Abstract
Myeloproliferative diseases (MPDs) represent the commonest cause of splanchnic vein thrombosis (SVT), including Budd-Chiari syndrome (BCS) and portal vein thrombosis (PVT), but their diagnosis is hampered by changes secondary to portal hypertension, while their influence in the outcome of SVT remains unclear. We assessed the diagnostic and prognostic value of JAK2 and MPL515 mutations in 241 SVT patients (104 BCS, 137 PVT). JAK2V617F was found in 45% of BCS and 34% of PVT, while JAK2 exon 12 and MPL515 mutations were not detected. JAK2V617F was found in 96.5% of patients with bone marrow (BM) changes specific for MPD and endogenous erythoid colonies, but also in 58% of those with only one feature and in 7% of those with neither feature. Stratifying MPD diagnosis first on JAK2V617F detection would have avoided BM investigations in 40% of the patients. In BCS, presence of MPD carried significantly poorer baseline prognostic features, required hepatic decompression procedures earlier, but had no impact on 5-year survival. Our results suggest that JAK2V617F testing should replace BM investigations as initial test for MPD in patients with SVT. Underlying MPD is associated with severe forms of BCS, but current therapy appears to offset deleterious effects of MPD on the medium-term outcome.
Introduction
Splanchnic vein thromboses (SVT), which include portal vein thrombosis (PVT) and thrombosis of the hepatic veins causing Budd-Chiari syndrome (BCS), are frequent presenting complications of undiagnosed Philadelphia (Ph)–negative myeloproliferative disorders (MPDs). MPDs represent the commonest cause of BCS and PVT, found in approximately 50% and 25% of the patients, respectively.1-3 In patients with SVT, portal hypertension is a virtually constant feature. The resulting hypersplenism and hemodilution decrease the accuracy of blood cell counts and splenomegaly for MPD diagnosis. The atypical peripheral blood picture of MPD in the setting of SVT has lead to a variety of denominations as “atypical,” “latent,” or “occult” MPD.2,4-7 In such patients, MPD diagnosis has therefore been based on bone marrow biopsy (BMB) findings and endogenous erythroid colony (EEC) formation assessment, both of which have limitations.2,7,8 Indeed, BMB is invasive and potentially harmful in SVT patients who may have coagulopathy or need for anticoagulation therapy, and its interpretation for MPD diagnosis requires semiquantitative and qualitative evaluation.9 With regard to EEC studies, they use nonstandardized techniques, are reliably performed only in few specialized centers, and their significance as unique anomaly in patients with SVT has been disputed.10,11 These limitations have hindered diagnosis of underlying MPD and assessment of its influence on the outcome of SVT patients.
The discovery of JAK2V617F mutation, found in approximately 90% of patients with polycythemia vera (PV), and in 50% of those with essential thrombocythemia (ET) or primary myelofibrosis (PMF), has modified the diagnostic and prognostic approach to MPD.12-15 Recently, several small series have shown that JAK2V617F detection could be of interest for diagnosing MPD in BCS or PVT patients.4,16-18
Lastly, W515L and W515K mutations in the thrombopoietin receptor, MPL, were recently identified in approximately 5% of patients with PMF or ET,19,20 and JAK2 exon 12 mutations in less than 5% of PV patients.21,22 Those mutations could represent new molecular markers for diagnosing MPD in JAK2V617F- negative patients.
The major aim of the present study was to assess the diagnostic and prognostic impact of JAK2 and MPL mutation detection in a large cohort of patients with splanchnic vein thrombosis.
Methods
Inclusion criteria
This retrospective study was performed in 3 centers of the European Network for Vascular Disorders of the Liver (EN-Vie): University Hospitals Beaujon (Paris, France), Clinic (Barcelona, Spain), and Erasmus (Rotterdam, The Netherlands). Inclusion criteria were (1) diagnosis of PVT or BCS; (2) exclusion of associated malignancy or cirrhosis; (3) DNA for molecular testing; and (4) patient informed consent obtained in accordance with the Declaration of Helsinki. Approval was obtained from the EN-Vie institutional review board for this study.
BCS was diagnosed according to previously published criteria.3 PVT was diagnosed in the presence of endoluminal material and absence of flow in the portal vein, or cavernous transformation of the vein as shown by duplex-Doppler ultrasound, or contrast-enhanced CT scan or magnetic resonance imaging.23 BCS and PVT will thereafter be collectively referred to as SVT.
According to those criteria, a total of 241 cases (130 in Paris, 70 in Barcelona, and 41 in Rotterdam) could be collected. The site of thrombosis was the hepatic veins (BCS) in 104 patients and the portal vein (PVT) in 137 patients. For each patient, the following data were collected: BMB (n = 236); screening using validated methods for pro-thrombotic states, including genotyping for factor V Leiden and factor II, C677T MTHFR mutation, protein C, protein S, and antithrombin deficiencies, antiphospholipid antibodies, and paroxysmal nocturnal hemoglobinuria (determined by flow cytometry methods) (available in all cases); investigations for MPD, including EEC assessment (n = 125), serum erythropoietin level (n = 142), and red cell mass (RCM) measurement (n = 121). In all subjects, clinical, hematologic data, and liver function tests were recorded.
Hematologic studies
Bone marrow biopsy slides were centrally reviewed using Thiele's criteria for MPD evaluation,24 blindly to the results of other investigations.
The JAK2V617F mutation was detected by single nucleotide polymorphism genotyping assays using real-time polymerase chain reaction (PCR)-based mutation detection as previously described (using both Taqman ABI Prism 7700 and LightCycler techniques).25,26 PCR was performed either on archival whole blood DNA (n = 177) or freshly collected granulocytic DNA (n = 80) obtained at time of SVT diagnosis. Sensitivity of those assays was 0.5% to 1% of HEL cell line DNA diluted in nonmutated DNA, and 2% to 4% of a homozygously mutated patient DNA diluted in normal DNA. They also allow quantification of the percentage of circulating JAK2-mutated alleles (%V617F), as described.25,26
MPL exon 10 mutations were detected by direct sequencing, as previously described.27 Sensitivity of mutation detection by this method was 5% to 10%.
In the 147 JAK2V617F-negative patients, sufficient amounts of DNA were still available after MPL sequencing in 123 patients for successful JAK2 exon 12 mutations screening by direct sequencing, as described.22
Impact of MPD on prognosis of splanchnic vein thrombosis
Overall survival and event-free survival (EFS) in BCS patients with and without MPD or JAK2V617F mutation were compared after adjustment on prognostic features at diagnosis. One liver disease-specific (Child-Pugh score)28 and 2 BCS-specific scores29,30 that incorporate to a various extent serum albumin, bilirubin, prothrombin, encephalopathy, and ascites were used. BCS-specific scores, designed in 2 of our centers before the extensive use of percutaneous endovascular treatments, have been shown to reflect liver transplantation-free survival regardless of surgical portocaval shunt.29,30 Lack of an available prognostic score for PVT patients did not allow adjustment of overall survival and EFS according to JAK2V617F on baseline characteristics. EFS was defined as survival until liver decompressive procedure (angioplasty of hepatic vein or inferior vena cava, surgical portosystemic shunt, or transjugular intrahepatic portosystemic stent shunt), liver transplantation, or death, whichever occurred first. All patients were managed with routine anticoagulation from diagnosis. Indications for decompressive procedures, liver transplantation, and specific therapy for underlying causes varied according to center and period. No adjustment attempt was made on these indications.
Statistical analysis
Quantitative data were expressed as median values and first (Q1) and third (Q3) quartiles, whereas percentages were used for qualitative data. Comparisons between groups used nonparametric Kruskal-Wallis test or Fisher exact test depending on the variable.
Overall survival and EFS were measured from the date of BCS or PVT diagnosis (ie, date when imaging demonstrated thrombosis). Similar endpoints were defined from date of JAK2 mutation screening. Such right-censored data were estimated using the Kaplan-Meier method, and comparison between groups was made using the log-rank test.
All statistical tests were 2-sided, with P values of .05 or less denoting statistical significance. Statistical analysis was performed on SAS 9.1 (SAS, Cary, NC) software package.
Results
Among the 241 patients enrolled, 104 (43%) had BCS and 137 (57%) had PVT. BCS patients were significantly younger at diagnosis of thrombosis (median age, 36 years; Q1-Q3, 27-46 years) than PVT patients (median age, 42 years; Q1-Q3, 30-57 years; P = .007). There was a female predominance (66.3%) in BCS, compared with PVT (43.8%, P < .001).
Prevalence of JAK2V617F mutation and relationship with laboratory features
JAK2V617F was detected in 94 of 241 (39%) of all patients. There was a trend for higher JAK2V617F prevalence in BCS compared with PVT (45.2% and 34.3%, respectively, P = .086). Conversely, the percentage of mutated circulating allele tended to be lower in BCS (median, 30%; Q1-Q3, 15%-35%) than in PVT (median, 35%; Q1-Q3, 25%-50%, P = .06). In 16 patients, a second blood sample, drawn after a median follow-up of 5 years (range, 2-11 years), was available for JAK2 mutation testing. Among 12 mutated patients, JAK2V617F was still detected during follow-up, with stable V617F allele proportion in 9 of them, and slightly increased proportion in the remaining 3. Of the 4 patients without JAK2 mutation on the first sample, 3 remained negative, whereas JAK2V617F was detected on the second sample taken 7 years later, with 30% V617F circulating allele in the last patient. This patient had been diagnosed as ET at the time of PVT diagnosis, based on a high platelet count, positive BMB, presence of EEC, and normal RCM.
As shown in Table 1, in the whole cohort, JAK2V617F was associated with significantly higher hemoglobin (Hb) levels, hematocrit, white blood cell and platelet counts, and lower mean corpuscular volume. However, peripheral blood cell counts remained in the normal range in most patients with JAK2 mutation. Among them, only 2 of 36 males (5.6%) and 6 of 58 females (10.3%) had Hb more than 18.5 g/dL and 16.5 g/dL, respectively, whereas 14 of 94 (14.9%) had platelets more than 450.109/L (cutoff values for PV and ET diagnosis according to recently revised World Health Organization criteria31 ). Patients with JAK2V617F also had significantly larger spleen size, lower serum erythropoietin levels, and higher measured RCM than unmutated patients (Table 1).
Characteristics associated with the JAK2V617F mutation in 241 patients with Budd-Chiari syndrome or portal vein thrombosis
| All patients . | Without JAK2V617F, n = 147 (median [Q1-Q3]) . | With JAK2V617F, n = 94 (median [Q1-Q3]) . | P . |
|---|---|---|---|
| Age, y | 44 [35-59.7] | 47 [35.2-58] | .78 |
| Males, % | 76 (51.7%) | 36 (38.3%) | .048 |
| Hemoglobin, g/dL | 13 [11.3-14.4] | 13.75 [12.3-15.1] | .012 |
| Hematocrit, % | 39 [33.8-44] | 43 [38.7-47] | <.001 |
| MCV, fL | 87.3 [82.9-91.3] | 85 [81-88] | .023 |
| WBC count, ×109/L | 5.7 [4.3-7.6] | 8.52 [5.9-11.5] | <.001 |
| ANC, ×109/L | 3.67 [2.7-4.9] | 5.25 [3.7-9] | <.001 |
| Platelet count, ×109/L | 159 [104-257] | 333.5 [239-456] | <.001 |
| Spleen size BCM, cm | 0 [0-2.25] | 5 [0-11.25] | <.001 |
| Serum EPO, mU/mL | 16.2 [10.6-35] | 12 [7.4-17.] | .002 |
| Measured RCM, mL/kg | 26.2 [23-31] | 32.9 [27-37.1] | <.001 |
| Predicted RCM, (mL/kg | 24.7 [22-26.3] | 24.81 [23-27.4] | .37 |
| RCM more than 125% of predicted | 11/59 (18.6%) | 38/62 (61.3%) | <.001 |
| All patients . | Without JAK2V617F, n = 147 (median [Q1-Q3]) . | With JAK2V617F, n = 94 (median [Q1-Q3]) . | P . |
|---|---|---|---|
| Age, y | 44 [35-59.7] | 47 [35.2-58] | .78 |
| Males, % | 76 (51.7%) | 36 (38.3%) | .048 |
| Hemoglobin, g/dL | 13 [11.3-14.4] | 13.75 [12.3-15.1] | .012 |
| Hematocrit, % | 39 [33.8-44] | 43 [38.7-47] | <.001 |
| MCV, fL | 87.3 [82.9-91.3] | 85 [81-88] | .023 |
| WBC count, ×109/L | 5.7 [4.3-7.6] | 8.52 [5.9-11.5] | <.001 |
| ANC, ×109/L | 3.67 [2.7-4.9] | 5.25 [3.7-9] | <.001 |
| Platelet count, ×109/L | 159 [104-257] | 333.5 [239-456] | <.001 |
| Spleen size BCM, cm | 0 [0-2.25] | 5 [0-11.25] | <.001 |
| Serum EPO, mU/mL | 16.2 [10.6-35] | 12 [7.4-17.] | .002 |
| Measured RCM, mL/kg | 26.2 [23-31] | 32.9 [27-37.1] | <.001 |
| Predicted RCM, (mL/kg | 24.7 [22-26.3] | 24.81 [23-27.4] | .37 |
| RCM more than 125% of predicted | 11/59 (18.6%) | 38/62 (61.3%) | <.001 |
Q1, first quartile; Q3, third quartile; MCV, mean cell volume; WBC, white blood cell; ANC, absolute neutrophil count; BCM, below costal margin; and RCM, red cell mass.
Associated prothrombotic factors (listed in “Inclusion criteria”) were found at similar frequency in patients with and without JAK2V617F. The most frequent were antiphospholipid antibodies (in 14% and 17%, respectively), factor V or factor II mutation (in 9.6% and 11%, respectively), and protein C, S or antithrombin deficiencies (in 10.6% and 9.5%, respectively). However, the mean number of associated prothrombotic factor was 0.71 plus or minus 0.84 in patients without JAK2V617F and 0.59 plus or minus 0.94 in patients with JAK2V617F (P = .08), and at least one associated prothrombotic factor was found more frequently in patients without JAK2V617F (75 of 147, 51%) than in patients with JAK2V617F (35 of 94, 37.2%; P = .036). In addition, in JAK2V617F patients, relative frequencies of associated prothrombotic factors differed according to the site of thrombosis: factor V Leiden (19%), antiphospholipid antibodies (16%), and protein C deficiency (15%) were the most frequent prothrombotic factors found in BCS, whereas in PVT, most frequently associated prothrombotic factors were antiphospholipid antibodies (11%), protein S deficiency (8%), and G20210A factor II gene mutation (5%).
Differences in hematologic features according to JAK2 mutational status found in the whole cohort were generally observed both in BCS and PVT patients (Tables 2,3), except that in PVT, red cell parameters (Hb, hematocrit, and mean corpuscular volume) did not significantly differ between patients with and without JAK2V617F, although measured RCM was significantly higher in patients with JAK2V617F. Aspartate aminotransferase, alanine transaminase, and serum bilirubin levels were significantly higher, and factor V level significantly lower in BCS patients with JAK2V617F, compared with unmutated BCS (Table 2). Among PVT patients, there were no differences in liver function tests according to JAK2V617F (Table 3). Only factor V level was significantly lower in PVT patients with JAK2V617F.
Characteristics and prognostic scores associated with the JAK2V617F mutation in 104 patients with Budd-Chiari syndrome
| BCS patients . | Without JAK2V617F, n = 57 (median [Q1-Q3]) . | With JAK2 V617F, n = 47 (median [Q1-Q3]) . | P . |
|---|---|---|---|
| Age, y | 40 [33-46] | 46 [35-51] | .2 |
| Males, % | 24 (42.1%) | 11 (23.4%) | .061 |
| Hemoglobin, g/dL | 13 [11.6-14.2] | 14.6 [13-16.1] | .003 |
| Hematocrit, % | 39.5 [34.7-46] | 46.4 [41.8-49.5] | .002 |
| MCV, fL | 88 [83-91.6] | 84 [79-86.8] | .003 |
| WBC count, ×109/L | 6.7 [5-9] | 9.85 [6.58-14.2] | <.001 |
| ANC, ×109/L | 4.03 [3.1-5.47] | 7.35 [4.5-10.1] | .004 |
| Platelet count, ×109/L | 185 [117-269] | 352.5 [284.5-456] | <.001 |
| Spleen size BCM, cm | 0 [0-1] | 1 [0-5] | .03 |
| Serum EPO, mU/mL | 22.8 [15.4-46.7] | 13.25 [8-23.1] | .008 |
| Measured RCM, mL/kg | 25.6 [21.8-29.1] | 31.15 [26.8-35.2] | .007 |
| Predicted RCM, mL/kg | 24 [21.25-28.22] | 23.45 [23-26.5] | .8 |
| RCM more then 125% of predicted | 0/18 (0%) | 18/30 (60.0%) | <.001 |
| AST, ULN | 38 [26.25-7] | 78.5 [39-258] | .002 |
| ALT, ULN | 38 [21.5-91.5] | 80 [33.2-239.8] | .015 |
| Serum bilirubin, μmol/L | 23.97 [16-42.3] | 43 [22-61.2] | .025 |
| Serum creatinine, μmol/L | 79 [61.9-86] | 75 [60-89] | .56 |
| Serum albumin, g/dL | 38 [31.5-41] | 33.5 [29.9-41] | .38 |
| Factor V, % | 74 [51-97] | 45 [30-59.5] | <.001 |
| Prognostic scores | |||
| Child-Pugh score | 9 [7-10.75] | 10 [9-11] | .004 |
| Clichy prognostic index | 5.63 [5.12-6.63] | 6.51 [5.83-7.4] | .006 |
| Rotterdam BCS score | 2.37 [1.37-2.43] | 2.47 [2.36-2.59] | .002 |
| BCS patients . | Without JAK2V617F, n = 57 (median [Q1-Q3]) . | With JAK2 V617F, n = 47 (median [Q1-Q3]) . | P . |
|---|---|---|---|
| Age, y | 40 [33-46] | 46 [35-51] | .2 |
| Males, % | 24 (42.1%) | 11 (23.4%) | .061 |
| Hemoglobin, g/dL | 13 [11.6-14.2] | 14.6 [13-16.1] | .003 |
| Hematocrit, % | 39.5 [34.7-46] | 46.4 [41.8-49.5] | .002 |
| MCV, fL | 88 [83-91.6] | 84 [79-86.8] | .003 |
| WBC count, ×109/L | 6.7 [5-9] | 9.85 [6.58-14.2] | <.001 |
| ANC, ×109/L | 4.03 [3.1-5.47] | 7.35 [4.5-10.1] | .004 |
| Platelet count, ×109/L | 185 [117-269] | 352.5 [284.5-456] | <.001 |
| Spleen size BCM, cm | 0 [0-1] | 1 [0-5] | .03 |
| Serum EPO, mU/mL | 22.8 [15.4-46.7] | 13.25 [8-23.1] | .008 |
| Measured RCM, mL/kg | 25.6 [21.8-29.1] | 31.15 [26.8-35.2] | .007 |
| Predicted RCM, mL/kg | 24 [21.25-28.22] | 23.45 [23-26.5] | .8 |
| RCM more then 125% of predicted | 0/18 (0%) | 18/30 (60.0%) | <.001 |
| AST, ULN | 38 [26.25-7] | 78.5 [39-258] | .002 |
| ALT, ULN | 38 [21.5-91.5] | 80 [33.2-239.8] | .015 |
| Serum bilirubin, μmol/L | 23.97 [16-42.3] | 43 [22-61.2] | .025 |
| Serum creatinine, μmol/L | 79 [61.9-86] | 75 [60-89] | .56 |
| Serum albumin, g/dL | 38 [31.5-41] | 33.5 [29.9-41] | .38 |
| Factor V, % | 74 [51-97] | 45 [30-59.5] | <.001 |
| Prognostic scores | |||
| Child-Pugh score | 9 [7-10.75] | 10 [9-11] | .004 |
| Clichy prognostic index | 5.63 [5.12-6.63] | 6.51 [5.83-7.4] | .006 |
| Rotterdam BCS score | 2.37 [1.37-2.43] | 2.47 [2.36-2.59] | .002 |
MCV indicates mean cell volume; WBC, white blood cell; ANC, absolute neutrophil count; BCM, below costal margin; and RCM, red cell mass.
Characteristics associated with the JAK2V617F mutation in 137 patients with portal vein thrombosis
| PVT patients . | Without JAK2V617F, n = 90 (median [Q1-Q3]) . | With JAK2 V617F, n = 47 (median [Q1-Q3]) . | P . |
|---|---|---|---|
| Age, y | 49 [37-62] | 53 [36-60] | .96 |
| Males, % | 52 (57.8%) | 25 (53.2%) | .72 |
| Hemoglobin, g/dL | 13 [10.8-14.5] | 12.9 [12.1-14.5] | .73 |
| Hematocrit, % | 38.2 [33-43] | 40 [37-45] | .06 |
| MCV, fL | 87.1 [82.2-91] | 86.2 [82.2-90] | .64 |
| WBC count, ×109/L | 5.35 [3.9-7.1] | 6.8 [4.9-9.6] | .005 |
| ANC, ×109/L | 3.48 [2.5-4.6] | 4.76 [3.4-6.5] | .016 |
| Platelet count, ×109/L | 141 [101-230] | 323 [214-437] | <.001 |
| Spleen size BCM, cm | 0 [0-3] | 8 [4-15] | <.001 |
| Serum EPO, mU/mL | 14 [8.2-27] | 10.5 [6.8-14.7] | .027 |
| Measured RCM, mL/kg | 26.9 [24-31.7] | 34.5 [30-38] | <.001 |
| Predicted RCM, mL/kg | 25 [23.2-26] | 26.4 [24-27.5] | .075 |
| RCM more than 125% of predicted | 11/41 (26.8%) | 20/32 (62.5%) | .004 |
| AST, ULN | 28 [19.5-40] | 30 [22.5-42.5] | .41 |
| ALT, ULN | 35 [20-52.5] | 34 [25-62.5] | .68 |
| Serum bilirubin, μmol/L | 15.4 [12.5-25.8] | 16 [13-25] | .71 |
| Serum creatinine, μmol/L | 74 [61.9-88] | 75 [66.5-79.8] | .84 |
| Serum albumin, g/dL | 38.5 [35.2-42.7] | 40 [38-45] | .054 |
| Factor V, % | 92 [63.2-109.5] | 63.5 [47.7-86] | .004 |
| PVT patients . | Without JAK2V617F, n = 90 (median [Q1-Q3]) . | With JAK2 V617F, n = 47 (median [Q1-Q3]) . | P . |
|---|---|---|---|
| Age, y | 49 [37-62] | 53 [36-60] | .96 |
| Males, % | 52 (57.8%) | 25 (53.2%) | .72 |
| Hemoglobin, g/dL | 13 [10.8-14.5] | 12.9 [12.1-14.5] | .73 |
| Hematocrit, % | 38.2 [33-43] | 40 [37-45] | .06 |
| MCV, fL | 87.1 [82.2-91] | 86.2 [82.2-90] | .64 |
| WBC count, ×109/L | 5.35 [3.9-7.1] | 6.8 [4.9-9.6] | .005 |
| ANC, ×109/L | 3.48 [2.5-4.6] | 4.76 [3.4-6.5] | .016 |
| Platelet count, ×109/L | 141 [101-230] | 323 [214-437] | <.001 |
| Spleen size BCM, cm | 0 [0-3] | 8 [4-15] | <.001 |
| Serum EPO, mU/mL | 14 [8.2-27] | 10.5 [6.8-14.7] | .027 |
| Measured RCM, mL/kg | 26.9 [24-31.7] | 34.5 [30-38] | <.001 |
| Predicted RCM, mL/kg | 25 [23.2-26] | 26.4 [24-27.5] | .075 |
| RCM more than 125% of predicted | 11/41 (26.8%) | 20/32 (62.5%) | .004 |
| AST, ULN | 28 [19.5-40] | 30 [22.5-42.5] | .41 |
| ALT, ULN | 35 [20-52.5] | 34 [25-62.5] | .68 |
| Serum bilirubin, μmol/L | 15.4 [12.5-25.8] | 16 [13-25] | .71 |
| Serum creatinine, μmol/L | 74 [61.9-88] | 75 [66.5-79.8] | .84 |
| Serum albumin, g/dL | 38.5 [35.2-42.7] | 40 [38-45] | .054 |
| Factor V, % | 92 [63.2-109.5] | 63.5 [47.7-86] | .004 |
MCV indicates mean cell volume; WBC, white blood cell; ANC, absolute neutrophil count; BCM, below costal margin; and RCM, red cell mass.
Relationship between JAK2V617F, BMB findings, EEC, and RCM
BMB and EEC results according to site of thrombosis and to JAK2V617F status are presented in Table 4. Histopathology showed bone marrow MPD features in 30.5% of patients (hereafter considered “positive” BMB). In the whole cohort, sensitivity and specificity of positive BMB for a diagnosis of JAK2V617F-positive MPD were 67.4% and 91.7%, respectively. JAK2V617F was detected in most patients with positive BMB (86.1%), but also in 18.3% of patients with negative BMB (P < .009). Among patients without JAK2V617F, 6.9% had positive BMB, including 8 of 57 BCS and 2 of 87 PVT patients. Thus, whereas BMB findings alone allowed diagnosis of MPD in 31% patients, JAK2V617F data allowed increasing the proportion of MPD diagnosis to 44%. This positive overall impact of JAK2 mutation detection was observed both in BCS (increasing MPD diagnosis rate from 26.4% to 44%) and PVT patients (increasing MPD diagnosis from 27.5% to 35%).
Correlation between JAK2V617F and bone marrow biopsy findings and EEC
| Positive or negative . | No. of patients with JAK2 mutation/total no. of patients (% with JAK2V617F) . | ||
|---|---|---|---|
| All patients . | BCS . | PVT . | |
| Bone marrow biopsy | |||
| Positive | 62/72 (86.1) | 27/35 (77.1) | 35/37 (94.6) |
| Negative | 30/164 (18.3) | 18/67 (26.9) | 12/97 (12.4) |
| EEC | |||
| Positive | 34/44 (77.2) | 16/19 (84.2) | 18/25 (72) |
| Negative | 15/59 (25.4) | 8/21 (38.1) | 7/38 (18.4) |
| Bone marrow biopsy and EEC | |||
| Both positive | 28/29 (96.5) | 13/13 (100) | 15/16 (93.7) |
| Both negative | 3/41 (7.3) | 3/12 (25) | 0/29 (0) |
| Discordant | 18/31 (58) | 8/15 (53) | 10/16 (62.5) |
| Positive or negative . | No. of patients with JAK2 mutation/total no. of patients (% with JAK2V617F) . | ||
|---|---|---|---|
| All patients . | BCS . | PVT . | |
| Bone marrow biopsy | |||
| Positive | 62/72 (86.1) | 27/35 (77.1) | 35/37 (94.6) |
| Negative | 30/164 (18.3) | 18/67 (26.9) | 12/97 (12.4) |
| EEC | |||
| Positive | 34/44 (77.2) | 16/19 (84.2) | 18/25 (72) |
| Negative | 15/59 (25.4) | 8/21 (38.1) | 7/38 (18.4) |
| Bone marrow biopsy and EEC | |||
| Both positive | 28/29 (96.5) | 13/13 (100) | 15/16 (93.7) |
| Both negative | 3/41 (7.3) | 3/12 (25) | 0/29 (0) |
| Discordant | 18/31 (58) | 8/15 (53) | 10/16 (62.5) |
EEC indicates endogenous erythroid colony formation.
A total of 103 patients (of 125 with EEC studies) had successful EEC assessment in addition to JAK2V617 testing. In the whole cohort, sensitivity and specificity of EEC for diagnosis of JAK2V617F-positive MPD were 79.0% and 74.1%, respectively. In patients with BCS or PVT, JAK2V617F was detected in 84.2% and 72% of patients with EEC, compared with 38.1% and 18.4% of patients without EEC, respectively (P < .009).
Combining BMB and EEC results in 101 patients with both tests available, 29 patients (28.7%) had both positive BMB and EEC, 41 (40.6%) had negative BMB and no EEC, and 31 (30.7%) had discordant results (positive BMB without EEC, or negative BMB with EEC). JAK2V617F was detected in 96.5% of patients with positive BMB and EEC, in 7.3% of patients with negative BMB and no EEC, and in 58% of patients with discordant BMB and EEC results. There were no significant differences between BCS and PVT patients in this regard (Table 4). However, none of the 29 PVT patients with negative BMB and no EEC had JAK2 mutation.
RCM, measured in 121 patients, was greater than 125% of the predicted value (identifying absolute erythrocytosis) in 49 patients (40%). JAK2V617F was detected in 38 of them (78%), who could therefore definitely be diagnosed with PV (Figure 1). JAK2V617F was also detected in 24 patients with observed RCM less than 125% of the predicted value, who were therefore suspected to have ET or PMF (Figure 1). RCM measurement demonstrated absolute erythrocytosis in 11 patients without JAK2V617F and no evidence for MPD in BMB, all of whom had PVT. As serum erythropoietin levels were elevated and EEC were negative in 10 patients, reactive erythrocytosis was suggested in these patients. Only one patient had EEC and normal serum erythropoietin level (but no MPD features on BMB) and could possibly have had JAK2V617F-negative latent PV.
Proposed algorithm for diagnosis and classification of Ph-negative myeloproliferative disease in patients with Budd-Chiari syndrome or portal vein thrombosis based on JAK2V617F testing, followed by RCM determination in JAK2V617F-positive patients, and BMB and EEC assessment in JAK2V617F-negative patients. Values in italics represent distribution of patients of this cohort.
Proposed algorithm for diagnosis and classification of Ph-negative myeloproliferative disease in patients with Budd-Chiari syndrome or portal vein thrombosis based on JAK2V617F testing, followed by RCM determination in JAK2V617F-positive patients, and BMB and EEC assessment in JAK2V617F-negative patients. Values in italics represent distribution of patients of this cohort.
JAK2V617F-negative MPD
Among 144 patients without JAK2V617F, 10 (6.9%) had histopathologic MPD characteristics on BMB (8 of 57 BCS and 2 of 87 PVT patients). BCS patients with JAK2V617F-negative MPD had significantly lower hemoglobin (P = .027) and RCM (P = .013), but no significantly different white blood cell and platelet counts than JAK2V617F-positive BCS. In addition, liver function tests and the 3 prognostic scores were similar in JAK2V617F-negative and -positive MPD patients with BCS.
Analysis of MPL and JAK2 exon 12 mutations
Screening of MPL exon 10 mutations was made in 212 patients and searched for JAK2 exon 12 mutations in 123 JAK2V617F-negative patients. No MPL or JAK2 additional mutation were found in any of the samples tested, including the 10 JAK2V617F-negative patients with MPD criteria on BMB.
Underlying MPD as a prognostic factor in BCS
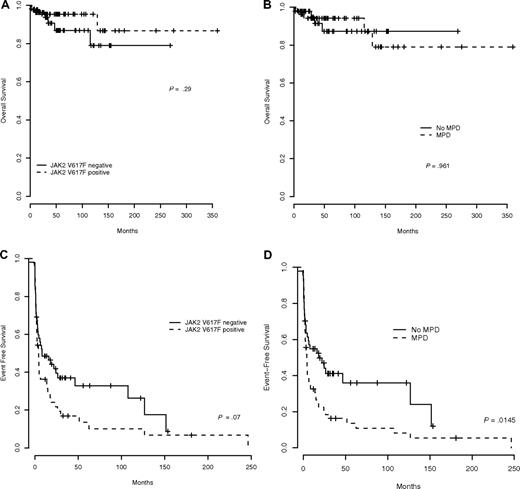
In BCS patients, all 3 prognostic scores assessed at the time of diagnosis (Child-Pugh score, Clichy prognostic index, and Rotterdam score)28-30 were significantly higher in patients with than in those without JAK2V617F (Table 2). With a median follow-up of 3.9 years, overall survival did not differ according to the presence or absence of JAK2V617F (P = .29, Figure 2A) or of an underlying MPD (defined by either JAK2V617F detection or by a positive BMB in JAK2V617F-negative patients; P = .961; Figure 2B). However, EFS tended to be shorter in BCS patients with JAK2V617F (P = .07, Figure 2C) and was significantly reduced in patients with MPD (P = .0145; Figure 2D). Similar results were obtained when endpoints were defined from the date of JAK2 mutation screening instead of date of thrombosis (with a median interval of 31 months between date of thrombosis and JAK2 screening, 25% of patients (first quartile) having been screened within 6 months of thrombosis).
Survival in Budd-Chiari syndrome patients. (A) Overall survival according to JAK2V617F. (B) Overall survival according to the existence of underlying MPD. (C) Event-free survival according to presence of JAK2V617F. (D) Event-free survival according to the existence of underlying MPD.
Survival in Budd-Chiari syndrome patients. (A) Overall survival according to JAK2V617F. (B) Overall survival according to the existence of underlying MPD. (C) Event-free survival according to presence of JAK2V617F. (D) Event-free survival according to the existence of underlying MPD.
In PVT patients, with a median follow-up of 5.5 years, overall survival and EFS were not significantly influenced by presence of JAK2V617F (P = .76 and P = .09, respectively) or underlying MPD (P = .71 and P = .22, respectively).
Discussion
This study in the largest BCS and PVT patient sample collected to date, with centralized assessment of all currently described MPD molecular markers, use of widely recognized histopathologic criteria, and appreciable length of follow-up, helped clarify 2 challenging and debated issues: the diagnostic criteria for, and the prognostic impact of, MPD in patients with BCS or PVT. We found that (1) MPD without typical peripheral blood changes was present in 40% to 50% of those patients; (2) JAK2 mutation-negative (including V617F and exon 12 mutations), MPL515 mutation-negative MPD still accounted for a proportion of those patients; (3) JAK2V617F testing represented an important advance in the diagnostic workup for the recognition of atypical MPD in BCS and PVT patients; and (4) BCS caused by MPD was more severe.
JAK2V617F was detected in 39% of patients in the whole population, including 45% of BCS patients and 34% of PVT patients. Combining JAK2V617F and BMB findings, the prevalence of MPD was 44% in the whole cohort, 53% in BCS patients, and 37% in PVT patients. These estimates in 104 BCS patients are consistent with data recently reported in smaller surveys where JAK2V617F was detected in 24 of 41 and 8 of 20 patients.16,32 In PVT, 2 recent Italian studies gave relatively discrepant estimates of 35.6% and 17.2% for JAK2V617F prevalence.32,33 Combining present data on JAK2, BMB, and EEC, with previously reported data on EEC and BMB in consecutive PVT patients,8 MPD prevalence in PVT can be estimated at 40%. Taken together, those studies confirm the strong association between MPD and SVT, an association that appears in this study to be almost equally tight for PVT as for BCS, and which has not yet been described for any other venous territory. Reasons underlying the link between MPD and BCS or PVT remain to be elucidated. Of note, however, is that 37% of our patients with MPD also had another predisposing factor for thrombosis (antiphospholipid antibodies, factor V Leiden, and protein C deficiency being the most frequent).
The present findings also confirmed the unusual features of MPD found in BCS and PVT patients,34,35 namely, younger age, female predominance in patients with PV, and generally normal blood counts. The present findings showed that, in addition to hypersplenism, hemodilution contributes to these atypical changes, as RCM was increased in many patients with normal hematocrit.4-8,18,34
JAK2V617F was not detected in 13.9% of patients with typical marrow findings of MPD, who also lacked the alternative JAK2 exon 12 or MPL515 mutations. These results are not surprising because JAK2 exon 12 mutations are present in less than 5% of PV,21 and JAK2V617F in only half,12-15 and MPL515 mutation in less than 5% of ET and PMF patients.19 These findings raise the issue of the optimal diagnostic test for MPD in unmutated JAK2 patients with BCS or PVT. The combination of concordant BMB findings and EEC, previously considered to constitute the most specific criteria in this regard,8 actually appeared to have limited usefulness. Indeed, JAK2V617F was virtually always detected when both tests were positive, whereas, when both tests were negative, JAK2V617F was still detected in 7% of patients. In the 30% patients with discordant BMB and EEC results, JAK2V617F was detected in 58% of cases. Accuracy of EEC for MPD diagnosis was less than that of BMB in this study. Furthermore, the significance of isolated EEC in the absence of positive BMB or JAK2V617F was unclear. Conversely, EEC is not a constant feature of PV, being present in 70% to 90% of cases.36 In addition, EEC results may vary according to the methodology used.10 Identification of other MPD associated mutations should facilitate their diagnosis, but our data indicate that screening for MPL515 mutations is of little help in SVT patients. JAK2 exon 12 mutations were not detected in this large cohort of patient but were very recently reported in 1 BCS and 1 PVT patient,37 suggesting that screening for additional molecular markers in JAK2V617F-negative patients could still be useful for diagnosing MPD in few cases.
Thus, a diagnostic algorithm for MPD can be recommended based first on JAK2V617F testing and second on BMB (Figure 1). Applying such strategy in the present cohort would have spared BMB and EEC in 39% of patients in whom underlying MPD could be established with JAK2 analysis. Once JAK2V617F is demonstrated, RCM determination allows further classification in PV versus ET or PMF.18 BMB remains necessary in patients without JAK2 mutation, particularly when they have BCS (14% of unmutated BCS had MPD vs 5% of PVT).
Several recent studies have shown that homozygosity for V617F mutation resulting from mitotic recombination (found in approximately 30% of PV and less than 5% of ET patients) plays a key role in the myeloproliferative disorders.38 Homozygosity promotes the development of PV,39,40 is associated with more frequent transformation to myelofibrosis,41 identifies patients with more symptomatic MPD, and is associated with higher risk of vascular events.42 In this study, only 16% of the patients had a percentage of circulating V617F mutant allele more than 50%, suggesting homozygosity. These findings may explain the low rate of evolution from “latent” to overt MPD reported in BCS and PVT patients.
A major issue in the field of SVT is the influence of etiology on clinical manifestations and outcome. Previous studies showed no impact of underlying causes, but they lacked statistical power because of the relatively small size of the samples.2,8,16,17,32,33,43,44 Moreover, a meta-analysis of these studies was difficult because diagnostic criteria for underlying MPD had been heterogeneous. The present study in BCS patients showed that liver dysfunction at the time of diagnosis was more severe in those with MPD or JAK2V617F. However, underlying MPD had no impact on overall survival, which is surprising because severity of liver dysfunction was consistently shown to represent the major independent prognostic factor for BCS. These discrepant findings can nevertheless be reconciled, considering that patients with underlying MPD had significantly shorter event-free survival, including shorter time to liver decompressive therapy (angioplasty, surgical or radiologic shunting) or transplantation. Thus, the prognostic influence of greater severity at presentation with BCS in MPD patients was offset by earlier relief of hepatic venous outflow block. If confirmed in other studies, this finding would justify including MPD among the arguments for early interventions to relieve the outflow block.45 In this regard, the facilitated diagnosis of MPD with JAK2V617F represents a major advance. In contrast to BCS, there was in PVT patients no discernible influence of underlying MPD or JAK2V617F on prognosis. Natural history of PVT (with no liver disease) is by far more favorable than that of BCS, and no prognostic score has yet been elaborated for PVT. Underlying MPD, in BCS and PVT patients, may play a deleterious role in the long term by late transformation of MPD rather than by their heralding, life-threatening but curable, vascular complications.8
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Jean-François Bernard for his help in data collection.
This work was supported in part by European Union (QLG1-CT-2002-01686).
Authorship
Contribution: J.-J.K., P.F., and D.C.V. designed research, analyzed data, and wrote the paper; J.-J.K. was the coordinator of the study; F.C., F.W.G.L., C.G., and S. Cereja collected, reviewed, and analyzed hematologic data; C.M., B.C., C.T., S.G., and N.C. performed molecular analysis; S. Chevret performed statistical analysis; D.C.-H. reviewed bone marrow biopsies; A.P., J.-C.G.-P., S.D.M., S.R., H.L.A.J., and B.C. collected, reviewed, and analyzed hepatologic data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Jacques Kiladjian, Service d'Hématologie Clinique, Hôpital Avicenne, 125 rue de Stalingrad, 93000 Bobigny, France; e-mail: jean-jacques.kiladjian@avc.aphp.fr.