Abstract
T- and natural killer (NK)–cell immunosuppression associated with ζ-chain down-regulation has been described in cancer, autoimmune, and infectious diseases. However, the precise stimuli leading to this bystander phenomenon in such different pathogen-dependent and sterile pathologies remained unresolved. Here, we demonstrate that Toll-like receptors (TLRs) play a major role in the induction of innate and adaptive immune system suppression; repetitive administration of single TLR 2, 3, 4, or 9 agonists, which do not exhibit any virulent or immune invasive properties, was sufficient to induce a bystander NK- and T-cell immunosuppression associated with ζ-chain down-regulation mediated by myeloid suppressor cells, as observed in the course of active pathologies. We identified a 35-amino acid (aa) region within the ζ-chain as being responsible for its degradation under TLR-mediated chronic inflammation. Furthermore, we provide evidence that ζ-chain levels could serve as a biomarker for chronic inflammation-dependent immunosuppression. Thus, although acute TLR-mediated activation could be beneficial in clearing pathogens or may serve as an immune adjuvant, such activation could be detrimental under sustained conditions.
Introduction
During a sustained response to inactivated pathogens, and in the course of cancer, infections, or autoimmune diseases, T and natural killer (NK) cells can become ζ-chain deficient and immunologically nonfunctional (reviewed in1 ). In our previous studies, we suggested that the causative link between these different pathologies is chronic inflammation and the ensuing accumulation of myeloid suppressor cells (MSCs), which lead to decreased ζ-chain expression and impaired T-cell function.2,3 However, several critical questions remain unresolved such as how is a similar immunosuppressed state induced during sterile and pathogen-dependent diseases, and what is the nature of the stimuli leading to the generation of massive inflammation resulting in such generalized immunosuppression?
We raised the hypothesis that in the course of the above-mentioned pathologies, chronic stimulation of the innate immune system via Toll-like receptors (TLRs) would lead to the induction of immunosuppression. TLRs recognize conserved pathogen-associated molecular patterns, which serve as TLR agonists/ligands (TLRLs).4-9 Moreover, recent studies suggest that endogenous, host-derived components, including fibrinogen, heat shock proteins, β-defensins, RNA, and DNA could also serve as TLRLs.5,10 TLRs are primarily expressed on cells of the innate immune system (myeloid and NK cells) and by some cells of the adaptive immune system (regulatory and activated T cells). Upon activation of any of the TLR signaling pathways, a primarily Th1-inflammatory response ensues, as reflected by the production of proinflammatory cytokines, chemokines, nitric oxide (NO), and other factors.1,11 Thus, TLRs could be activated during infections as well as in the course of sterile pathologies. It is well established that TLR-mediate acute activation is beneficial to the host because it leads to a critical response responsible for the clearance of pathogens and abnormal or damaged self-tissues. However, based on our observations, when TLR-mediated stimulation is sustained and chronic inflammation ensues, the appearance of immunosuppression is expected.
In the current study, we tested whether chronic exposure to a single TLRL, which does not exhibit any virulent or immune invasive properties, could induce immunologic nonresponsiveness characterized by reduced ζ-chain expression. To this end, we developed an in vivo experimental model system in which normal mice were repeatedly exposed to a single TLRL (specific for TLRs 2, 3, 4, or 9), encapsulated in multilamellar vesicles (MLVs). The effect of this treatment on the adaptive (T cell) and innate (NK cell) immune responses was assessed in conjunction with testing the expression levels of the ζ-chain. The ζ-chain, in both cell types, plays a crucial role in receptor expression and signaling function,12-14 and its expression is affected by the immunosuppressive conditions.
We demonstrate that chronic exposure to a single TLRL specific for TLR 2, 3, 4, or 9 is sufficient to induce T- and NK-cell immunosuppression mediated by MSCs, which is associated with ζ-chain degradation. A 35-amino acid (aa) region within the ζ-chain was identified as being responsible for its targeting to degradation under TLR-mediated chronic inflammation. Furthermore, because ζ-chain expression levels discriminate between acute and chronic inflammation characterized by immunosuppression, we suggest that reduced ζ-chain expression levels could serve as a biomarker for chronic inflammation-induced immunosuppression.
Methods
Mice
Female BALB/c, C3H/HeN, C3H/HeJ, B10.A, and C57BL/6 mice, 6 to 8 weeks of age, were bred at the Hebrew University specific pathogen-free facility. Transgenic mice encoding full-length (FL) ζ-chain and ζ-chain variants containing only a single distal immunoreceptor tyrosine-based activation motif (ITAM; distal) or no ITAMs (tail-less [TL]) were generated on the background of ζ−/− as described previously.15,16 Animal use followed protocols approved by the Hebrew University-Hadassah Medical School Institutional Animal Care and Use Committee.
Antigen, TLRLs, and immunization
Antigens and immune modulators were obtained as follows: ovalbumin grade 3, Zymosan A, poly(I:C), lipopolysaccharide (LPS) from Escherichia coli strain 055:B5 (cat.L-4524), all purchased from Sigma-Aldrich (St Louis, MO). Endotoxin-free ( ≤ 1 ng/mg DNA) phosphorothioate CpG-ODNs (5′-TGA CTGTGAACGTTCGAGATGA-3′), and the mutant (m)CpG-ODNs (5′-TGACTGTGAAGG TTAGAGATGA-3′) were kindly provided by Dr Eyal Raz from the University of California, San Diego. Mice were injected subcutaneously 3 times with specific TLR ligands (LPS [70 μg], Zymosan [200 μg], CpG-ODNs [70 μg], and Poly (I:C) [150 μg]) encapsulated in MLVs as described previously.17
Immunostaining and fluorescence-activated cell sorter analysis
Antibodies used for cell surface labeling were purchased from BD PharMingen (San Diego, CA) and included fluorescein isothiocyanate (FITC)–labeled anti-Thy-1.2, anti-Gr-1, and anti-bromodeoxyuridine (BrdU), phycoerythrin-labeled anti-CD4, anti-CD3, and anti-NK1.1. Biotinylated anti-CD3ε, anti–T-cell receptor (TCR)αβ, anti-Thy-1.2, and anti-Mac-1 (CD11b) were detected by streptavidin-Cy5 (Jackson Immunoresearch). Cells were precoated with anti-mouse CD16/CD32 (BD PharMingen), incubated for 30 minutes at 4°C with the specific labeled antibodies, washed, and then incubated with a second-step reagent. Intracellular staining of ζ and CD3ε-chains was performed as described previously.2 Samples were analyzed in a FACSCalibur apparatus using Cell Quest software (BD PharMingen).
6-Carboxyfluorescein succinimidyl ester staining and ex vivo proliferation assay
Splenocytes (20 × 106/mL) were incubated in phosphate-buffered saline (PBS) without Ca2+/Mg2+ containing 5 μM 6-carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) for 8 minutes at 25°C. At the end of this incubation, fetal calf serum (FCS) was added and the cells were washed in RPMI/8% FCS serum. CFSE-labeled splenocytes were activated with anti-CD3ε and anti-CD28 or phorbol-12-myristate-13-acetate (PMA) and Ca2+ ionophore, and the number of cell divisions of Thy1.2+ cells was determined by fluorescence activated cell sorter (FACS).
Intracellular BrdU staining
Splenocytes were activated as described in the previous section. They were then surface labeled with antiThy1.2, fixed as described previously,2 and stained with FITC-conjugated anti-BrdU antibodies. The cells were washed and analyzed by FACS. Nonpulsed activated cells were used as a control for the anti-BrdU staining.
Cell isolation and separation
For magnetic column separation, splenocytes were first labeled (30 minutes at 4°C) with biotin-conjugated antibodies. Negative selection using anti-CD11b and anti-B220 antibodies was performed for T-cell isolation ( > 90% purity), and positive selection was performed for the separation of Gr-1+Mac-1+ cells using anti-Gr-1 antibodies ( > 98% purity). The cells were then washed, labeled (45 minutes at 4°C) with anti-biotin antibodies conjugated to magnetic microbeads (Miltenyi Biotec, Auburn, CA), washed, and loaded onto a column placed in a magnetic field (Miltenyi Biotec).
Coincubation experiments
Hybridoma T cells or T cells isolated from the spleen were suspended in complete growing medium and coincubated for 16 hours at 37°C with a Gr1+Mac-1+–enriched cell population obtained from the spleen of LPS-treated mice. The cells were then harvested and the level of ζ-chain expression within the T-cell population was determined by FACS.
Cytotoxicity assays
For the in vitro assay, splenocytes were harvested from control or LPS-treated mice 18 hours on intraperitoneal injection of 200 μg poly(I:C) or PBS, and their killing activity was examined against target cells (YAC-1) that were pre-labeled with [35S]methionine. Various effector/target ratios were used in a cytotoxicity assay of 5-hour incubation at 37°C. Specific killing was calculated as described18 and normalized according to NK1.1+ cell percentage. For the in vivo assay, a published fluorescence labeling method19 was used. Briefly, splenocytes from BALB/C and C57BL/6 mice were stained with CFSE (Invitrogen, Carlsbad, CA) at a final concentration of 0.5 μM (CFSElow) and 5 μM (CFSEhigh), respectively. Cells (5 × 106) of each type were mixed and injected intravenously into recipient C57BL/6 mice (control, LPS-treated, and NK1.1-depleted mice). Peripheral blood lymphocytes (PBLs), lymph node, and spleen cells were harvested 18 hours after injection, and the ratio between the CFSEhigh and CFSElow populations was determined by FACS. The index of specific allogeneic cell clearance was calculated according to the formula: Index of specific allogeneic cell clearance = 1− BALB/C splenocytes (CFSElow) cells of total CFSE+ cells/ B6 splenocytes (CFSEhigh) cells of total CFSE+ cells.
Influenza virus infection and mice survival
Mice were infected by intranasal inoculation with the LD50 dose of influenza virus (A/PR/8/34) 2 days before the last LPS injection and mice survival was followed. LPS treatment itself did not affect mice survival.
Spleen immunohistochemistry
Five-micrometer, formalin-fixed, and paraffin-embedded sections from the spleens of control and LPS-treated mice were deparaffinized and rehydrated. After endogenous peroxidase quenching (H2O2), antigens were retrieved by boiling the sections in 10 mM citrate buffer (pH 6.0) or after pronase digestion. Immunostaining was then performed using the specific anti-Gr1 (clone RB6-8C5) or anti-CD3 antibodies, and the appropriate second reagents were applied (Zymed Laboratories, South San Francisco, CA) to visualize the labeled cells. Images of immunohistochemical staining were viewed with a Zeiss Axioplan 2 microscope and acquired with a Zeiss AxioCam digital camera (Zeiss, Oberkochen, Germany), using a 10×/0.30 NA Plan neofluar (Figures 4D,5Ci-iv) or a 20×/0.50 NA Plan neofluar (Figure 5Cv-vi) objective lens. Digitized images were processed using Adobe Photoshop 7 image processing and manipulation software (Adobe Systems, San Jose, CA).
Measurement of serum cytokines
Serum was obtained from control and LPS-treated (1 day after the third LPS injection) mice. Interleukin-4 (IL-4), IL-6, interferon-γ (IFN-γ), and tumor necrosis factor (TNF) concentrations in the serum were measured by enzyme linked immunosorbent assay (ELISA) according to manufacturer instructions (BD Biosciences PharMingen, San Diego, CA).
Constructs and transfection
FL and proximal ζ-chain constructs were generated by polymerase chain reaction and cloned into the expression vector pcDNA3.1. ζ-Deficient T-cell hybridoma MA5.8 cells16 were stably transfected with the FL and proximal ζ-chain constructs using the Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA) at a setting of 250 V and 950 microfarad with 10 μg of plasmid/1 × 107 cells. G418 resistant clones were screened for ζ-chain expression by FACS and Western blot analysis.
Results
Repeated LPS treatment induces TLR4-dependent impairment of T-cell function associated with ζ-chain down-regulation
We first analyzed the effect of sustained in vivo TLR4 activation on the adaptive immune system (T cells). This TLR is commonly activated because of its ability to recognize bacterial compounds, such as LPS, as well as endogenous proteins that are released from damaged tissues.20 In addition, TLR4 agonists are currently used as vaccine-adjuvants,21 and assessing their effect on the immune system is therefore critical. To this end, Tlr4 wild-type (C3H/HeN) and Tlr4 defective (C3H/HeJ) mice were repeatedly injected with highly purified LPS from E coli, encapsulated in MLVs (LPS-treated) to reduce its toxicity and enable its slow release, or injected with empty MLVs (control; Figure 1A), as described in “Antigen, TLRLs, and immunization.” We initially assessed the effect of this treatment on T-cell function. The ex vivo proliferation of splenic T cells from control and LPS-treated mice on TCR-mediated activation was evaluated by measuring cell divisions of gated CFSE-labeled T cells (Figure 1B) or BrdU incorporation (Figure 1C). Treatment with LPS was sufficient to induce significantly decreased TCR-mediated proliferation in splenic T cells of C3H/HeN-treated mice compared with that of cells derived from C3H/HeJ-treated mice or untreated control mice (Figure 1Bi-iii,C). However, when splenocytes derived from LPS-treated C3H/HeN mice were stimulated with a combination of PMA and Ca+2 ionophore, which bypasses TCR signaling, the T-cell proliferative response was partially rescued (up to 70%) relative to those isolated from the LPS-treated C3H/HeJ or control mice (Figure 1Biv-vi,C). Although both TCR-dependent and -independent signaling events are impaired in T cells derived from LPS-treated C3H/HeN mice, the effect on the TCR-mediated activation was by far more pronounced.
Chronic exposure to LPS leads to T-cell dysfunction associated with abnormal ζ-chain expression in mice with a functional TLR4. (A) Mice were treated 3 times with MLVs encapsulated with LPS as described in “Antigen, TLRLs, and immunization.” The mice were killed 2 days after the last treatment. (B) Splenocytes from control C3H/HeN (I,IV), LPS-treated C3H/HeN (II,V), and C3H/HeJ (III,VI) mice were labeled with CFSE and then activated ex vivo with anti-CD3 and anti-CD28 antibodies or with PMA and Ca2+ ionophore as described in “6-Carboxyfluorescein succinimidyl ester staining and ex vivo proliferation assay” (black histograms) or left untreated (white histograms). The proliferative response was assessed by monitoring cell divisions of gated CFSE-labeled Thy1.2+ T cells. (C) Splenocytes from control and LPS-treated mice were activated for 48 hours and specific T cell proliferation was measured by BrdU incorporation in Thy-1.2+ cells using FACS. The results are presented as the mean value of 3 independent experiments, and standard deviations are shown. *P < .02; **P < .001 (Student t test). (D) Splenic Thy1.2+ T cells from LPS-treated (black line) and control (gray area) C3H/HeN mice (I,II) or C3H/HeJ mice (III,IV) were analyzed for total ζ (I,III) and CD3ε (II,IV) expression levels by FACS. (E) Equal numbers of splenic T cells from LPS-treated and control mice were lysed and subjected to Western blot analysis. Expression of the protein tyrosine kinase ZAP-70, CD3ε and ζ-chain was assessed by immunoblotting (IB) using specific antibodies. A representative experiment is shown of 5 performed.
Chronic exposure to LPS leads to T-cell dysfunction associated with abnormal ζ-chain expression in mice with a functional TLR4. (A) Mice were treated 3 times with MLVs encapsulated with LPS as described in “Antigen, TLRLs, and immunization.” The mice were killed 2 days after the last treatment. (B) Splenocytes from control C3H/HeN (I,IV), LPS-treated C3H/HeN (II,V), and C3H/HeJ (III,VI) mice were labeled with CFSE and then activated ex vivo with anti-CD3 and anti-CD28 antibodies or with PMA and Ca2+ ionophore as described in “6-Carboxyfluorescein succinimidyl ester staining and ex vivo proliferation assay” (black histograms) or left untreated (white histograms). The proliferative response was assessed by monitoring cell divisions of gated CFSE-labeled Thy1.2+ T cells. (C) Splenocytes from control and LPS-treated mice were activated for 48 hours and specific T cell proliferation was measured by BrdU incorporation in Thy-1.2+ cells using FACS. The results are presented as the mean value of 3 independent experiments, and standard deviations are shown. *P < .02; **P < .001 (Student t test). (D) Splenic Thy1.2+ T cells from LPS-treated (black line) and control (gray area) C3H/HeN mice (I,II) or C3H/HeJ mice (III,IV) were analyzed for total ζ (I,III) and CD3ε (II,IV) expression levels by FACS. (E) Equal numbers of splenic T cells from LPS-treated and control mice were lysed and subjected to Western blot analysis. Expression of the protein tyrosine kinase ZAP-70, CD3ε and ζ-chain was assessed by immunoblotting (IB) using specific antibodies. A representative experiment is shown of 5 performed.
We next assessed whether the impaired TCR-mediated signaling function is associated with an abnormal TCR structure. Intracellular staining of splenic T cells (Figure 1D) and immunoblot analysis (Figure 1E) showed a reduced ζ-chain expression induced by LPS in C3H/HeN (Figure 1Di,E) but not in C3H/HeJ (Figure 1Diii,E) mice. Despite the loss of ζ-chain in splenic T cells from LPS-treated C3H/HeN mice, these cells expressed normal total levels of the CD3ε-chain (Figure 1Dii) as well as of the surface CD3ε and TCR αβ subunits (data not shown). The expression level of the protein tyrosine kinase, ζ-associated protein-70 (ZAP-70), a T-cell protein that is unrelated to the TCR subunits, was unaffected under these conditions (Figure 1E). Thus, persistent activation via a single TLR can induce the entire immunosuppressive milieu affecting all T cells similarly to that induced by an intact inactivated pathogen (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The bystander T-cell immunosuppression was observed in both CD4+ and CD8+ subpopulations (data not shown). The results showing that both TCR-dependent and -independent signaling pathways are affected on chronic LPS treatment suggest that the abnormal TCR structure is not the sole cause for the observed T-cell dysfunction.
Reduced antiviral response and impaired NK-cell function in LPS-treated mice
Because the immune response to viral infections involves a complex coordination between the innate and adaptive systems, we next examined the in vivo antiviral immunity of LPS-treated mice relative to controls. Mice were infected with an LD50 dose of influenza virus and monitored daily for mortality. The results revealed a reduced survival rate of LPS-treated mice relative to controls (Figure 2A). Because of the kinetics of an antiviral immune response in which NK-cell activity precedes the T-cell response, the expedited death of LPS-treated mice, 5 to 7 days after the viral infection, suggests that mortality is primarily attributable to an impaired NK-mediated innate immune response, although the initial involvement of T cells could not be excluded. These results demonstrate the suppressed antiviral activity of the innate and adaptive immune systems on repeated treatment of mice with LPS.
Reduced antiviral response and impaired NK-cell function associated with reduced ζ-chain expression in LPS-treated mice. (A) Mice were infected by intranasal inoculation with an LD50 dose of influenza virus (A/PR/8/34) 2 days before the third LPS treatment and monitored daily for mortality. The figure shows a representative survival curve, of 2 independent experiments performed, of control and LPS-treated mice after infection with influenza virus (n = 10). (B) Splenocytes from untreated and anti-NK1.1-treated mice (NK1.1-depleted mice) were stained for DX-5 and NK1.1 markers and analyzed by FACS. (C) In vivo cytotoxicity assay of CFSE-labeled allogeneic splenocytes was performed as described in “Cytotoxicity assays.” CFSE-labeled allogeneic (CFSElow) and syngeneic (CFSEhigh) cells were injected to untreated and NK1.1-depleted naive mice. After 24 hours, PBLs, lymph nodes (LNs), and splenocytes were analyzed to determine the ratio between allogeneic and syngeneic cells and the index of specific allogeneic cell clearance was calculated as described in “Cytotoxicity assays.” The mean value of 3 independent experiments with standard deviations is shown.* P < .005 (Student t test). (D) Representative plot of CFSE-labeled allogeneic (CFSElow) and syngeneic (CFSEhigh) cells within the spleen of control, LPS-treated or anti-NK1.1–treated mice 24 hours after administration (top panel). In the bottom panel, the results of 3 independent experiments are presented as the mean value plus or minus standard deviations (bottom panel). *P < .005 (Student t test). (E) Splenocytes were harvested from control and LPS-treated mice and subjected to in vitro NK-cytotoxicity assay with YAC-1 target cells in different effector to target ratios. Percentage of specific lysis was calculated as described in “Cytotoxicity assays.” *P < .01; **P < .001 (Student t test). (F) Before harvesting (24 hours), control and LPS-treated mice were injected intraperitoneally with poly (I:C); splenocytes were then harvested and subjected to in vitro NK-cytotoxicity assay as in E. (G) Total ζ-chain expression in splenic NK1.1+CD3− cells from LPS-treated and control C57BL/6 mice.
Reduced antiviral response and impaired NK-cell function associated with reduced ζ-chain expression in LPS-treated mice. (A) Mice were infected by intranasal inoculation with an LD50 dose of influenza virus (A/PR/8/34) 2 days before the third LPS treatment and monitored daily for mortality. The figure shows a representative survival curve, of 2 independent experiments performed, of control and LPS-treated mice after infection with influenza virus (n = 10). (B) Splenocytes from untreated and anti-NK1.1-treated mice (NK1.1-depleted mice) were stained for DX-5 and NK1.1 markers and analyzed by FACS. (C) In vivo cytotoxicity assay of CFSE-labeled allogeneic splenocytes was performed as described in “Cytotoxicity assays.” CFSE-labeled allogeneic (CFSElow) and syngeneic (CFSEhigh) cells were injected to untreated and NK1.1-depleted naive mice. After 24 hours, PBLs, lymph nodes (LNs), and splenocytes were analyzed to determine the ratio between allogeneic and syngeneic cells and the index of specific allogeneic cell clearance was calculated as described in “Cytotoxicity assays.” The mean value of 3 independent experiments with standard deviations is shown.* P < .005 (Student t test). (D) Representative plot of CFSE-labeled allogeneic (CFSElow) and syngeneic (CFSEhigh) cells within the spleen of control, LPS-treated or anti-NK1.1–treated mice 24 hours after administration (top panel). In the bottom panel, the results of 3 independent experiments are presented as the mean value plus or minus standard deviations (bottom panel). *P < .005 (Student t test). (E) Splenocytes were harvested from control and LPS-treated mice and subjected to in vitro NK-cytotoxicity assay with YAC-1 target cells in different effector to target ratios. Percentage of specific lysis was calculated as described in “Cytotoxicity assays.” *P < .01; **P < .001 (Student t test). (F) Before harvesting (24 hours), control and LPS-treated mice were injected intraperitoneally with poly (I:C); splenocytes were then harvested and subjected to in vitro NK-cytotoxicity assay as in E. (G) Total ζ-chain expression in splenic NK1.1+CD3− cells from LPS-treated and control C57BL/6 mice.
We next assessed the effect of sustained activation of TLR4 by LPS on the innate immune system, focusing on NK cells, given that the ζ-chain also plays a crucial role in the function of NK-cell activating receptors. In this set of experiments, we used C57B/6 mice because NK cells could be detected by anti-NK1.1 antibodies. To study the effect of LPS treatment on in vivo NK-cell function, we followed their capacity to clear fluorescently labeled allogeneic cells.19 To verify that allogeneic cell clearance depends on NK-cell activity, naive C57B/6 mice were first depleted of NK cells using anti-NK1.1 antibody or nondepleted (Figure 2B) and then were intravenously injected with a mixture of labeled allogeneic (CFSElow) and syngeneic (CFSEhigh) cells. The index of allogeneic cell clearance within the cell population of different lymphatic organs was then calculated. The results confirm that rejection of allogeneic splenocytes is mediated primarily by NK cells because the clearance index of the allogeneic cells was significantly reduced in mice that were depleted of NK1.1-expressing cells. Based on the results showing that the most pronounced effect was observed in the spleen (Figure 2C), we then analyzed the function of NK cells in the spleens of LPS-treated mice. The results revealed significantly impaired NK-cell function within LPS-treated mice, manifested by reduced clearance of allogeneic cells (Figure 2D, top and bottom panels). Furthermore, the dysfunction of NK cells isolated from LPS-treated mice was also apparent by their reduced in vitro cytotoxic activity (Figure 2E). Poly(I:C) treatment of mice 24 hours before cell harvest induced recovery of the NK-cell function (Figure 2F). As in the case of T cells, the impaired NK-cell function in the LPS-treated mice was associated with a dramatic loss of ζ-chain expression (Figure 2G). The approximate 5-fold increase in specific lysis activity of splenic NK cells derived from control or LPS-treated mice observed on treatment with poly(I:C) is mostly attributable to the in vivo priming of NK cells by type 1 IFNs22 and could result from activation of NK-cell receptors that do not require ζ-chain expression. Thus, chronic exposure to LPS immunosuppressed the innate immune system, as reflected by the impaired killing function of NK cells and the reduction in ζ-chain expression levels.
Repetitive treatment with TLR 2, 3, or 9 ligands affect T-cell function and ζ-chain expression
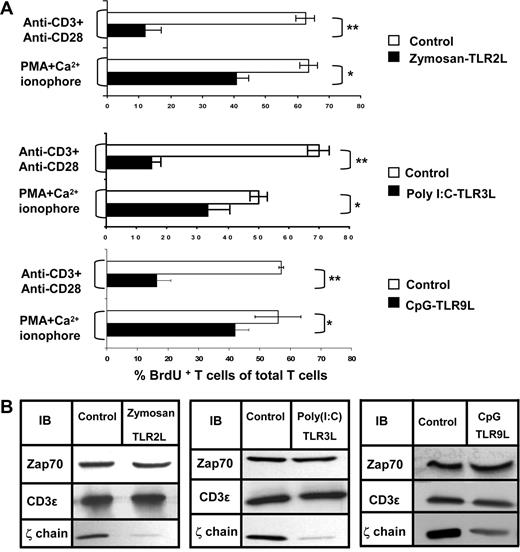
To evaluate whether TLRLs other than the TLR4L, LPS, could induce an immunosuppressive effect in our in vivo model, we repeatedly exposed normal mice to TLR2, 3, or 9 ligands encapsulated in MLVs. To evaluate the function of splenic T cells derived from these mice, ex vivo proliferation assays were performed using intracellular staining for BrdU. We found that the ex vivo T-cell proliferation in the TLRL-treated mice after TCR-mediated activation was impaired, whereas activation with PMA and Ca2+ ionophore partially overcame this defect (Figure 3A). Impaired NK-cell function within the TLRL-treated mice was also observed (data not shown). The immunosuppression was associated with ζ-chain down-regulation. In all cases, whereas ζ-chain expression was reduced, the expression of other TCR subunits (CD3ε) or signaling molecules (ZAP-70) was unaffected (Figure 3B). These results show that treatment with each of the TLRLs induced T-cell immunosuppression associated with reduced ζ-chain expression, similar to that generated by LPS (Figure 1) or by entire inactivated pathogens (Figure S1).
Treatment with TLR 2, 3, or 9 ligands induces impaired T-cell proliferation and ζ-chain down-regulation. Mice were injected subcutaneously 3 times with MLVs and encapsulated with one of the following TLRLs: Zymosan (TLR 2), poly (I:C) (TLR 3), or CpG (TLR 9), as described in “Antigen, TLRLs, and immunization.” (A) Proliferative response as determined by the percentage of BrdU+Thy1.2+ of total Thy1.2+ cells isolated from control mice (□) relative to TLRL-treated mice (■) after stimulation with anti-CD3 and anti-CD28 antibodies or with PMA and Ca2+ ionophore. The results are presented as the mean values of 3 independent experiments, and standard deviations are shown. *P < .005 (Student t test). (B) Equal numbers of splenic T cells from Zymosan, poly (I:C), and CpG-treated and control mice were lysed and subjected to Western blot analysis. Expression of the protein kinase ZAP-70, CD3ε, and ζ-chain was assessed by immunoblotting (IB) using specific antibodies.
Treatment with TLR 2, 3, or 9 ligands induces impaired T-cell proliferation and ζ-chain down-regulation. Mice were injected subcutaneously 3 times with MLVs and encapsulated with one of the following TLRLs: Zymosan (TLR 2), poly (I:C) (TLR 3), or CpG (TLR 9), as described in “Antigen, TLRLs, and immunization.” (A) Proliferative response as determined by the percentage of BrdU+Thy1.2+ of total Thy1.2+ cells isolated from control mice (□) relative to TLRL-treated mice (■) after stimulation with anti-CD3 and anti-CD28 antibodies or with PMA and Ca2+ ionophore. The results are presented as the mean values of 3 independent experiments, and standard deviations are shown. *P < .005 (Student t test). (B) Equal numbers of splenic T cells from Zymosan, poly (I:C), and CpG-treated and control mice were lysed and subjected to Western blot analysis. Expression of the protein kinase ZAP-70, CD3ε, and ζ-chain was assessed by immunoblotting (IB) using specific antibodies.
LPS treatment leads to an inflammatory response associated with abnormal spleen architecture
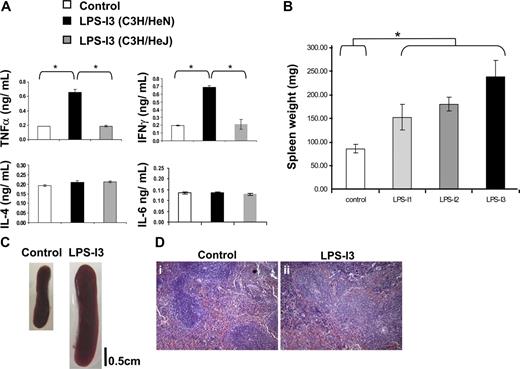
LPS treatment is known to induce an inflammatory response as reflected by the secretion of high levels of proinflammatory cytokines such as TNF-α, IL-1β, IL-8, and the induction of the synthesis of other proinflammatory proteins, such as inducible NO synthase.23 However, LPS was not previously shown to have any immunosuppressive effect on T- and NK-cell function or on ζ-chain expression. We therefore evaluated whether, under our experimental conditions, LPS treatment induces an inflammatory response and how this inflammation affects secondary lymphatic organs. The first indication for an inflammatory response was the elevated level of IFN-γ and TNF-α within LPS-treated C3H/HeN mice; no such elevation was seen in LPS-treated C3H/HeJ or control mice (Figure 4A). Moreover, splenomegaly was observed in LPS-treated C3H/HeN mice and was most pronounced after 3 LPS treatments (Figure 4B,C). In addition, the splenomegaly in the LPS-treated mice was associated with a distorted splenic architecture, as reflected by the expansion of the red and white pulp zones and the destruction of the follicular borders (Figure 4D). Similar inflammatory features were observed on treatment with the above-described TLRLs (data not shown).
LPS-induced inflammatory environment. (A) Cytokine profile as measured by ELISA of pooled sera from 5 mice. Sera from control C3H/HeN (□), LPS-treated C3H/HeN (■), and C3H/HeJ mice (■) were taken 2 days after the third LPS injection. A representative experiment is presented of 2 performed. (B) The weight of spleens from control and LPS-treated (1 to 3 treatments) mice was evaluated 2 weeks after the first injection. A summary of the results is presented as the mean value of 3 independent experiments, and standard deviations are shown. *P < .01 (Student t test). (C) Images of spleens after 3 LPS treatments were acquired using a Nikon COOLPIX 4300 digital camera (Nikon, Tokyo, Japan). A splenomegaly was observed in LPS-treated C3H/HeN mice relative to LPS-treated C3H/HeJ or control mice. (D) Paraffin-embedded sections from spleen of control (I) and LPS-treated (II) mice were stained with hematoxylin and eosin.
LPS-induced inflammatory environment. (A) Cytokine profile as measured by ELISA of pooled sera from 5 mice. Sera from control C3H/HeN (□), LPS-treated C3H/HeN (■), and C3H/HeJ mice (■) were taken 2 days after the third LPS injection. A representative experiment is presented of 2 performed. (B) The weight of spleens from control and LPS-treated (1 to 3 treatments) mice was evaluated 2 weeks after the first injection. A summary of the results is presented as the mean value of 3 independent experiments, and standard deviations are shown. *P < .01 (Student t test). (C) Images of spleens after 3 LPS treatments were acquired using a Nikon COOLPIX 4300 digital camera (Nikon, Tokyo, Japan). A splenomegaly was observed in LPS-treated C3H/HeN mice relative to LPS-treated C3H/HeJ or control mice. (D) Paraffin-embedded sections from spleen of control (I) and LPS-treated (II) mice were stained with hematoxylin and eosin.
ζ-chain as a biomarker for chronic inflammation-dependent immunosuppression induced by MSCs
We next characterized the pathway leading to ζ-chain down-regulation. The distorted spleen histology detected in mice with LPS-induced chronic inflammation (Figure 4D) was associated with the accumulation of Gr-1+Mac-1+ MSCs within the spleens of the TLR2, 3, 4, and 9 ligand-treated mice (Figure 5A). Ex vivo coincubation experiments revealed that Gr-1+Mac-1+ cells derived from LPS-treated mice induced ζ-chain down-regulation in normal T cells (Figure 5B) and ex vivo T-cell dysfunction (data not shown) in a ratio-dependent manner. MSCs isolated from mice treated with various TLRLs exhibited similar inhibitory effects on T cells (data not shown). To determine whether there is a correlation between the immunosuppressive function of MSCs and their in vivo localization relative to T cells in the spleen of LPS-treated mice, we performed immunohistochemistry on spleen sections using anti-CD3 and anti–Gr-1 antibodies (Figure 5C). The results revealed that relative to control mice, in spleens of LPS-treated mice, a more diffused CD3 staining (Figure 5Ci,ii) and an increased number of Gr-1–positive cells surrounding the T-cell zone (Figure 5Ciii,iv) were observed. Moreover, in some regions, an invasion of Gr-1–positive cells into the T cell zones was observed (Figure 5Cv,vi).
ζ-Chain expression serves as a biomarker for MSC-dependent T-cell immunosuppression. (A) Expansion of Gr-1+Mac-1+ cells in the spleens of control and TLRL-treated mice was determined by FACS analysis. The results are presented as the mean value of 3 independent experiments, and standard deviations are shown. *P < .001 (Student t test). (B) Normal splenic T cells were isolated and coincubated with Gr1+Mac-1+-enriched (95% purity) cells from LPS-treated mice at different ratios. After 16 hours, T cells were stained for ζ-chain and analyzed by FACS, and expression was compared with that of the originally separated T cells (cultured without MSCs, “1:0”). The histogram shows one experiment representative of 2 performed. (C) Paraffin-embedded sections of spleens from control (i,iii) or LPS-treated (ii,iv,v,vi) mice were stained with anti-CD3 or anti–Gr-1 antibodies. Photomicrographs of representative sections (I-IV;100×) and magnification of the boxed region (v,vi; 200×) are shown. (D) Kinetics of ζ-chain expression in the course of emergence and disappearance of the immunosuppressive environment. (E) Equal numbers of splenic T cells from control and LPS-treated (1 to 3 treatments; LPS I1-I3) mice (bottom panel) were lysed on days +2 and +8 and subjected to Western blot analysis. Expression of the ζ-chain and CD3ε was assessed by immunoblotting (IB) using specific antibodies. (F) Representative density plots of Gr-1+Mac-1+ cell distribution within the spleens of control and LPS-treated mice. (G) Splenocytes from control and LPS-treated mice from days +2 and +8 were activated with anti-CD3 and anti-CD28 antibodies; cell proliferation was then measured by BrdU incorporation in Thy-1.2 + cells using FACS analysis. Representative density plots of gated T cells are shown.
ζ-Chain expression serves as a biomarker for MSC-dependent T-cell immunosuppression. (A) Expansion of Gr-1+Mac-1+ cells in the spleens of control and TLRL-treated mice was determined by FACS analysis. The results are presented as the mean value of 3 independent experiments, and standard deviations are shown. *P < .001 (Student t test). (B) Normal splenic T cells were isolated and coincubated with Gr1+Mac-1+-enriched (95% purity) cells from LPS-treated mice at different ratios. After 16 hours, T cells were stained for ζ-chain and analyzed by FACS, and expression was compared with that of the originally separated T cells (cultured without MSCs, “1:0”). The histogram shows one experiment representative of 2 performed. (C) Paraffin-embedded sections of spleens from control (i,iii) or LPS-treated (ii,iv,v,vi) mice were stained with anti-CD3 or anti–Gr-1 antibodies. Photomicrographs of representative sections (I-IV;100×) and magnification of the boxed region (v,vi; 200×) are shown. (D) Kinetics of ζ-chain expression in the course of emergence and disappearance of the immunosuppressive environment. (E) Equal numbers of splenic T cells from control and LPS-treated (1 to 3 treatments; LPS I1-I3) mice (bottom panel) were lysed on days +2 and +8 and subjected to Western blot analysis. Expression of the ζ-chain and CD3ε was assessed by immunoblotting (IB) using specific antibodies. (F) Representative density plots of Gr-1+Mac-1+ cell distribution within the spleens of control and LPS-treated mice. (G) Splenocytes from control and LPS-treated mice from days +2 and +8 were activated with anti-CD3 and anti-CD28 antibodies; cell proliferation was then measured by BrdU incorporation in Thy-1.2 + cells using FACS analysis. Representative density plots of gated T cells are shown.
To evaluate whether the ζ-chain expression levels could serve as a biomarker for sensing changes in chronic inflammation-dependent immunosuppression, its emergence and disappearance, we performed kinetic experiments comparing the time frame of both processes (Figure 5D). Gradual ζ-chain down-regulation was observed as the inflammatory response progressed during the different intervals (1 to 3 LPS injections), with the most pronounced effect after the third LPS injection (Figure 5E). Interestingly, 8 days after the third LPS injection, a recovery of ζ-chain expression was observed relative to day + 2 (Figure 5E), correlating with normal percentages of MSCs (Figure 5F) and recovered in vitro TCR-mediated T-cell proliferation (Figure 5G). Thus, ζ-chain expression levels are reversible and are modified according to the inflammatory environment, suggesting its possible use as a biomarker to monitor chronic inflammation-dependent immunosuppression.
The mechanism for ζ-chain down-regulation under chronic inflammatory conditions
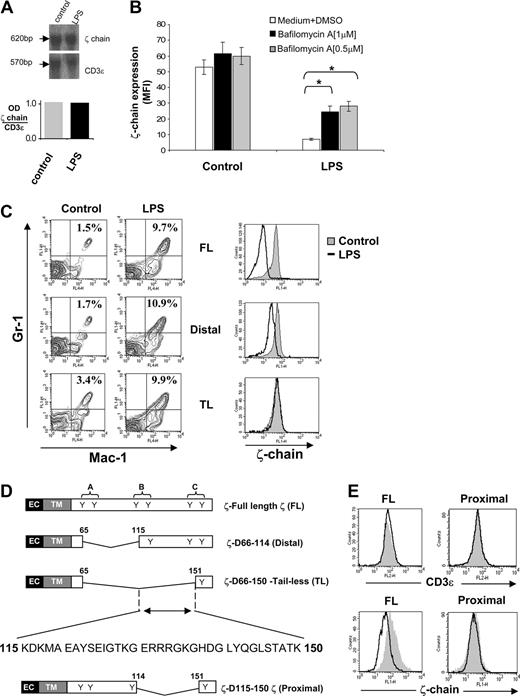
We next assessed at which level ζ-chain expression is regulated under chronic inflammatory conditions. Northern blot analysis of splenocytes revealed that ζ-chain mRNA levels of LPS-treated mice were similar to those of control mice (Figure 6A), suggesting that ζ-chain expression is regulated at the translational or post-translational level. Treatment of splenocytes isolated from LPS-treated mice with the lysosome inhibitor bafilomycin A increased ζ-chain expression up to 3-fold relative to ζ-chain down-regulated cells that were untreated and to approximately 60% of ζ-chain expression levels in control cells (Figure 6B). Furthermore, proteasomal inhibitors did not affect ζ expression under these conditions (data not shown). These results suggest a post-translational mechanism controlling ζ-chain expression under chronic inflammatory conditions.
A 35-aa region within the ζ-chain is responsible for its degradation after repeated LPS treatment. (A) RNA was extracted from spleens of control and LPS-treated mice and was analyzed by Northern blot. ζ-mRNA was detected after hybridization with a 32P-labeled ζ-specific cDNA probe (top panel). Hybridization with a 32P-labeled CD3ε-specific cDNA probe was used as a loading control (top panel). Densitometric analysis is shown as the ratio of ζ-chain to CD3ε mRNA (bottom panel). (B) Freshly isolated control and LPS-treated splenocytes were treated with or without bafilomycin A1 (1 μM or 0.5 μM) and then analyzed for total ζ-chain expression by FACS. The results are presented as the mean fluorescence intensity (MFI) of 2 independent experiments, and standard deviations are shown. (C) Representative density plots (left panel) of Gr-1+Mac-1+ cell distribution within the spleens of control and LPS-treated ζ-transgenic mice (described in panel D). Splenic T cells (Thy1.2+ cells) from LPS-treated ζ-transgenic mice were analyzed for ζ-chain expression levels by FACS (right panel). (D) Schematic representation of ζ-chain sequences in transgenic mice expressing FL and truncated ζ-chain (distal and TL). The extracellular (EC), transmembrane (TM), and cytoplasmic domains are depicted, and the location of ITAMs (I-III) in the cytoplasmatic domains of the ζ proteins and their tyrosine residues (Y) are indicated (D indicates deletion). A double-headed arrow represents the 35-aa ζ-chain region responsible for its targeting to lysosomal degradation. The aa sequence of this region is shown. A schematic representation of truncated ζ-chain (proximal) sequence, which lacks the above-mentioned 35-aa area. (E) ζ-deficient hybridoma T cells (MA5.8) were stably transfected with FL or truncated ζ-chain (proximal) as described in Materials and methods. T cell–transfected hybridoma T cells (2 × 105) were coincubated for 16 hours with 4 × 106 splenocytes derived from control (gray area) or LPS-treated (black line) B10.A mice. The hybridoma T cells were then analyzed for total CD3ε and ζ-chain expression levels by FACS.
A 35-aa region within the ζ-chain is responsible for its degradation after repeated LPS treatment. (A) RNA was extracted from spleens of control and LPS-treated mice and was analyzed by Northern blot. ζ-mRNA was detected after hybridization with a 32P-labeled ζ-specific cDNA probe (top panel). Hybridization with a 32P-labeled CD3ε-specific cDNA probe was used as a loading control (top panel). Densitometric analysis is shown as the ratio of ζ-chain to CD3ε mRNA (bottom panel). (B) Freshly isolated control and LPS-treated splenocytes were treated with or without bafilomycin A1 (1 μM or 0.5 μM) and then analyzed for total ζ-chain expression by FACS. The results are presented as the mean fluorescence intensity (MFI) of 2 independent experiments, and standard deviations are shown. (C) Representative density plots (left panel) of Gr-1+Mac-1+ cell distribution within the spleens of control and LPS-treated ζ-transgenic mice (described in panel D). Splenic T cells (Thy1.2+ cells) from LPS-treated ζ-transgenic mice were analyzed for ζ-chain expression levels by FACS (right panel). (D) Schematic representation of ζ-chain sequences in transgenic mice expressing FL and truncated ζ-chain (distal and TL). The extracellular (EC), transmembrane (TM), and cytoplasmic domains are depicted, and the location of ITAMs (I-III) in the cytoplasmatic domains of the ζ proteins and their tyrosine residues (Y) are indicated (D indicates deletion). A double-headed arrow represents the 35-aa ζ-chain region responsible for its targeting to lysosomal degradation. The aa sequence of this region is shown. A schematic representation of truncated ζ-chain (proximal) sequence, which lacks the above-mentioned 35-aa area. (E) ζ-deficient hybridoma T cells (MA5.8) were stably transfected with FL or truncated ζ-chain (proximal) as described in Materials and methods. T cell–transfected hybridoma T cells (2 × 105) were coincubated for 16 hours with 4 × 106 splenocytes derived from control (gray area) or LPS-treated (black line) B10.A mice. The hybridoma T cells were then analyzed for total CD3ε and ζ-chain expression levels by FACS.
Our next goal was to locate the region within the ζ-chain that is responsible for its targeting to degradation. To this end, we used transgenic mice expressing either FL ζ-chain (FL-ζ) or ζ-chain variants truncated in the intracytoplasmic domain, one containing only a single distal ITAM (distal) and the second containing no ITAMs (TL).15,16 These mice were treated with LPS as described in Materials and methods. Chronic inflammation was generated in all LPS-treated transgenic mice as shown by the elevation of MSCs within their spleen relative to the nontreated mice (Figure 6C left panel). Interestingly, analysis of ζ-chain expression in these mice revealed that although down-regulation was induced in the FL-ζ and distal transgenic mice after LPS treatment, ζ-chain expression levels in the TL mice were not altered in response to LPS (Figure 6C right panel). These results indicate that the region spanning 35 aa within the ζ-chain intracytoplasmic domain (115-150 aa; Figure 6D) is responsible for its targeting to degradation under the LPS-induced immunosuppressive conditions. To support this conclusion, ζ-deficient hybridoma T cells were stably transfected with FL or with a 35-aa truncated ζ-chain (proximal; Figure 6D) and cocultured with MSCs derived from LPS-treated syngeneic mice. The transfected cells were analyzed for total CD3ε and ζ-chain expression levels by FACS. The results revealed that whereas cells expressing FL ζ-chain are sensitive to the immunosuppressive environment and down-regulate the ζ-chain, cells expressing the proximal ζ-chain are resistant to the immunosuppressive environment and the truncated protein remains stable (Figure 6E). Thus, lack of the identified ζ-chain 35-aa region protects ζ-chain from degradation mediated by the MSCs derived from LPS-treated mice.
Discussion
In the current study, we highlight the role of TLRs in the induction of chronic inflammation-dependent immunosuppression, as observed during infections and sterile pathologies. We demonstrate that antigen-independent sustained in vivo activation through single TLRs, which does not involve virulent or immune invasive mechanisms, is sufficient to induce immunosuppression of the adaptive (T cells) and innate (NK cells) immune systems associated with ζ-chain down-regulation, as observed during chronic infections, cancer, and autoimmune disorders. Moreover, we provide evidence supporting the possible use of ζ-chain expression as a biomarker for chronic inflammation-induced immunosuppression.
The role of individual TLRs in the induction of a bystander immunosuppression in the course of chronic inflammation was tested by subjecting normal mice to a repeated administration of a single TLRL encapsulated in negatively charged MLVs.17 TLR2, 3, 4, or 9 ligands were encapsulated in MLVs to reduce their toxicity and enable their slow release and efficient entry into the cells, thus activating intracellular TLRs including TLRs 3 and 9. The TLRLs used in the current study are derived from or are constituents of various potential pathogens, including yeast (Zymosan A), viruses [Poly (I:C)] and bacteria (LPS and CpG-ODNs). We demonstrate that the sustained exposure of mice to each of the TLRLs resulted in the induction of both local and systemic inflammatory immune responses associated with a bystander T- and NK-cell immunosuppression, similar to those observed in the course of a variety of chronic pathologies. Moreover, we show that the induced immunosuppression is the consequence of a Th1-dependent chronic inflammatory response associated with a dramatic enlargement and disrupted architecture of the spleen attributable to the abnormal accumulation of MSCs, the cells responsible for ζ-chain down-regulation and impaired T-cell function. Immunohistochemistry staining of spleen sections from LPS-treated mice revealed that MSCs tightly border and in some regions invade the T-cell zones. The observed in vivo intimate interaction between MSCs and T cells in the affected spleens supports our ex vivo data demonstrating that MSCs confer their immunosuppressive effect on contact or close proximity with T cells; coincubation in the presence of a transwell abrogates the immunosuppressive effect of the MSCs.3 The observed immunosuppression could be caused by depletion of surrounding arginine as described previously.24 It has also been shown that MSCs can induce conversion of naive T cells to regulatory T cells (Tregs) that are known to have an immunosuppressive effect on T cell function.25 However, in our system, Tregs are less likely to be involved because separation of T cells from spleen of LPS-treated mice leads to their recovery within 4 hours (data not shown). Moreover, whereas MSC-dependent conversion of naive T cells to Tregs requires 5-day incubation,25 the immunosuppressive effect of MSCs in our system is observed within 16 hours. MSCs with immunosuppressive characteristics were also observed in tumor-bearing mice.26-30
The chronic inflammatory conditions generated on the TLRLs treatment not only affects ζ-chain expression and the ex vivo function of T and NK cells but also dramatically changes the general immune status of the treated mice, as reflected by reduced survival of LPS-treated mice after influenza virus infection relative to control mice. We suggest that the T helper function is also likely to be compromised during the induced chronic inflammation. This could lead to broad immunosuppression on sustained treatment with a single TLRL, which is not limited to T and NK cells but could also include B-cell dysfunction and decreased humoral immune responses. Indeed, it was demonstrated recently that repeated immunization with the TLRL CpG-ODN alters the morphology and function of mouse lymphoid organs, as reflected by reduced primary humoral responses. However, the effect of this treatment on T- and NK-cell function was not assessed.31
The strong and consistent association between ζ-chain down-regulation and the T- and NK-cell immunosuppression strongly suggests ζ-chain as a biomarker indicating the appearance of a chronic inflammation-dependent immunosuppressive environment. Moreover, the observed kinetics of ζ-chain expression, which correlates with the emerging chronic inflammation and its disappearance, support its measurement as an indicator of chronic inflammation accompanied by immunosuppression. Measurement of ζ-chain expression levels could be a valuable tool for the prevention/prediction of complications associated with pathologies characterized by chronic inflammation that eventually result in immunosuppression. We also demonstrate that ζ-chain down-regulation observed under TLR-induced chronic inflammatory conditions is controlled at the post-translational level, and by using ζ-transgenic mice expressing truncated ζ-chains, we identified a 35-aa region within the ζ-chain that is responsible for its targeting to degradation. Specific aa within this region could serve as targets for post-translational modifications. Alternatively, this region could serve as a site for association with protein(s) involved in targeting the ζ-chain to degradation.
Based on our data and the results of others, we propose a model (Figure 7) explaining the TLR-mediated T- and NK-cell immunosuppression and its association with ζ-chain down-regulation. Persistent antigen-independent activation of single or multiple TLRs, expressed on NK cells and MSCs, mediated via TLR agonists (pathogenic or endogenous), in conjunction or separately of an antigen-dependent T-cell activation mediated via the TCR, when stimulated, lead to an inflammatory response characterized by the production of IFN-γ. These activation pathways are characteristic of both sterile and infectious pathologies. Elevated levels of IFN-γ, together with TLRL persistence, lead to the recruitment/expansion and activation of MSCs within the affected tissue and lymphatic organs. The continuous presence of activated MSCs affects the surrounding T and NK cells, leading to their hyporesponsiveness, associated with the loss of ζ-chain expression. Thus, ζ-chain expression levels could serve as a biomarker “sensing” changes in the chronic inflammatory immunosuppressive environment and indicative of immunosuppression. Our results lead us to propose that the nature of the generated TLR-mediated inflammatory response, whether acute or chronic, will dictate whether its immunologic consequence is beneficial or harmful. Whereas an acute inflammatory response is vital for the immediate immune defense against pathogens and for the clearance of abnormal self-cells and molecules, a chronic inflammatory response could be detrimental to the host under conditions in which the host is unable to clear the pathogen because of the developing immunosuppression. In fact, TLRs have been shown to play a key role in the development of widespread pathologies.32 Because TLRs, and particularly TLR4, recognize endogenous molecules,5 TLRLs might have an important role in the induction of the local and systemic inflammation characterizing “sterile” pathologies, such as autoimmune diseases, cancer, and other pathologies characterized by chronic inflammation, in which the cause is unknown. We suggest that immunosuppression resulting from TLR-mediated chronic inflammation evolved to limit immunopathology in the context of chronic stimulation of the innate immune system and avoid tissue damage, sepsis, organ failure, and death. Thus, the delicate balance between inflammatory response and its extent is critical to a normal or immunosuppressed immune system.
Model depicting TLR-mediated T- and NK-cell immunosuppression. (A) Persistent Ag-independent activation of NK cells and MSCs by single or multiple TLRLs, typical of pathogens (infections) or endogenous compounds (cancer and autoimmune diseases), leads to an inflammatory response characterized by the production of IFN-γ. This can occur in conjunction with Ag-dependent T cell activation, which also contributes to the increased concentrations of IFN-γ. (B) Elevated levels of IFN-γ, together with TLRL persistence, lead to the recruitment/expansion and activation of MSCs within the affected tissue and lymphatic organs. (C) The continuous presence of activated MSCs affects the surrounding T and NK cells, leading to their hyporesponsiveness and loss of ζ-chain expression, which is a biomarker “sensing” changes in the chronic inflammatory immunosuppressive environment.
Model depicting TLR-mediated T- and NK-cell immunosuppression. (A) Persistent Ag-independent activation of NK cells and MSCs by single or multiple TLRLs, typical of pathogens (infections) or endogenous compounds (cancer and autoimmune diseases), leads to an inflammatory response characterized by the production of IFN-γ. This can occur in conjunction with Ag-dependent T cell activation, which also contributes to the increased concentrations of IFN-γ. (B) Elevated levels of IFN-γ, together with TLRL persistence, lead to the recruitment/expansion and activation of MSCs within the affected tissue and lymphatic organs. (C) The continuous presence of activated MSCs affects the surrounding T and NK cells, leading to their hyporesponsiveness and loss of ζ-chain expression, which is a biomarker “sensing” changes in the chronic inflammatory immunosuppressive environment.
Because of the key role of TLRLs in activation of the innate immune system leading mostly to a Th1-dependent inflammatory response, these agents are currently being tested as adjuvants for antimicrobial, antiallergic, and anticancer immunotherapy.5,33 However, based on our results, the feasibility of using TLRLs in a given therapy must be carefully investigated; the duration of such a treatment will dictate whether its effect is beneficial or harmful to the patient. Our description of the immunosuppressive process of the adaptive and innate immune systems triggered upon sustained activation of individual TLRs may allow a range of challenges in immunology and medicine to be addressed, with the goal of developing modalities that will block the inflammatory environment specifically induced by TLRLs. Identification of specific TLRs involved in the induction of chronic inflammation in a given pathology (eg, TLR9 in Lupus, TLR4 in LPS-mediated septic shock) could enable the development of controlled and disease-specific immunotherapy. Moreover, the mouse model system that we used in the current study might serve as a platform for testing new or currently used drugs for their ability to reduce chronic inflammation and to avoid complications resulting from the associated immunosuppression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the support of the Society of Research Associates of the Lautenberg Center, the Concern Foundation of Los Angeles, and the Harold B. Abramson Chair in Immunology. Thanks to Dr Elizabeth Shores from the FDA, National Institutes of Health, Bethesda, MD, for providing us with ζ-transgenic and ζ−/− mice. We thank Dr Eyal Raz from the University of California, San Diego, for providing the CpG-ODNs and (m)CpG-ODNs. Thanks also to Prof Eli Kedar and Dr Aviva Joseph for helping us with MLV preparation and to Prof Zichria Zakay-Rones and Roi Gazit for providing the influenza virus. Thanks to Dr Tali Nave for helping with Northern blot analysis. Thanks also to Efrat Manaster, Dr Shelley Shwarzbaum, Dr Steve Caplan, and Prof Rachel Ehrlich for critical reading of the manuscript.
This study was supported by the Israel Academy of Sciences and Humanities, the Israeli Ministry of Health, the Israel Cancer Association, the Joint German-Israeli Research Program (DKFZ), the Israel Cancer Research Fund (ICRF), and by the Joseph and Matilda Melnick Funds.
National Institutes of Health
Authorship
Contribution: I.V. designed and performed research and wrote the paper; L.B., R.G., and E.S. performed research; L.W. contributed vital new reagents; E.P. contributed vital new reagents or analytical tools and analyzed data; M.P. contributed vital new reagents or analytical tools; and M.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Michal Baniyash, Lautenberg Center for General and Tumor Immunology, Hebrew University-Hadassah Medical School, PO Box 12272, Jerusalem 91120, Israel; e-mail: baniyash@cc.huji.ac.il.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal