Autoimmune hemolytic anemia (AHA) is a common complication in chronic lymphocytic leukemia (CLL). The UK LRF CLL4 trial is the largest prospective trial in CLL to examine the prognostic impact of both a positive direct antiglobulin test (DAT) and AHA. Seven-hundred seventy-seven patients were randomized to receive chlorambucil or fludarabine, alone or with cyclophosphamide (FC). The incidence pretreatment of a positive DAT was 14%. Ten percent developed AHA. The DAT correctly predicted the development, or not, of AHA after therapy in 83% of cases, however only 28% of DAT-positive patients developed AHA. Of 299 patients tested both before and after treatment, those treated with single-agent fludarabine were most likely to remain DAT positive and to change from negative to positive. Patients treated with chlorambucil or fludarabine were more than twice as likely to develop AHA as those receiving FC. In a multivariate analysis, stage C disease and high β2 microglobulin were independent predictors of a positive DAT result. AHA, or a positive DAT, with or without AHA, independently predicted for reduced overall survival (OS). Four deaths, all on fludarabine monotherapy, were attributed to AHA. In conclusion, DAT status at the time of initiation of therapy provides a new prognostic indicator, although FC may protect against AHA. This trial was registered at http://isrctn.org as no. 58585610.
Introduction
Autoimmune hemolytic anemia (AHA) is a common complication in chronic lymphocytic leukemia (CLL), occurring in 10% to 25% of patients during the course of their disease.1,2 The direct antiglobulin test (DAT) may be positive at some time during the disease course in up to 35% of cases, but overt AHA occurs less frequently. Although AHA may occur in asymptomatic untreated CLL, it is more common in patients with advanced-stage disease, 10.5% in Binet stage B and C, compared with only 2.9% in stable stage A patients.1 A review of our data from MRC CLL Trials over the past 20 years shows an incidence of AHA of 8.6% in 1273 patients, rising to 11% after treatment, predominantly with alkylating agents.
A 1966 landmark paper first suggested that treatment with X-rays or alkylating agents might trigger the onset of AHA in CLL.3 Following the advent of purine analog therapy in the 1980s, there have been a number of single case reports and small series, involving more than 100 patients, suggesting that AHA may occur more commonly following treatment with these agents.4,,,,–9
The main results of the LRF CLL4 trial conducted in the United Kingdom between 1999 and 2004 have been reported,10 including the incidence of AHA. The trial provided an opportunity to study prospectively the incidence of a positive DAT and its relationship to the risk of developing AHA in 777 previously untreated CLL patients who received therapy with either chlorambucil or fludarabine given with or without cyclophosphamide. We present the findings from this study.
Methods
This study was approved by the Institutional Review Board of the Royal Marsden Hospital & Institute of Cancer Research and the Multi-Center Research Ethics Committee (MREC). The trial followed UK Medical Research Council Guidelines for good clinical practice. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients and trial design
LRF CLL4 was a prospective multicenter randomized controlled trial. The diagnosis of CLL was confirmed by central review of morphology and immunophenotype. Patients were eligible for trial entry if they had Binet stage C, B, or A progressive disease requiring treatment and had not received any prior therapy. Treatment was allocated on a 2:1:1 ratio, to chlorambucil (10 mg/m2 per day for 7 days), or to fludarabine alone (25 mg/m2 per day intravenously or 40 mg/m2 orally, for 5 days), or to fludarabine plus cyclophosphamide (FC; F 25 mg/m2 per day plus C 250 mg/m2 per day for 3 days intravenously or orally over 5 days with F 24 mg/m2 per day plus C 150 mg/m2 per day). Fludarabine was administered intravenously until February 2001 when the oral formulation became available and the protocol was modified to allow oral administration of the bioequivalent dose. All treatment was given every 4 weeks, F and FC for up to 6 cycles and chlorambucil up to 12 courses. The response recorded was the best achieved at any time, following first-line treatment.
Information regarding the result of the DAT test was requested at trial entry and at the time of maximum response to first treatment. Patients with a positive DAT, but without overt hemolysis, could be randomized in the trial with the recommendation that close monitoring for onset of hemolysis should be followed.
AHA was defined by standard criteria: a drop in hemoglobin, associated with a positive DAT and/or increased reticulocyte count and rise in indirect bilirubin with no other cause for anemia identified. In some cases, the diagnosis of AHA was supported by increased levels of lactate dehydrogenase (LDH) and low haptoglobin. Patients with AHA were eligible for the trial but received therapy for the hemolysis before initiating the allocated treatment. Cases of AHA occurring during first-line treatment were identified on the form completed at the end of first-line treatment. Pretreatment AHA was not reported.
Treatment of patients with AHA followed conventional guidelines for idiopathic AHA; namely prednisolone at 1 mg/kg body weight per day for 2 to 4 weeks tapered off over several weeks. Other therapies used included intravenous immunoglobulin, splenectomy, cyclosporine A, and rituximab. Details of treatment for AHA were not available. Concomitant treatments for patients entered into the trial included the following: allopurinol for the first 2 to 3 courses, cotrimoxazole throughout treatment and for 6 months after completion, and aciclovir in selected patients.
Other variables recorded at trial entry (either in all or in the majority of patients) and included in analyses were as follows: stage of disease, age, sex, β2 microglobulin, LDH, and absolute lymphocyte count (ALC). A range of other prognostic markers was also analyzed for the majority of trial patients11 : immunoglobulin heavy chain variable gene mutation status, cytogenetics by florescence in situ hybridization (FISH), and CD38 and ZAP-70 expression. A full analysis of these will follow in a separate report and is not included here.
Statistical methods
Overall survival was calculated from randomization to death from any cause, and progression-free survival (PFS) was time from randomization to relapse needing further therapy, progression, or death from any cause. For nonresponders and those with progressive disease (NR/PD), the date of progression was when NR/PD was recorded.
Comparisons between categoric variables were made using the chi-square test, and between quantitative variables using the Wilcoxon rank sum test. Logistic regression models were used to determine those factors that were independently associated with a positive DAT result and with the development of AHA. Survival functions have been estimated using the Kaplan-Meier method and groups compared with the log-rank test. Multivariate Cox regression analysis was used to determine whether prognostic factors were independently associated with outcome. A partial adjustment was made for missing values of β2 microglobulin, the variable with the largest proportion of missing data, using the median value. All P values are 2-sided.
Results
The trial recruited 783 patients between February 1999 and October 2004, with follow up to July 31, 2006. Six patients were excluded for consent or diagnostic reasons; 387 (50%) were allocated to receive chlorambucil, 194 fludarabine alone, and 196 FC. Two patients were lost to follow-up. Thirty percent of patients were older than 70 years.
DAT results
The DAT result in 637 patients (82%) was recorded at trial entry. In 107 patients, the test was not done and in 33 not recorded. After first treatment, 333 patients (43%) had test results, with 394 not done and 50 not recorded. At entry, 89 (14%) of 637 patients tested DAT positive and 44 (13%) of 333 after treatment. There were no significant differences among the 3 treatment groups in either the percentage of patients with test results available (range: 80%-85% at entry and 39%-48% after treatment) or the percentage of DAT-positive results (14% chlorambucil, 13% fludarabine, and 15% FC at entry, and 13%, 17%, and 10%, respectively, after treatment). Of the 299 patients tested at both time points, those treated with single-agent fludarabine were the most likely to remain DAT positive (82% vs 52% chlorambucil, 42% FC) and the most likely to change from negative to positive (9% vs 3%, 4%; Table 1). Thus fludarabine increased the net incidence of positivity in these patients by 4 cases (+ 5%), compared with 6 fewer cases after chlorambucil (− 4%) and 4 fewer after FC (− 5%), but these differences were not statistically significant (P > .05).
DAT results in 299 patients with results available both at entry and after treatment
| DAT result . | Chlorambucil, no. (%) . | Fludarabine, no. (%) . | Fludarabine plus cyclophosphamide, no. (%) . | Total, no. (%) . |
|---|---|---|---|---|
| Positive at entry | ||||
| Positive after treatment | 11 (52) | 9 (82) | 5 (42) | 25 (57) |
| Negative after treatment | 10 (48) | 2 (18) | 7 (58) | 19 (43) |
| Negative at entry | ||||
| Positive after treatment | 4 (3) | 6 (9) | 3 (4) | 13 (5) |
| Negative after treatment | 112 (97) | 62 (91) | 68 (96) | 242 (95) |
| DAT result . | Chlorambucil, no. (%) . | Fludarabine, no. (%) . | Fludarabine plus cyclophosphamide, no. (%) . | Total, no. (%) . |
|---|---|---|---|---|
| Positive at entry | ||||
| Positive after treatment | 11 (52) | 9 (82) | 5 (42) | 25 (57) |
| Negative after treatment | 10 (48) | 2 (18) | 7 (58) | 19 (43) |
| Negative at entry | ||||
| Positive after treatment | 4 (3) | 6 (9) | 3 (4) | 13 (5) |
| Negative after treatment | 112 (97) | 62 (91) | 68 (96) | 242 (95) |
Incidence of AHA
Among 759 patients with information available, 77 (10%) developed AHA: 47 (12%) receiving chlorambucil, 21 (11%) fludarabine alone, and 9 (5%) FC (P = .01). DAT results on treatment were available for 331 of these. Twenty (51%) of 39 with AHA were positive compared with 23 (8%) of 292 of those without AHA. Information on AHA was missing for 18 (2%) patients, of whom 10 died (4 within 40 days of entry) and none had a complete response recorded.
The positive predictive value of the test (chance that a DAT-positive patient will develop AHA) was 28% (38%, 29%, and 12% for chlorambucil, fludarabine, and FC, respectively). The negative predictive value of the test (chance that a DAT-negative patient will be AHA free) was 93% (92%, 90%, and 96%, for chlorambucil, fludarabine, and FC, respectively). Thus the DAT test at entry correctly predicted the development of AHA or not after therapy in 83% of cases (84%, 82% and 83% for chlorambucil, fludarabine, and FC, respectively). Information was not recorded regarding either the time elapsed from start of treatment until onset of AHA, or the incidence of AHA after second-line treatment.
Outcomes of AHA
Patients who developed AHA were less likely than others to have responded to treatment (43/65 [66% overall response rate] vs 530/654 [81%], P = .004) and less likely to have had a good (complete or nodular partial) response (12/65 [18%] vs 268/654 [41%], P < .001). Twelve AHA patients were not assessable for response (4 died less than 6 months after entry). Those patients who developed AHA did receive less therapy: 41% only had 1 to 3 courses of chlorambucil compared with 14% without AHA, only 38% completed 4 to 6 cycles of fludarabine compared with 73% of those without AHA, and 56% completed 4 to 6 cycles of FC compared with 74% non-AHA patients.
Four deaths were attributed to AHA during first-line treatment, all on fludarabine monotherapy. These account for 1.5% of the total deaths in the trial to July 2006, or 2% of deaths in which CLL was a cause. In patients treated with single-agent fludarabine these accounted for 6% of total deaths and 7% of CLL deaths. Survival times from randomization for these 4 patients were 1, 3, 3, and 5 months. Three were DAT positive, and one negative, at entry; 3 received oral and 1 intravenous fludarabine, for between 1 and 3 courses. Of the 4 patients, 3 presented with stage C disease and 1 with stage B, 1 patient had unmutated VH genes, 2 mutated, and 1 not known, and 1 patient had a p53 deletion in 85% of cells by FISH.
Survival at 5 years of patients without AHA was 58% (95% confidence interval [CI]: 53%-63%) compared with 37% (95% CI: 20%-53%) for patients who developed AHA, odds ratio (OR) = 0.40 (95% CI: 0.25–0.63); P < .001 (Figure 1A). PFS was significantly better for those who did not develop AHA (18% [95% CI: 14%-22%] at 5 years) compared with patients with AHA (9% [95% CI: 0%-18%] at 5 years); OR = 0.55 (95% CI: 0.40–0.75); P = .001 (Figure 1B). There was no statistically significant difference in the impact of AHA by treatment group for either PFS or OS (however numbers are small with only 9 AHA cases in the FC group).
The predictive value of AHA for survival and PFS. (A) Survival and (B) PFS by AHA or not, as recorded on end of first-line treatment form.
The predictive value of AHA for survival and PFS. (A) Survival and (B) PFS by AHA or not, as recorded on end of first-line treatment form.
Predictive value of DAT at trial entry for other outcomes
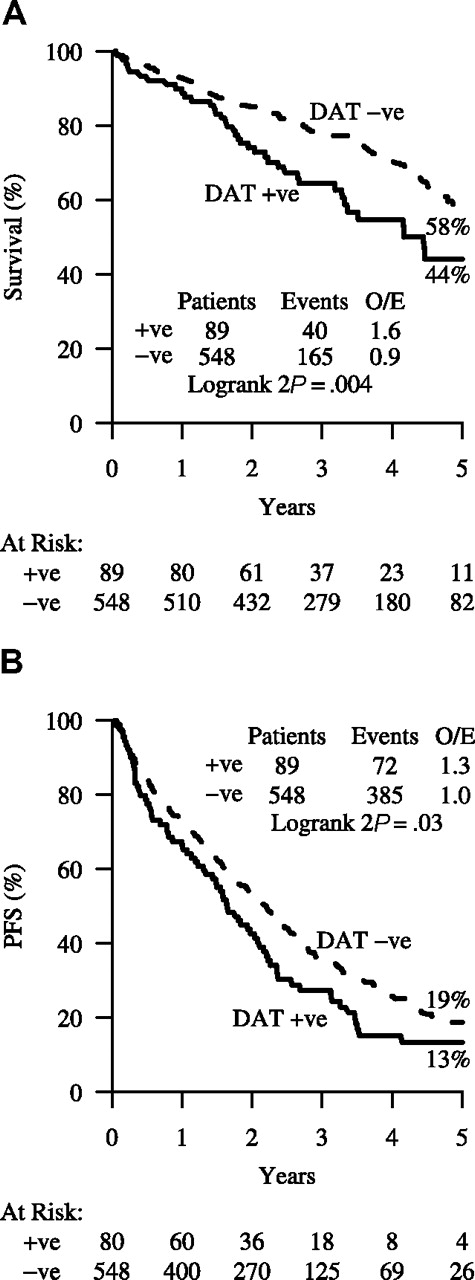
Comparing DAT-positive with DAT-negative patients, the former were just as likely to respond to treatment: 64 (77%) of 83 versus 404 (79%) of 512 (P = .7); but they had fewer good (complete or nodular partial) responses: 22 (27%) of 83 versus 198 (39%) of 512 (P = .03). Overall survival was significantly better for DAT-negative patients (58% [95% CI: 52%–63%] at 5 years) compared with DAT-positive patients (44% [95% CI: 31%-57%]; OR = 0.53 [95% CI: 0.35–0.79]; P = .004) (Figure 2A). Similarly, when considering PFS, the DAT-negative patients fared significantly better (19% [95% CI: 15%-24%] at 5 years) compared with the DAT-positive patients (13% [95% CI: 5%-21%]; OR = 0.72 [95% CI: 0.54–0.95]; P = .03; Figure 2B).
The predictive value of DAT positivity for survival and PFS. (A) Survival and (B) PFS by DAT test result at trial entry.
The predictive value of DAT positivity for survival and PFS. (A) Survival and (B) PFS by DAT test result at trial entry.
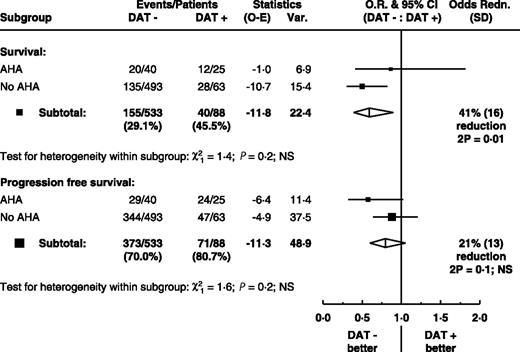
After exclusion of patients who developed AHA, DAT-negative patients still had better survival than DAT-positive patients (P = .01; Figure 3). Thus the difference in survival between DAT-positive and -negative patients is not accounted for by those who developed AHA. A similar trend is seen for PFS but is not statistically significant. For patients whose DAT result has been recorded, multivariate Cox regression analysis of variables including treatment allocation, AHA, DAT, and conventional risk criteria (ie, stage of disease, age, sex) showed both the DAT result and AHA independently and significantly associated with overall survival (both P = .03) and the DAT result, but not AHA, significantly associated with progression-free survival (P = .005). A multivariate analysis of all available covariates (which includes additionally β2 microglobulin, LDH, ALC, and percentage bone marrow lymphocytes) confirmed that the DAT result retained its independent prognostic value for PFS (hazard ratio [HR] for DAT-positive = 1.35, 95% CI: 1.03–1.78, P = .03), although β2 microglobulin could be only partially allowed for as the result was available for only 484 (76%) of patients. Moreover, both AHA and the DAT result retained their independent prognostic value for overall survival (HR for AHA = 1.52, 95% CI: 1.02–2.26, P = .04; HR for DAT positive = 1.46, 95% CI: 1.02–2.10, P = .04).
Survival and PFS: differences in event rates by entry DAT result within AHA subgroup. The squares and lines show the estimated odds ratios and their 95% CIs. The size of each square is proportional to the amount of information available in the subgroup. Overall estimates are shown by a diamond, with the width representing the 95% CI.
Survival and PFS: differences in event rates by entry DAT result within AHA subgroup. The squares and lines show the estimated odds ratios and their 95% CIs. The size of each square is proportional to the amount of information available in the subgroup. Overall estimates are shown by a diamond, with the width representing the 95% CI.
Factors predicting DAT results at trial entry and AHA development
Univariate analysis considered the following variables and their association with AHA status and with DAT result at entry: stage of disease, age, sex, treatment allocation, β2 microglobulin, absolute lymphocyte count (ALC), LDH, and percentage of bone marrow (BM) lymphocytes.
A DAT-positive test result at entry was associated with more advanced stage of disease, higher β2 microglobulin, and higher ALC (Table 2). Multivariate analysis showed that stage of disease and β2 microglobulin were the only clear independent predictors of DAT result, with stage C patients more likely to be DAT positive than stage A progressive or B patients (HR for stage C = 2.23,95% CI: 1.3–3.82, P = .004; HR for unit increase in β2 microglobulin = 1.11, 95% CI: 1.01–1.23, P = .03). A further analysis including genetic markers showed that immunoglobulin heavy chain mutation and FISH results (deletion at 11q23) were of only borderline significance (P = .05). However these results require careful interpretation because laboratory data are not available for all patients. A detailed analysis will be presented elsewhere.
Association between patient characteristics and DAT result at entry
| Factor* . | DAT negative . | DAT positive . | P . |
|---|---|---|---|
| Age, no. (%) | |||
| Younger than 60 y | 178 (86) | 28 (14) | .4 |
| 60 to 69 y | 210 (88) | 29 (12) | |
| 70 y or older | 160 (83) | 32 (17) | |
| Stage, no. (%) | |||
| A progressive | 146 (93) | 11 (7) | <.001 |
| B | 245 (89) | 31 (11) | |
| C | 157 (77) | 47 (23) | |
| Sex, no. (%) | |||
| Female | 142 (86) | 24 (14) | .9 |
| Male | 406 (86) | 65 (14) | |
| Treatment, no. (%) | |||
| Chlorambucil | 272 (86) | 43 (14) | .9 |
| Fludarabine | 135 (87) | 21 (13) | |
| FC | 141 (85) | 25 (15) | |
| B2M, mg/L, median (range), n=484 | 3.6 (0.0–29.4) | 4.5 (1.3–11.3) | .001† |
| ALC, ×109/L, median (range), n=619 | 74 (1–570) | 107 (2–519) | .007† |
| LDH, U/L, median (range), n=598 | 415 (73–1953) | 424 (4–1135) | .9† |
| Factor* . | DAT negative . | DAT positive . | P . |
|---|---|---|---|
| Age, no. (%) | |||
| Younger than 60 y | 178 (86) | 28 (14) | .4 |
| 60 to 69 y | 210 (88) | 29 (12) | |
| 70 y or older | 160 (83) | 32 (17) | |
| Stage, no. (%) | |||
| A progressive | 146 (93) | 11 (7) | <.001 |
| B | 245 (89) | 31 (11) | |
| C | 157 (77) | 47 (23) | |
| Sex, no. (%) | |||
| Female | 142 (86) | 24 (14) | .9 |
| Male | 406 (86) | 65 (14) | |
| Treatment, no. (%) | |||
| Chlorambucil | 272 (86) | 43 (14) | .9 |
| Fludarabine | 135 (87) | 21 (13) | |
| FC | 141 (85) | 25 (15) | |
| B2M, mg/L, median (range), n=484 | 3.6 (0.0–29.4) | 4.5 (1.3–11.3) | .001† |
| ALC, ×109/L, median (range), n=619 | 74 (1–570) | 107 (2–519) | .007† |
| LDH, U/L, median (range), n=598 | 415 (73–1953) | 424 (4–1135) | .9† |
Recorded on n = 637 patients unless otherwise stated.
Wilcoxon rank sum test.
Table 3 shows that the development of AHA is associated with stage C disease, positive DAT result, higher β2 microglobulin level, treatment with chlorambucil or fludarabine monotherapy, and older age. Multivariate analysis (Table 4) highlighted DAT test result, age, stage of disease, and treatment as independent predictors of AHA. Genetic markers were not significant in multivariate analysis. In the 621 patients for whom the DAT result at trial entry and AHA were recorded, those treated with chlorambucil or fludarabine monotherapy were roughly 3 times more likely to develop AHA compared with FC patients. Those with a negative DAT result at trial entry had a 77% reduced odds of developing AHA. The odds of developing AHA increased with age and were greater for patients with stage C disease than for earlier stage patients.
Association between patient characteristics and AHA
| Factor* . | No AHA . | AHA . | P . |
|---|---|---|---|
| Age, no. (%) | |||
| Younger than 60 y | 231 (93) | 17 (7) | .03 |
| 60 to 69 y | 256 (90) | 28 (10) | |
| 70 y or older | 195 (86) | 32 (14) | |
| Stage, no. (%) | |||
| A progressive | 176 (93) | 13 (7) | <.001 |
| B | 324 (94) | 20 (6) | |
| C | 182 (81) | 44 (19) | |
| Sex, no. (%) | |||
| Female | 180 (90) | 19 (10) | .8 |
| Male | 502 (90) | 58 (10) | |
| Treatment, no. (%) | |||
| Chlorambucil | 330 (88) | 47 (12) | .008 |
| Fludarabine | 169 (89) | 21 (11) | |
| FC | 183 (95) | 9 (5) | |
| DAT at entry, no. (%) | |||
| Positive | 63 (72) | 25 (28) | <.001† |
| Negative | 493 (92) | 40 (8) | |
| Unknown/missing | 126 (91) | 12 (9) | |
| B2M, mg/L, median (range), n = 546 | 3.6 (0.0–12.2) | 4.9 (1.3–12.7) | < .001‡ |
| ALC, ×109/L, median (range), n = 734 | 77 (1–568) | 94 (1–570) | .2‡ |
| LDH, U/L, median (range), n = 684 | 415 (69–1953) | 420 (4–1295) | .6‡ |
| Factor* . | No AHA . | AHA . | P . |
|---|---|---|---|
| Age, no. (%) | |||
| Younger than 60 y | 231 (93) | 17 (7) | .03 |
| 60 to 69 y | 256 (90) | 28 (10) | |
| 70 y or older | 195 (86) | 32 (14) | |
| Stage, no. (%) | |||
| A progressive | 176 (93) | 13 (7) | <.001 |
| B | 324 (94) | 20 (6) | |
| C | 182 (81) | 44 (19) | |
| Sex, no. (%) | |||
| Female | 180 (90) | 19 (10) | .8 |
| Male | 502 (90) | 58 (10) | |
| Treatment, no. (%) | |||
| Chlorambucil | 330 (88) | 47 (12) | .008 |
| Fludarabine | 169 (89) | 21 (11) | |
| FC | 183 (95) | 9 (5) | |
| DAT at entry, no. (%) | |||
| Positive | 63 (72) | 25 (28) | <.001† |
| Negative | 493 (92) | 40 (8) | |
| Unknown/missing | 126 (91) | 12 (9) | |
| B2M, mg/L, median (range), n = 546 | 3.6 (0.0–12.2) | 4.9 (1.3–12.7) | < .001‡ |
| ALC, ×109/L, median (range), n = 734 | 77 (1–568) | 94 (1–570) | .2‡ |
| LDH, U/L, median (range), n = 684 | 415 (69–1953) | 420 (4–1295) | .6‡ |
Recorded on n = 759 patients unless otherwise stated.
Test does not include DAT unknown/missing patients.
Wilcoxon rank sum test.
Multivariate logistic regression: independent factors associated with AHA (621 patients)
| . | OR* . | 95% CI . | P . |
|---|---|---|---|
| Treatment | |||
| FC | 1.00 | .02 | |
| Chlorambucil | 3.13 | 1.37–7.16 | |
| Fludarabine | 3.11 | 1.26–7.66 | |
| DAT result at entry | |||
| Positive | 1.00 | <.001 | |
| Negative | 0.23 | 0.12–0.42 | |
| Stage | |||
| A progressive | 1.00 | .002 | |
| B | 0.85 | 0.37–1.98 | |
| C | 2.47 | 1.15–5.32 | |
| Age | 1.06 | 1.03–1.10 | .001 |
| . | OR* . | 95% CI . | P . |
|---|---|---|---|
| Treatment | |||
| FC | 1.00 | .02 | |
| Chlorambucil | 3.13 | 1.37–7.16 | |
| Fludarabine | 3.11 | 1.26–7.66 | |
| DAT result at entry | |||
| Positive | 1.00 | <.001 | |
| Negative | 0.23 | 0.12–0.42 | |
| Stage | |||
| A progressive | 1.00 | .002 | |
| B | 0.85 | 0.37–1.98 | |
| C | 2.47 | 1.15–5.32 | |
| Age | 1.06 | 1.03–1.10 | .001 |
Odds ratio after adjustment for other 3 independent predictors.
Discussion
The UK LRF CLL4 trial is the largest prospective trial in CLL to examine the incidence and prognostic impact of a positive DAT and AHA in patients requiring therapy. We report an incidence of a positive DAT of 14% and AHA of 10%, levels that are consistent with previous studies in patients with active CLL.1,12,13
The number of patients with a positive DAT at trial entry was well balanced across all 3 treatment arms. Less than half of the patients were tested after completion of treatment, but of those tested at both time points 5% became DAT positive after therapy with the greatest risk (9%) occurring in the fludarabine monotherapy arm. For patients receiving the combination of fludarabine and cyclophosphamide, the percentage of DAT-positive individuals fell after treatment, while it increased in the fludarabine monotherapy arm. This suggests a tendency for FC treatment to reduce the rate of DAT positivity. In multivariate analysis of pretreatment parameters, a DAT-positive result was associated with more advanced stage of disease, as previously reported.1 In addition a strong link was shown between increased β2 microglobulin levels and a positive DAT. This observation has also been reported by Borthakur et al.14
The DAT result had an important independent association with the development of AHA. The approximate risk of a patient with a positive DAT developing AHA was 1 in 3. DAT negativity was a strong predictor (> 90%) for not developing AHA after therapy. Only 49% of reported AHA cases were DAT negative in this trial, in contrast with 82% in the results recently reported by Borthakur et al14 for CLL patients treated with FC plus rituximab. This finding may be related to the use of rituximab in the treatment regimen as they suggest, or may be related to the diagnostic definitions of AHA used.
The mechanism of AHA in CLL is still poorly understood. It usually results from production of high-affinity polyclonal IgG autoantibodies by the nonmalignant B lymphocytes. Possible mechanisms include aberrant antigen presentation by CLL B cells, defective T-cell function, and loss of regulatory T cells, with subsequent failure of control of autoreactive T cells.15,,–18 The latter may result from initiation of therapy and explain the frequent reports of AHA occurring in association with treatment for CLL. In LRF CLL4, the lowest incidence of AHA (5%) was seen in the FC arm. This level compares with that reported by Borthakur et al for the FCR regimen of 6.5%,14 suggesting that the combination of cyclophosphamide with fludarabine may have a protective effect against the development of AHA. Patients receiving chlorambucil or fludarabine monotherapy were 3 times more likely to develop AHA than those receiving the FC combination.
There were 4 deaths from autoimmune hemolysis after first-line therapy, accounting for 2% of all deaths in the trial. All these cases occurred in patients receiving fludarabine monotherapy. This study therefore agrees with the observation that AHA occurring in association with fludarabine treatment may be more severe.4,5 In contrast, both the incidence and the severity of AHA were lower for those patients receiving FC. There are a number of possible explanations for this. Patients achieving good remissions may be at less risk of developing AHA, and therefore the most effective treatment is likely to be associated with reduction in the incidence of AHA. The treatment responses in LRF CLL4, which have been reported elsewhere,10 showed that, although there was no significant difference in survival, the overall and complete response (CR) rates and the PFS were significantly better with FC than with either fludarabine alone or chlorambucil. The CR rate was 38% for FC, 15% for fludarabine, and 7% for chlorambucil. The German CLL and US Intergroup study groups have also reported superior response rates and PFS for FC compared with F alone.19,20 In the German study, there was a reduction in incidence of AHA in the FC arm, while in the US trial there was no difference in AHA between the 2 treatment arms. A second possibility is the total dose of fludarabine administered. In fludarabine monotherapy, the total dose was 200 mg/m2 compared with 120 mg/m2 when fludarabine was administered in combination with cyclophosphamide. If, as previously postulated by some,7 fludarabine is directly implicated in the development of AHA, there may be a dose relationship. Finally, one might propose that cyclophosphamide, an agent that is commonly used to treat autoimmune disease because of its immunosuppressive properties, could provide a protective effect when administered in combination with fludarabine.
The development of AHA was associated not only with a positive DAT and treatment modality, but also with more advanced disease stage and older age. Mauro et al12 and Barcellini et al13 have also reported age older than 65 years as a predictor of AHA, although this was not confirmed by Borthakur et al.14 The relationship between AHA and advanced stage has been a consistent finding across all studies. In our study, development of autoimmune hemolysis was associated with a poorer response to treatment and with both shorter PFS (9% vs 18%) and overall survival (37% vs 58%) at 5 years. In multivariate analysis, AHA retained independent prognostic value for OS but not for PFS. Three other studies have failed to show any independent effect of AHA on survival probability.12,13,17
Perhaps the most interesting, and somewhat surprising, observation in this study was the prognostic significance of a positive DAT, with or without associated AHA. Although overall response to treatment was the same regardless of DAT status, this did influence quality of remissions, with good (complete or nodular partial) response rates of 27% for DAT-positive patients versus 39% for DAT-negative patients (P = .03). PFS and overall survival were better for DAT-negative patients, even after exclusion of those who developed AHA. Multivariate analysis showed this to be a significant independent prognostic variable. This is the first report of the prognostic significance of the DAT test in CLL. The reason why a positive DAT should be a risk factor in CLL is not clear. It may be a surrogate marker for other poor prognostic features such as immunoglobulin heavy chain gene mutation status, and further studies will be needed to elucidate this.
In conclusion, the FC combination has a beneficial effect on both the DAT expression and the development of AHA during treatment of CLL. In addition, the DAT status at the time of initiation of therapy provides a new prognostic indicator for both PFS and OS. The DAT test remains, therefore, an important pretreatment assessment for all patients. Patients who are DAT positive may benefit from FC or FC plus rituximab rather than fludarabine or chlorambucil monotherapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to all physicians and research staff who participated in this trial.
LRF CLL4 was funded by a core grant from Leukemia Research UK. Laboratory studies were funded by an educational grant from Schering Health Care (UK) and Schering AG (Germany).
Authorship
Contribution: C.D. wrote the paper; R.W. performed statistical analysis; M.E. wrote part of the paper (“Results”); S.R. performed statistical analysis; D.M. and T.H. set up the trial; D.C. edited the paper and was trial coordinator.
A list of the members of the Studies Group and the Working Group is available as an appendix to the online version of this article (Document S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: C.D. received consulting and lecture fees from Schering AG; D.C. received consulting and lecture fees from Schering AG, Roche, and Genmab; the other authors declare no competing financial interests.
Correspondence: Claire Dearden, Royal Marsden NHS Foundation Trust, Downs Road, Sutton, Surrey, SM2 5PT, United Kingdom; e-mail: claire.dearden@rmh.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal