An international collaboration was set up to prospectively evaluate the role of allogeneic transplantation for adults with acute lymphoblastic leukemia (ALL) and compare autologous transplantation with standard chemotherapy. Patients received 2 phases of induction and, if in remission, were assigned to allogeneic transplantation if they had a compatible sibling donor. Other patients were randomized to chemotherapy for 2.5 years versus an autologous transplantation. A donor versus no-donor analysis showed that Philadelphia chromosome–negative patients with a donor had a 5-year improved overall survival (OS), 53% versus 45% (P = .01), and the relapse rate was significantly lower (P ≤ .001). The survival difference was significant in standard-risk patients, but not in high-risk patients with a high nonrelapse mortality rate in the high-risk donor group. Patients randomized to chemotherapy had a higher 5-year OS (46%) than those randomized to autologous transplantation (37%; P = .03). Matched related allogeneic transplantations for ALL in first complete remission provide the most potent antileukemic therapy and considerable survival benefit for standard-risk patients. However, the transplantation-related mortality for high-risk older patients was unacceptably high and abrogated the reduction in relapse risk. There is no evidence that a single autologous transplantation can replace consolidation/maintenance in any risk group. This study is registered at http://clinicaltrials.gov as NCT00002514.
Introduction
Treatment of acute lymphoblastic leukemia (ALL) has improved enormously in the last 40 years particularly in children in whom a long-term survival of 80% is now achieved.1,,–4 Adults have fared much worse; the survival in the best series may not even reach 35% to 40% for those younger than 60 years and less than 10% for those older than 60 years.5,,,–9
For adults, 2 separate approaches have been constructed, transplantation-based studies10,,,,–15 and attempts to optimize chemotherapy, reserving transplantation only for patients with the Philadelphia chromosome.14
The putative factors defining individual patients as “high risk” in adult ALL are numerous and include age, particularly older than 35 years,3,7,9,10,16,17 elevated white blood cell count (WBC), which may well differ in relation to a “high-risk” threshold between B- and T-cell ALL,7,11 cytogenetics,18,,,–22 and the presence or absence of minimal residual disease (MRD) at particular points in the early phases of treatment.23,24 Molecular complete remission (CR) rates currently achieved may be as low as 60%.25
The low incidence of the adult disease has meant that to study adequate numbers of patients, collaboration by large cooperative groups is required.
In 1993 collaboration from the UK Medical Research Council (MRC) and the Eastern Cooperative Oncology Group (ECOG) of the United States developed a large study to ask 2 fundamental questions regarding this disease.
(1)Given that the graft versus leukemia effect (GVL) was first described in adult ALL26 and given published data supporting allogeneic transplantation in first complete remission (CRI) for Ph+ and other high-risk patients,14 could the allogeneic effect improve the outcome for all suitable adult patients?
(2)Given the fact that protracted consolidation/maintenance therapy has been the mainstay of treatment for ALL, based on data mostly extrapolated from pediatric experience, could a single autologous transplantation be at least as effective?
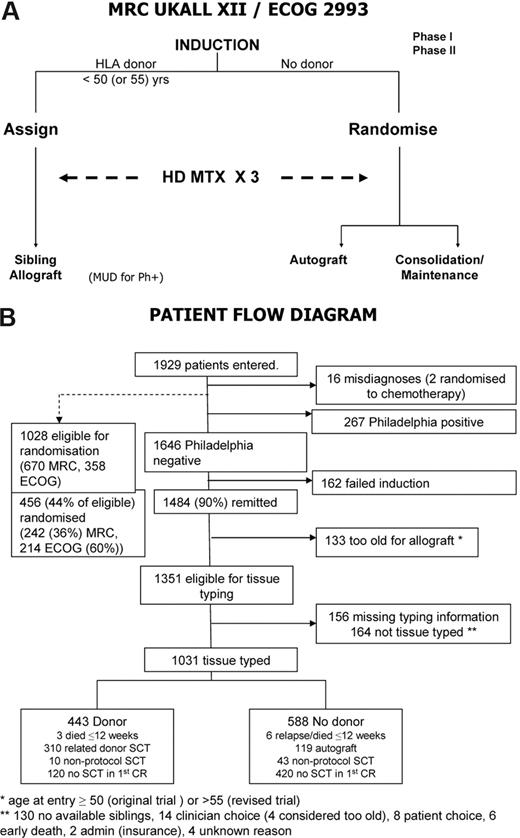
The design of the study was that all adult patients with a matched sibling donor would receive an allogeneic transplant, while those without would be randomized to an autologous transplantation versus consolidation maintenance/chemotherapy. The study design has been previously described in detail,27 and the simplified outline is shown in Figure 1A.
