Between February 1996 and December 2004, the German Leukemia Study Initiative registered 1766 consecutive patients for the acute myeloid leukemia (AML) 96 study, all of whom were diagnosed by central cytomorphology according to the French-American-British (FAB) and the new World Health Organization (WHO) classification. We focused our analysis on the prognostic impact of multilineage dysplasia (MLD) as a new parameter of the WHO classification for AML. We could not confirm the WHO statement that MLD occurs most frequently in older individuals, but we confirmed that MLD is often associated with an unfavorable cytogenetic profile (P < .001). In 1332 individuals receiving intensive AML therapy presence of MLD was negatively correlated with complete remission (P = .001) in univariate, but not in multivariate, analysis. Multivariate analysis of either event-free or overall survival again failed to show an independent prognostic significance of MLD besides age, cytogenetics, and, in part, NPM1/FLT3-ITD mutations. Our data support a reassessment of the WHO classification in the light of a more biologic understanding of AML. This study is registered at www.ClinicalTrials.gov as #NCT00180115.
Introduction
Morphology supported by cytochemistry has remained the cornerstone of diagnosis in acute myeloid leukemia (AML), which is still based on the first widely accepted classification introduced by French-American-British (FAB) hematologists.1,,,–5
For more than 30 years the clinical significance of morphological dysplastic (dys) features of hematopoietic cell lineages in AML has been examined with different and contradictory results.6,,,,,,–13,15,–17 Most studies have been performed in de novo AML.
In 1999 the World Health Organization (WHO) introduced a new classification for AML.18 The WHO classification was an important step forward on the path from morphology to a more biologic-orientated classification incorporating cytogenetics in the new system.19,20 This classification, however, defined the new morphologic entity “acute myeloid leukemia with multilineage dysplasia” (AML with MLD) occurring either de novo or following myelodysplastic syndrome (MDS) or a myelodysplastic/myeloproliferative disorder.
Our study started in 1996 and addressed the issue whether morphologic dysplasia (in 1, 2, or 3 cell lineages) in the pretreatment examination of peripheral blood and bone marrow smears has clinical relevance concerning remission rate and survival.
Besides the prospective evaluation of morphologic dysplasia, we analyzed cytogenetics that have been identified as the most important independent prognostic factor during the last 10 years.21,,,–25
Recently, FLT3-ITD and NPM1 mutations have been identified by our group and others as prognostically relevant molecular markers.26,,–29 For the first time we correlated MLD in our study with these molecular markers.
We also examined the WHO statement that “AML with multilineage dysplasia occurs most frequently in older individuals, is often associated with an unfavorable cytogenetic profile … and unfavorable response to therapy.”19 Our study is a prospective and comprehensive evaluation of MLD. Our large group of de novo and secondary AML patients was treated homogeneously and reflects the reality in AML, since many patients older than 60 years (median, 68 years) were incorporated in this study.
Methods
Patients
One thousand seven hundred sixty-six consecutive patients with AML (1387 with de novo AML, 307 with preceding MDS including RAEB-t, and 72 with therapy-related AML) were included and prospectively examined for dysplasia and cytogenetics. Only patients with a centrally performed complete morphologic diagnosis were included.
All patients eligible for intensive chemotherapy were treated according to the AML-96 multicenter protocol. A list of the participating study centers is given in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Patients were eligible if they had no prior chemotherapy for AML or MDS. Patient characteristics are listed in Table 1. We included 1766 patients for the analysis of dysplasia and cytogenetics. Finally, 1332 patients were eligible for intensive chemotherapy. Patients with promyelocytic leukemia (FAB M3/M3v) were not included and mostly treated within the European APL 93 study. Patients up to 60 years and patients over 60 years were treated according to different protocols. A detailed description of the study has been published previously.31 In patients up to 60 years, first induction therapy consisted of triple therapy with 10 mg/m2 mitoxantrone (days 4–8), 100 mg/m2 cytosine arabinoside (ara-C) (days 1–8), and 100 mg/m2 VP16 (days 4–8). Second induction consisted of 100 mg/m2 amsacrine (m-AMSA-days 1–5) and 2 × 1000 mg/m2 araC every 12 hours days 1 to 5 (MAMAC [m-AMSA and median dose ara-C]). In this protocol, postinduction was stratified according to cytogenetic risk groups for patients 60 years of age and younger. Patients could receive an allogeneic hematopoietic stem cell transplantation (HSCT), including the option of unrelated HSCT in the high-risk group.
Patient characteristics of the 2 groups
| . | All patients included . | Eligible patients for therapy . |
|---|---|---|
| All patients (n) | 1766 | 1332 |
| Median age (y, range | 60 (15–87) | 59 (15–87) |
| Male/female (n, %) | 916/850 (51.9/48.1) | 681/651 (51.1/48.9) |
| de novo AML (n, %) | 1387 (78.5) | 1045 (78.5) |
| Secondary AML (n, %) | 379 (21.5) | 287 (21.5) |
| History of MDS/RAEB-t (n, %) | 307 | 237 |
| t-AML (n) | 72 | 50 |
| dys 0 + 1 (n, %) | 1244 (70.4) | 923 (69.3) |
| MLD (dys 2+3) (n, %) | 522 (29.6) | 409 (30.7) |
| Cytogenetics (n, %) | 1618 (91.6) | 1253 (94.0) |
| Cytogenetic risk group* (n, %) | ||
| Favorable | 177 (10.0) | 96 (7.2) |
| Intermediate | 1224 (69.3) | 967 (72.6) |
| Unfavorable | 365 (20.7) | 269 (20.2) |
| FLT3-ITD pos/neg (n, %) | —– | 283 (22.9)/954 (77.1) |
| NPM1 mutant/wild type (n, %) | —– | 351 (29.0)/858 (71.0) |
| . | All patients included . | Eligible patients for therapy . |
|---|---|---|
| All patients (n) | 1766 | 1332 |
| Median age (y, range | 60 (15–87) | 59 (15–87) |
| Male/female (n, %) | 916/850 (51.9/48.1) | 681/651 (51.1/48.9) |
| de novo AML (n, %) | 1387 (78.5) | 1045 (78.5) |
| Secondary AML (n, %) | 379 (21.5) | 287 (21.5) |
| History of MDS/RAEB-t (n, %) | 307 | 237 |
| t-AML (n) | 72 | 50 |
| dys 0 + 1 (n, %) | 1244 (70.4) | 923 (69.3) |
| MLD (dys 2+3) (n, %) | 522 (29.6) | 409 (30.7) |
| Cytogenetics (n, %) | 1618 (91.6) | 1253 (94.0) |
| Cytogenetic risk group* (n, %) | ||
| Favorable | 177 (10.0) | 96 (7.2) |
| Intermediate | 1224 (69.3) | 967 (72.6) |
| Unfavorable | 365 (20.7) | 269 (20.2) |
| FLT3-ITD pos/neg (n, %) | —– | 283 (22.9)/954 (77.1) |
| NPM1 mutant/wild type (n, %) | —– | 351 (29.0)/858 (71.0) |
Patients older than 60 years received a different therapy: 2 induction cycles containing 45 mg/m2 daunorubicin (days 3–5) and 100 mg/m2 ara-C (days 1–7) were followed by postremission therapy consisting of MAMAC.
This study was approved by the ethics board of the Technical University Dresden. Informed consent was obtained in accordance with the Declaration of Helsinki.
Cytomorphology
Morphologic diagnosis was performed centrally based on light microscopic evaluation of Pappenheim-stained slides of peripheral blood and bone marrow smears. Cytochemistry was performed by myeloperoxidase reaction and nonspecific esterase using alphanaphtylacetate-esterase. Morphologic examination was performed independently by 2 hematologists with major experience in morphology. All bone marrow smears were reviewed for dysplastic features based on the proposed criteria by Goasguen et al7 and the WHO.19 The evaluation of dysplasia was documented according to WHO proposal as no MLD (dys 0 + 1) or as MLD (dys 2 + 3).
Cytogenetics and NPM1/FLT3-ITD mutations
Cytogenetic analysis was standardized and performed in the reference laboratories of the German Leukemia Study Initiative (DSIL). Cytogenetic data were classified according to the International System of Human Cytogenetic Nomenclature.32 Cytogenetic subgroups for the analysis were classified according to the Cancer and Leukemia Group B (CALGB).23
Definition of response
Statistics
Using the Kaplan-Meier method, event-free survival (EFS) and overall survival (OS) were calculated for all patients entering the AML 96 protocol. For comparison we used the log-rank test. For multivariate analysis of prognostic factors we used a Cox proportional hazard regression model. Stepwise forward selection was performed. Missing values were substituted by the median. Variables were added at a P value below .01 and deleted at a P value above .05.
Comparisons between different groups were made using 2-sided Fisher exact test. All calculations were performed using the SPSS software package, version 14.0 (SPSS, Chicago, IL).
Results
Patient groups
Pretreatment factors were investigated in all 1766 patients. Intensive chemotherapy was received by 1332 of 1766 patients (Table 1). Four hundred thirty-four (24.6%) patients were excluded from survival analysis due to the following reasons: FAB M3/M3v (16%), comorbidity and poor performance status (64%), prior AML-like therapy (4%), patient decision, and other causes (16%).
During consolidation therapy 383 (28%) patients received an autologous (12%) or allogeneic (16%) HSCT. We evaluated all transplant patients within the whole treatment group because the transplant option is a part of AML treatment today. Results obtained from survival analyses were not different whether we included or excluded transplant patients (data not shown).
Pretherapeutic characteristics and dysplasia in 1766 patients
Secondary AML showed as expected a significantly higher frequency of MLD compared with de novo AML (P < .001). When we compared the frequency of MLD between patients 60 years of age or younger and older patients, we could not find a significant difference (Table 2).
Frequencies of MLD in 1766 patients correlated with age, cytogenetic risk groups, NPM1/FLT3-ITD mutations, and history of AML
| . | All patients (n = 1766) MLD (%) . | P . |
|---|---|---|
| Age (≤ 60 vs > 60 years) | 28.7 vs 30.4 | NS (.4) |
| Cytogenetic risk group (favorable/intermediate/unfavorable) | 13.0/28.3/41.6 | < .001 |
| FLT3-ITD* (negative vs positive) | 34.1 vs 24.0 | < .001 |
| NPM1† (wild type vs mutation) | 31.5 vs 30.2 | NS (.7) |
| History of AML (de novo vs secondary) | 25.2 vs 45.4 | < .001 |
| . | All patients (n = 1766) MLD (%) . | P . |
|---|---|---|
| Age (≤ 60 vs > 60 years) | 28.7 vs 30.4 | NS (.4) |
| Cytogenetic risk group (favorable/intermediate/unfavorable) | 13.0/28.3/41.6 | < .001 |
| FLT3-ITD* (negative vs positive) | 34.1 vs 24.0 | < .001 |
| NPM1† (wild type vs mutation) | 31.5 vs 30.2 | NS (.7) |
| History of AML (de novo vs secondary) | 25.2 vs 45.4 | < .001 |
MLD indicates multilineage dysplasia according to WHO classification; NS, not significant.
Data available in 1237 patients.
Data available in 1209 patients.
Cytogenetic risk groups from favorable to intermediate and to unfavorable revealed a significantly increasing incidence of MLD (Table 2).
Overall, percentage of MLD in our study is well correlated with other studies (Table 3).
Frequencies of multi- or trilineage dysplasia in AML as published in large studies in comparison with our data
| Author . | No. of patients . | MLD (%) . | TLD (%) . | History of AML . |
|---|---|---|---|---|
| Brito-Bapapulle et al6 | 160 | — | 15 | de novo |
| Guasgen et al7 | 336 | — | 12 | de novo |
| Kariyama et al12 | 230 | — | 17 | de novo |
| Ballen et al15 | 106 | — | 9 | All types |
| Kariyama et al13 | 545 | — | 17 | de novo |
| Haferlach et al16 | 609 | 25 | 15 | de novo |
| Arber et al14 | 300 | 38 | — | All types |
| Miyazaki et al17 | 559 | — | 28 | de novo |
| Our study | 1766 | 30 | 9 | All types |
| Author . | No. of patients . | MLD (%) . | TLD (%) . | History of AML . |
|---|---|---|---|---|
| Brito-Bapapulle et al6 | 160 | — | 15 | de novo |
| Guasgen et al7 | 336 | — | 12 | de novo |
| Kariyama et al12 | 230 | — | 17 | de novo |
| Ballen et al15 | 106 | — | 9 | All types |
| Kariyama et al13 | 545 | — | 17 | de novo |
| Haferlach et al16 | 609 | 25 | 15 | de novo |
| Arber et al14 | 300 | 38 | — | All types |
| Miyazaki et al17 | 559 | — | 28 | de novo |
| Our study | 1766 | 30 | 9 | All types |
MLD (multilineage dysplasia) indicates dysplastic features in 2 or 3 lineages according to WHO classification; TLD (trilineage dysplasia) dysplastic features in 3 lineages; —, no data.
Dysplasia and NPM1/FLT3-ITD mutations
These mutations were analyzed retrospectively in all patients eligible for intensive chemotherapy and from whom we had enough cell material. FLT3-ITD analysis was performed in 93% and NPM1 analysis in 91% of patients (Table 1). Interestingly, MLD frequency was significantly higher in FLT3-ITD–negative compared with -positive patients, while MLD was equally distributed between patients with or without NPM1 mutation (Table 2).
We received comparable results calculating frequencies only for the subgroup of patients with normal karyotype where those mutations are prevalent.
Response to induction therapy in 1332 patients
Univariate analysis.
Complete remission was achieved in 67.6% of patients 60 years of age or younger and in 33.7% of patients older than 60 (P < .001).
Patients with de novo AML had a significantly better CR rate of 56.6%, compared with 35.5% for secondary AML (P < .001). This difference also was seen in the subgroup of patients older than 60 years (P = .001).
AML with MLD revealed a worse CR rate of 45.2% compared with 55.0% for patients with dys 0 + 1 (P = .001).
CR rates within the 3 cytogenetic risk groups differed significantly from each other: favorable, 82.3%; intermediate, 53.9%; and unfavorable, 34.6% (P < .001).
Multivariate analysis.
In multivariate analysis, age, history of AML, and cytogenetics remained independently predictive for CR, but MLD was discarded (P = .12; Table 4).
Multivariate analysis of clinical, morphologic, cytogenetic, and molecular variables
| . | All patients (n = 1332) . | Patients no older than 60 (n = 720) . | ||||||
|---|---|---|---|---|---|---|---|---|
| CR rate . | EFS . | OS . | ||||||
| P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | |
| Age ≤/> 37 | ||||||||
| Age (≤ 60 vs > 60) | < .001 | 0.27 (0.21–0.34) | < .001 | 1.80 (1.57–2.07) | < .001 | 1.97 (1.66–2.18) | < .001 | 1.68 (1.32–2.15) |
| Cytogenetic risk group | ||||||||
| Intermediate vs favorable | < .001 | 2.84 (1.62–4.97) | < .001 | 0.43 (0.31–0.60) | < .001 | 0.51 (0.36–0.70) | <.001 | 0.49 (0.33–0.73) |
| Intermediate vs unfavorable | < .001 | 0.46 (0.34–0.62) | < .001 | 1.66 (1.40–1.97) | < .001 | 1.71 (1.46–2.01) | < .001 | 1.55 (1.21–1.97) |
| NPM1-mut/FLT3-ITDneg | — | — | .003 | 0.73 (0.60–0.90) | NS | — | NS | — |
| NPM1-mut/FLT3-ITDpos | — | — | NS | — | NS | — | NS | — |
| NPM1-wt/FLT3-ITDpos | — | — | NS | — | NS | — | NS | — |
| History of AML (de novo vs secondary) | < .001 | 0.57 (0.42–0.76) | NS | — | NS | — | NS | — |
| Dysplasia (MLD vs dys 0+1) | NS | — | NS | — | NS | — | NS | — |
| . | All patients (n = 1332) . | Patients no older than 60 (n = 720) . | ||||||
|---|---|---|---|---|---|---|---|---|
| CR rate . | EFS . | OS . | ||||||
| P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | |
| Age ≤/> 37 | ||||||||
| Age (≤ 60 vs > 60) | < .001 | 0.27 (0.21–0.34) | < .001 | 1.80 (1.57–2.07) | < .001 | 1.97 (1.66–2.18) | < .001 | 1.68 (1.32–2.15) |
| Cytogenetic risk group | ||||||||
| Intermediate vs favorable | < .001 | 2.84 (1.62–4.97) | < .001 | 0.43 (0.31–0.60) | < .001 | 0.51 (0.36–0.70) | <.001 | 0.49 (0.33–0.73) |
| Intermediate vs unfavorable | < .001 | 0.46 (0.34–0.62) | < .001 | 1.66 (1.40–1.97) | < .001 | 1.71 (1.46–2.01) | < .001 | 1.55 (1.21–1.97) |
| NPM1-mut/FLT3-ITDneg | — | — | .003 | 0.73 (0.60–0.90) | NS | — | NS | — |
| NPM1-mut/FLT3-ITDpos | — | — | NS | — | NS | — | NS | — |
| NPM1-wt/FLT3-ITDpos | — | — | NS | — | NS | — | NS | — |
| History of AML (de novo vs secondary) | < .001 | 0.57 (0.42–0.76) | NS | — | NS | — | NS | — |
| Dysplasia (MLD vs dys 0+1) | NS | — | NS | — | NS | — | NS | — |
NPM1-wt/FLT3-ITDneg was set as the reference group.
dys 0+1 indicates dysplasia in 0 or 1 lineage; MLD, dysplasia in 2 or 3 lineages according to WHO classification19 ; NPM1-FLT3 group; mut, mutant; wt, wild type; NS, not significant; and —, no data.
Survival analysis in 1332 patients
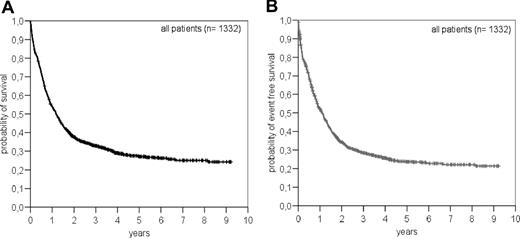
For all patients we have an OS of 24.3% and an EFS of 21.4% at 9 years (Figure 1). The median follow-up for patients alive was 4.2 years (range, 0.7–9.2).
Univariate analysis
OS and EFS differed significantly with respect to different age groups (P < .001), the defined cytogenetic risk profile (P < .001), and whether AML developed de novo or secondary (P < .001; figures not shown).
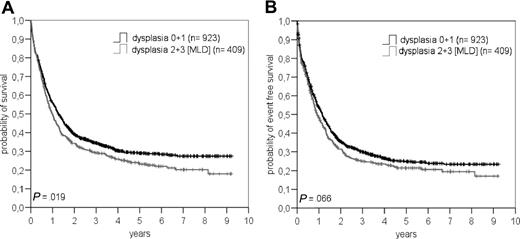
Concerning dysplasia as a single parameter, we saw also a significant difference in OS between patients with or without MLD (P = .019) but not in EFS (P = .07; Figure 2).
Overall survival (A) and event-free survival (B) in patients with or without multilineage dysplasia (MLD) according to WHO classification.
Overall survival (A) and event-free survival (B) in patients with or without multilineage dysplasia (MLD) according to WHO classification.
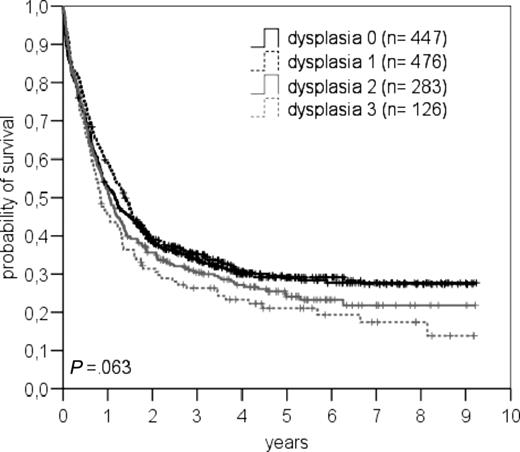
OS of patients with different numbers of dysplastic features (dys 0, 1, 2, 3) is shown in Figure 3.
Overall survival in patients with dysplastic features in 0, 1, 2, or 3 lineages.
Overall survival in patients with dysplastic features in 0, 1, 2, or 3 lineages.
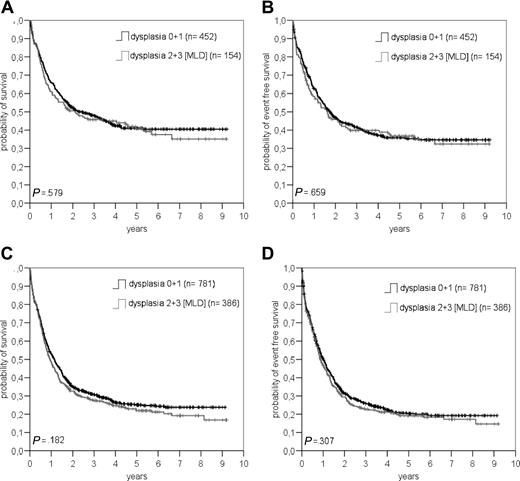
We also performed a subgroup analysis of patients no more than 60 years of age and de novo AML (n = 606) with or without MLD. Here the curves for OS and EFS (Figure 4A,B) were superimposable.
Comparision between patients with or without multilineage dysplasia (MLD) concerning overall survival (A+C) and eventfree survival (B+D) in patients ≤ 60 years with de novo AML (A+B) and in patients without t(8;21), inv(16), t(16;16), t(15;17) and (11q23) abnormalities according to the WHO classification (C+D).
Comparision between patients with or without multilineage dysplasia (MLD) concerning overall survival (A+C) and eventfree survival (B+D) in patients ≤ 60 years with de novo AML (A+B) and in patients without t(8;21), inv(16), t(16;16), t(15;17) and (11q23) abnormalities according to the WHO classification (C+D).
For further calculations, we followed the WHO classification and excluded all patients with t(8;21), inv (16), t(16/16), or 11q23 abnormalities (patients with t(15;17) were excluded a priori). When comparing MLD with non-MLD patients, no statistical difference was found for EFS and OS (P = .31 and .18, respectively; Figure 4C,D).
Finally, we performed an additional investigation of the subgroup of patients with normal karyotype (n = 673) because we wanted to know whether MLD (n = 203) can separate a group of patients with a worse prognosis within this largest cytogenetically not further defined group. However, we did not find a difference for EFS (P = .58) or OS (P = .37) (figures not shown).
Multivariate analysis
Since our group has recently shown in a retrospective investigation of our patients that NPM1 and FLT3-ITD mutations have a significant impact on survival as published by Thiede et al,28 we additionally included these data in our multivariate analysis besides age, cytogenetics, and history of AML.
As proven by our data (Table 4), MLD and history of AML did not remain independently significant factors. Age (≤ / > 60) and cytogenetic profile remained independent prognostic factors for EFS and OS, while the combination of NPM1 mutant/FLT3-ITD negative was an independent factor for EFS (Table 4).
When we focused our multivariate analysis of OS on patients no more than 60 years of age with an additional age separation at the median of younger patients of no more than/more than 37 years of age, still age and cytogenetics remained significant.
Discussion
This is a large study performed in AML patients focusing on prognostic relevance of myeloid, erythroid, and megakaryocytic dysplasia. It is a comprehensive evaluation of “AML with MLD” according to the WHO proposal, including an adequate number of younger and older patients as well as patients with de novo and secondary AML and performing a multivariate analysis of all major prognostic factors. In addition, we included the molecular markers NPM1/FLT3-ITD for the first time.
In the large cohort of 1766 consecutive patients we could clearly show that frequency of MLD was not significantly different (P = .43) between patients over the age of 60 (30.4%) and under the age of 60 (28.7%; Table 2). We therefore cannot confirm the statement of the WHO that MLD occurs most frequently in older individuals.19 Our study is repre-sentative of AML since the median age was 60 (15–87) years, and we included de novo as well as secondary AML patients. Miyazaki et al17 reported recently comparable frequencies of trilineage dysplasia in younger and older age groups of de novo AML patients.
We could confirm that MLD is significantly correlated with secondary AML (P < .001) and unfavorable cytogenetics (P < .001) as stated by the WHO. This is in parallel with other studies.8,9,16,17
For the first time we could show that MLD was surprisingly prevalent in FLT3-ITD–negative patients and equally distributed between patients with or without NPM1 mutation (Table 2). The assumed negative prognostic impact of MLD was not seen in conjunction with these molecular markers whether we looked to all patients or only to patients with normal karyotype where those markers are prevalent. On the contrary, FLT3-ITD mutation, which is correlated with worse prognosis, shows less often MLD features, while NPM1 mutation, which is correlated with good prognosis, shows the same frequency of MLD as NPM1 wild type.
The CR rate in our study for patients 60 years of age or younger (68%) and for the elderly (34%) was in the expected range.23,35,–37 CR rates were significantly worse in patients of older age, with secondary AML, with unfavorable cytogenetics (all P < .001), and also in patients with MLD (P = .001). In contrast, Haferlach et al16 could not find such a prognostic relevance in a younger group of patients with de novo AML. This might be explained by patient selection and the very intensive double-induction chemotherapy, including high dose ara-C (6 g/m2 per day × 3) and mitoxantrone used in this trial, which probably abrogated the negative MLD effect on CR rate seen in our and other studies.6,7,12,17
Despite the prognostic significance of MLD in predicting CR in univariate calculation, we were unable to show the same effect in multivariate analysis (Table 4).
The most important result obtained from our study is that MLD as defined by the WHO classification has no independent prognostic relevance for EFS and OS (Table 4).
In univariate analysis, already, OS differed between patients with or without MLD, solely, when we looked at the whole group of patients (Figure 2). This difference, however, was not seen anymore when we separately analyzed patients no more than 60 years of age with de novo AML, or the group of all patients without t(8;21), t(15;17), inv (16), t(16;16), or 11q23 abnormalities according to the WHO classification (Figure 4).
In the subgroup of patients with normal karyotype we found absolutely no difference between patients with or without MLD concerning EFS as well as OS (figures not shown).
Multivariate survival analysis performed in our study revealed cytogenetic risk profile as the most powerful prognostic factor besides age and, in part, the molecular markers NPM1/FLT3 (Table 4). History of AML lost its significance in multivariate analysis due to the fact that unfavorable cytogenetics were significantly correlated with secondary AML.
We are convinced that our results are solid and transferable to other AML populations worldwide because the treatment performed in the study can be regarded as standard therapy comparable to other studies.37,,–40 Major results of our multivariate analysis are in line with published data by Haferlach et al,16 but we could extend the conclusions to secondary AML and an older AML population. In addition, we showed new data concerning relation of molecular markers and MLD. Miyazaki et al17 and Arber et al14 published data on dysplasia in AML that are in part similar to our results, but they did not perform multivariate analyses of the major prognostic factors. Differences between our results and other publications are due to small patient groups, patient selection, or limited availability of cytogenetic data.6,,–9,12,14
Cytogenetics and age are unequivocally considered as major predictors of therapeutic outcome, however, prognosis cannot be considered independent of therapy. Striking examples of the impact of therapy on the prognosis of cytogenetic subgroups were given by the introduction of all-trans retinoic acid into the treatment of FAB M3 or the use of high-dose ara-C, particularly in AML with t(8;21) or inv (16), and t(16;16).24,41,42
The WHO classification was introduced in 1999 as a flexible system that will be adopted to new developments. The WHO opened the door of AML classification for cytogenetically defined entities for the first time. During the past 10 years we experienced a dramatic progress in cytogenetic and molecular understanding of AML, and therefore we should move forward to a more cytogenetically and biologically oriented classification of AML. In conclusion our data on morphologic dysplasia, particularly AML with MLD as defined by the WHO, clearly demonstrate that this category should no longer be accepted as a general and independent criterion for the classification of AML and for definition of prognostic subgroups. A subgroup classification of AML solely on the category of MLD may be misleading concerning therapeutic decisions and prognosis.
Morphology in AML is still very important for diagnosis as a rapid and inexpensive tool, but decisions toward a biologically directed therapy and prognosis should be based, besides clinical features of the patient, on cytogenetics and probably on gene expression and molecular markers in the near future.29,43
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Torsten Haferlach for reading the manuscript and for his comments. Thanks to the members of the DSIL group for participating in the AML 96 study; a complete list of participants can be found in Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article).
This work was supported in part by a grant from the Deutsche Krebshilfe (70-2210-Eh5).
Authorship
Author contribution: H.W. designed and performed research, analyzed data, and wrote the paper; U.S. designed and performed research and analyzed data; F.K. designed and performed research; G.P.-K. performed research; B.M. performed research; C.T. performed research and analyzed data; U.P. performed research; S.S. analyzed data and performed statistical analyses; M.S. designed research; G.E. designed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hannes Wandt, Medical Clinic 5, Hematology/Oncology, Klinikum Nürnberg Nord, Prof-Ernst-Nathan-Str 1, D-90419 Nürnberg, Germany; e-mail: wandt@klinikum-nuernberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal