Abstract
Recent studies have demonstrated that patients with myeloproliferative disorders (MPDs) frequently have acquired activating mutations in the JAK2 tyrosine kinase. A multikinase screen determined that lestaurtinib (formerly known as CEP-701) inhibits wild type JAK2 kinase activity with a concentration that inhibits response by 50% (IC50) of 1 nM in vitro. We hypothesized that lestaurtinib would inhibit mutant JAK2 kinase activity and suppress the growth of cells from patients with MPDs. We found that lestaurtinib inhibits the growth of HEL92.1.7 cells, which are dependent on mutant JAK2 activity for growth in vitro and in xenograft models. Erythroid cells expanded from primary CD34+ cells from patients with MPDs were inhibited by lestaurtinib at concentrations of 100 nM or more in 15 of 18 subjects, with concomitant inhibition of phosphorylation of STAT5 and other downstream effectors of JAK2. By contrast, growth of erythroid cells derived from 3 healthy controls was not significantly inhibited. These results demonstrate that lestaurtinib, in clinically achievable concentrations, inhibits proliferation and JAK2/STAT5 signaling in cells from patients with MPDs, and therefore holds promise as a therapeutic agent for patients with these disorders.
Introduction
Myeloproliferative disorders (MPDs) are clonal hematologic diseases characterized by excess production of one or more lineages of mature blood cells, a predisposition to bleeding and thrombotic complications, extramedullary hematopoiesis, and a variable progression to acute leukemia. MPDs are classified according to the hematopoietic lineage which is most prominently affected: chronic myelogenous leukemia (CML) is characterized by an increase in granulocytes, polycythemia vera (PV) by an expansion in red blood cell production, essential thrombocythemia (ET) by an isolated elevation in the platelet count, and chronic idiopathic myelofibrosis (CIMF) by a fibrotic bone marrow accompanied by either increased or decreased blood cell counts.1 Myelofibrosis can arise de novo, as CIMF, or can evolve from PV or ET as those diseases progress.
MPDs have provided the first and strongest examples of targeted therapeutics based on molecular pathogenesis. The discovery of the t(9;22) translocation in CML, which encodes a constitutively activated BCR-ABL tyrosine kinase,2 led directly to the development of small molecule inhibitors targeted to the ATP binding domain of the ABL kinase that have revolutionized the treatment and natural history of this heretofore fatal disease.3,4 In contrast, the molecular underpinnings of MPDs other than CML have, until recently, been largely unknown. Recently, a somatic activating mutation encoding a valine to phenylalanine substitution at position 617 (V617F) in Janus kinase 2 (JAK2) has been identified in more than 90% of patients with PV and in 40% to 70% of patients with ET and IMF.5-8 JAK2 is a nonreceptor tyrosine kinase that signals between cytokine receptors and downstream targets, including the transcription factors STAT3 and STAT5. JAK2 V617F expressed in cell lines confers cytokine-independent cell growth or hyper-responsiveness to cytokines and constitutive activation of STAT5.6-8 Similarly, progenitor cells from patients with the V617F mutation exhibit erythropoietin-independence in vitro.5 Several groups have demonstrated that the introduction of the V617F mutant in a retroviral bone marrow transplant model is sufficient to induce the polycythemic phenotype in mice, including progression to myelofibrosis.9,10 Taken together, these data suggest that aberrant activation of JAK2 plays a pivotal role in the pathophysiology of MPDs.
Effective inhibition of activated tyrosine kinases can have dramatic clinical effects in hematologic malignancies. As noted, imatinib, an ABL kinase inhibitor, is highly effective in patients with CML.11 Lestaurtinib (initially designated CEP701) is a potent, orally available fms-like kinase (FLT3) inhibitor that was developed and first tested in patients with a subset of acute myelogenous leukemia (AML) bearing activating mutations in FLT3.12 The compound has clinical activity in phase 1/2 studies of relapsed, FLT3 mutation–positive AML patients.13,14 Recent studies have further shown that lestaurtinib's inhibitory activity is not limited to FLT3.
The identification of a JAK2 mutation as a common molecular lesion in patients with MPDs raises the possibility that a small molecule inhibitor of JAK2 could provide significant clinical benefits in this group of disorders. No JAK2-targeted therapy is currently available for patients with MPDs. As part of a recently broadened kinase inhibition screen, lestaurtinib was identified as a potent JAK2 kinase inhibitor in vitro. Here, we show that lestaurtinib is a potent V617F JAK2 inhibitor that can suppress the growth and JAK/STAT signaling in primary erythroid cells from subjects with MPDs, but has only marginal growth-inhibitory activity against erythroid precursors derived from healthy bone marrows.
Methods
In vitro kinase screen
The kinase activity of baculovirus-expressed JAK2 was assayed by using time-resolved fluorescence (TRF). Ninety-six–well Costar high binding plates were coated with 100 μL/well of 10 μg/mL Neutravidin (Pierce, Rockford, IL) in Tris-buffered saline at 37°C for 2 hours, followed by 100 μL/well of 1 μg/mL 15-mer peptide substrate (biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide; Infinity Biotech Research and Resource, Aston, PA) at 37°C for another hour. The JAK2 assay mixture consisting of 20 mM HEPES (pH 7.2), 0.2 μM ATP, 1 mM MnCl2, 0.1% bovine serum albumin (BSA), increasing concentrations of CEP-701, and JAK2 were then added to the assay plate and assay continued at room temperature for 20 minutes. Detection of the phosphorylated product was performed using europium-labeled antiphosphotyrosine antibody in TRF readout. Inhibition curves were generated in replicates and plotted as percent inhibition versus log10 of the concentration of compound. Data were analyzed by nonlinear regression by fitting to the sigmoidal dose-response (variable slope) equation in GraphPad Prism version 4 (GraphPad Software, San Diego, CA).
Sample acquisition and processing
Peripheral blood and bone marrow samples were obtained from the Stem Cell Core facility at the University of Pennsylvania through a University of Pennsylvania Institutional Review Board–approved protocol. Mononuclear cells (MNC) from marrow or blood were isolated on a density gradient (Ficoll-Paque PLUS; GE Healthcare, Little Chalfont, United Kingdom) within 24 hours of collection. CD34+ cells were positively selected using immunomagnetic beads (Miltenyi Biotec, Auburn, CA). Purity of this selection method is consistently greater than 5%. In addition, lysed whole blood cells, MNC, granulocytes and/or CD34+ cells were collected for genotyping.
Genotyping
Genomic DNA was isolated from mononuclear cells, granulocytes, white blood cells, CD34+ or CD34− cells using Promega's Wizard Genomic DNA Purification kit (Madison, WI) following the manufacturer's instructions. Allele-specific PCR and BsaXI restriction analysis were done as previously described using 100 ng and 200 ng of DNA, respectively.5
Cell culture
HEL 92.1.7 (ATCC, Manassas, VA) were maintained in RPMI 1640 supplemented with 10% bovine serum albumin (BSA) at a concentration of 106 cells/mL. Primary human CD34+ cells were plated at a density of 2 to 5 × 105 cells/mL in Iscove Modified Dulbecco Medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 20% BIT 9500 (StemCell Technologies, Vancouver, BC) containing 50 mg/mL BSA, 50 μg/mL insulin, and 1 mg/mL iron-saturated human transferrin; 10 ng/mL IL-3, 10 ng/mL IL-6, and 25 ng/mL stem cell factor (SCF); 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were maintained/expanded at a density of 3 to 5 × 105 cells/mL, and the medium was refreshed every 48 hours. On the fifth day of culture, the medium was supplemented with 2 U/mL erythropoitin (EPO). All cells were incubated at 37°C in a fully humidified incubator supplemented with 5% CO2. Assays were performed between days 7 and 10 of culture.
For cytokine stimulation experiments, cells were expanded for 9 days as in the preceding paragraph and washed twice in PBS; cells were resuspended at 106 cells/mL in a 6-well plate in IMDM/0.5% FBS and incubated at 37°C for 4 hours; followed by the addition of either: 100 U/mL EPO, 100 ng/mL thrombopoietin (TPO), or control and incubated for 10 minutes at 37°C and immediately placed on ice. For stimulation, cytokines were added directly to the plate.
XTT and MTS assays
Cells (5 × 104 cells/well) were plated in a flat-bottom 96-well plate in 100 μL culture medium in the presence of 30, 100, or 300 nM lestaurtinib or no drug/DMSO vehicle control, such that 4 mM stock CEP-701 in DMSO was always diluted more than 6000-fold. Plates were read in a 96-well plate reader (EL800; Bio-Tek Instruments, Winooski, VT) at 515 nM. When more than one time point was measured for a given sample, data presented for that sample at each dose level are reported as the mean at all time points.
Xenografts
Cells (107 HEL92.1.7) were mixed with Matrigel and injected subcutaneously into a flank of nude (nu/nu) mice. When tumors reached approximately 150 mm,3 animals were divided into control and treated group (10/group), which were dosed subcutaneously twice a day with the vehicle or 30 mg/kg of lestaurtinib, respectively. Tumor volumes were determined every 3 to 4 days.
Western blot analysis
HEL92.1.7 cells or primary erythroid cultures were incubated with the concentration of lestaurtinib indicated in the Figures for 1 hour or 24 hours and whole-cell extracts were prepared in Triton-based lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with protease inhibitors (Roche Applied Science, Indianapolis, IN). Proteins (10-25 μg/lane) were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes, which were probed with specific antibodies, as recommended by manufacturer. For analysis of JAK2 phosphorylation, extracts (250 μg) were immunoprecipitated overnight with JAK2 antibody (Upstate Biotechnology, Charlottesville, VA), followed by a Western blot using a tyrosine-specific 4G10 antibody (Upstate Biotechnology). Blots were stripped and reprobed with JAK2 antibody. All other antibodies, except for cyclinD1/D2 (Upstate Biotechnology), were from Cell Signaling Technology.
For the washout experiment, cultures (day 9) of healthy bone marrow cells and bone marrow cells from a subject with ET were washed free of cytokines and incubated in IMDM supplemented with 0.5% BSA at 37°C/5% CO2 for 4 hours, followed by a 10-minute incubation with 100 U/mL EPO, 100 ng/mL TPO, or no cytokines. Western blots were performed as described in the preceding paragraph.
Morphologic analysis
Cytospin preparations of cells were stained with a modified Wright-Giemsa stain and evaluated under a 100× oil objective on a Leica DM LB2 microscope (Leica Microsystems, Wetzlar, Germany). Images were acquired with a Leica DC300 camera and Leica DCTwain, v5.1.10 acquisition software (Leica Microsystems) and Adobe Photoshop CS (Adobe Systems, San Jose, CA).
Statistical analysis
Means were compared between 2 or 3 groups using a 2-sided Student t test or analysis of variance (ANOVA), respectively, with the aid of JMP IN (SAS Institute, Cary, NC) software.
Results
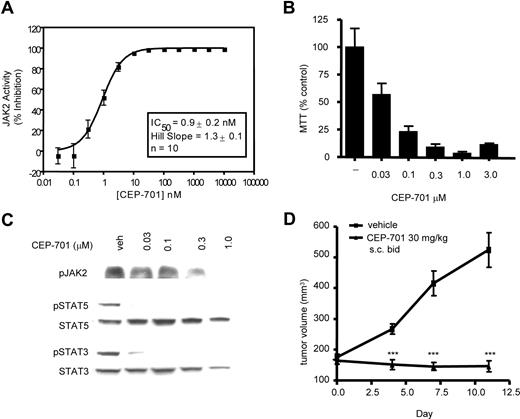
Lestaurtinib inhibits wild-type JAK2 activity and mutant JAK2 activity in vitro and in vivo
Lestaurtinib is currently in clinical trials in AML based on its FLT3 inhibitory activity. As part of a counterscreen for additional activities, we identified wild type JAK2 kinase as another target, with an in vitro concentration that inhibits response by 50% (IC50) of 0.9 (± 0.2) nM (Figure 1A). The discovery of JAK2 V617F as the prevalent molecular lesion in MPDs prompted us to evaluate the efficacy of lestaurtinib against this mutant. Initially, we studied the human erythroleukemia cell line HEL92.1.7, which is homozygous for the V617F mutation.15 Lestaurtinib strongly inhibited phosphorylation of V617F JAK2 and its downstream targets STAT5 and STAT3 (Figure 1C), with an IC50 for inhibition of STAT5 phosphorylation of 10 to 30 nM. Down-regulation of JAK/STAT signaling resulted in inhibition of growth of HEL92.1.7 cells with an IC50 of 30 to 100 nM (Figure 1B). Finally, lestaurtinib significantly suppressed the growth of HEL92.1.7 tumor xenografts in nude mice compared with vehicle-treated animals (Figure 1D). Pharmacodynamic analysis revealed a strong, time-dependent inhibition of STAT5 phosphorylation, which correlated with increased concentrations of lestaurtinib in tumors (not shown). In addition, Western blot of HEL92.1.7 cells demonstrated that these cells do not express FLT3, eliminating the possibility that the effects seen were secondary to FLT3 inhibition (see “Discussion”). Taken together, these data demonstrated that lestaurtinib could potently inhibit activation of JAK2 V617F in cell culture and in animal models.
Lestaurtinib is a potent inhibitor of the wild-type and the V617F mutant JAK2. (A) In in vitro kinase assay lestaurtinib inhibited activity of the WT JAK2 with an average (n = 10) IC50 of 0.9 nM (± 0.2 nM). Lestaurtinib suppressed JAK2/STAT signaling (B), proliferation in cultures (C) and growth in vivo (D) of HEL92.1.7 human erythroleukemia cells, which are homozygous for the V617F mutation. (B) HEL92.1.7 cells were incubated with increasing concentrations of lestaurtinib, as indicated, and effects on JAK2, STAT5 and STAT3 activation were evaluated by Western blot using phospho-specific antibodies. (C) HEL92.1.7 cultures were incubated with increasing concentrations of lestaurtinib for 72 hours and cell proliferation was assessed by MTS assay. All samples were done in triplicate. (D) Lestaurtinib (30 mg/kg, subcutaneous delivery twice a day) suppressed growth of HEL92.1.7 tumor xenografts in nude mice. Nude mice were injected with HEL92.1.7 cells; when tumors reached approximately 150 mm3, animals (10/group) were divided into treated and control groups, which were dosed with lestaurtinib or vehicle, respectively. Tumor volumes were measured every 3 to 4 days. Results were statistically significant (***) with P < .01. Error bars represent SD.
Lestaurtinib is a potent inhibitor of the wild-type and the V617F mutant JAK2. (A) In in vitro kinase assay lestaurtinib inhibited activity of the WT JAK2 with an average (n = 10) IC50 of 0.9 nM (± 0.2 nM). Lestaurtinib suppressed JAK2/STAT signaling (B), proliferation in cultures (C) and growth in vivo (D) of HEL92.1.7 human erythroleukemia cells, which are homozygous for the V617F mutation. (B) HEL92.1.7 cells were incubated with increasing concentrations of lestaurtinib, as indicated, and effects on JAK2, STAT5 and STAT3 activation were evaluated by Western blot using phospho-specific antibodies. (C) HEL92.1.7 cultures were incubated with increasing concentrations of lestaurtinib for 72 hours and cell proliferation was assessed by MTS assay. All samples were done in triplicate. (D) Lestaurtinib (30 mg/kg, subcutaneous delivery twice a day) suppressed growth of HEL92.1.7 tumor xenografts in nude mice. Nude mice were injected with HEL92.1.7 cells; when tumors reached approximately 150 mm3, animals (10/group) were divided into treated and control groups, which were dosed with lestaurtinib or vehicle, respectively. Tumor volumes were measured every 3 to 4 days. Results were statistically significant (***) with P < .01. Error bars represent SD.
Growth of primary cells from subjects with MPDs
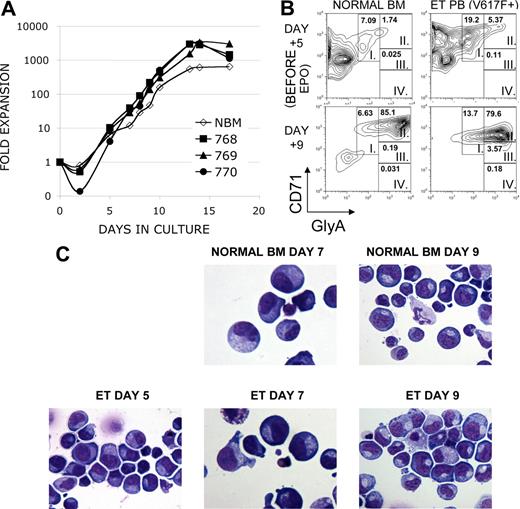
To test the antiproliferative activity of lestaurtinib against primary erythroid cells isolated from patients with MPDs, primary progenitor cells were expanded in a liquid culture system to obtain sufficient numbers of cells.16 Because patients with MPDs are known to have increased numbers of circulating stem and progenitor cells, we used CD34+ cells from peripheral blood as starting material.17 Our culture system allowed for significant expansion of predominantly erythroid cells (Figure 2). Overall, cells from subjects with MPDs expanded approximately 1000-fold during 14 days of culture while healthy controls expanded less in the more terminal stages, as might be expected by the clinical phenotype seen in myeloproliferative disorders (Figure 2A). The log phase of growth followed the addition of erythropoietin (EPO) on day 5; the absolute number of cells peaked between days 10 and 14 and subsequently declined. Cells cultured from subjects with MPDs were enriched for early erythroid precursors before the addition of EPO, compared with controls cells grown in parallel from healthy bone marrow donors, as shown by the expression of the erythroid markers CD71 and glycophorin A (mean 33.7% vs 6.6%, P = .017, Figure 2B,C and data not shown). After the addition of EPO, both disease samples and controls were predominantly erythroid (Figure 2B,C; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, we are able to obtain a large population of early erythroid cells expanded from patient material for our studies.
Erythroid expansion and phenotype of cultured primary cells from subjects with MPDs. CD34+ cells were isolated from peripheral blood from a subject with postthrombocythemic myeloid metaplasia (sample no. 768) and peripheral blood (sample no. 770) and bone marrow (sample no. 769) from a subject with ET. Cells were cultured in parallel with CD34+ cells isolated from a healthy bone marrow donor (NBM). Both MPD subjects had the JAK2 V617F+ mutation. Cells were cultured in the presence of IL-3, IL-6 and SCF, and EPO was added on day 5 of culture. (A) Cells were cultured in the presence of IL-3, IL-6 and SCF, and EPO was added on day 5 of culture. Cell number is presented as fold-expansion over the starting number of CD34+ cells. (B) CD71 and Glycophorin A expression was analyzed on cultured cells. Maturation-specific erythroid gates were drawn in accordance with flow cytometry models of murine and human erythroid development, with high expression of CD71 and moderate expression of glycophorin A in the earliest erythroblasts (group I) with successive increases in glycophorin A expression and loss of CD71 as the cells mature (groups II-IV). Shown are a representative MPD sample and NBM sample, EPO-independent erythroid differentiation in analyzed MPD samples (mean 33.7%, n = 3) was significantly greater than NBM samples (mean 6.6%, n = 2, P = .017). (C) Morphologic characterization of cultured cells. Cytospins of cultured cells were stained with a modified Wright-Giemsa stain. Note that before the addition of EPO on day 5, cells from a subject with ET possessed features characteristic of erythroblasts: round nuclei with clumped chromatin, royal blue cytoplasm and a lack of granulation. These features are more prominent following EPO in both healthy and MPD samples.
Erythroid expansion and phenotype of cultured primary cells from subjects with MPDs. CD34+ cells were isolated from peripheral blood from a subject with postthrombocythemic myeloid metaplasia (sample no. 768) and peripheral blood (sample no. 770) and bone marrow (sample no. 769) from a subject with ET. Cells were cultured in parallel with CD34+ cells isolated from a healthy bone marrow donor (NBM). Both MPD subjects had the JAK2 V617F+ mutation. Cells were cultured in the presence of IL-3, IL-6 and SCF, and EPO was added on day 5 of culture. (A) Cells were cultured in the presence of IL-3, IL-6 and SCF, and EPO was added on day 5 of culture. Cell number is presented as fold-expansion over the starting number of CD34+ cells. (B) CD71 and Glycophorin A expression was analyzed on cultured cells. Maturation-specific erythroid gates were drawn in accordance with flow cytometry models of murine and human erythroid development, with high expression of CD71 and moderate expression of glycophorin A in the earliest erythroblasts (group I) with successive increases in glycophorin A expression and loss of CD71 as the cells mature (groups II-IV). Shown are a representative MPD sample and NBM sample, EPO-independent erythroid differentiation in analyzed MPD samples (mean 33.7%, n = 3) was significantly greater than NBM samples (mean 6.6%, n = 2, P = .017). (C) Morphologic characterization of cultured cells. Cytospins of cultured cells were stained with a modified Wright-Giemsa stain. Note that before the addition of EPO on day 5, cells from a subject with ET possessed features characteristic of erythroblasts: round nuclei with clumped chromatin, royal blue cytoplasm and a lack of granulation. These features are more prominent following EPO in both healthy and MPD samples.
Lestaurtinib inhibits growth of cells from subjects with MPDs
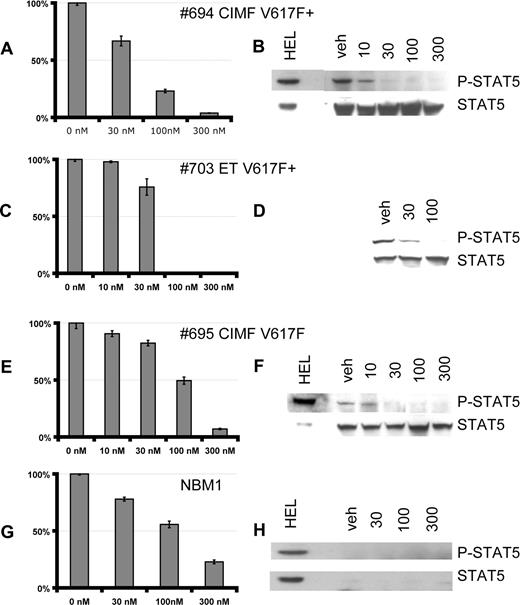
The response to lestaurtinib of cultured primary erythroid cells from MPD patients (Table 1) and healthy bone marrow was evaluated. Between days 7 and 10, when in the log-phase of growth, cells were incubated with increasing concentrations of lestaurtinib for 24 to 72 hours, and proliferation was assayed using the tetrazolium dye XTT. Compared with treatment with vehicle alone (normalized in these studies to 100%), proliferation of cells from subjects with V617F+ ET and CIMF was markedly inhibited at 100 nM of lestaurtinib (Figure 3A,C). There was substantial heterogeneity in the response of individual subject samples, as illustrated by a sample from one subject with V617F− CIMF that was only moderately inhibited at 100 nM (Figure 3E). In contrast, control samples from healthy bone marrow were only partially inhibited even at the 300 nM dose level. Note that healthy control cells were obtained from bone marrow, whereas patient samples were obtained from peripheral blood and we cannot exclude the possibility that the differences seen are due to the comparison of bone marrow to peripheral blood CD34+ cells as starting material. All MPD samples, irrespective of the extent of their growth inhibition, exhibited a marked decrease in STAT5 phosphorylation after the 1-hour treatment with lestaurtinib (Figure 3B,D,F and Figure S2). A similar dose response was obtained for inhibition of AKT phosphorylation (Figure S2). Lestaurtinib does not directly inhibit AKT or MAPK at these concentrations. Control bone marrow samples had a reproducibly low baseline level of expression and phosphorylation of STAT5 (explored in more detail below). As with HEL92.1.7 cells, Western blots demonstrated no expression of FLT3 in these cells, demonstrating that these effects are not secondary to FLT3 inhibition (Figure 6A and data not shown). These results suggested that lestaurtinib could preferentially inhibit the growth of cells from MPD subjects over healthy controls.
Subject characteristics and therapy
| Sample . | Diagnosis . | Age, y . | WBC, ×109/L . | Hg B, g/dL . | HCT, % . | Platelets, ×109/L . | Cytogenetics . | Rx . | V617F . | Proliferation at 100 nM, % . | Inhibited . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 648 | CIMF | 66 | 24.2 | 8.2 | 28 | 455 | 46,XY,del(13)(q12q14)[10]/46,XY[7] | THAL/PRED | ++ | 47.20 | I |
| 694 | CIMF | 66 | 23.8 | 9.2 | 28 | 353 | 46,XY,del(13)(q12q14)[10]/46,XY[7] | EPO | ++ | 37.63 | I |
| 695 | CIMF | 41 | 7 | 10.8 | 33 | 582 | 46,XX | ASA | − | 66.90 | N |
| 699 | CIMF | 45 | 3.5 | 8 | 24 | 123 | ND | None | − | 31.92 | I |
| 692 | ET | 40 | 5.6 | 13.9 | ND | 409 | 46,XX[11] | HU | + | 21.56 | I |
| 703 | ET | 47 | 8.4 | 14 | 41 | 563 | 46,XX[25] | HU | + | 20.75 | I |
| 708 | ET | 67 | 6.3 | 13.5 | 38 | 186 | ND | HU, ASA | + | 39.21 | I |
| 713 | ET | 52 | 5.2 | 11.4 | 34 | 509 | ND | HU, ASA | + | 4.22 | I |
| 777 | ET | 73 | 9.8 | ND | 48 | 1065 | 46,XY[8] | None | + | 14.80 | I |
| 675 | ET | 73 | 4.4 | 13.2 | 39 | 383 | 46,XX[25] | HU | − | 31.86 | I |
| 688 | ET | 75 | 7.5 | 14.1 | 41 | 1143 | 46,XY | ASA | − | 43.47 | I |
| 704 | ET | 53 | 6 | 12.1 | 35 | 575 | 46,XX | HU, ASA | − | 34.16 | I |
| 707 | ET | 57 | 4.5 | 14 | 259 | ND | HU | − | 20 | I | |
| 706 | ET | 41 | 8 | 11.6 | 36 | 602 | ND | HU, ASA | − | 85.45 | N |
| 700 | PV | 69 | 8 | 11.7 | 33 | 732 | ND | HU, ASA | + | 27.89 | I |
| 739 | PV | 56 | 6.3 | 13.2 | 39 | 369 | ND | HU, PB, ASA | + | 22.42 | I |
| 724 | PV | 76 | 8.3 | 15 | 44 | 372 | ND | PB, ASA | + | 57.75 | N |
| 742 | PV→ AML | 63 | 53.5 | 8.7 | 27 | 549 | ND | HU | ++ | 15.38 | I |
| NBM1 | CONTROL | ND | ND | ND | ND | ND | ND | ND | − | 60.57 | N |
| NBM2 | CONTROL | ND | ND | ND | ND | ND | ND | ND | − | 65.49 | N |
| NBM3 | CONTROL | ND | ND | ND | ND | ND | ND | ND | − | 89.38 | N |
| Sample . | Diagnosis . | Age, y . | WBC, ×109/L . | Hg B, g/dL . | HCT, % . | Platelets, ×109/L . | Cytogenetics . | Rx . | V617F . | Proliferation at 100 nM, % . | Inhibited . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 648 | CIMF | 66 | 24.2 | 8.2 | 28 | 455 | 46,XY,del(13)(q12q14)[10]/46,XY[7] | THAL/PRED | ++ | 47.20 | I |
| 694 | CIMF | 66 | 23.8 | 9.2 | 28 | 353 | 46,XY,del(13)(q12q14)[10]/46,XY[7] | EPO | ++ | 37.63 | I |
| 695 | CIMF | 41 | 7 | 10.8 | 33 | 582 | 46,XX | ASA | − | 66.90 | N |
| 699 | CIMF | 45 | 3.5 | 8 | 24 | 123 | ND | None | − | 31.92 | I |
| 692 | ET | 40 | 5.6 | 13.9 | ND | 409 | 46,XX[11] | HU | + | 21.56 | I |
| 703 | ET | 47 | 8.4 | 14 | 41 | 563 | 46,XX[25] | HU | + | 20.75 | I |
| 708 | ET | 67 | 6.3 | 13.5 | 38 | 186 | ND | HU, ASA | + | 39.21 | I |
| 713 | ET | 52 | 5.2 | 11.4 | 34 | 509 | ND | HU, ASA | + | 4.22 | I |
| 777 | ET | 73 | 9.8 | ND | 48 | 1065 | 46,XY[8] | None | + | 14.80 | I |
| 675 | ET | 73 | 4.4 | 13.2 | 39 | 383 | 46,XX[25] | HU | − | 31.86 | I |
| 688 | ET | 75 | 7.5 | 14.1 | 41 | 1143 | 46,XY | ASA | − | 43.47 | I |
| 704 | ET | 53 | 6 | 12.1 | 35 | 575 | 46,XX | HU, ASA | − | 34.16 | I |
| 707 | ET | 57 | 4.5 | 14 | 259 | ND | HU | − | 20 | I | |
| 706 | ET | 41 | 8 | 11.6 | 36 | 602 | ND | HU, ASA | − | 85.45 | N |
| 700 | PV | 69 | 8 | 11.7 | 33 | 732 | ND | HU, ASA | + | 27.89 | I |
| 739 | PV | 56 | 6.3 | 13.2 | 39 | 369 | ND | HU, PB, ASA | + | 22.42 | I |
| 724 | PV | 76 | 8.3 | 15 | 44 | 372 | ND | PB, ASA | + | 57.75 | N |
| 742 | PV→ AML | 63 | 53.5 | 8.7 | 27 | 549 | ND | HU | ++ | 15.38 | I |
| NBM1 | CONTROL | ND | ND | ND | ND | ND | ND | ND | − | 60.57 | N |
| NBM2 | CONTROL | ND | ND | ND | ND | ND | ND | ND | − | 65.49 | N |
| NBM3 | CONTROL | ND | ND | ND | ND | ND | ND | ND | − | 89.38 | N |
A sample was inhibited (I) if proliferation at 100 nM was less than 50% control (vehicle treated) cells, and not inhibited (N) if proliferation was more than 55% control. By this determination, 15 of 18 MPD samples are inhibited by lestaurtinib versus 0 of 3 control samples. All samples were from different subjects with the exception of samples 648 and 694, which were from the same subject at different points in time. The presence of the JAK2 V617F substitution was determined for each sample, and is listed in column 10.
WBC indicates white blood cells; Hg B, hemoglobin B; HCT, hematocrit; Rx, prescription; −, a sample where the mutation was not detected; +, a sample positive for the mutation; ++, a sample considered homozygous for the V617F mutation, as determined by a complete resistance to restriction enzyme digestion with BsaXI; I, inhibited; and N, not inhibited.
Lestaurtinib inhibits growth and JAK/STAT signaling in cultured erythroid precursors. Effects of lestaurtinib on proliferation (A, C, E, G) and STAT5 phosphorylation (B, D, F, H) in erythroid precursors derived from CD34+ progenitors purified from peripheral blood of MPD patients (A-F) and from a bone marrow of the healthy subject (G, H) were evaluated. Cultures were counted and replated at 5 × 105 cells/mL and then treated with increasing concentrations of lestaurtinib, as indicated, for 48 to 72 hours and cell proliferation was analyzed by XTT assay (A, C, E, G). To assess effects of lestaurtinib on JAK/STAT signaling (B, D, F, H), cultures were treated with increasing concentrations of lestaurtinib for 1 hour, and activation of STAT5 was analyzed by Western blot using phospho-specific STAT5 antibody. Blots were stripped and reprobed with a total STAT5 antibody. The status of the V617F mutation was determined in mononuclear cells by allele-specific PCR and BsaX1 restriction analysis. Stripping and reprobing of blot in H confirmed equal protein loading (data not shown). Vertical lines have been inserted to indicate a repositioned gel lane. Error bars in panels A, C, E, and G represent SE.
Lestaurtinib inhibits growth and JAK/STAT signaling in cultured erythroid precursors. Effects of lestaurtinib on proliferation (A, C, E, G) and STAT5 phosphorylation (B, D, F, H) in erythroid precursors derived from CD34+ progenitors purified from peripheral blood of MPD patients (A-F) and from a bone marrow of the healthy subject (G, H) were evaluated. Cultures were counted and replated at 5 × 105 cells/mL and then treated with increasing concentrations of lestaurtinib, as indicated, for 48 to 72 hours and cell proliferation was analyzed by XTT assay (A, C, E, G). To assess effects of lestaurtinib on JAK/STAT signaling (B, D, F, H), cultures were treated with increasing concentrations of lestaurtinib for 1 hour, and activation of STAT5 was analyzed by Western blot using phospho-specific STAT5 antibody. Blots were stripped and reprobed with a total STAT5 antibody. The status of the V617F mutation was determined in mononuclear cells by allele-specific PCR and BsaX1 restriction analysis. Stripping and reprobing of blot in H confirmed equal protein loading (data not shown). Vertical lines have been inserted to indicate a repositioned gel lane. Error bars in panels A, C, E, and G represent SE.
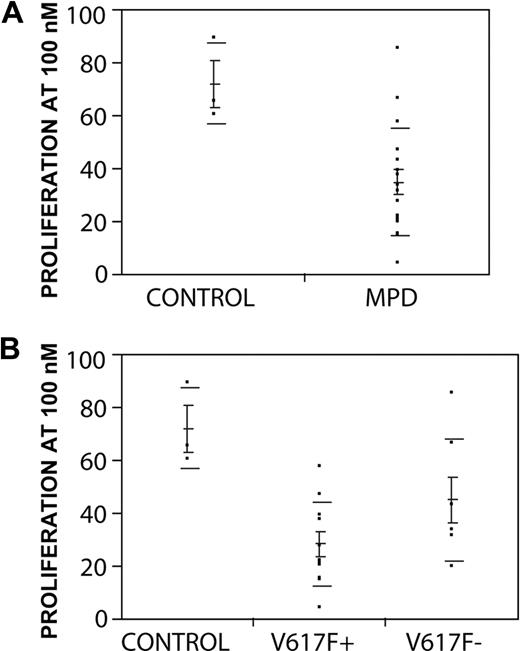
To determine the range of response of patient samples, a larger cohort of subject samples was screened for sensitivity to lestaurtinib (Table 1). At the 100 nM dose level, growth of 15 of 18 samples from subjects with MPDs was inhibited more than 50% compared with untreated cells, whereas 3 control samples were less than 50% inhibited. By specific MPD subtype, 3 of 4 samples from 3 subjects with CIMF were inhibited, 9 of 10 samples from subjects with ET were inhibited, and 3 of 4 samples from subjects with a history of PV were inhibited. Of interest, the inhibitory effect of lestaurtinib was noted in a PV sample that had transformed to acute leukemia at the time of sample collection (Table 2). MPD samples were significantly more inhibited by lestaurtinib than controls: the mean proliferation at 100 nM from samples from subjects with MPDs (n = 18) was 34.6% (± 4.7%), whereas control samples from healthy donors (n = 3) had a mean proliferation of 71.8% (± 8.9%; P = .03; Figure 4A). Virtually all samples showed some growth inhibition at 300 nM and again MPD samples were more inhibited than healthy donor controls, although the difference did not meet statistical significance (means 11.76% ± 4.07% vs 28.57% ± 8.14%; P = .08). Importantly, samples from MPD subjects with or without the JAK2 V617F mutation were both inhibited by lestaurtinib. Cells from patients with the JAK2 V617F mutation were inhibited to a greater degree than were samples without the mutation, but this difference did not achieve statistical significance (P = .08; Figure 4B). For one subject with JAK2 V617F+ ET, we were able to compare marrow and peripheral blood cells and found they were both sensitive to inhibition by lestaurtinib (Figure S1B). Taken together, these data demonstrate that lestaurtinib is a potent and selective inhibitor of proliferation of primary erythroid cells from subjects with MPDs.
Primary erythroid cells from MPD samples are selectively inhibited by lestaurtinib. (A) Proliferation at 100 nM: comparison of means between MPD samples (34.6% ± 20.1%) and NBM controls (71.8% ± 8.9%) is significant (P = .03). (B) Proliferation at 100 nM: comparison of means between MPD samples stratified by presence (V617F+, n = 11, mean 28.1% ± 4.8%) or absence (V617F-, n = 7 mean 44.8% ± 8.7%) of JAK2 versus NBM controls (P = .006 by ANOVA). The difference of means between V617F+and V617− samples did not reach statistical significance (P = .08 Student t-test). Means, standard error of the mean (shorter horizontal lines), and standard deviation (longer horizontal lines) are depicted for each group.
Primary erythroid cells from MPD samples are selectively inhibited by lestaurtinib. (A) Proliferation at 100 nM: comparison of means between MPD samples (34.6% ± 20.1%) and NBM controls (71.8% ± 8.9%) is significant (P = .03). (B) Proliferation at 100 nM: comparison of means between MPD samples stratified by presence (V617F+, n = 11, mean 28.1% ± 4.8%) or absence (V617F-, n = 7 mean 44.8% ± 8.7%) of JAK2 versus NBM controls (P = .006 by ANOVA). The difference of means between V617F+and V617− samples did not reach statistical significance (P = .08 Student t-test). Means, standard error of the mean (shorter horizontal lines), and standard deviation (longer horizontal lines) are depicted for each group.
Lestaurtinib inhibits STAT5 activation and other downstream effectors of JAK2/STAT signaling
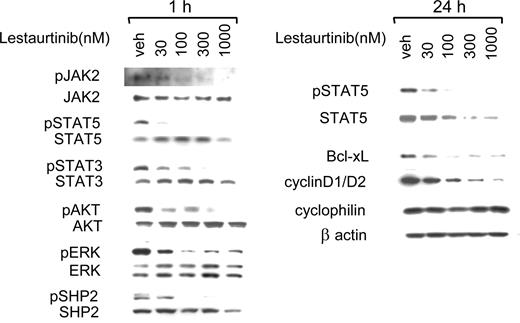
To assess in greater detail the effects of lestaurtinib-mediated JAK2 inhibition on signaling pathways in erythroid precursors, cultured cells derived from a JAK2 V617F+ CIMF patient were analyzed. After 1 hour exposure, lestaurtinib inhibited phosphorylation of JAK2 and its downstream targets, STAT5 and STAT3, in a dose-dependent manner, with significant inhibition observed at 30 nM (Figure 5). Similar inhibition of JAK/STAT signaling was observed in all analyzed MPD samples, independent of their JAK2 V617F status (Figure S2). Lestaurtinib also suppressed phosphorylation of AKT and ERK, whose activation is driven by JAK2 (Figure 5). Inhibition of AKT and ERK exhibited a dose response similar to that demonstrated for the inhibition of JAK2/STAT signaling, in accordance with the data obtained for other MPD samples (Figure S2). Transmission of the activating signal from JAK2 to PI3-K and MAPK pathways involves activation of various scaffolding and adaptor molecules.18 Lestaurtinib inhibited phosphorylation of 2 such molecules, SHP2 and Gab2, suggesting that it may disrupt the multiprotein signaling complex that forms after JAK2 activation. Lestaurtinib-mediated inhibition of JAK2-triggered signaling pathways resulted in down-regulation of expression of downstream effectors including Bcl-xL and cyclin D1/D2 at 24 hours (Figure 5). Twenty-four–hour exposure to lestaurtinib also suppressed expression of nonphosphorylated STAT5 in erythroid precursors. Note that lestaurtinib does not inhibit AKT or ERK kinase directly at micromolar concentrations in in vitro kinase assays (data not shown). Lestaurtinib inhibits JAK3 with an IC50 of 3 nM (data not shown), but JAK3 is not thought to play a role in signal transduction in erythroid cells. Taken together, these data demonstrate that growth inhibition of hematopoietic cells from subjects with MPDs correlates with the inhibition of JAK2 dependent signal transduction pathways.
Lestaurtinib inhibits STAT5 phosphorylation and other downstream effectors of JAK/STAT signaling. Lysates from cultured cells from a subject with CIMF (homozygous for the V617F mutation) exposed to various concentrations of lestaurtinib for 1 hour and 24 hours. Phosphorylation of JAK2, STAT5, STAT3, AKT, ERK, and SHP2 was assayed by Western blot from samples treated for 1 hour (left panel). Blots were stripped and reprobed with antibodies against total protein for controls. Right Panel: After a 24-hour exposure, phosphorylation of STAT5 and expression of STAT5, Bcl-xL, and cyclin D1 and D2 were assessed by Western blot. Cyclophilin and β-actin served as loading controls.
Lestaurtinib inhibits STAT5 phosphorylation and other downstream effectors of JAK/STAT signaling. Lysates from cultured cells from a subject with CIMF (homozygous for the V617F mutation) exposed to various concentrations of lestaurtinib for 1 hour and 24 hours. Phosphorylation of JAK2, STAT5, STAT3, AKT, ERK, and SHP2 was assayed by Western blot from samples treated for 1 hour (left panel). Blots were stripped and reprobed with antibodies against total protein for controls. Right Panel: After a 24-hour exposure, phosphorylation of STAT5 and expression of STAT5, Bcl-xL, and cyclin D1 and D2 were assessed by Western blot. Cyclophilin and β-actin served as loading controls.
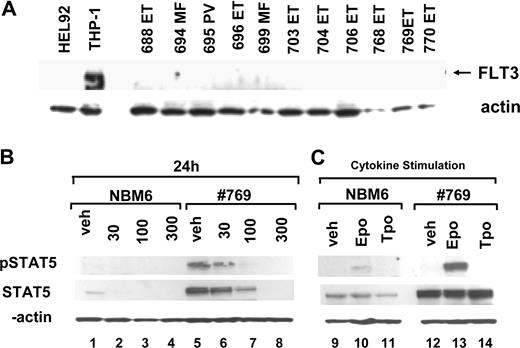
Lestaurtinib has been previously demonstrated to inhibit FLT3. To confirm that FLT3 was not the target of inhibition in HEL92.1.7 cells or primary erythroid cultures from patients, we analyzed these cells for expression of FLT3 by Western blotting (Figure 6A). FLT3 was clearly expressed in the THP1 cell line. However, no FLT3 protein was detectable in HEL92.1.7 cells or in any of the subject samples. Given this, it is unlikely that FLT3 inhibition plays a role in the results described.
Healthy bone marrow donors have lower levels of total STAT5 expression and STAT5 phosphorylation than MPD samples. Cells from bone marrow CD34+ cells from a subject with ET (769, V617F+) and a healthy donor (NBM6) were grown in parallel for 9 days in the presence of IL-3 (10 ng/mL), IL-6 (10 ng/mL), SCF (25 ng/mL) and EPO (2 U/mL starting on day 5). (A) Cells were counted and replated at 5 × 105 cell/mL and then exposed for 24 hours to lestaurtinib at different concentrations, lysed, and expression of phosphorylated STAT5 and total STAT5 was assayed by Western blotting. (B) After expansion, cells were washed twice in PBS and replated in media lacking all cytokines for 4 hours and then restimulated for 10 minutes with EPO (100 U/mL), TPO (100 ng/mL), or no cytokine control. Cells were collected and lysed. Lysates were analyzed for phospho-STAT5 and total STAT5 expression.
Healthy bone marrow donors have lower levels of total STAT5 expression and STAT5 phosphorylation than MPD samples. Cells from bone marrow CD34+ cells from a subject with ET (769, V617F+) and a healthy donor (NBM6) were grown in parallel for 9 days in the presence of IL-3 (10 ng/mL), IL-6 (10 ng/mL), SCF (25 ng/mL) and EPO (2 U/mL starting on day 5). (A) Cells were counted and replated at 5 × 105 cell/mL and then exposed for 24 hours to lestaurtinib at different concentrations, lysed, and expression of phosphorylated STAT5 and total STAT5 was assayed by Western blotting. (B) After expansion, cells were washed twice in PBS and replated in media lacking all cytokines for 4 hours and then restimulated for 10 minutes with EPO (100 U/mL), TPO (100 ng/mL), or no cytokine control. Cells were collected and lysed. Lysates were analyzed for phospho-STAT5 and total STAT5 expression.
Erythroid precursors from healthy bone marrow donors reproducibly expressed lower levels of STAT5 than MPD samples and exhibited minimal, if any, STAT5 phosphorylation. This result was confirmed in 4 separate bone marrow samples (data not shown). To compare directly the expression and activation of STAT5 in erythroid cells derived from bone marrow of a healthy donor and a bone marrow sample from a subject with a V617F+ MPD, samples were expanded simultaneously. Cells obtained from a healthy donor showed markedly lower total STAT5 expression than the ET sample (Figure 6, compare lane 1 to lane 5). Constitutive phosphorylation of STAT5 was readily detectable in the ET subject sample, but was not detectable in the healthy donor sample (Figure 6). To determine whether STAT5 could be activated in healthy erythroid cells, cells collected on day 9 of culture were washed and incubated in a cytokine-free medium for 4 hours and then stimulated for 10 minutes with high doses of EPO or TPO.19 STAT5 phosphorylation was moderately induced after EPO stimulation but not after TPO stimulation in cultures obtained from healthy bone marrow, suggesting that these cells expressed a functional EPO receptor but not a TPO receptor, as would be expected by the cell culture conditions (Figure 6, lanes 10 and 11). In contrast, EPO more strongly induced phosphorylation of STAT5 (Figure 6, lane 13) and AKT (not shown) in the ET-derived sample. The removal of cytokines appreciably, but not fully, reduced constitutive phosphorylation of STAT5 in the MPD sample, suggesting that the V617F mutation in JAK2 confers cytokine hypersensitivity but is not sufficient alone to achieve maximal activation of STAT5. These results suggest that the tonic activation of a JAK/STAT signaling pathway may up-regulate both expression and activation of STAT5 in patient samples compared with healthy controls.
Discussion
MPDs are strongly associated with constitutive JAK/STAT signaling. The activating V617F mutation in JAK2 has been identified as the predominant molecular lesion in non-CML MPDs.1-4 Here we present evidence that lestaurtinib is a potent JAK2 inhibitor and may effectively suppress the increased blood cell production associated with these diseases. A primary screen demonstrated that lestaurtinib inhibited wild-type JAK2 at low-nanomolar concentrations. Initial analysis using an erythroleukemia cell line homozygous for the V617F mutation revealed that lestaurtinib was also active against mutant JAK2, potently inhibiting JAK/STAT signaling and cell growth in cell culture and in tumor xenografts.
We hypothesized that primary cells from subjects with MPDs would be similarly sensitive to JAK2 inhibition. A liquid erythroid cell culture system was established from progenitor cells obtained from peripheral blood samples, and yielded adequate numbers of cells to analyze. Morphologically and immunophenotypically these cells differentiate from blasts through later normoblast and nucleated red blood cell stages in a manner consistent with healthy human erythroid development and similar to control cells grown from CD34+ cells from healthy marrow donors.20 These features make this a feasible, rational and clinically relevant ex vivo system for studying the biology of MPDs. Lestaurtinib preferentially inhibited the growth of erythroid precursors from subjects with MPDs. Inhibition was dose-dependent; at 100 nM lestaurtinib suppressed cell proliferation more than 50% in 15/18 tested MPD samples, compared with 0/3 samples from healthy donors. The inhibitory effect was independent of V617F mutation status and MPD subtype. Lestaurtinib induced modest apoptosis in primary erythroid precursors and HEL 92.1.7 cultures, but only at the highest concentrations (data not shown), indicating that lestaurtinib functions predominantly as a suppressor of proliferation.
To define more precisely the antiproliferative mechanism of lestaurtinib inhibition, multiple signaling pathways were analyzed. All samples from MPD patients, independent of the V617F mutation status and disease diagnosis, exhibited high levels of constitutive JAK/STAT and AKT signaling. Lestaurtinib markedly inhibited STAT5 and AKT phosphorylation in all MPD samples, with strong down-regulation observed at 30 nM for most samples. Signaling through JAK/STAT, PI3-K/AKT and MAPK in response to EPO is initiated by activation of JAK2—all of these pathways were suppressed by lestaurtinib. Furthermore, the multiprotein signaling complex that forms after JAK2 activation and transmits signals to PI3-K/AKT and MAPK pathways was inhibited by lestaurtinib, as demonstrated by the reduction in phosphorylation of 2 such proteins, SHP1 and Gab2.18,21 These data demonstrate that lestaurtinib inhibits JAK2 and its downstream signaling pathways. Finally, analysis of expression of known downstream effectors of JAK2 signaling in erythroid precursors revealed that lestaurtinib strongly down-regulated expression of cyclin D1/D2 and Bcl-xL, both known to be involved in erythroid proliferation and survival.20,22,23 Garcon et al have recently demonstrated that elevated expression of Bcl-xL alone is sufficient to generate EPO independent erythroid colonies (EEC), a hallmark of MPDs.22 These data, together with reported increased expression of Bcl-xL in PV erythroblasts, suggest that constitutively active JAK2 might mediate EEC formation via EPO-independent induction of Bcl-xL.23 In this context, the down-regulation of Bcl-xL expression by lestaurtinib suggests a molecular mechanism of growth inhibition. Extended exposure to lestaurtinib also resulted in dose-dependent inhibition of STAT5 in HEL 92.1.7 cells and primary erythroid precursors from MPD subjects. The down-regulation of STAT5 by JAK inhibitors may be mediated by a STAT5 positive feedback loop, or could be targeted by activated caspases; both mechanisms have been observed for STAT3.24 Whatever the actual mechanism, down-regulation of STAT5 expression by lestaurtinib would likely amplify and extend inhibitory effects in mutated clones. In summary, our data demonstrate that erythroid cells from patients with MPDs have increased expression and activation of multiple EPO dependent signaling pathways that can be suppressed by lestaurtinib, providing a molecular rationale for testing JAK2 inhibitors in subjects with MPDs.
Our results reveal important differences between healthy and MPD samples that may broaden the therapeutic window for JAK2 inhibitors. The finding that all 4 healthy bone marrow samples analyzed expressed low levels of STAT5 and exhibited minimal, if any, STAT5 phosphorylation suggests inherent differences between erythroid precursors from healthy subjects and those with MPDs. The dramatic difference in EPO-mediated activation of STAT5 and AKT in cytokine-deprived cultures between healthy and MPD precursors further supports this notion. The markedly higher levels of STAT5 expression and phosphorylation provide a molecular explanation for increased sensitivity of MPD-derived erythroid precursors to lestaurtinib. Within MPD samples, it is not surprising that inhibition of the JAK2 pathway is not specific to samples bearing the JAK2 V617F mutation. The clinical phenotypes of patients with MPDs with or without the V617F mutation are not distinct, and a molecular lesion has yet to be characterized in a significant proportion of patients with CIMF and ET. Four additional gain-of-function mutations in exon 12 of JAK2 have recently been identified in the minority of PV patients without the V617F mutation; the discovery of activating mutations in the TPO receptor (MPL) in patients with ET and CIMF also supports the premise that MPDs are generally diseases of JAK/STAT hyperactivation, although the mechanism of activation may be variable.25-27 Our results support a model whereby up-regulation of the JAK2 signaling pathway is a common pathophysiologic mechanism underlying MPDs. Taken together, these data suggest a potential for lestaurtinib to preferentially eradicate mutated clones, without affecting the expansion of healthy precursors.
The studies by Druker et al of imatinib in CML showed definitively that molecularly targeted therapy can have a profound impact in diseases with well-defined molecular signatures.28,29 An unexpected benefit of the compound was its efficacy in other diseases characterized by activation PDGFR or c-KIT. Rare cases of nonclassical MPDs with a chromosomal translocation involving PDGFR were found to be responsive to imatinib, and gastrointestinal stromal tumors, which almost uniformly involve the activation of KIT, were correctly hypothesized to have a high probability of responding to imatinib.30,31 Later, the empiric observation that the hypereosinophilic syndrome (HES) responded to low doses of imatinib led to a larger study of the drug, together with a more systematic molecular hypothesis that HES might involve activation of ABL, KIT, or PDGFR.32 Subsequently, a novel fusion involving PDGFRA was identified in HES.33 These examples demonstrate that kinase inhibitors are powerful tools both to further the understanding of and treatment of malignant diseases.
Lestaurtinib, which has been extensively studied in the clinic and has an excellent safety record, is orally bioavailable and effective in inhibiting FLT3 kinase activation in AML. Lestaurtinib is not a specific FLT3 inhibitor, but rather an inhibitor of multiple kinases. Although it inhibits JAK3 in vitro, safety data thus far have not demonstrated significant immunosuppression. The preclinical data presented here suggest that it could have at least comparable efficacy to FLT3 for inhibiting JAK2, and is therefore an attractive drug candidate for a targeted therapy in patients with MPDs. Lestaurtinib may exemplify a class of therapeutic molecules with potentially broad therapeutic activity.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients who donated their cells to support this research. Thanks to A. Perl and J. Thompson for critical reading of the manuscript, and to Andrea Miltiades for help with data collection.
This work was performed in part by Cephalon Oncology, which has a commercial interest in lestaurtinib. M.C. is a recipient of the Leukemia & Lymphoma Society of America Clinical Scholar Award. E.O.H. is supported by National Institutes of Health grant number K12 RR017625 in Patient Oriented Research.
National Institutes of Health
Authorship
Contribution: E.O.H. designed the study, performed experiments, analyzed data, and wrote the manuscript. C.S., M.J., C.R.S., C.R., S.Y., and T.A. performed experiments and analyzed data. S.G.E., M.C., B.R., and P.D. designed the study and edited the manuscript.
Conflict-of-interest disclosure: M.C. has received research support from Cephalon Oncology. C.S., M.J., C.R., S.Y., T.A., B.R., and P.D. are employees of Cephalon Oncology. All other authors declare no competing financial interests.
Correspondence: Pawel Dobrzanski, Cephalon Inc, 145 Brandywine Pkwy, West Chester, PA 19380; e-mail: pdobrzan@cephalon.com.