Methods
Statistics
The target recruitment was based on 550 patients randomized, giving 95% power to detect an absolute difference of 15% in event-free survival (EFS) between autologous transplantation and chemotherapy. The randomization rate was lower than anticipated, and in 2006 the coordinators asked for disclosure of the results to plan the next trial. As the recruitment period had already been extended, the Steering Committee, in ignorance of the results, agreed to close the trial.
All patients were centrally registered at the Clinical Trial Service Unit in Oxford, for MRC patients, or at the ECOG operations office for ECOG patients. Randomization between autograft and chemotherapy was done at these 2 centers, with minimization (MRC) used to balance over sex, age (< 20, 20–29, 40–49, 50 + years), WBC count (< 10 × 109/L, 10–49 × 109/L, ≥50 × 109/L), and Ph status, or balanced blocks (ECOG) within strata by age (< 50, 50+ years), time to remission (< 4 weeks, > 4 weeks), and Ph status.
The primary outcome measure was overall survival (OS). Other outcomes analyzed were (1) EFS, defined as the time to relapse or death, (2) relapse rate, and (3) nonrelapse mortality, defined as time to death censored at relapse. Kaplan-Meier curves were used and all treatment comparisons were by intention to treat. Comparisons between autologous transplantation and chemotherapy were made by the log-rank method. All event times were measured from the diagnosis, or from randomization for the randomized comparison of autologous transplantation and chemotherapy.
The comparison between those with versus those without a matched related donor was used as an unbiased assessment of the effect of matched related donor transplantation. This comparison included only patients younger than 50 or 55 years, commensurate with the upper age limit for related donor transplantation. Differences between the Kaplan-Meier curves at 5 years were tested with a chi-square test. All P values are 2-sided. Subgroup analysis has been performed for risk groups based on reports from other trials and interim prognostic factor analyses from this trial.27
Patients
The trial recruited patients between 1993 and 2006. All patients from 15 to 59 years of age with newly diagnosed ALL, including Ph positive, received identical induction therapy, irrespective of risk assessment, including central nervous system (CNS) prophylaxis and treatment of CNS disease, if present at diagnosis. In 2003, the upper age limit of the study was raised to 64 years and for an allogeneic transplantation was raised to 54 from 49 years. All patients who had an HLA-matched sibling donor were assigned to receive an allogeneic transplant. Patients with the Philadelphia chromosome could also receive a matched unrelated donor transplant. Those who did not have an HLA-matched sibling donor, or were older than 50 years or (later) 55 years, were randomized to receive a single autologous transplant or consolidation/maintenance therapy. Prior to receiving the assigned or randomized therapy, all patients received intensification with high-dose methotrexate. In this study, patients older than 35 years or those with a high WBC count at presentation (≥ 100 × 109/L for B lineage and ≥ 30 × 109/L for T lineage) along with all patients with the Philadelphia chromosome were deemed to be high risk. All the others were classified as standard risk. Time to remission was not an independent risk factor in this study.27 The study was approved by the relevant Institutional Review Boards of each center (Document S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article) and informed consent was obtained in accordance with the Declaration of Helsinki. The precise details of the study regimen have been previously reported.27
Results
A total of 1929 patients were registered to this study of whom 16 were excluded as misdiagnoses (Figure 1B). The median follow-up is 4 years 11 months (range, 1 month to 13 years 11 months).
Outline of study and treatment demographics. (A) Simplified overall schema of the study. MUD indicates matched unrelated donor transplantation; HD MTX, high-dose methotrexate. (B) Patient flow diagram. All randomized patients with the exception of 2 misdiagnoses were included in the comparison of autologous transplantation and chemotherapy. A large number of patients were lost, as in all transplantation studies, in the period from initial accrual to randomization. Only Ph-negative patients were eligible for the intent-to-treat donor versus no-donor analysis as Ph-positive patients without a donor were to have unrelated donor transplantation if possible.
Outline of study and treatment demographics. (A) Simplified overall schema of the study. MUD indicates matched unrelated donor transplantation; HD MTX, high-dose methotrexate. (B) Patient flow diagram. All randomized patients with the exception of 2 misdiagnoses were included in the comparison of autologous transplantation and chemotherapy. A large number of patients were lost, as in all transplantation studies, in the period from initial accrual to randomization. Only Ph-negative patients were eligible for the intent-to-treat donor versus no-donor analysis as Ph-positive patients without a donor were to have unrelated donor transplantation if possible.
Overall survival
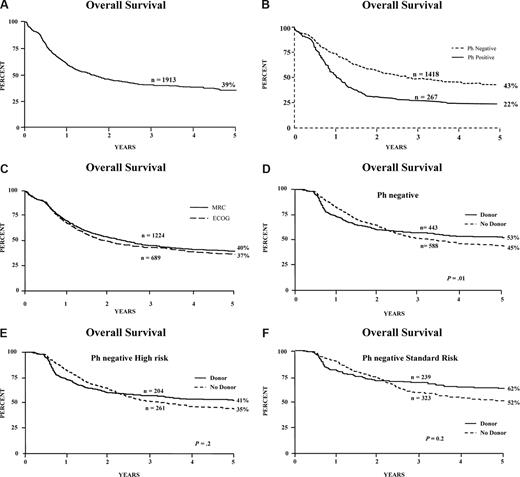
The OS of all 1913 patients at 5 years was 39% (Figure 2A); it was 43% for patients who were Ph negative (Figure 2B), and there was no difference in survival between patients entered through MRC or ECOG (Figure 2C). Details of the Ph-positive patients have been presented28 and are being published separately.
Survival of patients and donor versus no-donor analysis. Overall survival from diagnosis for (A) all patients entered on the study, including Ph-positive; (B) Ph-negative and Ph-positive patients; (C) patients entered via MRC or ECOG; (D) donor versus no-donor for all Ph-negative patients; (E) donor versus no-donor for Ph-negative patients with high risk; and (F) donor versus no-donor for Ph-negative patients with standard risk.
Survival of patients and donor versus no-donor analysis. Overall survival from diagnosis for (A) all patients entered on the study, including Ph-positive; (B) Ph-negative and Ph-positive patients; (C) patients entered via MRC or ECOG; (D) donor versus no-donor for all Ph-negative patients; (E) donor versus no-donor for Ph-negative patients with high risk; and (F) donor versus no-donor for Ph-negative patients with standard risk.
Donor versus no-donor analysis for Philadelphia chromosome–negative patients
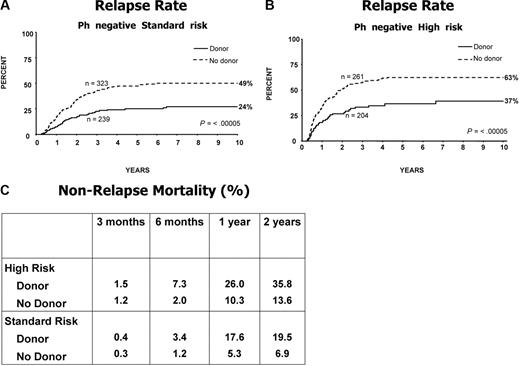
Figure 1B is a flowchart of patients in the study. Of the 1646 Ph-negative patients, there were 1351 patients who were younger than 50 years or younger than 55 years and achieved a complete remission on protocol. In 1031 patients, HLA-typing information was available. Figure 2D demonstrates the overall survival benefit when all patients on study are included in this analysis with a 5-year survival of 53% (95% CI = 48%-58%) for patients with a donor versus 45% (95% CI = 40%-49%) for patients without a donor (P = .01). Patients at high risk had an OS of 41% versus 35% for donor versus no donor, respectively, which was not significantly different (P = .2; Figure 2E); however, the OS was significantly improved among patients at standard risk—62% versus 52% for donor versus no donor, respectively (P = .02), for survival at 5 years (Figure 2F), although an interaction test was not significant (P = .4). The relapse rate was significantly reduced in both risk groups, demonstrating the potent graft-versus-leukemia effect in an allogeneic transplantation (Figure 3A,B).
Relapse on study and mortality not associated with relapse. Relapse rate for (A) Ph-negative patients at standard risk; (B) Ph-negative patients at high risk; and (C) nonrelapse mortality for high-risk and standard-risk patients. Note the underlying mortality at 1 and 2 years among the no-donor group.
Relapse on study and mortality not associated with relapse. Relapse rate for (A) Ph-negative patients at standard risk; (B) Ph-negative patients at high risk; and (C) nonrelapse mortality for high-risk and standard-risk patients. Note the underlying mortality at 1 and 2 years among the no-donor group.
Nonrelapse mortality
Figure 3C illustrates the high mortality rate after transplantation among high-risk patients at 1 and 2 years, mostly due to graft-versus-host disease and infection. At 2 years, 36% of high-risk patients with a donor had died from nonrelapse causes compared with 14% of patients who did not have a donor. Among standard-risk patients, the nonrelapse mortality at 2 years was 20%, bearing in mind that among patients who did not have a donor there was also a significant nonrelapse mortality of 7% at 2 years. Surprisingly the nonrelapse mortality did not change over the 13 years of this study.
Chemotherapy versus autologous transplantation randomization for all patients, including Ph-positive patients
Among the 456 patients randomized to chemotherapy vs autologous transplantation, 16 were Ph positive. Patients randomized to chemotherapy had significantly improved 5-year EFS (41% vs 32%; P = .02); and OS (46% [95% CI = 39%-53%] vs 37% [95% CI = 31%-44%]; P = .03; Figure 4A,B).
Randomized chemotherapy versus autologous transplantation, measured from time of randomization. (A) Event-free survival for all patients. (B) Overall survival for all (C) high-risk patients and (D) standard-risk patients. (E) Nonrelapse mortality for all patients undergoing chemotherapy or autologous transplantation.
Randomized chemotherapy versus autologous transplantation, measured from time of randomization. (A) Event-free survival for all patients. (B) Overall survival for all (C) high-risk patients and (D) standard-risk patients. (E) Nonrelapse mortality for all patients undergoing chemotherapy or autologous transplantation.
Among patients at high risk, the 5-year survival for chemotherapy versus autologous transplantation was 37% versus 31%, respectively, and for patients at standard risk this was 56% versus 46%, respectively (interaction P = .8; Figure 4C,D).
In contrast to the donor versus no-donor analysis, there was no difference in the nonrelapse mortality between autologous transplantation and chemotherapy (Figure 4E), irrespective of the risk group (data not shown).
Effect of allogeneic transplantation versus chemotherapy: donor versus no-donor analysis for Ph-negative patients censoring at autologous transplantation
As patients randomized to chemotherapy had a better outcome than those undergoing autologous transplantation, an analysis was made comparing patients with a donor versus those without a donor, censoring at autologous transplantation, to assess the effect of sibling donor transplantation versus chemotherapy only.
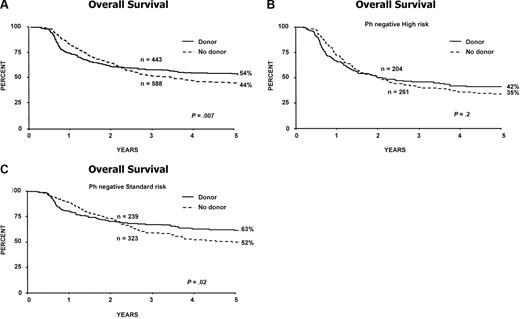
Figure 5A demonstrates the superior OS for patients with a donor. As with the primary donor versus no-donor analysis, this superiority could not be demonstrated among patients at high risk, but was significant for patients at standard risk with a 5-year survival of 63% for those with a donor versus 52% for those without a donor (interaction P = .6; Figure 5B,C).
Overall survival from diagnosis for donor versus no-donor for Ph-negative patients censoring at first remission autologous transplantation. Estimation of the effect of sibling donor transplant versus chemotherapy in (A) all patients; (B) high-risk patients; and (C) standard-risk patients.
Overall survival from diagnosis for donor versus no-donor for Ph-negative patients censoring at first remission autologous transplantation. Estimation of the effect of sibling donor transplant versus chemotherapy in (A) all patients; (B) high-risk patients; and (C) standard-risk patients.
Discussion
The overall CR rate of 90% (Figure 1B) is very high and confirms the efficacy of the induction regimen, with relatively low toxicity allowing a very high percentage of patients to proceed to receive postremission therapy.27 The rate of randomization was higher in ECOG at 60% than in the MRC at 36% (Figure 1B), possibly reflecting somewhat different cultural practices in the trial groups regarding adherence to randomization rules and potential penalties. There was no difference in survival between the MRC versus the ECOG patients (Figure 2C), indicating that the study was well balanced in all aspects on each side of the Atlantic.
The study originally included patients between 15 and 55 years although the age threshold, if there is one, at which “adult” ALL begins and “childhood” ALL ceases is a matter of current controversy. Indeed there may be evidence that patients up to the age of 20 to 25 years or beyond might in 2007 achieve better outcomes on pediatric protocols.28,–30
Hitherto, allogeneic stem-cell transplantation has been accepted as being of value in second remission and for high-risk patients in first remission.9,10,12,–14 In this study, outcome of allogeneic transplantation for all patients should best be estimated from a donor versus no-donor analysis for patients between 15 and 50 years. This eliminates selection bias that would operate if only those who underwent transplantation were compared with those who did not. We regarded 50 years as the upper age limit initially for allogeneic transplantation, at the beginning of the study in 1993. This may not be the case 14 years on. Survival at 5 years of 53% for those Ph-negative patients with a donor, versus 45% for those without, (P = .02) indicates the superiority of allogeneic transplantation overall while eliminating the biases of selection of transplantation or not for those who have a donor.
Surprising though it might appear, the high-risk patients benefit less from having a donor than the standard-risk patients in terms of overall survival. For high-risk patients, the donor versus no-donor comparison shows a 5-year 41% versus 35% OS, which is not significantly superior (P = .2; Figure 2E). This is an important finding since there is often a view that high-risk patients should proceed immediately to allogeneic transplantation. In fact, the relapse rate for high-risk patients is much reduced by the availability of a donor (Figure 3B), 63% versus 37% at 5 years (P ≤ .001), so it is the toxicity of the transplant that is largely responsible for the lack of significantly improved survival for those patients who have a donor.
Somewhat unexpectedly, the significant overall benefits of having a donor are better for the standard-risk Ph-negative patient who has a significantly superior survival at 5 years if he/she has a donor (62% vs 52%; P = .02; Figure 2D). Relapse rates for these patients with a donor are also reduced as for high-risk patients (49% vs 24%; P ≤ .001; Figure 3A). With a donor, the nonrelapse mortality is far greater for high-risk than for standard-risk patients (Figure 3C). The nonrelapse mortality was also higher for high-risk patients compared with standard-risk among those not undergoing a transplantation (14% vs 7%; Figure 3C). Therefore a not insignificant number of patients were lost from treatment-related causes other than transplantation.
In the randomized group comparing chemotherapy and autologous transplantation, the EFS and OS were superior for chemotherapy (P = .02 and P = .03, respectively). Differences were not statistically significant within subgroups, but these comparisons lack statistical power.
When comparing patients with a donor to the best no-donor groups (ie, by removing the effect of autologous transplantation by censoring), the OS is once again significantly superior for standard-risk patients with a donor but not for high-risk patients (Figure 5A-C).
This study shows that the most potent antileukemia effect for adults with ALL derives from an allogeneic transplantation as demonstrated by the significantly reduced relapse rate. This confirms the original hypothesis of a potential allogeneic effect and survival benefit therefrom. However, the transplantation-related mortality for high-risk patients—due to the older age group—was unacceptably high. Although the size of the improvement in survival for the donor group was not statistically significantly different between the standard- and high-risk groups, this much higher mortality rate suggests that the difference is real. Thus benefit was confined to patients with standard-risk disease for whom undergoing an allogeneic transplantation demonstrates significant survival advantages over those undergoing conventional therapy.
For the younger patients with poor risk features, a matched unrelated donor (MUD) allogeneic transplantation may become a real option for the future,30,,,,–35 ahead of conventional consolidation/maintenance chemotherapy
Several relatively small, variously designed studies of autologous transplantation in ALL have been reported.10,,–13,36 The overall data suggest that autologous transplantation offers little, if any, antileukemia benefit over conventional chemotherapy. However, as in no study was there a worse outcome for autologous transplantation; its short duration offers a potentially significant benefit. In this large study, the outcome for autologous transplantation was not equivalent and therefore it cannot be suggested as the preferred modality.
Adult ALL is a relatively uncommon disorder with perhaps fewer advances made in the last 2 decades than for other major hematologic malignancies. It has taken a very large collaborative study lasting many years to demonstrate the value of a sibling allograft in this disease and the lack of additional value of an autograft over conventional consolidation/maintenance chemotherapy for those without a donor.
It is not easy to consider how to go forward. The very high remission rate on the present protocol makes the achievement of a higher “conventional” remission rate very difficult, and it may be that interventions that attempt to reduce the amount of MRD are the way forward. This might potentially be achieved, as in so many other hematologic disorders, by the addition of monoclonal antibody to chemotherapy given that both the CD20 and CD22 antigens are variably expressed in a considerable number of adult ALL patients37
Despite the high transplant-related mortality in older high-risk patients, the GVL remains the single most potent strategy, and efforts must be made to reduce the toxicity. There are only rare data regarding the use of reduced-intensity transplantation conditioning in ALL.38 Nevertheless, some form of less toxic transplantation must be studied in this high-risk group of patients.
The toxicity of the induction phases for many adults might be reduced using pegylated asparaginase39 and perhaps with the induction of hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone)40 or other alternative chemotherapy and studying whether delays in treatment that sometimes occur in the early phases on this protocol can be reduced or abrogated.
Because of the paucity of significant new agents for this disease, it seems likely that the MUD transplantation as a potential curative option will be offered to more patients in any new study with a risk-adapted approach; perhaps an ablative transplantation for those younger than 40 years without a sibling and a nonablative approach for those 40 years or older.
Sibling donor allogeneic transplantation is the treatment of choice for adults with standard-risk ALL in remission providing the greatest chance for a long-term survival. Autologous transplantation has a less favorable outcome than consolidation/maintenance chemotherapy for those without a donor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to acknowledge with thanks the assistance of Alli Lewis and Sonia Kamenetsky in the preparation of the manuscript.
Authorship
Contribution: A.H.G. and J.M.R. designed and performed research and wrote the paper; S.M.R., G.B., and J.D. analyzed data; H.M.L., M.S.T., A.K.F., A.K.B., R.C., P.H.W., M.R.L., A.M., I.M.F., S.L., N.C., and D.I.M. performed research; L.F. and E.P. contributed vital data.
A list of the participating centers of the United Kingdom Medical Research Council Adult Leukemia Working Party and Eastern Cooperative Oncology Group can be found in Document S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anthony H. Goldstone, Director of Services, North London Cancer Network, UCLH, 6th Floor Rosenheim Wing, 25 Grafton Way, London WC1 E 6DB, United Kingdom; e-mail: anthony.goldstone@uclh.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal