Abstract
Glycogen storage disease type Ib (GSD-Ib) is caused by a deficiency in the glucose-6-phosphate (G6P) transporter (G6PT) that works with a liver/kidney/intestine–restricted glucose-6-phosphatase-α (G6Pase-α) to maintain glucose homeostasis between meals. Clinically, GSD-Ib patients manifest disturbed glucose homeostasis and neutrophil dysfunctions but the cause of the latter is unclear. Neutrophils express the ubiquitously expressed G6PT and G6Pase-β that together transport G6P into the endoplasmic reticulum (ER) lumen and hydrolyze it to glucose. Because we expected G6PT-deficient neutrophils to be unable to produce endogenous glucose, we hypothesized this would lead to ER stress and increased apoptosis. Using GSD-Ib mice, we showed that GSD-Ib neutrophils exhibited increased production of ER chaperones and oxidative stress, consistent with ER stress, increased annexin V binding and caspase-3 activation, consistent with an increased rate of apoptosis. Bax activation, mitochondrial release of proapoptotic effectors, and caspase-9 activation demonstrated the involvement of the intrinsic mitochondrial pathway in these processes. The results demonstrate that G6P translocation and hydrolysis are required for normal neutrophil functions and support the hypothesis that neutrophil dysfunction in GSD-Ib is due, at least in part, to ER stress and increased apoptosis.
Introduction
Glycogen storage disease type I (GSD-I) is a group of autosomal recessive disorders caused by a deficiency in the glucose-6-phosphatase-α (G6Pase-α) complex that consists of a glucose-6-phosphate transporter (G6PT, also known as SLC37A4) and the hydrolytic enzyme G6Pase-α (also known as G6PC).1,2 Between meals, blood glucose homeostasis is maintained by glucose produced in the terminal steps of gluconeogenesis and glycogenolysis,1,2 via G6Pase-α–mediated hydrolysis of glucose-6-phosphate (G6P). Both G6Pase-α3 and G6PT4 are anchored in the endoplasmic reticulum (ER) by multiple transmembrane domains with G6PT transporting cytoplasmic G6P across the ER membrane to the G6Pase-α active site situated inside the ER lumen.5 A deficiency of G6PT causes GSD type Ib (GSD-Ib, MIM2322206 ) and a deficiency in G6Pase-α causes GSD type Ia (GSD-Ia, MIM2322006). Because G6PT and G6Pase-α must be functionally coupled to transport and hydrolyze G6P to glucose,7,8 a detrimental mutation in either protein prevents the other from functioning efficiently and leads to a common metabolic phenotype of disturbed glucose homeostasis manifested initially by changes in the glucose and lipid profiles of blood, and in the longer term with liver and kidney disease.1,2 This profile is consistent with the expression pattern of G6Pase-α, which is restricted primarily to the liver, and kidney, with a low level of expression in the small intestine9 and G6PT, which is expressed ubiquitously.10 Although G6Pase-α is not expressed in myeloid cells, GSD-Ib patients do manifest symptoms of neutropenia and neutrophil dysfunctions.1,2,11,12 Their neutrophils exhibit apoptosis, characterized by increased annexin V binding and caspase-3 activation.13 The underlying cause of these dysfunctions and the relationship to glucose homeostasis is unclear.
Recently a second G6P hydrolase activity, G6Pase-β (also known as G6PC3), was identified.14,15 G6Pase-β shares similar kinetic properties,15 active site structure,15,16 and membrane localization16 with G6Pase-α. The G6Pase-β also couples functionally with G6PT15 to form an active complex that hydrolyses G6P to glucose. More interestingly, G6Pase-β, like G6PT, is expressed ubiquitously.14 This suggests that the G6PT-G6Pase-β complex is the counterpart of the G6PT-G6Pase-α complex in nongluconeogenic organs and that endogenous glucose might be produced to some extent in nongluconeogenic cells and tissues, including neutrophils. Therefore, it is reasonable to hypothesize that the myeloid defects in the G6PT-deficient GSD-Ib patients are caused by the loss of endogenous glucose production in the myeloid tissues. If this is correct, mice deficient in either G6PT or G6Pase-β should exhibit the same myeloid dysfunctions.
In earlier work, we generated G6PT-deficient GSD-Ib mice and demonstrated that they manifest all known metabolic and myeloid dysfunctions characteristics of the human disorder,17 and that G6PT expression in bone marrow and neutrophils is required for normal myeloid functions.18 More recently we generated mice deficient in the G6Pase-β catalytic unit.19 As predicted, these mice manifest neutropenia along with neutrophil dysfunctions in Ca2+ mobilization, respiratory burst, and chemotaxis,19 mimicking the myeloid dysfunction seen in G6PT-deficient GSD-Ib patients1,2,11,12 and mice.17 Therefore, hydrolysis of G6P in the ER of neutrophils appears critical for normal neutrophil functions.
The lumen of the ER serves as a critical site in protein maturation and its biochemical environment is uniquely designed to facilitate optimal posttranslational modification, folding, and assembly of proteins destined for the cell membrane or secretion.20 When cells experience conditions that alter ER homeostasis, such as glucose deprivation, a series of signal transduction cascades are activated that are collectively termed the unfolded protein response (UPR). This is characterized by the induction of expression of ER chaperones, such as the glucose-regulated proteins (GRPs) and protein disulfide isomerase (PDI) that assist in protein folding and assembly.21-23 If the stress is prolonged, it results in apoptotic cell death.23-26 We have recently shown that the G6Pase-β–deficient neutrophils, which are unable to hydrolyze endolumenal G6P to glucose, exhibit both ER stress and an enhanced rate of apoptosis.19
In this study, we have undertaken a more detailed study to delineate the underlying cause of GSD-Ib using G6PT-deficient neutrophils isolated from GSD-Ib mice. We show that these neutrophils exhibit an increased rate of apoptosis similar to that seen in the human GSD-Ib neutrophils.13 We present evidence that neutrophil apoptosis results from increased oxidative stress and is mediated, at least in part, by the intrinsic mitochondrial pathway. We also show that G6PT-deficient mouse neutrophils exhibit increased expression of GRPs and PDI, consistent with ER stress.21-23 These findings are in agreement with those seen in the G6Pase-β–deficient mouse neutrophils.19 Together these findings are consistent with the hypothesis that deficiencies of either G6PT or G6Pase-β lead to the same neutrophil dysfunction, strongly supporting the hypothesis that the underlying cause of neutrophil dysfunction in GSD-Ib is the disruption of endogenous glucose production in the ER.
Methods
G6PT-deficient GSD-Ib mice
All animal studies were conducted under an animal protocol approved by the NICHD Animal Care and Use Committee. A glucose therapy, which consists of intraperitoneal injection of 20 to 200 μL of 15% glucose every 12 hours, was initiated on the first postnatal day for GSD-Ib mice, as described previously.17 Mice that survived weaning were given glucose therapy in addition to unrestricted access to Mouse Chow (Zeigler Bros, Gardners, PA). GSD-Ib is an autosomal recessive disorder.1,2 Because the phenotypes of wild-type and heterozygous mice are identical, with both exhibiting normal development, metabolic, and myeloid functions,17 we use the designation “control” or “unaffected” to refer to both wild-type and heterozygous mice.
Western-blot analysis
Neutrophils were isolated from peritonea of 6- to 7-week-old GSD-Ib mice after thioglycollate-stimulation, as described previously.17 Glucose therapy was discontinued in GSD-Ib mice the evening before neutrophils isolation. For Western blot analysis, peritoneal neutrophil lysates were electrophoresed through a 10% or 16% polyacrylamide-SDS gel and transblotted onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were incubated with one of the following primary antibodies: a goat polyclonal antibody against a gelatinase peptide (Santa Cruz Biotechnology, Santa Cruz, CA); a rat monoclonal antibody against Ly-6G/Gr-1 or Smac/Diablo (BD Pharmingen, San Diego, CA); a mouse monoclonal antibody against KDEL (Stressgen, BC) that detects GRP78; a mouse monoclonal antibody against PDI (Novus Biologicals, Littleton, CO); a mouse monoclonal antibody against Bax, Omi/HtrA2, or β-actin (Santa Cruz Biotechnology); a rabbit polyclonal antibody against GRP17027 ; a rabbit polyclonal antibody against GAPDH (Santa Cruz Biotechnology); or a rabbit polyclonal antibody against the manganese superoxide dismutase (Mn-SOD; Millipore). The membranes were then incubated with the appropriate horseradish peroxidase-conjugated second antibody and the immunocomplex visualized using the Immobilon western chemiluminescent HRP substrate from Millipore. At least 4 separate experiments were conducted for each protein in which each mouse was assessed individually.
Oxyblot analysis
The OxyBlot Protein Oxidation Detection Kit (Chemicon, Temecula, CA) was used to detect proteins modified by oxygen free radicals and other reactive species. The carbonyl groups introduced into the proteins by oxidative modification are derivatized to 2,4-dinitrophenylhydrazone (DNP-hydrazone) by reaction with 2,4-dinitrophenylhydrazine (DNPH) and the DNP-derivatized proteins detected by Western blot analysis. Briefly, neutrophil lysates (15 μg in 5 μL) were combined with 5 μL 12% SDS and 10 μL 1× DNPH solution. After incubation at 25°C for 15 minutes, 7.5 μL neutralization solution was added and the samples were resolved by electrophoresis through a 10% polyacrylamide-SDS gel and transblotted onto PVDF membranes. The membranes were incubated with a rabbit anti-DNP antibody, and then with a horseradish peroxidase-conjugated goat antirabbit second antibody. Proteins that underwent oxidative modification were identified as bands only in the derivatized samples. As a negative control, the DNPH solution in the derivatization reaction was replaced with the derivatization-control solution (Chemicon).
Annexin V binding and caspase-3 activity assays
Apoptosis of thioglycollate-elicited peritoneal neutrophils was examined by flow cytometry and immunofluorescent analyses, using the annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit (BioVision, Mountain View, CA) according to manufacturer's instructions. For flow cytometric analysis, neutrophils (5 × 105 cells) were incubated for 10 minutes with annexin V-FITC and propidium iodide (PI). The green fluorescence of FITC-conjugated annexin V and the red fluorescence of DNA-bound PI were monitored using a FACSCalibur (BD Biosciences, San Jose, CA). The data were analyzed using both WinMDI and FlowJo software (TreeStar, Ashland, OR). Immunofluorescent analysis was performed on neutrophils that were settled onto glass slides by cytospin and stained with FITC-conjugated annexin V. After incubation at 25°C for 20 minutes in the dark, cells were fixed with 2% (vol/vol) formaldehyde solution for 15 minutes. Nuclei were stained with Hoechst 33342 (Invitrogen, Carlsbad, CA). Fluorescence was visualized using the Axioskop2 plus fluorescence microscope (Carl Zeiss, Thornwood, NY) and 40×/0.75 NA or 100×/1.35 NA optical lenses (Carl Zeiss). Photographs were taken with an Axiocam HRc digital camera (Carl Zeiss v r1.4) and the images were digitalized with AxioVision 4.3 software (Carl Zeiss). Annexin V+ neutrophils were counted in 8 fields (400× magnification) that were selected from GSD-Ib and control mice in a nonprejudiced fashion and reported as the mean average.
The DEVD-cleaving activity of active caspase-3 was measured using labeled Asp-Glu-Val-Asp-p-nitroanilide (DEVD-pNA) as substrate and a caspase-3 colorimetric assay kit from BioVision, following the manufacturer's protocol. The pNA light emission was quantified using a microtiter plate reader at a wavelength of 405 nm.
Reactive oxygen species measurements
Levels of cytosolic reactive oxygen species (ROS) were assessed by the conversion of carboxy-2,7-dichlorodihydrofluorescein diacetate (H2OCFDA) to carboxy-2,7-dichlorofluorescein (DCF) using the Image-iT Live Green Reactive Oxygen Species Detection Kit (Invitrogen) and analyzed by flow cytometry and immunofluorescence. Approximately 5 × 105 of freshly isolated peritoneal neutrophils were incubated at 37°C for 30 minutes in the dark in Hank balanced salt solution (HBSS) buffer containing Ca2+, Mg2+, and carboxy-H2DCFDA; and the conversion to fluorescent carboxy-DCF analyzed using the FACSCalibur and FlowJo software. Immunofluorescence analysis was performed on thioglycollate-elicited peritoneal neutrophils that were placed onto the glass slides by cytospin and incubated with carboxy-H2DCFDA at 37°C for 30 minutes in the dark. Nuclei were stained with Hoechst 33342 and fluorescence visualized using the Axioskop2 plus fluorescence microscope, and digitalized with the AxioVision 4.3. Carboxy-DCF–positive neutrophils were counted in 8 fields (400× magnification) that were selected from GSD-Ib and control mice in a nonprejudiced fashion and reported as the mean average.
The mitochondrial localization of ROS was assessed by the conversion of dihydrorhodamine (DHR)-123 to the fluorescent derivative rhodamine-123 (R-123) and analyzed by flow cytometry and immunofluorescence. Approximately 5 × 105 of freshly isolated peritoneal neutrophils were incubated at 37°C for 15 minutes in the dark in HBSS buffer containing Ca2+, Mg2+, and 2 μM DHR-123; and the conversion to R-123 analyzed using the FACSCalibur and FlowJo software. Immunofluorescence analysis was performed on peritoneal neutrophils that were settled onto glass slides by cytospin, incubated with 2 μM DHR-123 in a HBSS buffer containing Ca2+ and Mg2+ at 37°C for 15 minutes in the dark. Nuclei were stained with Hoechst 33342 and fluorescence visualized. R-123 positive neutrophils were counted in 8 fields (400× magnification) that were selected from GSD-Ib and control mice in a nonprejudiced fashion and reported as the mean average.
Immunofluorescence analysis
For caspase determination, the thioglycollate-elicited peritoneal neutrophils were settled onto the glass slides by cytospin immediately after isolation. For Bax, the second mitochondria-derived activator of caspase (Smac; also known as direct IAP binding protein with low pI [Diablo]), and the serine protease Omi/high temperature requirement A2 (HtrA2) determination, peritoneal neutrophils were first incubated with MitoTracker Red CMXRos probe (Invitrogen) to label the mitochondria following the instruction of the manufacturer. Briefly, peritoneal neutrophils were incubated with 100 nM of MitoTracker Red CMXRos for 30 minutes at 37°C in Tris-buffered saline (TBS) containing 1% bovine serum albumin (BSA), pelleted, and washed at 37°C in TBS-1% BSA and settled onto the glass slides by cytospin. Then, neutrophils on glass slides were fixed for 10 minutes at 25°C in 3.75% paraformaldehyde, and permeabilized for 5 minutes at 25°C in 0.2% Triton X-100. The permeabilized neutrophils were blocked with TBS-1% BSA for 1 hour at 25°C, followed by incubation overnight at 4°C with a rabbit monoclonal antibody that detects cleaved caspase-3 (Asp170; Cell Signaling, Danvers, MA), a rabbit monoclonal antibody that detects cleaved caspase-9 (Asp353; Cell Signaling), a mouse monoclonal antibody to Bax (Santa Cruz Biotechnology), a rabbit monoclonal antibody to Smac/Diablo (Santa Cruz Biotechnology), or a mouse monoclonal antibody to Omi/HtrA2 (Santa Cruz Biotechnology). After 3 consecutive washes with TBS, the neutrophils were incubated for 1 hour at 25°C with a goat antirabbit or a goat antimouse IgG-FTIC, followed by washing in TBS. The cells were then mounted with an antifade, water-based mounting medium (Vector Laboratories, Burlingame, GA). Nuclei were stained with Hoechst 33342 and fluorescence was visualized using the Axioskop2 plus fluorescence microscope, and digitalized with the AxioVision 4.3. Active caspase-3, active caspase-9, Bax, Smac/Diablo, and Omi/HtrA2 positive neutrophils were counted in 8 fields (400× magnification) that were selected from GSD-Ib and control mice in a nonprejudiced fashion and reported as the mean average.
Statistical analysis
The unpaired t test was performed using the GraphPad Prism Program, version 4 (GraphPad Software, San Diego, CA). Values were considered statistically significant at P less than .05.
Results
The GSD-Ib neutrophils exhibit ER stress and enhanced apoptosis
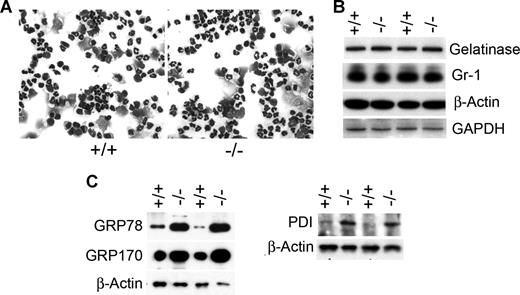
Murine G6PT-deficient neutrophils were isolated from the peritoneal space of 6- to 7-week-old GSD-Ib and their unaffected littermates after thioglycollate-induced peritonitis. We have previously shown that neutrophil accumulation in the G6PT-deficient peritoneum is decreased to 33% of the control levels.17 The cause of the impaired neutrophil trafficking was attributed to the reduced local production of chemokines, combined with the intrinsic resistance of GSD-Ib neutrophils to chemoattractants.17 Despite reduced neutrophil accumulation in the peritoneum, staining of GSD-Ib and control neutrophil populations showed that both contain band and segmented nucleophilic cells (Figure 1A) and both express similar levels of the granule protein gelatinase and the myeloid differentiation antigen Gr-1 (Figure 1B). Because gelatinase is a marker of terminal neutrophil differentiation28 and Gr-1 is a marker of neutrophil maturation,29 the results indicate that both wild type and G6PT-deficient peritoneal neutrophils are of similar maturity.
Increase in the expression of ER chaperones in neutrophils of GSD-Ib mice. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Hema 3–stained cytospins of peritoneal neutrophils. (B) Western-blot analysis of protein extracts of peritoneal neutrophils using antibodies against gelatinase, Gr-1, β-actin, or GAPDH. Each lane contains 50 μg protein. (C) Western-blot analysis of protein extracts of neutrophils using antibodies against GRP78, GRP170, PDI, or β-actin. Each lane contains 50 μg protein.
Increase in the expression of ER chaperones in neutrophils of GSD-Ib mice. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Hema 3–stained cytospins of peritoneal neutrophils. (B) Western-blot analysis of protein extracts of peritoneal neutrophils using antibodies against gelatinase, Gr-1, β-actin, or GAPDH. Each lane contains 50 μg protein. (C) Western-blot analysis of protein extracts of neutrophils using antibodies against GRP78, GRP170, PDI, or β-actin. Each lane contains 50 μg protein.
If translocation of G6P into the lumen of the ER is critical for neutrophil glucose homeostasis and normal neutrophil function, then GSD-Ib neutrophils should show evidence of ER stress. We therefore compared the level of expression of molecular chaperones in the UPR signal transduction pathway21-23 between neutrophils of GSD-Ib and control mice. Western-blot analysis showed that the production of molecular chaperones, GRP78/Bip,21-23 GRP170,21,22 and PDI,22,23 were increased in neutrophils of GSD-Ib mice over their unaffected littermates (Figure 1C).
The presence of apoptosis in the control and G6PT-deficient neutrophils was assessed using 2 apoptotic markers-the externalization of phosphatidylserine on the plasma membrane,30 and the activation of caspase-3.31,32 Flow cytometric analysis showed that a significantly elevated number of peritoneal neutrophils from GSD-Ib mice stained positive for annexin V compared with neutrophils from the control mice (Figure 2A), suggesting there was an enhanced rate of apoptosis in murine GSD-Ib neutrophils. Likewise, immunofluorescence analysis showed that 15.2% (± 1.2%) of peritoneal neutrophils from GSD-Ib mice stained positive for annexin V while 5.0% (± 0.6%) of neutrophils from control littermates bound to annexin V (Figure 2B). Consistent with this, 16.0% (± 1.8%) of GSD-Ib peritoneal neutrophils stained positive to antibody against active caspase-3, while 1.2% (± 0.2%) neutrophils from unaffected mice stain positive for active caspase-3 (Figure 2C). Moreover, activity assays demonstrated that levels of active caspase-3 were increased in GSD-Ib neutrophils (Figure 2D).
GSD-Ib neutrophils display increased rate of apoptosis. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative flow cytometric analysis of neutrophil annexin V (x-axis) binding and PI (y-axis) uptake. Numbers on the plots are percentages of total cells. (B) Quantification of annexin V–positive neutrophils determined by immunofluorescence staining. (C) Quantification of caspase-3–positive neutrophils determined by immunofluorescence staining. (D) The DEVD-cleaving activity of active caspase-3 in protein extracts of peritoneal neutrophils. Data represent the means (± SEM) of 3 independent experiments. **P < .001.
GSD-Ib neutrophils display increased rate of apoptosis. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative flow cytometric analysis of neutrophil annexin V (x-axis) binding and PI (y-axis) uptake. Numbers on the plots are percentages of total cells. (B) Quantification of annexin V–positive neutrophils determined by immunofluorescence staining. (C) Quantification of caspase-3–positive neutrophils determined by immunofluorescence staining. (D) The DEVD-cleaving activity of active caspase-3 in protein extracts of peritoneal neutrophils. Data represent the means (± SEM) of 3 independent experiments. **P < .001.
Increased ROS accumulation in GSD-Ib neutrophils
One consequence of ER stress-triggered cell death is the accumulation of ROS,33,34 which can be measured by the conversion of the ROS-sensitive reactant carboxy-H2DCFDA to the fluorescent carboxy-DCF derivative. Neutrophils from both GSD-Ib and control mice were loaded with carboxy-H2DCFDA and the conversion to fluorescent carboxy-DCF derivative was examined by flow cytometry (Figure 3A) and immunofluorescence (Figure 3B) analyses. Both showed that a significantly elevated number of peritoneal neutrophils from GSD-Ib mice exhibited strong fluorescent signals compared with neutrophils from the control mice, consistent with the oxidative stress.
GSD-Ib neutrophils exhibit oxidative stress. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative flow cytometric analysis of neutrophil carboxy-DCF staining. (B) Quantification of carboxy-DCF–positive neutrophils determined by immunofluorescence staining. Data represent the means (± SEM) of 3 independent experiments. ***P < .001. (C) Oxyblot analysis of carbonyl groups in oxidative modified proteins. Each lane contains 50 μg protein. The vertical line has been inserted to indicate a repositioned gel lane. (D) Representative flow cytometric analysis of neutrophil R-123 staining. (E) Quantification of R-123–positive neutrophils determined by immunofluorescence staining. Data represent the means (± SEM) of 3 independent experiments. **P < .001. (F) Western blot analysis of protein extracts of neutrophils using antibodies against Mn-SOD or β-actin. Each lane contains 50 μg protein.
GSD-Ib neutrophils exhibit oxidative stress. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative flow cytometric analysis of neutrophil carboxy-DCF staining. (B) Quantification of carboxy-DCF–positive neutrophils determined by immunofluorescence staining. Data represent the means (± SEM) of 3 independent experiments. ***P < .001. (C) Oxyblot analysis of carbonyl groups in oxidative modified proteins. Each lane contains 50 μg protein. The vertical line has been inserted to indicate a repositioned gel lane. (D) Representative flow cytometric analysis of neutrophil R-123 staining. (E) Quantification of R-123–positive neutrophils determined by immunofluorescence staining. Data represent the means (± SEM) of 3 independent experiments. **P < .001. (F) Western blot analysis of protein extracts of neutrophils using antibodies against Mn-SOD or β-actin. Each lane contains 50 μg protein.
Peptides, proteins, and free amino acids are targets for oxidative attack by ROS.34,35 An oxyblot analysis (Figure 3C) showed GSD-Ib neutrophils contained significantly more oxidatively modified proteins than the control neutrophils, consistent with the observation of ROS accumulation, indicative of oxidative stress.
The production of ROS was further evaluated by the conversion of DHR-123 to the fluorescent R-123, which selectively accumulates in the mitochondria of living cells.36,37 Flow cytometric (Figure 3D) and immunofluorescence (Figure 3E) analyses showed that significantly more GSD-Ib neutrophils stained positive with R-123 compared with the control neutrophils. This indicated that more ROS accumulate within the neutrophil mitochondrial network of the GSD-Ib mice than the controls.
The mitochondrial antioxidant enzyme Mn-SOD that decomposes superoxide to hydrogen peroxide38,39 is induced by elevated concentrations of ROS. Western blot analysis showed that expression of Mn-SOD in neutrophils of GSD-Ib mice was markedly elevated over their unaffected littermates (Figure 3F), supporting the presence of mitochondrial oxidative stress in GSD-Ib neutrophils.
The intrinsic mitochondrial pathway plays a role in GSD-Ib neutrophils apoptosis
Upon the induction of apoptosis, Bax, a proapoptotic protein of the Bcl-2 family, is oligomerized and translocated to the outer mitochondrial membrane, leading to membrane permeabilization.40,41 Immunofluorescence analysis using an antibody to Bax showed that colocalization of Bax with the mitochondria-specific MitoTracker Red was found in significantly more neutrophils from GSD-Ib mice, compared with neutrophils form the control mice (Figure 4A), indicating that Bax translocation to the mitochondria was enhanced in GSD-Ib neutrophils. In addition to mitochondrial Bax translocation, the expression levels of Bax in neutrophils of GSD-Ib mice were marked elevated compared with their unaffected littermates. In GSD-Ib mice, 35.6% (± 2.7%) of peritoneal neutrophils stained positive to antibody to Bax, while 2.5 (± 0.4%) neutrophils from control mice stain positive for Bax (Figure 4A). Western blot analysis confirmed the increase in Bax proteins (Figure 4B) in neutrophils of GSD-Ib mice compared with the unaffected littermates.
Bax activation in GSD-Ib neutrophils. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative immunofluorescence of Bax staining (green fluorescence), Hoechst 33342 nuclei staining (blue fluorescence), and MitoTracker Red mitochondrial staining (red fluorescence), magnification, ×1000, and quantification of Bax-positive neutrophils in unaffected and GSD-Ib mice. Data represent the means (± SEM) of 3 independent experiments. ***P < .001. (B) Western blot analysis of protein extracts of neutrophils using antibodies against Bax, β-actin, or GAPDH. Each lane contains 50 μg protein.
Bax activation in GSD-Ib neutrophils. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative immunofluorescence of Bax staining (green fluorescence), Hoechst 33342 nuclei staining (blue fluorescence), and MitoTracker Red mitochondrial staining (red fluorescence), magnification, ×1000, and quantification of Bax-positive neutrophils in unaffected and GSD-Ib mice. Data represent the means (± SEM) of 3 independent experiments. ***P < .001. (B) Western blot analysis of protein extracts of neutrophils using antibodies against Bax, β-actin, or GAPDH. Each lane contains 50 μg protein.
Neutrophil mitochondria express several proapoptotic effectors including Smac/Diablo and Omi/HtrA2, which are released into the cytosol in response to apoptotic stimuli when membrane permeabilization is increased.42-44 Immunofluorescence analyses showed that the release of Smac/Diablo and Omi/HtrA2 into the cytosol was increased in GSD-Ib neutrophils, compared with the controls (Figure 5A,C). Moreover, GSD-Ib neutrophils also exhibited increased production of both proteins. Neutrophils from GSD-Ib mice stained 7.5-fold higher than controls for Smac/Diablo (Figure 5A) and 7.9-fold higher than controls for Omi/HtrA2 (Figure 5C). Western blot analysis confirmed the increase in Smac/Diablo (Figure 5B) and Omi/HtrA2 (Figure 5D) proteins in neutrophils of GSD-Ib mice compared with the unaffected littermates.
Increased accumulation and release of proapoptotic factors in GSD-Ib neutrophils. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative immunofluorescence of Smac/Diablo staining (green fluorescence), Hoechst 33342 nuclei staining (blue fluorescence), and MitoTracker Red mitochondrial staining (red fluorescence), magnification, ×1000, and quantification of Smac/Diablo-positive neutrophils in unaffected and GSD-Ib mice. (B) Western-blot analysis of protein extracts of neutrophils using antibodies against Smac/Diablo, β-actin or GAPDH. Each lane contains 50 μg protein. (C) Representative immunofluorescence of Omi/HtrA2 staining (green fluorescence), Hoechst 33 342 nuclei staining (blue fluorescence), and MitoTracker Red mitochondrial staining (red fluorescence), magnification, ×1000, and quantification of Omi/HtrA2-positive neutrophils in unaffected and GSD-Ib mice. Data represent the means (± SEM) of 3 independent experiments. ***P < .001. (D) Western blot analysis of protein extracts of neutrophils using antibodies against Omi/HtrA2, β-actin, or GAPDH. Each lane contains 50 μg protein.
Increased accumulation and release of proapoptotic factors in GSD-Ib neutrophils. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Representative immunofluorescence of Smac/Diablo staining (green fluorescence), Hoechst 33342 nuclei staining (blue fluorescence), and MitoTracker Red mitochondrial staining (red fluorescence), magnification, ×1000, and quantification of Smac/Diablo-positive neutrophils in unaffected and GSD-Ib mice. (B) Western-blot analysis of protein extracts of neutrophils using antibodies against Smac/Diablo, β-actin or GAPDH. Each lane contains 50 μg protein. (C) Representative immunofluorescence of Omi/HtrA2 staining (green fluorescence), Hoechst 33 342 nuclei staining (blue fluorescence), and MitoTracker Red mitochondrial staining (red fluorescence), magnification, ×1000, and quantification of Omi/HtrA2-positive neutrophils in unaffected and GSD-Ib mice. Data represent the means (± SEM) of 3 independent experiments. ***P < .001. (D) Western blot analysis of protein extracts of neutrophils using antibodies against Omi/HtrA2, β-actin, or GAPDH. Each lane contains 50 μg protein.
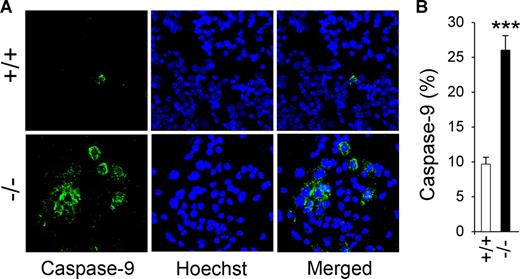
The mitochondrial, stress-induced route of apoptosis involves the activation of caspase-9.31,32 In GSD-Ib mice, 26.0% (± 2.1%) of peritoneal neutrophils stained positive to antibody to active caspase-9, while 9.7% (± 1.0%) neutrophils from unaffected mice stain positive for active caspase-9 (Figure 6), demonstrating that levels of active caspase-9 were increased in GSD-Ib neutrophils.
Caspase-9 activation in GSD-Ib neutrophils. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Immunofluorescence of active caspase-9 staining (green fluorescence) and Hoechst 33342 nuclei staining (blue fluorescence), magnification ×400. (B) Quantification of caspase-9–positive neutrophils in unaffected and GSD-Ib mice. Data represent the means (± SEM) of 3 independent experiments. ***P < .001.
Caspase-9 activation in GSD-Ib neutrophils. Peritoneal neutrophils were isolated from 6- to 7-week-old unaffected (+/+) and GSD-Ib (−/−) mice during thioglycollate-elicited peritonitis. (A) Immunofluorescence of active caspase-9 staining (green fluorescence) and Hoechst 33342 nuclei staining (blue fluorescence), magnification ×400. (B) Quantification of caspase-9–positive neutrophils in unaffected and GSD-Ib mice. Data represent the means (± SEM) of 3 independent experiments. ***P < .001.
Discussion
Neutrophils are capable of endogenous glucose production in their ER through the hydrolysis of G6P.19 This capability depends upon the coordinated functioning of 2 proteins, the G6P transporter, G6PT1,2 and the G6P hydrolase, G6Pase-β.14,15 GSD-Ib patients1,2,11,12 and mice,17 deficient in G6PT, manifest neutropenia and neutrophil dysfunctions. Similarly, G6Pase-β–deficient mice manifest myeloid dysfunctions mimicking G6PT deficiency.19 Because a deficiency in either G6PT or G6Pase-β leads to the same phenotype it suggests that the basis of the neutrophil dysfunctions is a disruption of endogenous glucose production in the ER of neutrophils. When ER homeostasis is perturbed by glucose deprivation, cells experience ER stress, which activates the UPR.21-23 This response is characterized by the increased expression of ER chaperones that alleviate protein misfolding or aggregation. Prolonged activation of the UPR is proapoptotic.23-26 Therefore, if endogenous glucose production in the ER of the neutrophils is critical, any deficiency in the G6PT-G6Pase-β complex should lead to ER stress and increased apoptosis. In neutrophils isolated from human GSD-Ib patients, there are signs of apoptosis.13 Furthermore, in neutrophils of G6Pase-β deficient mice,19 ER stress and increased apoptosis are observed. To tie these observations together back to GSD-Ib and the G6PT deficiency we have characterized the neutrophils from GSD-Ib mice. In particular we have sought to demonstrate the presence of both ER stress and increased apoptosis in the murine GSD-Ib neutrophils and understand the mechanism behind these processes.
At a morphological level, the neutrophils from the GSD-Ib mice and their unaffected littermates appear similar. They express similar levels of neutrophil maturation markers, gelatinase28 and Gr-1,29 showing that the deficiencies observed are not simply a reflection of different developmental stages of the cells. Like the G6Pase-β–deficient neutrophils,19 the G6PT-deficient murine neutrophils exhibit a marked increase in apoptotic cell numbers, evidenced by an increase in annexin V binding and caspase-3 activation. This was also similar to the observations of human GSD-Ib neutrophils,13 tying the common function of G6Pase-β and G6PT, namely endogenous production of glucose, to apoptosis.
In neutrophils, apoptosis is exquisitely regulated because it is essential for the successful resolution of inflammation. Neutrophil apoptosis can be initiated by 2 main pathways, namely, the extrinsic death receptor pathway that responds to extracellular stimuli and the intrinsic mitochondrial pathway that is controlled by the Bcl-2 family proteins.23 Because the G6Pase-β–deficient mouse neutrophils had shown increased levels of ER stress19 and the mitochondrial pathway is predominant during spontaneous neutrophil apoptosis,45 we looked for evidence of similar stress in the G6PT-deficient GSD-Ib mouse neutrophils and the involvement of the intrinsic mitochondrial pathway in apoptosis.
The G6PT-deficient murine neutrophils exhibited an increased level of ER stress similar to that seen in the G6Pase-β–deficient neutrophils.19 This was evidenced by the enhanced production of molecular chaperones, GRP78/Bip,21-23 GRP170,21,22 and PDI22,23 in GSD-Ib neutrophils compared with neutrophils of their unaffected littermates (Figure 1C). One consequence of ER stress can be the increased production of ROS. These species can attack DNA, proteins, lipids, and carbohydrate to produce DNA strand break, protein oxidation and lipid peroxidation.33-35 Under normal physiologic conditions, ROS act as important cellular signals, and excess formation is prevented by the endogenous antioxidant defense system, which includes Mn-SOD. Mn-SOD, which is localized in the mitochondria,38 is induced by elevated concentrations of ROS and acts to decompose superoxide to hydrogen peroxide.39 However, when excess ROS production is unchecked, ROS can disrupt vital metabolic pathways and initiate apoptotic cell death.33-35 In the murine GSD-Ib neutrophils, ROS production was stably elevated over the control neutrophils, despite the induction of Mn-SOD expression (Figure 3), suggesting that at least one component of the increased apoptosis came through activation of the mitochondrial pathway. Another contribution to ROS production in the GSD-Ib neutrophils may be via the oxidation and reduction of disulfide bonds in the ER23,33 as a result of the up-regulation of PDI that catalyzes thiol-disulfide exchange.23
During neutrophil apoptosis, the proapoptotic Bax protein normally localized in the cytosol46,47 is translocated to the mitochondria, resulting in the permeabilization of the outer membrane and the release of Smac/Diablo and Omi/HtrA2 from the mitochondrial intermembrane space to the cytoplasm.42-44 There, they bind to the inhibitor of apoptosis proteins,48 disrupting their binding to caspases and eliminating caspase inhibition. As a result, a cascade of caspase activation occurs allowing these cysteine proteases to degrade the cellular components that lead to the cell death. In the murine GSD-Ib neutrophils, levels of Bax are elevated in the mitochondrial membrane over those seen in the control neutrophils (Figure 4) and consistent with the anticipated increase in membrane permeability, there is an increased release of both Smac/Diablo and Omi/HtrA2 into the cytosol of the GSD-Ib neutrophils compared with the control neutrophils (Figure 5). Both caspase-3 and caspase-9 activation are also greater in the GSD-Ib neutrophils. Therefore the intrinsic mitochondrial pathway is implicated strongly in the apoptosis seen in the GSD-Ib neutrophils. Moreover, the similarities seen between the G6Pase-β- and G6PT-deficient mouse neutrophils argue strongly that the neutrophil dysfunction seen in GSD-Ib is not a result of a novel activity of G6PT in nongluconeogenic organs, but rather a deficiency in the endogenous glucose production caused by a defective G6Pase-β-G6PT complex.
It is interesting to note that UPR-dependent apoptosis is also responsible for cyclic neutropenia and severe congenital neutropenia resulted from mutations in the neutrophil elastase ELA2 gene.49,50 These findings show that intracellular accumulation of misfolded mutant elastases in the cytoplasm induces UPR and that the subsequent UPR-dependent apoptosis leads to impaired myeloid precursor cell differentiation and chronic neutropenia.
While the above mechanisms can explain the myeloid problems in GSD-Ib, other mechanisms could also contribute. A second major role of G6P in the ER of neutrophils is as a substrate for the endoluminal pentose phosphate pathway. Hydrolysis of G6P by hexose-6-phosphate dehydrogenase51,52 generates NADPH, which regulates the activity of 11β-hydroxysteroid dehydrogenase-1.53 This ER-associated oxoreductase generates cortisol/corticosterone locally to modulate immune responses, and as such could also contribute to myeloid dysfunctions.
In conclusion, we have investigated a murine model of GSD-Ib which accurately reflects the metabolic and myeloid aspects of the human disease,17 to elucidate potential mechanisms underlying the neutrophil dysfunctions. We have shown that neutrophils from mice defective in G6P transport across the ER membrane have the identical characteristics as neutrophils previously characterized from mice with a defect in ER endoluminal G6P hydrolysis.19 The mechanism underlying this includes ER stress and increased apoptosis mediated at least in part via the intrinsic mitochondrial pathway. These findings support the hypothesis that the myeloid dysfunctions observed in GSD-Ib are due, at least in part, to a lack of endogenous glucose production, tying glucose homeostasis to immune function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This research was supported by the Intramural Research Programs of the NICHD, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S.Y.K. and H.S.J. designed and performed research, analyzed data, and contributed to writing the paper; P.A.M. performed research, B.C.M. analyzed data and contributed to writing the paper; J.Y.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janice Y. Chou, Building 10, Room 9D42, NIH, 10 Center Drive, Bethesda, MD 20892-1830; e-mail: chouja@mail.nih.gov.
References
Author notes
*S.Y.K. and H.S.J. contributed equally to this work.