Abstract
Human leukocyte antigen class I molecules expressed by tumor cells play a central role in the regulation of natural killer (NK) cell–mediated immune responses. The proteasome inhibitor bortezomib has demonstrated significant activity in multiple myeloma (MM). We hypothesized that treatment of MM with bortezomib results in the reduction of cell-surface expression of class I and thereby sensitizes MM to NK cell–mediated lysis. Here we report that bortezomib down-regulates class I in a time- and dose-dependent fashion on all MM cell lines and patient MM cells tested. Downregulation of class I can also be induced in vivo after a single dose of 1.0 mg/m2 bortezomib. Bortezomib significantly enhances the sensitivity of patient myeloma to allogeneic and autologous NK cell–mediated lysis. Further, the level of decrease in class I expression correlates with increased susceptibility to lysis by NK cells. Clinically relevant bortezomib concentrations do not affect NK-cell function. Our findings have clear therapeutic implications for MM and other NK cell–sensitive malignancies in the context of both allogeneic and autologous adoptively transferred NK cells.
Introduction
Multiple myeloma (MM) is a prototypic B-cell malignancy with overall survival varying from a few months to more than 15 years with melphalan-based autologous stem cell transplantation.1 It is well established that patients with abnormal, especially hypodiploid karyotypes, and high lactic dehydrogenase have a worse prognosis.2 Recently, we have reported that approximately 13% of MM patients have a high-risk prognostic score defined by the differential expression of 70 key genes, many of which are located on chromosome 1.3 These patients have a poor outcome compared with low-risk patients, with inferior actuarial event-free survival (18% vs 60%, hazard ratio (HR) = 4.51) and overall survival (28% vs 78%, HR = 5.16) at 5 years despite intensive treatment on total therapy tandem transplantation regimens and the incor-poration of novel agents, such as bortezomib into up-front management.3 There is therefore a need for novel treatment approaches that target the melphalan-refractory myeloma stem cell pool in high-risk patients and synergize with currently available cytoreductive regimens
Killer-cell immunoglobulin-like receptor (KIR)-ligand (KIR-L) mismatched natural killer (NK) cells have potent antileukemic effects in the setting of heavily T cell–depleted haplo-identical allogeneic transplantation, but the majority of MM patients cannot tolerate the associated toxicity of such regimens.4 However, we have recently demonstrated in a pilot trial that T cell–depleted haplo-identical KIR-L mismatched NK cells can be safely given to high-risk MM patients in the setting of an autologous transplantation without evidence of graft-versus-host disease.5 Unfortunately, not all NK cells transfused are alloreactive; indeed, the majority are inhibited by patient human leukocyte antigen (HLA) class I, particularly HLA-C and -Bw4 molecules.6 NK-cell activity is regulated by a dynamic balance between inhibitory and activating receptors that recognize ligands on target cells, with the inhibitory signals typically being dominant to prevent destruction by NK cells of healthy cells.7-11 It has been postulated that mature NK cells express at least one inhibitory receptor for autologous HLA class I, thus preserving self-tolerance. In contrast, NK cells avidly lyse tumor cells that do not display such inhibitory KIR-L. A classic example is the cell line K562, which does not express HLA class I.
HLA class I molecules consist of a major histocompatibility complex–encoded heavy chain, the light chain β2-microglobulin (β2M),12 and a third subunit, a peptide of 8 to 10 amino acids.12,13 In humans, class I heavy chains are encoded by 3 loci, HLA-A, -B, and -C. The proteasome is responsible for the generation of peptides, which are transported to the endoplasmic reticulum. There they combine with HLA class I and β2M, and are transported to the cell surface via the Golgi apparatus.14 Binding of peptides to HLA class I is essential for the stability of HLA class I at the cell surface. HLA class I molecules/β2M complexes, without peptide, are retained in the endoplasmic reticulum and Golgi apparatus. Some empty HLA class I molecules/β2M complexes “fall off” the cell surface, as is demonstrated by the T2 cell line, which has a defect in transporting peptides from the cytosol into the endoplasmic reticulum.15-17 Proteasome inhibition reduces the amount of peptide available to bind to HLA class I and consequently decreases HLA class I present at the cell surface.18 We hypothesized that bortezomib, a partial proteasome inhibitor that is clinically approved for the treatment of refractory/relapsed myeloma,19-21 could reduce HLA expression on MM cells. Herein we report a novel mechanism of action of bortezomib and suggest that bortezomib can be used as an immunomodulating agent to sensitize MM cells to NK cell–mediated killing.
Methods
Cells
The following cell lines were obtained from American Type Culture Collection (Manassas, VA): U266, RPMI 8226, H929, JJN3, EJM, MM-S1, OCI-My5, K562, Caki-1, Caki-2, MDA-231, and MCT. ARP-1 and ARK were developed in our institute. L-363 was kindly provided by Protein Design Labs BioPharma (Fremont, CA). The 8226/R5 bortezomib-resistant MM cell line22 was a kind gift from Dr R. Buzzeo (H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL). Peripheral blood samples and bone marrow samples were obtained from patients or healthy donors after informed consent in accordance with the Declaration of Helsinki. Approval was obtained by the University of Arkansas for Medical Sciences Institutional Review Board for these studies. CD56+/CD3− NK cells, CD138+ MM cells, monocytes, B cells and CD34+ progenitor cells were isolated from peripheral blood or bone marrow using magnetic bead selection (Miltenyi Biotech, Auburn, CA). Dendritic cells were generated from monocytes as described.23 All selected cells were routinely more than 95% pure.
Reagents and antibodies
Clinical grade bortezomib (Velcade) was purchased from or donated by Millennium Pharmaceuticals (Cambridge, MA). The proteasome-specific inhibitors, lactacystin (lac) and adaAhx3L3VS (adamantane-acetyl-(6-aminohexanoyl)3-(leucinyl)3-vinyl-(methyl)-sulfone, AdaA; EMD Biosciences, La Jolla, CA) were stored as a 5 mM stock solution in DMSO at −80°C. Cell-surface protein expression was determined by standard flow cytometry using unconjugated or fluorescein isothiocyanate (FITC), phycoerythrin, allophycocyanin-conjugated antibodies to: CD3, CD14, CD19, CD45, CD56, CD83, CD138 (BD PharMingen, San Diego, CA), HLA-ABC (clone B9.12.1), CD34 (Immunotech, Marseille, France), MICA/B (Serotec, Raleigh, NC), TRAIL-R1/death receptor (DR)4, TRAIL-R2/DR5 (Biolegend, San Diego, CA), UL 16 binding protein (ULBP)1, ULBP2, and ULBP3 (R&D Systems, Minneapolis, MN). FITC-conjugated goat anti–mouse IgG (BD, San Jose, CA), FITC-conjugated F(ab′)2 fragment of rabbit antihuman IgM (Dako, Carpinteria, CA), FITC-conjugated antihuman IgG (Zymed Laboratories, Carlsbad, CA), and antihuman HLA-C mAb (clone WK4C11, gift of Dr A. Mulder, Leiden University Medical Center, Leiden, the Netherlands) were also used. The rNKp30/human IgG1 Fc chimera, rNKp44/human IgG1 Fc chimera, and rNKp46/human IgG1 Fc chimera were purchased from R&D Systems. Human IgG was purchased from Caltag Laboratories (Burlingame, CA). The procedure for staining cells with immunoglobulin fusion proteins has been described.24 The anti-HLA class I antibody used in blocking experiment, clone B9.12.1, was purchased from Immunotech. Control mAb, antihuman TRAIL mAb (Biolegend), anti-hNKG2D, anti-hNKp30, anti-hNKp44, and anti-hNKp46 mAbs (R&D Systems) were used in NK receptor blocking experiments. All monoclonal antibodies were present at the final concentration of 10 μg/mL. The HLA-C binding peptides (peptide 1, EGDCAPEEK; peptide 2, KAAVDLSHFL; peptide 3, RYPPVIVAY; Bio-Synthesis, Lewisville, TX) were added into cell culture media at a concentration of 100 μg/mL. Peptides 1 and 2 have been reported to bind HLA-C07 and HLA-C08, respectively.23,25 The design of peptide 3 was based on published optimal binding motifs26 and the HLA-C0702 binding score obtained with BIMAS HLA peptide binding prediction software.27 The irrelevant peptides (peptide 4, HIVB35, HPDIVIYQY; peptide 5, A1B35, EVDPIGHLY; Bio-Synthesis) were used as control. Human β2M (Sigma-Aldrich, St Louis, MO) was added in peptide experiments at 2 μg/mL. Annexin V was obtained from the Vybrant apoptosis assay kit (Invitrogen, Carlsbad, CA) and propidium iodide (PI) from Sigma-Aldrich.
In vitro treatment, acid stripping, and flow cytometry
MM, breast cancer, renal carcinoma cell lines, and patient MM cells were treated with various concentrations of bortezomib at indicated times to study down-regulation of HLA class I and specifically HLA-C on the cell surface. Controls were incubated in medium alone. Half of the cells were immediately stained for flow cytometry and/or immunofluorescence analysis. The other half was recultured without bortezomib for an additional 6 days. Acid treatment of JJN3 was performed to remove HLA class I molecules from the cell surface as previously described.28 Cells after acid stripping were recultured in the presence or absence of 1 μM bortezomib and incubated for indicated hours at 37°C to study HLA class I reexpression. Plasma cells were purified from BM aspirates collected before and 48 hours after a single dose of bortezomib (1.0 mg/m2). These samples were immediately cryopreserved, subsequently simultaneously thawed, treated identically, and analyzed in the same experiment. In all experiments, cells were analyzed for HLA class I and HLA-C expression after gating on PI negative or PI and annexin V double negative cells. The percentage of HLA expression decrease was calculated as 100 × [mean fluorescence intensity (MFI) of control − MFI of treated cells]/MFI of control.
Immunofluorescence analysis by confocal microscopy
JJN3 cells were labeled in suspension with antihuman HLA-ABC (clone W6/32; Serotec, Oxford, United Kingdom) at 4°C for 30 minutes. After washing with ice-cold phosphate-buffered saline (PBS), cells were incubated with Alexa fluor 594-conjugated antimouse IgG secondary antibody (Molecular Probes, Eugene, OR) at 4°C for 30 minutes. Cells were washed twice with ice-cold PBS, fixed with 4% paraformaldehyde at 4°C for 10 minutes, and then immobilized on glass slides. 4,6 Diamidino-2-phenylindole (Sigma-Aldrich) 1 μg/mL in PBS was used for staining nuclei. Slides were viewed with a Zeiss Axiovert 200M (Carl Zeiss, Jena, Germany) using a 63×/1.4 NA objective lens and Immersol 518F (Zeiss, Oberkochen, Germany). Spinning-disk confocal image stacks were acquired using a Retiga EXi camera (QImaging, Burnaby, BC) fitted with a BD Bioimaging CARVII spinning-disc confocal accessory (BD Bioimaging, Rockville, MD) with microscope, as described previously.29 Images were deconvolved with Hugens Essential 2.7 software (Scientific Volume Imaging, Hilversum, the Netherlands) from mid-cell image planes, processed with Adobe Photoshop version CS2 software (Adobe Systems, San Jose, CA), and were analyzed for the cell-surface expression of class I using lPLab version 3.9.5 software (BD Bioimaging). The fluorescence intensity was corrected by subtracting the blank field intensity. A total of 30 cells per group from 3 independent experiments were considered, and the mean intensity (+ SD) was plotted.
51Cr release assay
Cytotoxicity mediated by NK cells was determined using a standard 4-hour 51Cr release assay at the indicated effector/target (E/T) ratios as reported.30 The NK cell-sensitive cell line K562 was used as positive control. Specific lysis percentage was calculated as (test release − spontaneous release)/(maximal release − spontaneous release) × 100. All experiments were performed in triplicate wells and at least 3 independent experiments were completed.
Statistical analysis
Results of experimental points obtained from multiple experiments were reported as mean. Significance levels were determined by Student t test analysis. P values of .05 or less were considered significant.
Results
Proteasome inhibition down-regulates HLA-ABC and HLA-C on MM cell lines and patient MM cells in vitro
Bortezomib concentrations used in vitro experiments were based on known pharmacokinetics. Bortezomib at 1.0 mg/m2 inhibits the proteasome by 60% with proteasome function returning to baseline by 72 hours.31,32 Bortezomib is rapidly cleared from the plasma; 24 hours after a dose of 1.3 mg/m2, residual plasma levels are approximately 2.5 nM.33 Down-regulation of HLA-ABC after 10 nM bortezomib treatment was observed in all myeloma cell lines tested (n = 10) except for the bortezomib-resistant cell line 8226/R5 (Figure 1A), by a median of 49% (range, 31%-70%). Bortezomib-sensitive JJN3 cells showed persistent reduction of HLA class I during reculture without bortezomib for 3 days followed by slow restoration of HLA class I with recovery of baseline levels at 6 days (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). HLA-ABC down-regulation was also observed in 10 to 50 nM bortezomib-treated primary MM cells (n = 6, Figure 1B). The median reduction of HLA class I among 6 patient primary MM samples was 47% (range, 24%-55%). Three of the primary MM cells (subjects 3, 5, and 6) were obtained from patients who were refractory to multiagent chemotherapy regimens containing bortezomib. We did not observe down-regulation of HLA class I on 2 renal cell carcinoma and 2 breast cancer cell lines, which is consistent with the findings of other investigators.34 We also tested healthy cells, including B cells, monocytes, dendritic cells, and CD34+ progenitor cells, and bortezomib did not induce down-regulation of HLA class I (data not shown). These results suggest that MM cells are particularly sensitive to the effects of bortezomib, which is in keeping with its remarkable clinical activity in MM.
MM cells exposed to bortezomib have decreased HLA class I expression on the cell surface. (A) Bortezomib induced identical down-regulation of HLA class I on MM cell lines after gating on PI negative or PI and annexin V double negative cells. MM cell lines were exposed to 10 nM bortezomib for 24 hours, and then stained with annexin V FITC/HLA-ABC allophycocyanin/PI. Class I expression on the cell surface was analyzed by flow cytometry; 8226/R5 was a bortezomib–resistant MM cell line. The data represent one of 3 individual experiments. The percentage of HLA class I decrease was determined by: 100 × (MFI of control − MFI of treated cells)/MFI of control. (B) Reduction of HLA class I expression was tested on patient MM after bortezomib treatment. The patient MM cells were treated with 10 to 50 nM bortezomib for 16 to 24 hours. UPN indicates unique patient number. (C) Viability of MM cell lines after exposure to bortezomib. MM cells were incubated with 10 nM bortezomib for 24 hours. Cells were stained with annexin V and PI and analyzed by flow cytometry. One of 3 representative experiments was shown. The percentages are shown of cells that are annexin V and PI double negative (lower left quadrant), double positive (upper right quadrant), solely annexin V positive (lower right quadrant), and solely PI positive (upper left quadrant).
MM cells exposed to bortezomib have decreased HLA class I expression on the cell surface. (A) Bortezomib induced identical down-regulation of HLA class I on MM cell lines after gating on PI negative or PI and annexin V double negative cells. MM cell lines were exposed to 10 nM bortezomib for 24 hours, and then stained with annexin V FITC/HLA-ABC allophycocyanin/PI. Class I expression on the cell surface was analyzed by flow cytometry; 8226/R5 was a bortezomib–resistant MM cell line. The data represent one of 3 individual experiments. The percentage of HLA class I decrease was determined by: 100 × (MFI of control − MFI of treated cells)/MFI of control. (B) Reduction of HLA class I expression was tested on patient MM after bortezomib treatment. The patient MM cells were treated with 10 to 50 nM bortezomib for 16 to 24 hours. UPN indicates unique patient number. (C) Viability of MM cell lines after exposure to bortezomib. MM cells were incubated with 10 nM bortezomib for 24 hours. Cells were stained with annexin V and PI and analyzed by flow cytometry. One of 3 representative experiments was shown. The percentages are shown of cells that are annexin V and PI double negative (lower left quadrant), double positive (upper right quadrant), solely annexin V positive (lower right quadrant), and solely PI positive (upper left quadrant).
Bortezomib induces apoptosis in MM cells, which potentially may be accompanied by loss of surface molecule expression. We therefore compared the analysis of HLA class I expression by gating on PI and annexin V double negative cells (to exclude early apoptotic cells) and PI single negative cells and obtained identical results in terms of reduction in HLA class I expression (Figure 1A). The proportion of live cells and the kinetics of apoptosis after exposure to bortezomib are depicted in Figures 1C and 2G, respectively.
Bortezomib reduces HLA class I expression on JJN3 and primary MM in a dose- and time-dependent fashion. MM cells were incubated with various doses of bortezomib for indicated times. PI-negative cells were gated and analyzed for class I expression. (A) Dose response: JJN3 was exposed to increasing doses of bortezomib for 24 hours. (B) Time course: JJN3 cells were incubated at 10 nM bortezomib for increasing durations of time. (C) Dose response: Patient MM was exposed to increasing doses of bortezomib for 24 hours. (D) Time course: Patient MM was incubated at 10 nM bortezomib for increasing durations of time. Class I expression was also tested on JJN3 after bortezomib by spinning-disc confocal microscopy. (E) Dose response: JJN3 was exposed to increasing doses of bortezomib for 24 hours. (F) Time course: JJN3 cells were incubated at 10 nM bortezomib for increasing durations of time. Images were acquired using a Zeiss Axiovert 200M microscope (Carl Zeiss) fitted with a BD Bioimaging CARV II spinning-disc confocal accessory. Mid-cell confocal images were presented (original magnification, ×250). The fluorescence intensity was quantified using IPLab version 3.9.5 software and corrected by substracting the blank field intensity. A total of 30 cells per group from 3 independent experiments were considered and the mean intensity (± SD) was plotted. (G) The kinetics of apoptosis on JJN3 after drug treatment. JJN3 was treated with 10 nM bortezomib for indicated times. Cells were stained with annexin V and PI and analyzed by FACS. The data represent 1 of 3 individual experiments. The percentages are shown of cells that are annexin V and PI double negative (lower left quadrant), double positive (upper right quadrant), solely annexin V positive (lower right quadrant), and solely PI positive (upper left quadrant).
Bortezomib reduces HLA class I expression on JJN3 and primary MM in a dose- and time-dependent fashion. MM cells were incubated with various doses of bortezomib for indicated times. PI-negative cells were gated and analyzed for class I expression. (A) Dose response: JJN3 was exposed to increasing doses of bortezomib for 24 hours. (B) Time course: JJN3 cells were incubated at 10 nM bortezomib for increasing durations of time. (C) Dose response: Patient MM was exposed to increasing doses of bortezomib for 24 hours. (D) Time course: Patient MM was incubated at 10 nM bortezomib for increasing durations of time. Class I expression was also tested on JJN3 after bortezomib by spinning-disc confocal microscopy. (E) Dose response: JJN3 was exposed to increasing doses of bortezomib for 24 hours. (F) Time course: JJN3 cells were incubated at 10 nM bortezomib for increasing durations of time. Images were acquired using a Zeiss Axiovert 200M microscope (Carl Zeiss) fitted with a BD Bioimaging CARV II spinning-disc confocal accessory. Mid-cell confocal images were presented (original magnification, ×250). The fluorescence intensity was quantified using IPLab version 3.9.5 software and corrected by substracting the blank field intensity. A total of 30 cells per group from 3 independent experiments were considered and the mean intensity (± SD) was plotted. (G) The kinetics of apoptosis on JJN3 after drug treatment. JJN3 was treated with 10 nM bortezomib for indicated times. Cells were stained with annexin V and PI and analyzed by FACS. The data represent 1 of 3 individual experiments. The percentages are shown of cells that are annexin V and PI double negative (lower left quadrant), double positive (upper right quadrant), solely annexin V positive (lower right quadrant), and solely PI positive (upper left quadrant).
Next we established that bortezomib reduced HLA class I expression in a dose- and time-dependent fashion on the JJN3 cell line and primary MM cells using flow cytometry (Figure 2A-D). Reduction of HLA class I expression on the plasma membrane of JJN3 cells was further confirmed by immunofluorescence analysis and confocal microscopy (Figure 2E,F). HLA-C is the principal inhibitory KIR-L. We therefore demonstrated that, with an HLA-C specific antibody, there was also equivalent down-regulation of HLA-C (data not shown).
Reduced provision of peptides resulting from proteasome inhibition by bortezomib is responsible for down-regulation of HLA-C
Bortezomib has a multiplicity of effects on cells, and it is therefore important to establish a mechanistic explanation for the observed down-regulation of HLA class I. We first reproduced down-regulation of HLA class I with the highly specific proteasome inhibitors lactacystin and the peptide vinyl sulfone AdaA (Figure S2A,B). We next studied in detail the ability of bortezomib to inhibit the generation of HLA class I at the cell surface by acid stripping HLA class I from the cell surface and examining the reexpression of HLA class I in the presence or absence of bortezomib. The kinetics of HLA reexpression in the presence or absence of bortezomib after acid stripping is presented in Figure S3. Immediately after treatment of JJN3 with acid, the expression of class I (MFI = 144) was nearly undetectable compared with untreated controls (MFI = 3096). In the absence of bortezomib, cells reexpressed 34% (MFI = 1053) of the pretreatment level of HLA class I in 10 hours, whereas in the presence of 1 μM bortezomib, only 17% was reexpressed (MFI = 524). These data indicate that bortezomib affects the expression of newly formed HLA class I molecules at the cell surface. Finally, we examined whether exogenous HLA-C binding peptides could stabilize HLA-C at the cell surface of bortezomib-treated cells in analogy to classic peptide binding experiments performed with the transporter associated with antigen processing–deficient cell line T2.15 JJN3 is HLA-C0702, 0802 to which peptide 1, peptide 2, and peptide 3 bind. JJN3 cells cocultured in the presence of the HLA-C binding peptides during treatment with bortezomib retained normal expression of HLA-C, in direct contrast to JJN3 cells incubated with bortezomib without the HLA-C binding peptides, which exhibited reduced levels of HLA-C expression (Figure 3A). The addition of irrelevant control peptides did not have this effect (data not shown). These results indicate that provision of exogenous HLA-C binding peptides rescued expression of HLA-C during bortezomib treatment and stabilized HLA-C at the cell surface, thus establishing a mechanism of action. We next used bortezomib-treated and untreated cells as targets and performed cytotoxicity assays. We found that provision of exogenous HLA-C binding peptides, but not irrelevant peptides, prevented the bortezomib-mediated increase in NK cell–mediated lysis (Figure 3B). Taken together, these data support the hypothesis that changes in HLA class I expression after bortezomib treatment may enhance NK cell–mediated lysis of MM cells.
Provision of exogenous HLA-C binding peptides rescues expression of HLA-C during bortezomib treatment and also prevents the bortezomib–mediated increase in NK cell–mediated lysis. (A) JJN3 cells were cocultured in the presence or absence of the HLA-C binding peptides during 16 hours treatment with bortezomib. Human β2M was added in the medium. Cells were gated on PI–negative cell population and analyzed by flow cytometry for HLA-C expression. The percentage HLA-C expression was shown. Data were reported as means (± SD) of 3 individual experiments. (B) JJN3 cells were treated with 50 nM bortezomib for 18 hours in the presence or absence of the HLA-C binding peptides or irrelevant peptides. Treated and untreated cells were then used as targets in a standard 4-hour 51Cr release assay. K562 was used as a positive control for maximum lysis. Data were reported as means (± SD).
Provision of exogenous HLA-C binding peptides rescues expression of HLA-C during bortezomib treatment and also prevents the bortezomib–mediated increase in NK cell–mediated lysis. (A) JJN3 cells were cocultured in the presence or absence of the HLA-C binding peptides during 16 hours treatment with bortezomib. Human β2M was added in the medium. Cells were gated on PI–negative cell population and analyzed by flow cytometry for HLA-C expression. The percentage HLA-C expression was shown. Data were reported as means (± SD) of 3 individual experiments. (B) JJN3 cells were treated with 50 nM bortezomib for 18 hours in the presence or absence of the HLA-C binding peptides or irrelevant peptides. Treated and untreated cells were then used as targets in a standard 4-hour 51Cr release assay. K562 was used as a positive control for maximum lysis. Data were reported as means (± SD).
Down-regulation of class I expression by bortezomib also occurs in vivo in primary MM cells
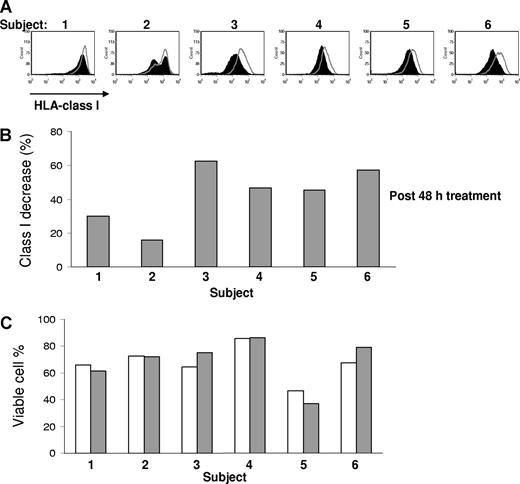
We next studied whether down-regulation of class I molecules on patient MM cells could also be induced in vivo after administration of bortezomib. We therefore analyzed the HLA class I surface expression by flow cytometry on purified patient MM cells pretreatment and 48 hours after a single dose of 1.0 mg/m2 bortezomib. As shown in Figure 4, surface level of HLA class I was significantly decreased (P = .002). Bortezomib treatment down-regulated class I by a median of 47% (range, 16%-63%, n = 6) in vivo, compared with pretreated cells. In currently used dose schedules, there is no cumulative inhibition of proteasome function by bortezomib, and we therefore did not study the effect of repeated dosing on HLA class I expression.35
Bortezomib down-regulates HLA class I in vivo on patient MM cells. Comparison of surface expression of HLA class I on the pretreatment and posttreatment of primary MM cells from 6 patients. The paired samples were cryopreserved, simultaneously thawed, and analyzed in the same experiment. PI-negative cells were analyzed for HLA class I expression. Open curves were before bortezomib. The black curves were 48 hours after a single dose of bortezomib 1.0 mg/m2. The percentage of class I expression decrease was determined by 100 × (MFI of pretreatment sample − MFI of posttreatment sample)/MFI of pretreatment sample. Statistically significant difference of class I expression between the pre- and post- bortezomib treatment samples was present (P = .002, Student t test). The viability of pretherapy (▭) and after therapy ( ) samples after thawing was similar.
) samples after thawing was similar.
Bortezomib down-regulates HLA class I in vivo on patient MM cells. Comparison of surface expression of HLA class I on the pretreatment and posttreatment of primary MM cells from 6 patients. The paired samples were cryopreserved, simultaneously thawed, and analyzed in the same experiment. PI-negative cells were analyzed for HLA class I expression. Open curves were before bortezomib. The black curves were 48 hours after a single dose of bortezomib 1.0 mg/m2. The percentage of class I expression decrease was determined by 100 × (MFI of pretreatment sample − MFI of posttreatment sample)/MFI of pretreatment sample. Statistically significant difference of class I expression between the pre- and post- bortezomib treatment samples was present (P = .002, Student t test). The viability of pretherapy (▭) and after therapy ( ) samples after thawing was similar.
) samples after thawing was similar.
Bortezomib treatment enhances the sensitivity of patient MM cells to allogeneic and autologous NK cell–mediated lysis in vitro
We have shown that bortezomib concentrations of approximately 10 to 50 nM can down-regulate HLA class I inhibitory KIR-L on primary MM cells. Because decreased HLA class I expression is associated with increased sensitivity of cells to NK cell–mediated lysis, we evaluated whether bortezomib treatment would augment the sensitivity of primary MM cells to allogeneic NK cell–mediated cytolysis in vitro. The tumor cells were treated with 10 to 50 nM bortezomib for 16 to 24 hours. Treated and untreated cells were then used as targets in a standard 4-hour 51Cr release assay. Exposure to bortezomib was able to significantly enhance the sensitivity of these cells to lysis by allogeneic NK cells. There was a median increase of 3.4-fold (range, 2.6-9.6, n = 4) in cytotoxicity after bortezomib treatment, compared with untreated controls (Figure 5; Table 1). In one experiment, targets expressed all known inhibitory KIR-L; hence, there was low NK-cell alloreactivity against untreated targets. The treatment of this patient's MM cells with bortezomib produced a 9.6-fold increase in allogeneic NK cell–mediated lysis. Lactacystin treatment of JJN3 cells yielded a similar result, indicating that enhancement of NK cell–mediated toxicity was because of proteasome inhibition (Figure S2C).
Bortezomib treatment enhances the sensitivity of patient MM to allogeneic and autologous NK cell–mediated lysis. The patient MM cells were treated with 10 to 50 nM bortezomib for 16 to 24 hours. Treated and untreated cells were then used as targets in a standard 4-hour 51Cr release assay. UPN indicates unique patient number.
Bortezomib treatment enhances the sensitivity of patient MM to allogeneic and autologous NK cell–mediated lysis. The patient MM cells were treated with 10 to 50 nM bortezomib for 16 to 24 hours. Treated and untreated cells were then used as targets in a standard 4-hour 51Cr release assay. UPN indicates unique patient number.
Enhanced cytotoxicity of NK cells against bortezomib-treated patient MM cells
| Patient . | Untreated lysis plus or minus SD, % . | Treated lysis plus or minus SD, % . | Fold increase, no. . | E/T ratio . |
|---|---|---|---|---|
| Allogenic NK | ||||
| 1 | 13.9 ± 2.8 | 39.6 ± 3.9 | 2.8 | 15:1 |
| 2 | 8.6 ± 6.6 | 22.7 ± 5.1 | 2.6 | 14:1 |
| 3 | 6.4 ± 3.0 | 61.5 ± 7.2 | 9.6 | 20:1 |
| 4 | 4.8 ± 2.7 | 19.0 ± 1.7 | 4.0 | 20:1 |
| Autologous NK | ||||
| 5 | 2.6 ± 3.7 | 27.6 ± 4.4 | 10.6 | 15:1 |
| 6 | 0.2 ± 3.4 | 45.9 ± 7.7 | 229.5 | 9:1 |
| Patient . | Untreated lysis plus or minus SD, % . | Treated lysis plus or minus SD, % . | Fold increase, no. . | E/T ratio . |
|---|---|---|---|---|
| Allogenic NK | ||||
| 1 | 13.9 ± 2.8 | 39.6 ± 3.9 | 2.8 | 15:1 |
| 2 | 8.6 ± 6.6 | 22.7 ± 5.1 | 2.6 | 14:1 |
| 3 | 6.4 ± 3.0 | 61.5 ± 7.2 | 9.6 | 20:1 |
| 4 | 4.8 ± 2.7 | 19.0 ± 1.7 | 4.0 | 20:1 |
| Autologous NK | ||||
| 5 | 2.6 ± 3.7 | 27.6 ± 4.4 | 10.6 | 15:1 |
| 6 | 0.2 ± 3.4 | 45.9 ± 7.7 | 229.5 | 9:1 |
Next, we determined whether bortezomib treatment would sensitize patient tumor cells to killing by autologous NK cells in vitro. Patient CD138+ plasma cells and CD56+ NK cells were isolated from bone marrow and peripheral blood mononuclear cells, respectively. The MM cells were exposed to 10 to 50 nM bortezomib for 16 to 24 hours. The cells were then washed and used in cytotoxicity assays. We found that bortezomib significantly increased the sensitivity of primary MM cells (n = 2) to autologous NK cell–mediated killing (Figure 5; Table 1). Bortezomib at 10 nM, which is expected to be a higher level than 48 hours after a dose of intravenous bortezomib 1.0 mg/m2, did not affect NK-cell viability and function (data not shown). This supports the notion that the function of adoptively transferred NK cells will not be inhibited by residual bortezomib.
Blocking with HLA-antibody increases sensitivity to NK cell–mediated lysis to similar levels achieved with bortezomib
The reduction in HLA class I expression observed after bortezomib therapy varied from approximately 15% to 60% observed in vitro and in vivo. We next studied whether this amount of HLA class I reduction is physiologically relevant and is indeed compatible with increased sensitivity to NK cell–mediated lysis. We reproduced our findings with bortezomib by treating JJN3 MM cells with a HLA blocking antibody at increasing concentrations before chromium release assays with NK effectors. The percentage of unblocked, “free,” HLA class I, able to interact with KIR receptors on NK cells, was measured (Figure 6A). Figure 6B clearly shows that there is a gradual concentration–dependent increase in NK cell–mediated lysis with addition of HLA class I blocking antibody. The degree of HLA down-regulation was therefore positively correlated with the degree of NK cell–mediated killing. Pretreatment with bortezomib down-regulated HLA class I on JJN3 by 60% (Figure 6C), which resulted in increased NK cell–mediated lysis (Figure 6D). This cytotoxicity curve was remarkably similar to the increase in NK cell–mediated lysis obtained by blocking 65% of HLA class I with HLA class I antibody. Figure 6B and D pertains to the same experiment. These findings suggest that the amount of HLA class I down-regulation mediated by bortezomib may be indeed physiologically relevant and not an epiphenomenon.
Reduced HLA class I on the myeloma cell surface results in NK cell–mediated recognition and lysis. (A) Cells were incubated with increasing concentrations of HLA-ABC blocking or isotype control antibody. PI-negative cells were gated for analysis of HLA class I expression. The percentages indicate the amount of HLA class I blocked. (B) A chromium release cytotoxicity assay demonstrated increased recognition of targets with HLA blocked. Data were reported as mean (± SD). (C) Bortezomib treatment (10 nM for 24 hours) induced a 60% decrease in cell-surface HLA class I. PI–negative cells were analyzed for HLA class I expression. The percentage of HLA class I reduction was determined by: 100 × (MFI of control − MFI of HLA-blocked or bortezomib-treated cells)/MFI of control. (D) Class I reduction by bortezomib treatment resulted in increasing killing of JJN3. Interestingly, the level of lysis was comparable between the untreated targets with 65% blocking of HLA class I and the bortezomib-treated targets, which had a 60% decrease in cell-surface HLA class I. The HLA class I devoid line K562 and bortezomib-treated JJN3 cells with 100% blocking of HLA class I were used as positive controls for maximum lysis. The “0% blocked” targets were incubated with an isotype control antibody. Panels B and D pertain to the same experiment. Data were reported as mean (± SD).
Reduced HLA class I on the myeloma cell surface results in NK cell–mediated recognition and lysis. (A) Cells were incubated with increasing concentrations of HLA-ABC blocking or isotype control antibody. PI-negative cells were gated for analysis of HLA class I expression. The percentages indicate the amount of HLA class I blocked. (B) A chromium release cytotoxicity assay demonstrated increased recognition of targets with HLA blocked. Data were reported as mean (± SD). (C) Bortezomib treatment (10 nM for 24 hours) induced a 60% decrease in cell-surface HLA class I. PI–negative cells were analyzed for HLA class I expression. The percentage of HLA class I reduction was determined by: 100 × (MFI of control − MFI of HLA-blocked or bortezomib-treated cells)/MFI of control. (D) Class I reduction by bortezomib treatment resulted in increasing killing of JJN3. Interestingly, the level of lysis was comparable between the untreated targets with 65% blocking of HLA class I and the bortezomib-treated targets, which had a 60% decrease in cell-surface HLA class I. The HLA class I devoid line K562 and bortezomib-treated JJN3 cells with 100% blocking of HLA class I were used as positive controls for maximum lysis. The “0% blocked” targets were incubated with an isotype control antibody. Panels B and D pertain to the same experiment. Data were reported as mean (± SD).
Bortezomib-mediated enhancement of lysis of MM correlates with down-regulation of class I, rather than interactions between MM cells and NK-cell receptors not belonging to the KIR family
Although we have mechanistically linked bortezomib with HLA class I down-regulation and demonstrated that the degree of class I down-regulation achieved is physiologically important, it still cannot be ruled out that interactions between other receptor families than KIR and MM cells may be of importance as well. We therefore examined the effect of bortezomib therapy on the interaction of MM cells with TRAIL, NKG2D, and the NCRs on NK cells. It has previously been reported that bortezomib up-regulates DR4 and DR5 expression on renal cell carcinoma and thereby enhances TRAIL-mediated apoptosis.34 We confirmed that bortezomib up-regulates DR4 and DR5 on MM cells (Figure 7A). Although we observed a notable increase in expression of DR4 and DR5 on JJN3 with bortezomib, blocking of TRAIL on NK cells did not influence the amount of increased killing by NK cells of treated JJN3 cells (Figure 7B). In contrast, the extent of down-regulation of HLA class I on JJN3 correlated with enhanced sensitivity to NK cell–mediated cytolysis (Figure 7C). These experiments suggest that the effect of bortezomib on HLA class I rather than on DR4 and DR5 expression is the dominant mechanism for enhancing NK cell–mediated killing of the MM cell line JJN3.
Enhanced NK-cell cytotoxicity against bortezomib-treated JJN3 correlates with down-regulation of class I, rather than interactions between MM cells and NK-cell receptors not belonging to the KIR family. (A) After exposure to bortezomib (10 nM) for 14 hours, HLA class I was down-regulated, and DR4 and DR5 were up-regulated. (B) Increased NK-cell killing was not abolished by blocking TRAIL on NK cells. JJN3 was exposed to 10 nM bortezomib for 14 hours. 51Cr labeled JJN3 was incubated at 37°C with NK cells at different ratios. The supernatants were collected after 4 hours and analyzed by gamma count. (C) Augmented NK-cell cytotoxicity correlated with decreased class I. JJN3 exposed to 10 nM bortezomib for 12 hours and 14 hours were used as targets in a 4-hour 51Cr release assay. (D) Bortezomib did not affect expression of NKG2D ligands, such as MICA/B, ULBP-1, -2, and -3 on JJN3. (E) Blocking of NKG2D on effectors did not have a major effect on the increased NK-cell killing of bortezomib-treated JJN3. JJN3 cells were treated with 10 nM bortezomib for 20 hours. (F) There was no significant up-regulated expression of NCR ligands on JJN3 after treatment. (G) Blocking of NCRs on NK cells did not have a major effect on the enhanced NK-cell lysis of bortezomib-treated JJN3. JJN3 was exposed to 10 nM bortezomib for 20 hours. Data were reported as mean (± SD) for panels B,C,E, and G.
Enhanced NK-cell cytotoxicity against bortezomib-treated JJN3 correlates with down-regulation of class I, rather than interactions between MM cells and NK-cell receptors not belonging to the KIR family. (A) After exposure to bortezomib (10 nM) for 14 hours, HLA class I was down-regulated, and DR4 and DR5 were up-regulated. (B) Increased NK-cell killing was not abolished by blocking TRAIL on NK cells. JJN3 was exposed to 10 nM bortezomib for 14 hours. 51Cr labeled JJN3 was incubated at 37°C with NK cells at different ratios. The supernatants were collected after 4 hours and analyzed by gamma count. (C) Augmented NK-cell cytotoxicity correlated with decreased class I. JJN3 exposed to 10 nM bortezomib for 12 hours and 14 hours were used as targets in a 4-hour 51Cr release assay. (D) Bortezomib did not affect expression of NKG2D ligands, such as MICA/B, ULBP-1, -2, and -3 on JJN3. (E) Blocking of NKG2D on effectors did not have a major effect on the increased NK-cell killing of bortezomib-treated JJN3. JJN3 cells were treated with 10 nM bortezomib for 20 hours. (F) There was no significant up-regulated expression of NCR ligands on JJN3 after treatment. (G) Blocking of NCRs on NK cells did not have a major effect on the enhanced NK-cell lysis of bortezomib-treated JJN3. JJN3 was exposed to 10 nM bortezomib for 20 hours. Data were reported as mean (± SD) for panels B,C,E, and G.
Bortezomib treatment did not significantly affect expression of the NKG2D ligands MICA/B and ULPB-1, -2, and -3 (Figure 7D). Further blocking of NKG2D did not significantly influence the increase in NK cell–mediated lysis of bortezomib-treated JJN3 (Figure 7E). We next examined the expression of NCR ligand on JJN3 using fusion proteins of NCRs with IgFc for NKp30, NKp44, and NKp46. Figure 7F demonstrates that there was no significant increase of NCR ligands on JJN3 after treatment with bortezomib. Further blocking of NCRs did not influence the augmented NK cell–mediated killing of bortezomib-treated cells (Figure 7G).
Discussion
Bortezomib is a partial proteasome inhibitor that selectively targets the 26S subunit36 and has activity in relapsed/refractory MM.19-21 Bortezomib has well-documented antiproliferative, proapoptotic, and antiangiogenic effects.36-39 However, bortezomib also has immunomodulatory activities. Bortezomib-induced apoptosis results in increased heat shock protein 90 on the cell surface, improving cross-presentation of tumor antigens to dendritic cells.40
The proteasome is the major source of proteolytic activity involved in the generation of peptides for presentation by major histocompatibility complex class I molecules.41 We report the new observation that bortezomib down-regulates HLA class I on MM cells, resulting in increased NK cell–mediated lysis. The lack of down-regulation of HLA class I on CD34+ and healthy cells is consistent with reports that MM is significantly more sensitive to proteasome inhibition compared with CD34+ bone marrow progenitor cells.42,43 A single dose of bortezomib also down-regulated HLA class I on CD138-purified MM cells in vivo. We confirmed that HLA-C was equally down-regulated because HLA-C is the principal inhibitory ligand for KIR on NK cells. Two well-characterized highly specific proteasome inhibitors, lactacystin and AdaA, were used to substantiate that the effects observed with bortezomib were the result of proteasome inhibition and not caused by inhibition of other proteases.44 Other investigators have also shown that the generation of the stable heterodimers of class I molecules was markedly inhibited in cells treated with proteasome inhibitors.45,46
We could reproduce the increase in sensitivity of MM cells to NK cell–mediated lysis using HLA class I antibodies. The extent of HLA class I down-regulation achieved by bortezomib and HLA class I blocking antibody produced very similar increases in NK cell–mediated cytolysis, supporting the notion that the degree of HLA class I down-regulation may be indeed physiologically relevant. Further, provision of HLA-C binding peptides to bortezomib-treated cells abrogated the increased lysis of MM. HLA-C binding peptides also rescued HLA-C expression on MM, thus providing a mechanistic explanation for the effect of bortezomib. Finally, we ruled out that bortezomib did not alter interaction of MM cells with TRAIL, NKG2D, and NCRs on NK cells.
Haplo-identical donor NK cells have been transfused in combination with an autograft for MM or after immunosuppression for acute leukemia or renal cell carcinoma.5,47 These studies have demonstrated activity of haplo-identical KIR-L mismatched NK cells, but therapeutic efficacy has been hampered because only a subpopulation of transferred NK cells is allo-reactive. The bortezomib-induced down-regulation of HLA class I and enhanced NK cell–mediated killing indicate that bortezomib can recruit nonalloreactive NK-cell populations. Limited NK-cell persistence caused by active rejection by residual host lymphocytes also reduced therapeutic efficacy. Bortezomib can potentially promote NK-cell persistence by specifically depleting alloreactive T lymphocytes.48 Both effects suggest that bortezomib may play an important role in potentiating the antitumor activity of allogeneic KIR-L mismatched NK cells.
Bortezomib also sensitized primary MM cells to killing by autologous NK cells. Under normal circumstances, MM cells display self–HLA-molecules, which inactivate autologous NK cells. This also helps to explain the relative lack of activity of adoptively transfused autologous NK cells in the solid tumor setting.49 Bortezomib can overcome KIR-L inhibition of NK cells by MM and can make autologous adoptive NK-cell therapy more effective and widen its application. The half-life of bortezomib is short, and serum levels present 48 hours after a dose of bortezomib did not adversely affect NK-cell function.
Previous reports have indicated that bortezomib up-regulates the surface expression of the death receptors, including Fas, TNFR1, TRAIL-R1/DR4, TRAIL-R2/DR5, DR3, and DR6, on tumor cells. Renal cell carcinoma seems to be especially sensitive to this action of bortezomib.34,50-53 Interestingly, we did not observe down-regulation of HLA class I on renal cell and breast carcinoma cell lines. We confirmed that bortezomib up-regulates DR4 and DR5 on MM cells. However, in the case of myeloma death, receptor-mediated apoptosis is not the dominant mechanism of action because antibody-blocking of TRAIL on NK cells, which interacts with DR4 and DR5 on MM, did not abrogate the potentiating effect of bortezomib. However, down-regulation of HLA class I on MM cells correlated with increased lysis, which indicates that in the case of MM, cell-surface modulation of HLA class I is the prevailing mechanism regulating NK-cell activity. One could nevertheless take advantage of the up-regulation of DR4 and DR5 and combine bortezomib with TRAIL-receptor specific antibodies.
Bortezomib resistance has been well described and is the result of the up-regulation of various heat shock proteins, which suppress both intrinsic and extrinsic apoptotic pathways.54 It is encouraging that HLA could be down-regulated in bortezomib-refractory MM cells, but ultimately clinical studies have demonstrated that the combination of bortezomib and adoptively transferred NK cells can truly target highly chemotherapy refractory MM cells.
In conclusion, we have demonstrated that the proteasome inhibitor bortezomib down-regulates class I and enhances the sensitivity of myeloma to NK cell–mediated lysis. Our findings have important therapeutic implications for MM and other NK cell–sensitive malignancies in the context of both allogeneic and autologous adoptively transferred NK cells and combination therapy with monoclonal antibodies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grant CA55819 and Senior Award 28-06 from the Multiple Myeloma Research Foundation.
National Institutes of Health
Authorship
Contribution: J.S., G.J.T., B.B., and F.v.R. conceptualized the work and wrote the paper; J.S., T.K.G., P.A.M., S.M.S., R.E.K., and J.D.S. performed research; A.M. contributed vital new reagents; B.S. supervised core microscopy; and B.B., G.J.T., and F.v.R. enrolled and treated patients on research protocols.
Conflict-of-interest disclosure: B.B. has received research funding from Millennium Pharmaceuticals. Bortezomib was provided for these studies by Millennium Pharmaceuticals. All other authors declare no competing financial interests.
Correspondence: Frits van Rhee, University of Arkansas for Medical Sciences, Myeloma Institute for Research and Therapy, 4301 West Markham, No. 776, Little Rock, AR 72205; e-mail: vanrheefrits@uams.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal