cell cycling and reduced function
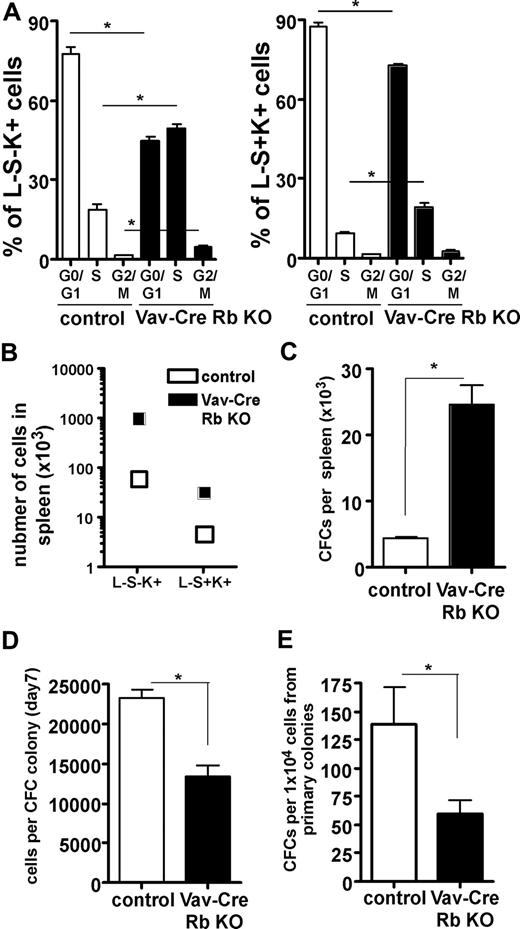
One possible interpretation of the data presented so far is that under replicative stress, the frequency of primitive hematopoietic cells from Vav-Cre Rb KO animals in S-phase is increased, and at the same time, the function of these cells is impaired. Although the mouse spleen is a hematopoietic organ even at baseline conditions, the increased hematopoiesis in the spleen of Vav-Cre Rb KO animals reflects stress hematopoiesis, especially on face of their anemia.33 Consistent with the interpretation of the data here, both HSCs and HPCs showed a significant increase in the frequency of cells in the S-phase of the cell cycle in Vav-Cre Rb KO animals (Figure 7A). Interestingly, while we observed an overall dramatic increase in the total number of HSCs (L−S+K+ cells) and HPCs (L−S−K+ cells) in the spleen (Figure 7B), we also observed a significant decrease of the relative frequency of HSCs (L−S+K+) in the spleens of Vav-Cre Rb KO animals and a more than 2-fold increase of the frequency of HPCs (L−S−K+), suggesting that under stress conditions, Vav-Cre Rb KO HSCs might be skewed in their differentiation ability (Figure S4). The increase in the number of L−S−K+ HPCs was consistent with a significant increase in the number of CFCs in the spleen of Vav-Cre Rb KO animals (Figure 7C). CFCs from Vav-Cre Rb KO animals were significantly impaired in their proliferative activity, as they presented with a reduced number of cells per colony (Figure 7D). Vav-Cre Rb KO CFCs from the spleen were also significantly impaired in their self-renewal activity upon replating (Figure 7E). In summary, these data support that under conditions of replicative stress, HPCs deficient in Rb show an increase in cells in S-phase of the cell cycle, which correlates with impaired self-renewal.
Stress hematopoiesis in spleen in Vav-Cre Rb KO animals results in an increased frequency of HSCs and HPCs in cell cycle and reduced function of HPCs. (A) Animals were pulse-labeled with BrdU in vivo, and the frequency of splenic HPCs (L−S−K+ cells) and splenic HSCs (L−S+K+) in the G0/G1, S, and G2/M phases of the cell division cycle analyzed by flow cytometry (n = 9 for both control and Vav-Cre Rb KO). (B) Total number of HPCs (L−S−K+) and HSCs (L−S+K+) in spleens of control and Vav-Cre Rb KO animals (n = 5 for control and n = 7 for Vav-Cre Rb KO). (C) Colony assays were performed to determine the frequency of committed progenitor cells (n = 4 for control and n = 5 for Vav-Cre Rb KO). (D) Number of cells per CFC colony to determine the proliferative potential of the CFCs (n = 4 for control and n = 5 for Vav-Cre Rb KO). (E) Number of colonies per 1 × 104 cells from primary colonies to determine the self-renewal potential of CFCs (n = 4 for control and n = 5 for Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM; *P < .05.
Stress hematopoiesis in spleen in Vav-Cre Rb KO animals results in an increased frequency of HSCs and HPCs in cell cycle and reduced function of HPCs. (A) Animals were pulse-labeled with BrdU in vivo, and the frequency of splenic HPCs (L−S−K+ cells) and splenic HSCs (L−S+K+) in the G0/G1, S, and G2/M phases of the cell division cycle analyzed by flow cytometry (n = 9 for both control and Vav-Cre Rb KO). (B) Total number of HPCs (L−S−K+) and HSCs (L−S+K+) in spleens of control and Vav-Cre Rb KO animals (n = 5 for control and n = 7 for Vav-Cre Rb KO). (C) Colony assays were performed to determine the frequency of committed progenitor cells (n = 4 for control and n = 5 for Vav-Cre Rb KO). (D) Number of cells per CFC colony to determine the proliferative potential of the CFCs (n = 4 for control and n = 5 for Vav-Cre Rb KO). (E) Number of colonies per 1 × 104 cells from primary colonies to determine the self-renewal potential of CFCs (n = 4 for control and n = 5 for Vav-Cre Rb KO). Shown are mean values plus or minus 1 SEM; *P < .05.
Discussion
Using a mouse model system in which the retinoblastoma tumor suppressor gene (Rb) is constitutively deleted in all hematopoietic cells, we determined the consequences of loss of Rb activity for hematopoiesis and the hematopoietic stem cell compartment. Although Vav1 is primarily expressed in hematopoietic cells, based on published reports and our own data (Figure S1), we cannot exclude though that the Rb allele is not deleted in nonhematopoietic cells.
Loss of Rb in Vav-Cre Rb KO animals resulted in a reduced frequency of erythrocytes and B cells, as well as an increased number of myeloid cells, with extreme extramedullary hematopoiesis in the spleen. However, this phenotype does not qualify as a myeloproliferative disorder (MPO), since the PB of Vav-Cre Rb KO animals did not present with an overall increase in the white blood cell count, even after months of follow-up. Surprisingly, we detected no change in T-cell numbers in Vav-Cre Rb KO animals, in contrast to the severe impact on B-cell generation. A common theme in Rb-deficient cells is that they are impaired in their terminal differentiation ability.34,35 Therefore, additional in-depth functional studies will be necessary to determine whether the myeloid cells in Vav-Cre Rb KO animals represent fully differentiated and thus functional cells.
In summary, the data presented here are at large consistent with data in the literature on the consequences of loss or impaired Rb function on mature hematopoietic cell lineages. Impaired erythroid differentiation in fetal liver cells derived from Rb−/− embryos has been reported by several laboratories.35,–37 Walkey et al reported impaired B-cell differentiation of adult hematopoietic cells deficient in Rb.17 Finally, Bergh et al demonstrated enhanced neutrophil lineage commitment in CD34+ HPCs in which RB levels were reduced by antisense Rb nucleotides.38 In agreement with data from Walkley et al,39 we also found that loss of Rb resulted in a higher frequency of HPCs in PB.
Our analyses also revealed that under steady-state conditions, even in the presence of anemia, loss of Rb does not affect the number and cycling parameters of HSCs and HPCs in BM, which is also consistent with sustained contribution to hematopoiesis of the competitively transplanted Vav-Cre Rb KO cells even after 27 weeks (Figure 4E). Based on the data from (1) the CAFC assay; (2) our transplantation, long-term reconstitution experiments, and secondary transplantations; and (3) the analysis of hematopoiesis in the spleen, we conclude that during stress hematopoiesis, loss of Rb severely impairs the self-renewal ability of HPCs and HSCs. The data obtained with the CAFC assay also further support the notion that the observed defect in Vav-Cre Rb KO cells in our analyses is at least partially stem cell intrinsic.
Whereas the data from the in vivo repopulation experiments as well as the cell-cycle analyses after 5-FU indicate that Rb plays a similar role in HSCs as well as HPCs, data from the CAFC assay seems to contradict this conclusion, as it indicates that HSCs are selectively affected by the loss of Rb. A coordinated loss of “cell potential” in primitive Rb−/− cells at any stage of their potential could in theory explain the pattern in the change in frequencies in the CAFC assay. Another explanation to reconcile these findings might be that depending on the type of stress, Rb might play distinct roles in HSCs and HPCs.
Published data on the role of loss of the retinoblastoma tumor suppressor gene in HSPCs are inconsistent, with the major controversy arising around whether the phenotypes associated with loss of Rb in the hematopoietic system are intrinsic or extrinsic to HSCs and HPCs. Our findings are in part in accordance with data published by Spike et al8 as well as Hu et al,40 which report that mice that receive transplants of Rb−/− fetal liver cells develop splenomegaly, indicating that this phenotype is intrinsic to Rb−/− hematopoietic cells. Spike et al further described impaired function of Rb−/− fetal HSCs upon transplantation, which is also supported by our analyses. In contrary to these and our data, however, Wakley et al identified that stem-cell mobilization, splenomegaly, and myeloproliferation as well as impaired HSC function are strictly dependent on both the deletion of Rb in the microenvironment as well as in the myeloid lineage, but are independent of the status of Rb in HSCs.39
These somewhat contradictory results might be explained by differences in the experimental approaches. (1) Genetically different Rb−/− or Rbflox/flox strains were used in these experiments, in addition to acute versus chronic deletion of Rb in hematopoietic cells. Thus, epistatic factors might influence the phenotype of Rb−/− hematopoietic cells. (2) HSCs and HPCs from different developmental stages (fetal versus adult) were analyzed, which would imply altered epigenetic imprinting of HSCs in the absence of Rb. (3) Our data supports that stress is an important factor to elicit some of the phenotypes associated with loss of Rb in HSCs and HPCs. Thus, distinct levels of stress inferred upon HSCs and HPCs in the individual experimental setups might result in differences in outcomes. (4) Vav1-Cre–driven deletion of Rb might result also in the deletion of Rb in nonhematopoietic cells in Vav-Cre Rb KO mice. It is thus a possibility that some of the phenotypes observed in our experiments might actually be a consequence of the deletion of Rb in nonhematopoietic cells. On the other hand, the phenotypes observed in Vav-Cre Rb KO animals do not fully recapitulate the phenotype reported for animals in which Rb was deleted in both the hematopoietic system as well as the microenvironment.39
Loss of Rb results in an impaired ability of retinal precursor cells, erythroid precursor cells, and postnatal auditory supporting cells to go through developmental stage–specific cell-cycle exit.8,34,41 This cell-cycle exit inability has been associated in each of these cases with impaired function of these cells. Our data further suggest that a similar mechanism might work in Rb−/− HPCs and HSCs when put under replicative stress. Although loss of Rb is not sufficient to cause elevated levels of cell-cycle entry in HSCs and HPCs (Figures 5 and 6), the elevated frequency of Rb-deficient HSCs and HPCs in cycle at 4 days after 5-FU treatment or in the spleen might be due to impaired cell-cycle exit. Impaired cell-cycle exit in response to stress or proliferative stimulation might thus be a unifying theme in primary cells lacking Rb, independent of their origin or differentiation status.
In contrast to the retina and auditory supporting cells, in the hematopoietic system, stress is necessary to elicit prolonged cell-cycle activity. This might imply that the extrinsic signals necessary for a correct cell-cycle exit provided by the niche in BM are independent of Rb function, whereas outside of the BM niche, like in the spleen or under stress, Rb is central for a correct cell-cycle exit. Another possibility might be that other pocket proteins, known to be able to differentially compensate for the loss of Rb,42 differentially compensate for Rb in BM, but not in the spleen and/or under stress.
What might be the molecular mechanisms linking loss of Rb, impaired cell-cycle regulation, and impaired stem-cell function? Rb interacts with members of the E2F family of transcription factors and mediates repression of E2F-regulated gene products.3 One possibility thus might be that genes that are normally repressed by E2Fs are expressed throughout the entire cell cycle, and that these gene products result in altered cell-cycle exit as well as in impaired stem-cell function. Rb also exerts its function, at least in part, through the recruitment of histone deacetylases, which are associated with gene repression, and histone and DNA methyltransferases.2 Another possibility is that the impaired function might be due insufficient epigenetic repression of specific genes in Rb−/− cells. Thus, an interesting hypothesis to test might be whether loss of Rb results in altered epigenetic marking of stem cells.
Under proliferative stress, Rb-deficient hematopoietic cells exhibit an increased number of cells in cycle, a hallmark of cancer. This would predict that treatments that result in a replicative burden on leukemic cells (which most chemotherapeutic regimens do) might actually, in contradiction to the intended effects, result in enhanced proliferative activity of Rb−/− cells and thus render such leukemias more difficult to treat. In good agreement with this hypothesis is the finding that multiple myelomas in which Rb is inacvtivated are associated with a poor prognosis.43 Thus, novel treatment regimens might be necessary for leukemias in which Rb or members of the Rb pathway are inactivated.
Rb−/− hematopoietic cells do not develop spontaneous leukemia, which was also observed by Hu et al.40 This indicates that deletion of Rb is only one out of probably multiple mutations/lesions necessary to cause leukemia. Based on our data, we predict that additional mutations that either establish self-renewal activity or further inhibit terminal differentiation in Rb−/− cells might be a cooperating partner in leukemogenesis in Rb−/− hematopoietic cells, a hypothesis open to further experimental analyses. In summary, our data indicate that Rb is critical for hematopoiesis and HSC function, especially under situations of stress.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Susanne Wells, Jose Cancelas, and David Williams for critical reading of the manuscript and Andrew Roberts for providing vav1Cre+ animals.
This work was supported by National Institutes of Health grant R01 HL076604 to H.G. and a grant from Cancer Free Kids to H.G.
National Institutes of Health
Authorship
Contribution: M.-D.F. and T.K. designed and performed research; E.S.K. contributed vital new reagents; R.F. and Z.L. performed research; and H.G. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hartmut Geiger, Division of Experimental Hematology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: hartmut.geiger@cchmc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal