Phase 1 testing of SGN-30, a chimeric monoclonal antibody for the treatment of CD30+ malignancies, was conducted in a multicenter study. To explore the safety profile and establish the maximum tolerated dose (MTD), 24 patients with refractory or relapsed Hodgkin lymphoma or CD30+ non-Hodgkin lymphoma received 6 weekly doses of intravenous SGN-30 at 4 dose levels (2, 4, 8, or 12 mg/kg). Serum concentrations of SGN-30 rose rapidly and were dose dependent. Adverse events were mild, with nausea, fatigue, and fever attributed to study treatment. One episode of hypersensitivity rash was reported. The MTD was not reached. Serious adverse events included herpes zoster (n = 2), influenza, and pneumonia. One patient with cutaneous anaplastic large cell lymphoma (8 mg/kg) achieved a complete response. Six patients, of whom 4 had Hodgkin lymphoma, achieved stable disease with durations ranging from 6 to 16 months. The pharmacokinetic profile of SGN-30 showed a biphasic disposition, and estimated half-lives ranging between 1 to 3 weeks. The 6 weekly infusions of SGN-30 resulted in approximately 2- to 3-fold accumulation in serum exposures consistently across the dose range. These results demonstrate that weekly administration of SGN-30 is safe and has modest clinical activity in patients with CD30+ tumors. This trial is registered at http://www.ClinicalTrials.gov as no. NCT00051597.
Introduction
Advances in the use of combination chemotherapy regimens have resulted in higher remission and cure rates for patients with lymphoma. Despite these advances, many patients with Hodgkin lymphoma (HL) or aggressive non-Hodgkin lymphomas (NHLs) will either fail to achieve an initial complete response (CR) or will subsequently relapse, requiring additional therapy.1 These patients are typically treated with salvage chemotherapy regimens followed by high-dose myeloablative chemotherapy with stem cell rescue, which may offer a 30% to 50% cure rate.2,3 Patients who fail to respond to salvage therapy or relapse after stem cell transplantation are rarely cured and will require novel treatment approaches.4,5 CD30, a member of the tumor necrosis factor receptor family, is expressed on a variety of malignant cells of hematopoietic origin, but is rarely found on nonmalignant cells, making it a good target for cancer therapy. In healthy individuals, CD30 is expressed only on a small number of activated B and T cells.6 CD30 plays an important role in some biologic functions, including regulation of cell growth and survival, and cytokine and chemokine secretion.7 Within hematopoietic malignancies, it is highly expressed by the malignant Reed-Sternberg (RS) cells of HL, and anaplastic large cell lymphoma (ALCL), but can also be expressed by immunoblastic B-cell lymphoma, cutaneous T-cell lymphoma, and multiple myeloma. In recent years, a variety of immunotherapies has been explored to target not only CD30+ tumors, but also a broader range of lymphomas.8,,,,,,–15 SGN-30 is a newly developed CD30-specific chimeric antibody constructed from the variable regions of the anti-CD30 murine monoclonal AC10 and the human gamma 1 heavy chain and kappa light chain constant regions. It binds to a number of CD30+ cancer cell lines and activated T cells but does not react with other human tissues.16,,,–20 Preclinical studies of SGN-30 have demonstrated antitumor activity in both in vitro and in vivo models. In vivo testing against HL xenograft models in severe combined immunodeficient mice produced significant increases in survival and a dose-dependent reduction in tumor mass.7 Using the combination effects method to quantify interactions of SGN-30 and individual cytotoxic agents it was found that significant synergies resulted in combination with doxorubicin, bleomycin, vinblastine, etoposide, and cytarabine.21 SGN-30 has also shown in vitro and in vivo activity against CD30-expressing ALCL cell lines and tumor xenograft models (unpublished data, Seattle Genetio, 2001) and Tian et al showed activity of the M44 and He Fi-1 antibodies (both targeting CD30) in similar in vitro and in vivo settings.7,22
SGN-30 was well tolerated at single and repeated doses (up to 100 mg/kg) in preclinical monkey models with no observed dose-limiting toxicity (DLT). Further, a single-dose phase 1 clinical study revealed no grade 3 nonhematologic or hematologic toxicities at doses up to 15 mg/kg.23 Here we report the results of a phase 1 multidose escalation study designed to identify the maximum tolerated dose (MTD) and proposed dose for phase 2 studies.
Methods
Patients
After informed consent was obtained in accordance with the Declaration of Helsinki, 24 patients with histologically confirmed CD30+ hematologic malignancies were entered in the trial and were treated at 8 centers in the United States between August 2002 and October 2003. This study was approved by the Institutional Review Boards of all participating institutions. (Washington University, M. D. Anderson Cancer Center, Norris Cancer Center, Cornell University, University of Rochester, University of Nebraska Medical Center). Eligible patients had refractory HL or anaplastic large cell lymphoma and had refused, were ineligible for, or had failed high-dose chemotherapy with stem cell rescue, or had other CD30+ malignancies that were beyond first remission or that were refractory to frontline chemotherapy. Patients were older than 18 years of age, had a life expectancy of more than 3 months, measurable disease, an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, and were a minimum of 4 weeks from prior chemotherapy or radiotherapy. Required laboratory values at study entry were as follows: absolute neutrophil count of 1 × 109/L (1000/μL) or higher, platelet count of 75×109/L (75 000/μL) or higher, serum bilirubin level of 1.5 × or less the upper limit of normal, serum creatinine of 1.5 × or less the upper limit of normal, and BUN of 1.5 × or less the upper limit of normal. Patients with symptomatic cardiac disease, multiple primary malignancies, active infection, allergy to recombinant human or mouse proteins, titers to previous monoclonal antibody treatments, symptomatic brain disease, and concurrent therapy with other antineoplastic agents, corticosteroids, or other experimental agents were excluded from the trial.
Study design
Cohorts of 6 patients were sequentially assigned to receive intravenous SGN-30 at 4 dose levels: 2, 4, 8, and 12 mg/kg administered weekly for 6 consecutive weeks, followed by a 4-week follow-up period during which patients were monitored for adverse effects. Dose escalation would occur following the completion of all doses by the 6 patients at each dose level and continued either until the MTD was identified or a dose of 12 mg/kg was reached. The decision to escalate dosing and the determination of the MTD were based upon the occurrence of a drug-related DLT, defined as follows: grade 3 or greater neutropenia or thrombocytopenia, grade 2 or greater febrile neutropenia, or grade 3 or greater nonhematologic toxicity (with the exception of hypersensitivity reactions). The MTD was defined as the dose level at which 2 of 6 patients experienced a DLT. No further escalation was to occur after defining the MTD, but a cohort of up to 6 additional patients was to be treated at that dose level to further elucidate DLTs.
Study drug was withheld in instances of grade 2 toxicity without recovery before the next scheduled dose. If the patient resumed treatment at the next scheduled time point, then the missed dose was added in week 7. If the toxicity persisted for an additional week (ie, 2 missed doses), the patient was removed from the study.
Drug formulation and administration
SGN-30 was supplied to the study sites by Seattle Genetics, as 100 mg (10 mg/mL) of sterile, preservative-free, monoclonal antibody in single-use vials. Patient doses were drawn and diluted to a final concentration of 1 to 5 mg/mL in an infusion bag containing either 0.9% sodium chloride USP, or 5% dextrose in water USP. Study drug was administered at an initial rate of 100 mg/h for the first 30 minutes, and patients were closely monitored for hypersensitivity or infusion reactions. If no adverse responses were noted, the remainder of the drug dose was infused over the next 90 minutes for a total infusion time of 2 hours. Prophylactic premedications were not prescribed, but diphenhydramine and acetaminophen were provided, as needed, to treat mild to moderate infusion reactions. Patient vital signs were monitored immediately prior to, and at 5, 15, and 30 minutes, as well as at completion of the infusion.
Assessments
Baseline assessments included the following: medical history and physical examination; chemistry, thyroid function, urine, and hematology analyses; serum soluble CD30 and tumor CD30 expression analyses; human antichimeric antibody (HACA) analysis; radiographic scans; and bone marrow biopsy.
Safety
Patients were monitored throughout the study by physical examination, laboratory analyses, and assessment of adverse events. Adverse events were graded according to the National Cancer Institute–Common Toxicity Criteria (NCI-CTC), Version 2 (NCI, Bethesda, MD). Analyses of CBC with differential and platelet counts were conducted weekly and blood chemistry, every 2 weeks. Physical examinations, thyroid testing, hematology, and serum chemistry were repeated at the end-of-treatment assessment (day 64).
Efficacy
Antitumor responses were measured using the Cotswold criteria for patients with HL and the International Workshop Standardized Response Criteria for patients with NHL.24,25 Appropriate radiologic assessments and other scans were also performed at baseline, day 64, and at other time points, as needed.
SGN-30 pharmacokinetics
Blood samples were collected at specific time intervals after infusion for pharmacokinetic assessment of SGN-30 in patients. After collection of samples, sera were isolated and stored at −70°C until assayed at a central laboratory (PPD Development, Richmond, VA). A validated enzyme-linked immunosorbent sandwich assay (ELISA) method was used to measure SGN-30 concentrations in serum. In brief, microtiter plates precoated with murine anti-idiotype antibody against SGN-30 were incubated with diluted serum samples, and bound SGN-30 was detected using a secondary horseradish peroxidase (HRP)–conjugated goat anti–human IgG–specific antibody. Standard and quality control samples of SGN-30 in normal human serum were included in each analytic run. The intra-assay and interassay precision and accuracy of the quality control samples in all the analytic runs were within plus or minus 10%. The lower limit of quantitation for the assay was 1.56 μg/mL.
Pharmacokinetic assessment was limited to estimation of maximum concentration (Cmax), area under the concentration versus time curve (AUCtau), and observed trough concentration (Cmind7) following first (cycle 1) and last (cycle 6) dose. The specified pharmacokinetic parameters were calculated by a validated noncompartmental method using WinNonLin version 4.0 program (Pharsight, Mountain View, CA). The relationships of derived pharmacokinetic parameters with duration of treatment were assessed by calculating accumulation indices for each parameter. An accumulation index is the ratio of value of a parameter between cycle 6 and cycle 1. In addition, the relationship of the pharmacokinetic parameters with dose was also explored.
Human antichimeric antibody (HACA) response
Serum samples were collected prior to each weekly dose of SGN-30 on days 1, 15, 29, and at 30 days after treatment (day 64). Sera were frozen at −20°C or below and shipped for analysis at the conclusion of testing for each dosing cohort. Human antichimeric antibody responses were measured by a validated ELISA assay developed at PPD Development. Briefly, microtiter plates, precoated with SGN-30, were incubated with dilutions of serum samples, and bound anti–SGN-30 antibodies were detected with a biotinylated SGN-30 conjugate. Streptavidin-conjugated HRP served as a colorimetric indicator. HACA titers were assessed for positive samples based on dilutions with signals above a cutpoint determined from negative control values.
Soluble antigen (sCD30) detection
Assays for serum soluble CD30 levels using a commercially purchased ELISA (Bender Medsystems, Burlingame, CA) were performed at baseline, day 36, and day 64. Briefly, microtiter plate wells coated with anti-sCD30 were incubated with dilutions of serum samples and bound sCD30 was detected with an anti–sCD30-HRP conjugate. Serum concentration of sCD30 was derived from values obtained by a standard curve measured at 450 nm.
Statistics
It was anticipated that up to 30 patients would be enrolled in the trial, with accrual completed within 12 months. Descriptive statistics were used in the analysis of all observations in this study.
Results
Patient characteristics
Twenty-four patients were enrolled in the study, 11 male and 13 female. Age ranged from 23 to 79 years (median: 38 years) and the majority of subjects were white (67%). HL was the most common primary diagnosis (87.5%). Only 3 patients (12.5%) had diagnoses of NHL. Most patients had been heavily pretreated, with the median number of prior treatment regimens being 5 (range: 2 to 10 regimens). Seventy-one percent of the patients received 5 or more systemic regimens, 83% had prior bone marrow or stem-cell transplantation, and 83% had undergone prior radiotherapy. Patients were assessed for either ECOG or Karnofsky performance scores. The median ECOG was 0, and the median Karnofsky was 90. The elapsed time since initial diagnosis ranged from 2 to 13 years (median: 3 years; Table 1).
Patient demographics
| Characteristic . | Dose . | ||||
|---|---|---|---|---|---|
| 2 mg/kg, n = 6 . | 4 mg/kg, n = 6 . | 8 mg/kg, n = 6 . | 12 mg/kg, n = 6 . | Total, n = 24 . | |
| Mean age, y | 34.5 | 40.8 | 40.0 | 41.2 | 39.1 |
| Sex, M/F | 4/2 | 4/2 | 2/4 | 1/5 | 11/13 |
| Primary diagnosis | |||||
| HD | 5 | 5 | 5 | 6 | 21 |
| NHL | 1 | 1 | 1 | 0 | 3 |
| Mean interval since diagnosis, y | 3.4 | 6.8 | 5.7 | 3.9 | 5.0 |
| Prior surgery | 0 | 3 | 2 | 1 | 6 |
| Prior radiotherapy | 4 | 5 | 6 | 5 | 20 |
| Prior transplantation | 6 | 5 | 5 | 4 | 20 |
| Prior chemotherapy regimens | |||||
| 2 to 4 | 2 | 1 | 1 | 3 | 7 |
| 5 to 7 | 3 | 3 | 2 | 3 | 11 |
| 8 to 10 | 1 | 2 | 3 | 0 | 6 |
| Characteristic . | Dose . | ||||
|---|---|---|---|---|---|
| 2 mg/kg, n = 6 . | 4 mg/kg, n = 6 . | 8 mg/kg, n = 6 . | 12 mg/kg, n = 6 . | Total, n = 24 . | |
| Mean age, y | 34.5 | 40.8 | 40.0 | 41.2 | 39.1 |
| Sex, M/F | 4/2 | 4/2 | 2/4 | 1/5 | 11/13 |
| Primary diagnosis | |||||
| HD | 5 | 5 | 5 | 6 | 21 |
| NHL | 1 | 1 | 1 | 0 | 3 |
| Mean interval since diagnosis, y | 3.4 | 6.8 | 5.7 | 3.9 | 5.0 |
| Prior surgery | 0 | 3 | 2 | 1 | 6 |
| Prior radiotherapy | 4 | 5 | 6 | 5 | 20 |
| Prior transplantation | 6 | 5 | 5 | 4 | 20 |
| Prior chemotherapy regimens | |||||
| 2 to 4 | 2 | 1 | 1 | 3 | 7 |
| 5 to 7 | 3 | 3 | 2 | 3 | 11 |
| 8 to 10 | 1 | 2 | 3 | 0 | 6 |
Drug delivery
Of the 24 patients enrolled, 22 patients received all 6 doses of study drug. Two patients terminated the study early as a result of disease progression, one of whom received only 4 doses of SGN-30. Nineteen patients received their weekly SGN-30 on time. Three patients had dose delays including one patient with herpes zoster, one patient with chest pain, and one patient with a false laboratory reading that was normal on redraw. One patient had temporary interruption of SGN-30 infusion due to flushing. The infusion was stopped and restarted after symptoms resolved without any additional problems. All patients received full doses at their assigned dosing level. Total exposure to SGN-30 ranged from 127 mg at the lowest dose level to 1356 mg in the highest dose group.
Toxicity
Treatment was well tolerated with no treatment-related deaths. Only 4 grade 3/4 events were observed in 4 patients: anemia (8 mg/kg), pneumonia (8 mg/kg), fatigue (12 mg/kg), and herpes zoster (12 mg/kg). Grade 3 anemia in a patient was the only grade 3/4 event thought to be possibly related to treatment and did not meet the protocol definition of DLT. The patient entered the study with a hemoglobin level of 1.83 mmol/L (11.8 g/dL) and 1 week after the sixth infusion it had dropped to 1.22 mmol/L (7.9 g/dL). White count and platelet count were normal. The patient had previously been treated with 4 chemotherapy regimens and a stem-cell transplantation. Transfusion was given and treatment with erythropoetin alfa was commenced.
There was 1 death during the study and another 2 patients experienced a total of 4 nonfatal serious adverse events. One patient treated at 8 mg/kg died 19 days after the last dose of SGN-30 due to radiologically documented disease progression and severe bacterial or fungal chest infection. The serious adverse events were considered unlikely to be related to SGN-30. The first of the 2 patients (treated at 4 mg/kg SGN-30) developed pneumonia, influenza, and herpes zoster. Following treatment with antibiotics and antivirals, these infections resolved completely. The second patient treated at 12 mg/kg was hospitalized with herpes zoster after 2 doses of SGN-30. She was treated with acyclovir and was able to complete her remaining 4 doses of SGN-30.
Twenty-three (96%) of 24 patients experienced adverse events during the study with an approximately equal distribution among the dosage levels tested. The majority of these events were mild or moderate in severity with only 4 patients (17%) experiencing grade 3/4 events. Five patients (21%) had grade 1 events and 14 (58%) had grade 2 events. In only 11 patients (46%) was the causality thought to be possibly, probably, or definitely related to SGN-30. Events that were most commonly associated with SGN-30 were nausea, fatigue, and fever (Table 2), and the majority of these events were categorized as mild. One episode of hypersensitivity (rash) was noted during infusion (2 mg/kg) at day 15.
Number of patients experiencing adverse events classified as related to treatment with SGN-30, by dose level
| Adverse event . | Dose . | ||||
|---|---|---|---|---|---|
| 2 mg/kg, n = 6 . | 4 mg/kg, n = 6 . | 8 mg/kg, n = 6 . | 12 mg/kg, n = 6 . | Total (%), n = 24 . | |
| Nausea | 2 | 0 | 1 | 0 | 3 (13) |
| Fatigue | 1 | 1 | 0 | 1 | 3 (13) |
| Pyrexia | 2 | 0 | 1 | 0 | 3 (13) |
| Anorexia | 1 | 0 | 1 | 0 | 2 (8) |
| Myalgia | 1 | 0 | 1 | 0 | 2 (8) |
| Headache | 1 | 1 | 0 | 0 | 2 (8) |
| Pruritis | 0 | 1 | 0 | 1 | 2 (8) |
| Anemia | 0 | 0 | 1 | 0 | 1 (4) |
| Diarrhea | 1 | 0 | 0 | 0 | 1 (4) |
| Fecal abnormality | 1 | 0 | 0 | 0 | 1 (4) |
| Oral pain | 0 | 0 | 0 | 1 | 1 (4) |
| Infusion site warmth | 0 | 0 | 1 | 0 | 1 (4) |
| Hepatic pain | 0 | 1 | 0 | 0 | 1 (4) |
| Herpes zoster | 0 | 0 | 0 | 1 | 1 (4) |
| Dizziness | 1 | 0 | 0 | 0 | 1 (4) |
| Dysgeusia | 0 | 1 | 0 | 0 | 1 (4) |
| Pharyngitis | 1 | 0 | 0 | 0 | 1 (4) |
| Rash | 1 | 0 | 0 | 0 | 1 (4) |
| Sweating | 0 | 0 | 1 | 0 | 1 (4) |
| Hot flushes | 0 | 0 | 0 | 1 | 1 (4) |
| Adverse event . | Dose . | ||||
|---|---|---|---|---|---|
| 2 mg/kg, n = 6 . | 4 mg/kg, n = 6 . | 8 mg/kg, n = 6 . | 12 mg/kg, n = 6 . | Total (%), n = 24 . | |
| Nausea | 2 | 0 | 1 | 0 | 3 (13) |
| Fatigue | 1 | 1 | 0 | 1 | 3 (13) |
| Pyrexia | 2 | 0 | 1 | 0 | 3 (13) |
| Anorexia | 1 | 0 | 1 | 0 | 2 (8) |
| Myalgia | 1 | 0 | 1 | 0 | 2 (8) |
| Headache | 1 | 1 | 0 | 0 | 2 (8) |
| Pruritis | 0 | 1 | 0 | 1 | 2 (8) |
| Anemia | 0 | 0 | 1 | 0 | 1 (4) |
| Diarrhea | 1 | 0 | 0 | 0 | 1 (4) |
| Fecal abnormality | 1 | 0 | 0 | 0 | 1 (4) |
| Oral pain | 0 | 0 | 0 | 1 | 1 (4) |
| Infusion site warmth | 0 | 0 | 1 | 0 | 1 (4) |
| Hepatic pain | 0 | 1 | 0 | 0 | 1 (4) |
| Herpes zoster | 0 | 0 | 0 | 1 | 1 (4) |
| Dizziness | 1 | 0 | 0 | 0 | 1 (4) |
| Dysgeusia | 0 | 1 | 0 | 0 | 1 (4) |
| Pharyngitis | 1 | 0 | 0 | 0 | 1 (4) |
| Rash | 1 | 0 | 0 | 0 | 1 (4) |
| Sweating | 0 | 0 | 1 | 0 | 1 (4) |
| Hot flushes | 0 | 0 | 0 | 1 | 1 (4) |
White blood cell counts, platelet counts, creatinine levels, total bilirubin levels, and liver enzymes remained constant throughout the course of treatment. Peripheral lymphocyte analysis was performed on CD3, CD3/4, CD3/8, CD16, and CD19 subsets, and while the majority of patients had low baseline counts, most likely as a result of prior therapy, no significant drug-induced decline in any subset was noted. Baseline hemoglobin levels were low (grade 2 or 3) in 6 patients, and each of these patients required transfusions during the study as did an additional 3 patients who entered with normal and grade 1 hemoglobin levels.
Testing for thyroid function was performed because a tissue cross-reactivity study showed binding of the murine antibody to thyroid epithelial cells of cynomolgus monkeys. The results revealed a great deal of variation that was commonly attributed to euthyroid sick syndrome. Only 5 patients had normal T4, T3, and TSH at baseline. Two patients entered the study on replacement thyroid hormones for presumed hypothyroidism. Eleven patients, including the 2 on thyroid hormone replacement, demonstrated a decrease in free T4 after therapy with minimal changes in TSH. In only one patient treated at 12 mg/kg was there an increase in TSH at end of study, although T3 remained unchanged from baseline.
Decline in performance status was reported in 7 patients and no improvements were observed after therapy. None of these declines was assessed by the treating physician as treatment related. Vital signs were monitored before, during, and after each infusion. While drops in systolic blood pressure of up to 20 mmHg were noted during the infusion in 6 patients, recovery by the end of the infusion to baseline was noted and supportive measures were not needed.
SGN-30 pharmacokinetics
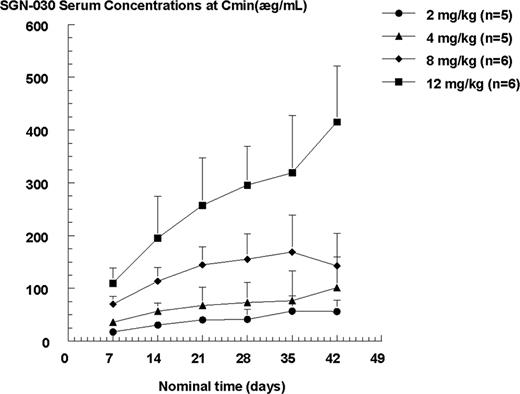
Dose-related increases in pharmacokinetic parameters, namely Cmax, AUCtau, and Cmind7, were observed within the dose range (2–12 mg/kg) studied. The summary statistics of the pharmacokinetic parameters for SGN-30 are shown in Table 3. The SGN-30 exposures (Cmax, AUCtau, and Cmind7) generally increased proportionally to the dose increment after both first and last infusion. Based on the observed trough concentrations, SGN-30 did not appear to reach steady state after 6 weekly infusions. Figure 1 illustrates the mean Cmin concentrations versus time profiles for each dose level. There was a consistent (2- to 3-fold) accumulation in SGN-30 exposures observed at each dose level following 6 weekly infusions compared with the first infusion.
Summary statistics of SGN-30 pharmacokinetic parameters following first and last infusion in patients with refractory or recurrent CD30+ hematologic malignancies
| Dose group, mg/kg . | Cmax, μg/mL . | AUCtau, μg*d/mL . | Cmind7, μg/mL . | AIAUC* . | AICmax* . | AICmin* . |
|---|---|---|---|---|---|---|
| Cycle 1 | ||||||
| 2, n = 6 | 46.1 (31) | 185.9 (17) | 16.91 (21) | NA | NA | NA |
| 4, n = 6 | 89.1 (19) | 366.1 (22) | 33.97 (20) | NA | NA | NA |
| 8, n = 5 | 181.5 (16) | 705.5 (21) | 68.61 (24) | NA | NA | NA |
| 12, n = 4 | 265.0 (28) | 1123 (18) | 107.1 (21) | NA | NA | NA |
| Cycle 6 | ||||||
| 2, n = 4 | 100.5 (26) | 525.7 (29) | 57.58 (36) | 2.6 (34) | 2.0 (32) | 3.0 (41) |
| 4, n = 4 | 200.2 (25) | 991.6 (36) | 115.2 (38) | 2.6 (31) | 2.2 (17) | 3.2 (27) |
| 8, n = 4 | 265.2 (13) | 1324 (32) | 130.9 (43) | 2.0 (49) | 1.5 (17) | 2.0 (57) |
| 12, n = 4 | 592.3 (27) | 3381 (25) | 397.9 (31) | 2.7 (19) | 2.0 (8) | 3.5 (29) |
| Dose group, mg/kg . | Cmax, μg/mL . | AUCtau, μg*d/mL . | Cmind7, μg/mL . | AIAUC* . | AICmax* . | AICmin* . |
|---|---|---|---|---|---|---|
| Cycle 1 | ||||||
| 2, n = 6 | 46.1 (31) | 185.9 (17) | 16.91 (21) | NA | NA | NA |
| 4, n = 6 | 89.1 (19) | 366.1 (22) | 33.97 (20) | NA | NA | NA |
| 8, n = 5 | 181.5 (16) | 705.5 (21) | 68.61 (24) | NA | NA | NA |
| 12, n = 4 | 265.0 (28) | 1123 (18) | 107.1 (21) | NA | NA | NA |
| Cycle 6 | ||||||
| 2, n = 4 | 100.5 (26) | 525.7 (29) | 57.58 (36) | 2.6 (34) | 2.0 (32) | 3.0 (41) |
| 4, n = 4 | 200.2 (25) | 991.6 (36) | 115.2 (38) | 2.6 (31) | 2.2 (17) | 3.2 (27) |
| 8, n = 4 | 265.2 (13) | 1324 (32) | 130.9 (43) | 2.0 (49) | 1.5 (17) | 2.0 (57) |
| 12, n = 4 | 592.3 (27) | 3381 (25) | 397.9 (31) | 2.7 (19) | 2.0 (8) | 3.5 (29) |
Pharmacokinetic parameter data are summarized as geometric means (%CV).
NA indicates not applicable. AI indicates accumulation index, calculated as ratio of respective parameter value between cycle 6 and cycle 1.
Mean SGN-30 serum concentrations at trough (Cmind7) versus time profiles for each cycle. The data are illustrated as arithmetic mean + SD.
Mean SGN-30 serum concentrations at trough (Cmind7) versus time profiles for each cycle. The data are illustrated as arithmetic mean + SD.
Since the blood samples for pharmacokinetics were collected only for 4 weeks after the last dose, accurate estimates of terminal half-life could not be obtained. However, using the available data, the terminal half-life estimates ranged between 1 to 3 weeks. The pharmacokinetic parameters such as steady-state volume of distribution (Vss), clearance (CL), and mean residence time (MRT) that rely on the accurate estimation of the first order terminal rate constant were not reported.
Tumor response
One patient (8 mg/kg) achieved a CR. This 69-year-old male with a history of cutaneous ALCL was previously treated with acitretin, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, prednisone), and oral methotrexate. He had lesions on the arm, thigh, and back, the largest of which measured 2.4 × 1.7 cm. He achieved a CR by week 5 of treatment and remained in remission for 26 months after a single 6-week course. Confirmatory biopsies of residual hyperpigmented macules 2 months after completion of treatment showed no evidence of lymphoma. At the time of recurrence, he received 6 additional doses of SGN-30 on a compassionate use protocol and again achieved a CR, which lasted 22 months. He achieved a third CR with 6 additional doses of SGN-30, again on a compassionate use protocol, which is ongoing at 3 months. Both recurrences involved skin only. There were no partial responses (PRs). Six patients achieved stable disease (SD), 4 of the 6 had HL. One patient treated at 2 mg/kg had a greater than 40% decrease in disease volume that was maintained for 16 months, 2 patients treated at 8 mg/kg had durations of SD of 7+ and 8 months, and the fourth patient treated at 12 mg/kg underwent a transplantation after a stable disease duration of 6 months and subsequently achieved a complete remission. Seventeen patients demonstrated disease progression while on study.
Tumor measurements were recorded at baseline and at day 64. More patients assigned to the 2-mg/kg dosing level developed new lesions at the day-64 assessment than did patients in the other dosing levels. New lesions appeared in 4 patients in the 2-mg/kg dosing group and in 1 patient each in the 4-, 8-, and 12-mg/kg dosing levels.
Antibody response
Few HACA titers were detected. Four patients developed human antichimeric antibodies while on study with higher titers in patients receiving lower doses of SGN-30 (2 mg/kg and 4 mg/kg; Table 4). In the patient treated at 2 mg/kg, the titer was persistent at the end of treatment evaluation and follow-up samples to determine the subsequent kinetics were not obtained. One additional patient began treatment with a transient low HACA titer of 1:20. This patient provided no medical history of previous treatment with monoclonal antibodies, and assays that followed SGN-30 administration were negative. Three of the 4 subjects who developed HACA responses to SGN-30 displayed lower than expected serum concentrations of SGN-30 at various points throughout the study.
HACA titers
| . | Dose . | |||
|---|---|---|---|---|
| 2 mg/kg, n = 6 . | 4 mg/kg, n = 6 . | 8 mg/kg, n = 6 . | 12 mg/kg, n = 6 . | |
| HACA responders | 1 | 2 | 1 | 1 |
| Baseline | neg | neg; neg | neg | 1:20 |
| Before cycle 2 | ND | 1:100; neg | neg | neg |
| Before cycle 4 | 1:100 | 1:100; 1:20 | neg | neg |
| Day 64 | 1:500 | 1:20; neg | 1:20 | ND |
| . | Dose . | |||
|---|---|---|---|---|
| 2 mg/kg, n = 6 . | 4 mg/kg, n = 6 . | 8 mg/kg, n = 6 . | 12 mg/kg, n = 6 . | |
| HACA responders | 1 | 2 | 1 | 1 |
| Baseline | neg | neg; neg | neg | 1:20 |
| Before cycle 2 | ND | 1:100; neg | neg | neg |
| Before cycle 4 | 1:100 | 1:100; 1:20 | neg | neg |
| Day 64 | 1:500 | 1:20; neg | 1:20 | ND |
ND indicates not done.
Soluble CD30 detection
Assays were conducted to measure sCD30 levels in the serum of patients at baseline, day 36, and day 64. Twenty patients provided either all 3 samples or baseline and one other sample. Median baseline value in these 20 patients was 104 (range: 39–659) U/mL. Median posttreatment value was 572 (range: 92–2960) U/mL. One patient at the 8-mg/kg dose who achieved a complete response, had soluble CD30 levels of 91 U/mL at baseline and 92 U/mL at the end of treatment. All other patients, regardless of whether they achieved stable disease or progressive disease, experienced significant rises between baseline and day 64. Median sCD30 levels on day 36 were 2- to 4-fold (range: 0.8- to 6.9-fold) higher than baseline and on day 64 were 3- to 6-fold (range: 1- to 8.9-fold) higher than baseline. No sCD30 levels were drawn after day 64. The increases in sCD30 were not dose related and did not correlate with SGN-30 serum concentrations.
Discussion
In this phase 1 study, adverse events were common, but were primarily mild or moderate in intensity; severe events were few and did not define an MTD. As a result, all 4 dose levels of study drug were explored and no patients were withdrawn from the study due to adverse events. With the exception of thyroid function, laboratory parameters, vital signs, and findings at physical examination remained generally stable throughout the study. Six patients (25%) did experience drops in blood pressure of between 10 and 20 mmHg during the course of the infusion, but the majority had recovered to baseline by the end of the infusion and were without clinically significant sequelae. The most common thyroid function abnormality detected after treatment was a decrease in free T4, but lack of significant changes in TSH did not support a diagnosis of hypothyroidism. It is most likely that these patients were affected by the euthyroid sick syndrome. The most common adverse events that were felt to be related to SGN-30 administration were nausea, fatigue, and anorexia occurring in 13% of patients. These events were generally mild to moderate in severity and responded to standard symptomatic treatments. They are similar to infusion reactions noted during treatments with other monoclonal antibodies (eg, alemtuzumab and rituximab).
Since CD30 is expressed on activated lymphocytes, lymphocyte subset analysis was performed. No evidence of lymphocyte depletion was seen. Functional assays were not performed. However no clinical data suggested that these already immunocompromised patients had worsening of their immune function. Two cases of herpes zoster were observed in patients with advanced HL, but were felt to be related to advanced and progressive disease rather than SGN-30.
SGN-30 appeared to exhibit linear pharmacokinetics within the dose range (2–12 mg/kg), as evident by the dose proportional increases in exposures after 6 weekly infusions. The estimated half-life values were similar to published values for a monoclonal IgG antibody.26 Based on observed decline in the serum concentrations after the dose, SGN-30 appeared to have a biphasic disposition. The observed accumulation in serum SGN-30 exposures with once weekly dosing is believed to be contributed by longer (1–3 weeks) estimated half-life of the antibody. The long half-life of the drug raises the possibility that the dosing frequency could be decreased from once weekly to once every 3 or 4 weeks, while still maintaining what are believed to be therapeutic serum concentrations. In fact, preliminary results from a phase 2 study with SGN-30 in patients with cutaneous ALCL where the drug is dosed once per month suggest that this is a viable dosing schedule.27 If SGN-30 is to be combined with chemotherapy, less frequent dosing may also be advantageous to integrate with chemotherapy scheduling.
Soluble CD30 levels generally regarded as a prognostic factor and as a surrogate marker for disease activity were found to rise with treatment regardless of whether the patient had progressive or stable disease.28 The mechanism(s) responsible for the rise in soluble CD30 after treatment with SGN-30 are unknown, but could reflect SGN-30–soluble CD30 complexes that persist in circulation longer than uncomplexed soluble CD30. Future studies will be needed to elucidate the process by which sCD30 levels are increased in the blood. Until this issue is resolved, it will not be possible to use soluble CD30 as a marker of activity of SGN-30. Only baseline measurements in the absence of SGN-30 can be used with any confidence as a prognostic marker. Schnell et al in a phase 1 study with an anti-CD30 ricin A-chain immunotoxin in patients with refractory CD30+ lymphoma found that the immunotoxin bound to soluble CD30 and suggested that prolonged persistence of complexes in the blood might be a factor contributing to toxicity.29
SGN-30 is a chimeric monoclonal and hence has the potential to generate a HACA response. The data from this study do not suggest that this response is significant. However, the patient population studied is not fully immunocompetent and may not be able to mount a full immune response against the drug, and the HACA assay, which is a bridging ELISA, may be interfered with in the presence of high concentrations of SGN-30. There may also be interference with the PK ELISA for SGN-30 when HACAs are present. There was a suggestion that the HACA response was more marked at the lower doses of SGN-30, but this observation might be the result of assay interference by higher concentrations of SGN-30.
Treatment responses were assessed using published guidelines.24,25 One CR was attained by day 36 in a patient who was enrolled with cutaneous NHL. The patient maintained a remission for 26 months and was retreated at the time of relapse and again attained CR. Stable disease was attained by 6 (25%) patients and 17 (71%) progressed. Four of the 21 patients with HL obtained stable disease as their best response. All 4 had decreases in tumor volume ranging from 5% to 40% and the durations of stable disease ranged from 6+ to 16 months. All patients had undergone prior stem-cell transplantation, and the number of prior treatment regimens ranged from 4 to 10. All patients entered the study with progressive disease, so even small decreases in disease volume provide an indication of the biologic activity of the drug.
SGN-30, in addition to inducing apoptosis in tumor cell lines via signaling mechanisms, also has the potential to kill tumor cells by antibody-dependent cellular phagocytosis (ADCP) immune effector function (unpublished data).7,21 This mechanism of action has been well demonstrated for anti-CD20 immunotherapies.30 Because all patients on study had been heavily pretreated, it is possible that the resultant immunosuppression could have compromised the ADCP activity of the drug. The lack of objective responses may also relate to the fact that in HL, the CD30-expressing malignant RS cells targeted by SGN-30 constitute only a small proportion of the tumor bulk, most of which is an inflammatory infiltrate. The relationship between the RS cells and the surrounding infiltrate is complex. On the one hand, the RS cells secrete a number of chemokines (particularly TARC and MDC) that attract inflammatory cells to the tumor.31 On the other hand, the cellular infiltrate seems to play an important part in the maintained survival of RS cells by perpetuating an environment supporting growth and survival of RS cells.32 The infiltrate does not express CD30 and will not be a target for SGN-30. Thus any beneficial effects of the antibody in directly targeting the RS cells may be in part counteracted by the survival signals emanating from the surrounding infiltrate. Combining a chemotherapeutic agent with SGN-30 could address both cellular populations. In vitro data showing marked synergy with a number of chemotherapy agents support testing this approach.21,33 The lack of significant hematologic and biochemical toxicities with SGN-30 make it ideal to combine with currently used chemotherapy regimens such as ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine).
Disease stabilization for prolonged periods with SGN-30 in some patients with refractory HL may have clinical significance. The protocol did not allow more than 6 doses of SGN-30, and it is possible that prolonged treatment might have improved the objective response rate and/or prolonged the time to disease progression. The data also provide a rationale for phase 2 studies using the drug as monotherapy with consideration of a more prolonged course of therapy or in combination with chemotherapy.
Preliminary results of a multicenter phase 2 study of SGN-30 in refractory or recurrent HL revealed no responders in 15 patients treated with 6 weekly 6-mg/kg doses.34 Data are not yet available for the cohort of HL patients receiving the higher 12-mg/kg dose. Another CD30-directed monoclonal antibody, MDX-060, a fully human anti-CD30 antibody, has also been tested in relapsed HL. In a phase 1/2 study, 63 patients with HL were treated at doses between 0.1- and 15-mg/kg weekly for 4 weeks.35 Four patients responded, including 2 CRs and 2 PRs. All of the responders were heavily pretreated and responses did not appear to be dose related. An additional 10 patients had stable disease for at least 6 months, and 5 patients had stable disease for at least 12 months following treatment with MDX-060.
The second major malignancy expressing CD30 is ALCL, which is characterized by sheets of large pleomorphic tumor cells with homogeneous expression of CD30.36 From the perspective of a targeted therapy, this tumor represents a very attractive prospect, and preliminary clinical results with both SGN-30 and MDX-060 confirm this with objective responses seen in phase 2 trials in both systemic and cutaneous variants of the disease.27,34,37 Twenty-nine patients with systemic ALCL received at least 6 weekly doses of SGN-30, 24 at 6 mg/kg per week and 15 at 12 mg/kg per week.37 Five patients with stable disease or objective response received 2 or more cycles. Objective responses were seen in 8 patients (21%) including 2 CRs and 6 PRs. Patients on this study had received a median of 3 prior therapies and 85% were ALK-1 negative. Two of 7 patients with ALCL treated with MDX-030 responded.35
In absence of a discernable MTD, dose-dependent toxicity, or dose-dependent response, a weekly dose of 6 mg/kg was initially investigated in phase 2 studies of SGN-30. Based on PK modeling, 6 mg/kg was considered to be the lowest dose that achieved sufficiently high peak concentrations (≥ 120 μg/mL) after each dose and maintained trough values (20 μg/mL) that were associated with efficacy in mouse xenograft models. Lack of response in the first 15 patients treated at this dose resulted in treating additional patients at 12 mg/kg per week.
In summary, this phase 1 study confirmed the safety and tolerability of SGN-30. Preliminary results of phase 2 studies of SGN-30 and MDX-060 confirm modest efficacy of anti-CD30 monoclonal antibodies in HL and ALCL. Ongoing studies include investigation of antibody drug conjugates (ADCs) and combinations of SGN-30 and chemotherapy. A novel synthetic antimitotic agent, monomethyl auristatin E, has been conjugated to SGN-30 and has achieved complete tumor regressions in mice bearing L540cy HL xenografts at doses as low as 2 mg/kg every 4 days for 4 total doses.38 Phase 1 studies of this antibody-drug conjugate in CD30+ malignancies are under way. In addition, based on preclinical synergy studies, phase 1 and 2 studies of SGN-30 in combination with chemotherapy are being conducted in collaboration with the NCI-CTEP for HL and systemic ALCL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors would like to thank Tracy Wrey for her editorial assistance.
Authorship
Contribution: N.L.B. and A.Y. performed research, analyzed data, and wrote the paper; M.H.C., A.F., J.D.R., J.P.L., S.H.B., and R.G.B. performed research and edited the paper; J.M.L. analyzed data; B.W.H. and J.B. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.M.L., B.W.H., and J.B. are employees of Seattle Genetics. All other authors declare no competing financial interests.
Correspondence: Nancy L. Bartlett, Siteman Cancer Center, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8056, St Louis, MO 63110; e-mail: nbartlet@im.wustl.edu.