Based on their ability to control T-cell homeostasis, Foxp3+CD4+CD25+ regulatory T cells (Tregs) are being considered for treatment of autoimmune disorders and acute graft-versus-host disease (aGVHD). When combining Tregs with the immunosuppressant rapamycin (RAPA), we observed reduced alloreactive conventional T-cell (Tconv) expansion and aGVHD lethality compared with each treatment alone. This synergistic in vivo protection was paralleled by intact expansion of polyclonal Tregs with conserved high FoxP3 expression. In contrast to Tconv, activation of Tregs with alloantigen and interleukin-2 preferentially led to signal transducer and activator of transcription 5 (STAT5) phosphorylation and not phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway activity. Expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a negative regulator of the PI3K/Akt/mTOR pathway, remained high in Tregs but not Tconv during stimulation. Conversely, targeted deletion of PTEN increased susceptibility of Tregs to mTOR inhibition by RAPA. Differential impact of RAPA as a result of reduced usage of the mTOR pathway in Tregs compared with conventional T cells explains the synergistic effect of RAPA and Tregs in aGVHD protection, which has important implications for clinical trials using Tregs.
Introduction
Immune-mediated disorders often present as a combination of destructive tissue damage and variable systemic organ manifestations based on an overwhelming T- and/or B-cell reaction. There has been great interest recently in targeting the interleukin-2 (IL-2) pathway by specific immunosuppressive drugs and the use of CD4+CD25+Foxp3+ regulatory T cells (Tregs) for the treatment of such inflammatory conditions.1,2 To specifically modulate aberrant immune responses such as acute graft-versus-host disease (aGVHD), allograft rejection, or autoimmune disorders by immunosuppressive therapy, while sparing Treg activity, a more detailed insight into the biologic differences between Tconv and Tregs is crucial. One critical requirement for Treg function and expansion in preventing aGVHD is calcineurin-dependent IL-2 production,3 which is affected by the immunosuppressant cyclosporin A (CSA). In light of the finding that the interaction between NFATc and Foxp3 is required for the suppressive effects of Treg cell4,5 interference with nuclear factor of activated T cells (NFAT) by CSA may also contribute to the observed effects of CSA on Treg biology. We and others have shown that rapamycin (RAPA) but not CSA allows for Treg expansion and function in mice3,6,7 and in humans.8,9
In contrast to CSA, RAPA inhibits mammalian target of rapamycin (mTOR) pathway activity, which is downstream of IL-2/phosphatidylinositol 3-kinase (PI3K) signaling.10 This pathway is negatively regulated by PTEN (phosphatase and tensin homolog deleted on chromosome 10), a phosphoinositol 3,4,5-triphosphatase that catalyzes the reverse reaction of PI3K.11 Other pathways that are activated in response to IL-2 signaling that may be preferentially used when mTOR is inhibited include the Janus tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT) and the mitogen-activated protein kinase (MAPK) pathway. Recent data have demonstrated an essential role for IL-2 receptor (R)β-dependent STAT5 signaling for the development of Tregs.12
Here we describe the in vivo impact of mTOR inhibition on expansion, migration, Foxp3 expression, and activity of Tregs after allogeneic hematopoietic cell transplantation (aHCT). From a mechanistic standpoint, we studied the differential impact of RAPA on Tconv and Tregs with respect to PI3K/mTOR and STAT5 pathway activity.
Methods
Mice
C57BL/6 (H-2kb, Thy-1.2), FVB (H-2kq), BALB/c (H-2kd, Thy-1.2), and C57BL/6 PTENtm1Hwu mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA). C57BL/6Lck-Cre mice were a kind gift from Dr J. Crabtree (Stanford University, Stanford, CA). Mice were used between 6 and 12 weeks of age. Only sex-matched combinations were used for transplant experiments. The luciferase-expressing (luc+) transgenic FVB/N L2G85 were described previously13 and backcrossed for more than 10 generations to the C57BL/6 background. All animal protocols were approved by the University Committee on Use and Care of Laboratory Animals at Stanford University.
Flow cytometric analysis
The following antibodies were used for flow cytometric analysis: anti-CD16/32 (2.4G2), CD4 (RM4-5), CD8α (53-6.7), CD25 (PC61), CD11c (M1/70), H-2Kq (KH114), H-2Kd (34-2-12), CTLA-4 (UC10-4B9), GITR (DTA-1), CD27 (LG.7F9), CD103 (2E7), CD62L (MEL-14), CCR7 (4B12), all antibodies from BD Pharmingen (San Diego, CA), and eBiosciences (San Diego, CA), rabbit anti–mouse CCR4 (Novus Biologicals, Littleton, CO), secondary anti–rabbit FITC (Santa Cruz Biotechnology, Santa Cruz, CA), CCR5 (HM-CCR5) (BioLegend, San Diego, CA). Foxp3 staining was performed using the intracellular Foxp3 staining kit (antibody: FJK-16s) as described in the protocol (eBiosciences). Staining was performed in the presence of purified anti-CD16/32 at saturation to block nonspecific staining. Propidium iodide (Sigma-Aldrich, St Louis, MO) was added before analysis to exclude dead cells. For Phospho flow analysis, the following antibodies were used: phycoerythrin (PE) anti-phos-Stat5 (pY694, clone 47, 611964; BD Biosciences Pharmingen, San Diego, CA), anti-phospho-p70S6 kinase (Thr421/Ser424, rabbit), anti-phospho-4E-BP1 (236B4, rabbit, both Cell Signaling Technology, Danvers, MA) with secondary goat-antirabbit PE (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were lysed and fixed with 1× PhosphoFlow Lyse/Fix Buffer for 15 minutes at 37°C, then permeabilized with PhosFlow Perm Buffer III on ice for 1 hour (all Phosflow reagents from BD Biosciences) as described previously.14 For Vβ TCR usage analysis, the screening panel that recognizes mouse Vβ 2, 3, 4, 5.1 and 5.2, 6, 7, 8.1 and 8.2, 8.3, 9, 10b, 11, 12, 13, 14, and 17a T-cell receptors was used (BD Pharmingen). All analytical flow cytometry was done on a modified dual laser LSRScan (BD Immunocytometry Systems, San Diego, CA) in the Shared FACS Facility, Center for Molecular and Genetic Medicine at Stanford University using FlowJo software (TreeStar, Ashland, OR) for data analysis.
Immunosuppressive treatment
For in vivo studies, RAPA (Wyeth-Ayerst, Princeton, NJ) was dissolved in carboxymethylcellulose sodium salt (C-5013; Sigma-Aldrich) and polysorbate 80 (P-8074; Sigma-Aldrich). RAPA stock solution was stored at 4°C in the dark in distilled water according to the manufacturer's instructions. Intraperitoneal injections were given once daily and started on d0. Dosage was adjusted to the body weight every other day. The RAPA dosage employed in vivo of 0.5 or 1.5 mg/kg per day corresponds to blood levels that were reported to reach 6 to 15 ng/mL.15 Immunosuppressive treatment was continued until death or end of the observation period (day 100).
Western blot analysis
T cells that were isolated from in vitro cultures with or without RAPA were counted and resuspended in an appropriate volume (10 μL/106 cells) of radioimmunoprecipitation assay buffer (Sigma-Aldrich). Proteinase inhibitor cocktail (BD Pharmingen) and phosphatase inhibitor cocktail (Pierce Chemical, Rockford, IL) were included in the lysis buffer. After 45 minutes' incubation on ice, the sample viscosity was decreased by centrifuging through a QiaShredder column (QIAGEN, Valencia, CA). The concentration of proteins in each sample was determined using a BCA assay kit (Pierce Chemical). Each protein sample was suspended in sodium dodecyl sulfate (SDS) sample buffer (4× buffer; Invitrogen, Carlsbad, CA) and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) electrophoresis using precast Tris-HCl Ready-Gels (4%-12%; Invitrogen). Proteins were transferred to polyvinylidene diflouride membranes (Millipore, Temecula, CA) and immunoblotting was performed using conventional methods. The following primary antibodies were used: anti-PTEN (Y184; Abcam, Cambridge, MA), anti-p70S6 kinase (49D7), anti-4E-BP-1 (236B4), anti-mTOR (7C10) antibodies (Cell Signaling Technology).
aGVHD model
Acute GVHD was induced as described previously.3 In brief, recipients were exposed to lethal irradiation with 800 cGy on day 0 followed by intravenous injection of 5 × 106 TCD-BM cells after. To induce aGVHD 1.6 × 106 or 106 CD4+/CD8+ T cells in the FVB/N→BALB/c or C57BL/6→BALB/c model, respectively, were injected intravenously on day 2. Tregs were derived from donors of the same genetic background as the conventional donor T cells and injected intravenously on day 0 at the dosage indicated for the respective experiment. To track Treg/Tconv expansion 5 × 105 Treg or Tconv from luc+ C57BL/6 donors were given where indicated. Mice were fed antibiotic water (sulfamethoxazole-trimethoprim; Schein Pharmaceuticals, Dartmore, CT).
In vivo bioluminescence imaging
In vivo bioluminescence imaging (BLI) was performed as described previously.16 In brief, mice were injected intraperitoneally with luciferin (10 μg/g of body weight, intraperitoneally). Ten minutes later, mice were imaged using an IVIS200 charge-coupled device (CCD) imaging system (Xenogen, Alameda, CA) for 5 minutes. Imaging data were analyzed and quantified with Living Image Software (Xenogen, Alameda, CA) and IgorProCarbon (WaveMetrics, Lake Oswego, OR).
Histopathology
Tissues were fixed with 10% formalin and embedded in paraffin, and sections of 5 μm thickness were mounted on positively charged precleaned microscope slides (Superfrost/Plus; Fisher Scientific, Hampton, NH). Hematoxylin/eosin staining of paraffin-embedded tissue sections was performed according to standard protocols. Evaluation of the stained tissue sections was performed on a Nikon microscope (Eclipse TE 300; Nikon, Melville, NY). Standard magnifications were 200×/0.45 NA and 400×/0.60 NA. Microscopic photos were obtained using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI). Histopathology scoring was performed by an experienced pathologist (N.K.) in a blinded fashion according to a previously published system.17
Cell isolation and sorting
Single cell-suspensions from cervical lymph nodes, axillary lymph nodes, inguinal lymph nodes, mesenteric lymph nodes, and spleens were enriched for CD25+ cells after sequential staining with anti-CD25 PE (BD Phar-Mingen) and anti-PE magnetic beads using the autoMACS system (Miltenyi Biotec, Auburn, CA). CD25+ cells were then stained with anti-CD4 APC and sorted on a MoFlow cell sorter (BD Biosciences, Mountain View, CA) for the CD25high population (15%-20% of the enriched CD25+ cells). This sorting strategy yielded at least 96% CD4+CD25+Foxp3+ cells.
T cell–depleted bone marrow (TCD BM) was obtained through negative depletion using anti-CD4 and anti-CD8 magnetic beads (Miltenyi Biotech). For transplantation of conventional and luc+ T-cell subsets, splenic single-cell suspensions from FVB-L2G85 mice were enriched with CD4/CD8-conjugated magnetic beads using the AutoMACS system (Miltenyi Biotech). After enrichment, CD4+ and CD8+ T cells reached more than 90% purity.
Proliferation assay
CD4+CD25− and CD4+CD25high T cells from luciferase transgenic animals were cultured in flat-bottomed, 96-well plates and stimulated when indicated with 2 or 5 μg/mL each of αCD3 (clone 145-2C11; BD Biosciences) and αCD28 (clone 37.51; BD Biosciences), IL-2 (100 IU/mL), at a concentration of 2 × 105 cells/well. Rapamycin (Sigma-Aldrich) was dissolved in DMSO (Sigma-Aldrich) and added at the concentrations of 10 ng/mL or 100 ng/mL when indicated. Culture medium consisted of RPMI 1640 supplemented with l-glutamine (2 mM), penicillin (100 U/mL), streptomycin (0.1 mg/mL), 2-mercaptoethanol (5 × 10−5 M), and 10% fetal calf serum. After 48 hours cultured T cells were analyzed by BLI or harvested and analyzed by FACS or Western blot. For studies on Treg/Tconv expansion, cells from wild-type (wt) and PTEN−/− donors were used as indicated for the respective experiments. Expansion of luciferase transgenic T cells was quantified by adding 6 μg/mL luciferin per well. Five minutes later the 96-well plate was imaged using an IVIS200 CCD imaging system (Xenogen) for 2 minutes. Expansion is quantified in photons per second per square centimeter.
Mixed leukocyte reaction for Treg suppressor function
Tregs (CD4+CD25high+H-2kb+Thy-1.1+) were incubated for 96 hours with γ-irradiated (30 Gy) antigen-presenting cells (APCs) (CD11c+H-2kd+), and CFSE+-labeled conventional T (Tconv)-cells (CD4+CD25−H-k2b+Thy-1.2+) each population 2 × 105 cells. Cell division was monitored by levels of CFSE dilution or thymidine incorporation. Cultures were evaluated in triplicate in 96-well flat-bottomed plates in a total volume of 200 μL.
Generation of animals with PTEN−/− CD4 T cells
As PTEN deficiency results in embryonic lethality, we generated mice with targeted deletion of PTEN specific to the T-cell compartment. Mice homozygous for the Ptenflox allele were crossed with LckCre transgenic mice (both C57BL/6). Resulting F1 litters had PTEN-deficient T cells, which results in the development of T-cell lymphomas by the age of 10 to 12 weeks18 ; therefore, animals were killed before that age. C57BL/6 littermate wt mice were used for control studies.
Adoptive transfer of CFSE labeled wt versus PTEN −/− Tregs
For in vivo cell proliferation analysis, Tregs (106/mL) isolated from wt or PTEN-deficient donor animals were resuspended in plain phosphate-buffered saline (PBS) and stained with Vybrant carboxyfluorescein diacetate (CFSE), succinimidyl ester tracer kit (Invitrogen) at a final concentration of 5 μM for exactly 6 minutes at 37°C. Immediately after staining, cells were washed in 5 volumes of ice-cold RPMI plus 10% fetal bovine serum (Invitrogen) twice, resuspended in PBS, and counted before intravenous injection into BALB/c mice that had received irradiation with 800 rad and were transplanted with T cell-depleted bone marrow (TCD-BM). On day 3 after transfer donor type (H-2kb), Tregs were analyzed by fluorescence-activated cell sorting (FACS) for CFSE dilution or phospho-flow analysis.
Statistical analysis
Differences in animal survival (Kaplan-Meier survival curves) were analyzed by log-rank test. Differences in proliferation of luc transgenic T cells in vitro or in vivo MFI, histopathology scores, Vβ usage, numbers of CD4 and CD8 H-2kq cells were analyzed using the 2-tailed Student t test. Error bars indicate the standard deviation from the geometric mean. A P value less than .05 was considered statistically significant.
Results
Synergistic protective effect of Treg and RAPA against aGVHD-related morbidity and mortality was based on reduced expansion of conventional T cells after BMT
To investigate whether RAPA could increase Treg suppressor function in vivo, we combined partially protective Treg:Tconv ratios (1:4, 1:8) with a RAPA dose (0.5 mg/kg of body weight) that was previously titrated in the allogeneic BMT model.3 aGVHD lethality was significantly reduced when Tregs were added but not when recipients were treated with the nonprotective RAPA dose (Figure 1A,C). Weight loss as a surrogate parameter for aGVHD severity (Figure 1B,D) and lethality (Figure 1A,C) was reduced when animals received Tregs in combination with RAPA compared with PBS. Histologic evaluation demonstrated reduced aGVHD severity in the Treg/RAPA animals compared with any other group that received Tconv along with TCD-BM (Figure 1E,i-v). Cumulative histopathologic evaluation of large bowel, small bowel, and liver demonstrated reduced aGVHD histopathology score when Tregs and RAPA were combined compared with each treatment alone (Figure 1Evi).
Combined rapamycin/Treg treatment hada synergistic protective effect against aGvHD. BALB/c mice were injected with 5 × 106 TCD-BM cells alone (●) or together with 1.6 × 106 CD4+/CD8+ (1:4) T cells and Tregs when indicated (both H-2kq) after lethal irradiation at 800 cGy. (A) Percentage survival of animals receiving conventional T cells (Tconv) + PBS (△, n = 10), T cells and rapamycin (RAPA) 0.5 mg/kg (*, n = 10), Treg:Tconv (1:8) + PBS (□, n = 10), or Treg:Tconv (1:8) + RAPA (○, n = 10). The combination of Tregs with RAPA compared with Tregs with PBS improves survival (○ versus □, P = .003). Survival data from 2 independent experiments are combined. (B) Weight change of animals in the different groups. Animals receiving Treg:Tconv (1:8) in combination with RAPA compared with PBS experience less-pronounced weight loss and recover to their baseline weight. (C) Survival of animals receiving conventional T cells (Tconv) only (△, n = 10), Tconv and RAPA 0.5 mg/kg (*, n = 10), Treg:Tconv (1:4) + PBS (□, n = 10), or Treg:Tconv (1:4) + RAPA (○, n = 10). The combination of Tregs with RAPA compared with Tregs with PBS improves survival (○ versus □, P = .007). Survival data from 2 independent experiments are combined. (D) Animals receiving Treg:Tconv (1:4) in combination with RAPA compared with PBS experience less weight loss after transplantation. (E) Twelve days after transplantation, 5 mice from each group were killed and samples of liver, small bowel, and large bowel were analyzed for evidence of pathologic damage. Hematoxylin and eosin stains of colon tissue obtained from these mice are shown. Tissue section from the colon of mice receiving TCD-BM (i), with Tconv (ii), Tconv and RAPA (0.5 mg/kg) (iii), Treg:Tconv (1:8) + PBS (iv), Treg:Tconv (1:8) + RAPA (v). Colon tissue from TCD-BM (i) and Treg:Tconv (1:8) + RAPA (v) displays intact crypts with goblet cells. The other groups have crypt abscesses (black arrows), sloughing of the colonic mucosa (blue arrows), destruction of the crypt, and loss of goblet cells. (vi) Cumulative GVHD histopathology scoring for large bowel, small bowel, and livers of animals from the indicated groups, *P < .05.
Combined rapamycin/Treg treatment hada synergistic protective effect against aGvHD. BALB/c mice were injected with 5 × 106 TCD-BM cells alone (●) or together with 1.6 × 106 CD4+/CD8+ (1:4) T cells and Tregs when indicated (both H-2kq) after lethal irradiation at 800 cGy. (A) Percentage survival of animals receiving conventional T cells (Tconv) + PBS (△, n = 10), T cells and rapamycin (RAPA) 0.5 mg/kg (*, n = 10), Treg:Tconv (1:8) + PBS (□, n = 10), or Treg:Tconv (1:8) + RAPA (○, n = 10). The combination of Tregs with RAPA compared with Tregs with PBS improves survival (○ versus □, P = .003). Survival data from 2 independent experiments are combined. (B) Weight change of animals in the different groups. Animals receiving Treg:Tconv (1:8) in combination with RAPA compared with PBS experience less-pronounced weight loss and recover to their baseline weight. (C) Survival of animals receiving conventional T cells (Tconv) only (△, n = 10), Tconv and RAPA 0.5 mg/kg (*, n = 10), Treg:Tconv (1:4) + PBS (□, n = 10), or Treg:Tconv (1:4) + RAPA (○, n = 10). The combination of Tregs with RAPA compared with Tregs with PBS improves survival (○ versus □, P = .007). Survival data from 2 independent experiments are combined. (D) Animals receiving Treg:Tconv (1:4) in combination with RAPA compared with PBS experience less weight loss after transplantation. (E) Twelve days after transplantation, 5 mice from each group were killed and samples of liver, small bowel, and large bowel were analyzed for evidence of pathologic damage. Hematoxylin and eosin stains of colon tissue obtained from these mice are shown. Tissue section from the colon of mice receiving TCD-BM (i), with Tconv (ii), Tconv and RAPA (0.5 mg/kg) (iii), Treg:Tconv (1:8) + PBS (iv), Treg:Tconv (1:8) + RAPA (v). Colon tissue from TCD-BM (i) and Treg:Tconv (1:8) + RAPA (v) displays intact crypts with goblet cells. The other groups have crypt abscesses (black arrows), sloughing of the colonic mucosa (blue arrows), destruction of the crypt, and loss of goblet cells. (vi) Cumulative GVHD histopathology scoring for large bowel, small bowel, and livers of animals from the indicated groups, *P < .05.
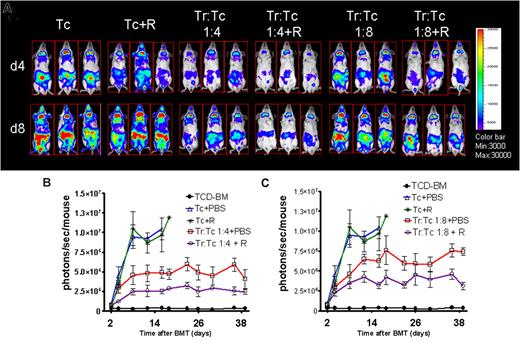
Combining a suboptimal Tconv:Treg ratio with a nonprotective RAPA dose reduced expansion of luciferase expressing (luc+) Tconv significantly more than Tregs + PBS (P = .02) or RAPA alone (P = .003) indicative for a synergistic in vivo effect of RAPA and Tregs as demonstrated for representative timepoints (Figure 2A). Quantification of the photons per second over total body area demonstrated lower intensities for the Treg/RAPA compared with Treg/PBS combinations at 2 different Tconv:Treg ratios (Figure 2B,C).
RAPA/Tregs protected from aGVHD by reducing alloreactive T-cell expansion in vivo. (A) Single timepoints showing the expansion of luciferase transgenic (luc+) donor T cells in 3 representative BALB/c mice (of 10 animals per group) receiving T cells in combination with Tregs at different ratios (1:4 and 1:8) combined with RAPA as indicated. T-cell expansion is significantly reduced when Tregs are combined with RAPA (column 3 vs 4 and 5 vs 6 from the left). (B) Expansion of luciferase-labeled T cells as quantified in emitted photons over total body area at serial timepoints after BMT. BLI signal intensity of mice receiving TCD-BM (●, n = 10), with T cells (△, n = 15), T cells and RAPA 0.5 mg/kg (*, n = 10), Treg:T cells (1:4) + PBS (□, n = 12) Treg:T cells (1:4) + RAPA (○, n = 12). Signal intensity is significantly higher in animals receiving Treg:T cells (1:4) + PBS compared with Treg:T cells (1:4) + RAPA (□ vs ○, P < .05). (C) BLI signal intensity of mice receiving TCD-BM (●, n = 10), with T cells (△, n = 15), T cells and RAPA 0.5 mg/kg (*, n = 10), Treg:T cells (1:8) + PBS (□, n = 12) Treg:T cells (1:9) + RAPA (○, n = 12). Signal intensity was significantly higher in animals receiving Treg:T cells (1:8) + PBS compared with Treg:T cells (1:8) + RAPA (□ vs ○, P < .05).
RAPA/Tregs protected from aGVHD by reducing alloreactive T-cell expansion in vivo. (A) Single timepoints showing the expansion of luciferase transgenic (luc+) donor T cells in 3 representative BALB/c mice (of 10 animals per group) receiving T cells in combination with Tregs at different ratios (1:4 and 1:8) combined with RAPA as indicated. T-cell expansion is significantly reduced when Tregs are combined with RAPA (column 3 vs 4 and 5 vs 6 from the left). (B) Expansion of luciferase-labeled T cells as quantified in emitted photons over total body area at serial timepoints after BMT. BLI signal intensity of mice receiving TCD-BM (●, n = 10), with T cells (△, n = 15), T cells and RAPA 0.5 mg/kg (*, n = 10), Treg:T cells (1:4) + PBS (□, n = 12) Treg:T cells (1:4) + RAPA (○, n = 12). Signal intensity is significantly higher in animals receiving Treg:T cells (1:4) + PBS compared with Treg:T cells (1:4) + RAPA (□ vs ○, P < .05). (C) BLI signal intensity of mice receiving TCD-BM (●, n = 10), with T cells (△, n = 15), T cells and RAPA 0.5 mg/kg (*, n = 10), Treg:T cells (1:8) + PBS (□, n = 12) Treg:T cells (1:9) + RAPA (○, n = 12). Signal intensity was significantly higher in animals receiving Treg:T cells (1:8) + PBS compared with Treg:T cells (1:8) + RAPA (□ vs ○, P < .05).
Rapamycin inhibited Treg and Tconv in vitro expansion differentially and allows for the maintenance of high Foxp3 protein expression and suppressor function
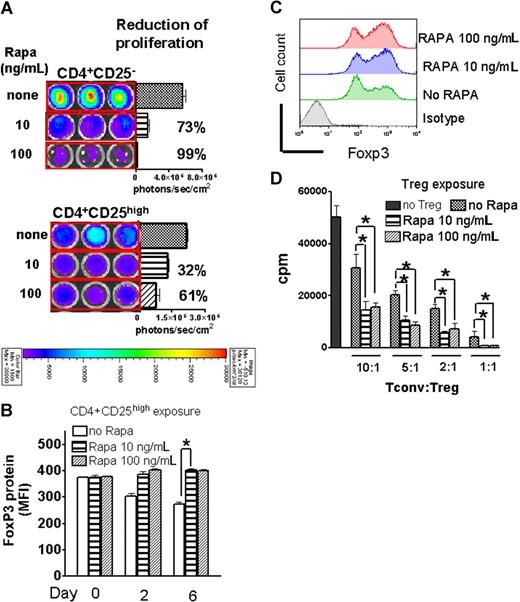
To investigate whether the observed synergistic in vivo effects of RAPA and Tregs were based on expansion differences, we exposed luciferase transgenic Tregs and Tconv to CD3/CD28 stimulation in the presence of IL-2. Tregs proliferated less than Tconv, which is compatible with their hyporesponsive phenotype in vitro (Figure 3A). Addition of RAPA to the culture resulted in a greater reduction of signal intensity on expanding luciferase transgenic Tconv (CD4+CD25−) compared with Tregs (CD4+CD25high) with 73 (± 6%) versus 32% (± 3%) (P = .012) at 10 ng/mL RAPA and 99% (± 7%) versus 61% (± 3%) (P = .014) at 100 ng/mL RAPA signal reduction, respectively (Figure 3A). To further delineate the influence of RAPA on Foxp3 expression and Treg suppressor function, Tregs that had been exposed to RAPA for different timepoints were analyzed by FACS (Figure 3B) or used in functional assays (Figure 3D). We found that Foxp3 expression declined in activation cultures in the absence of RAPA, possibly due to an overgrowth of conventional T cells with more vigorous expansion kinetics in response to CD3/CD28 stimulation compared with Tregs. The decline of Foxp3 protein expression was prevented by the addition of RAPA to the activation culture (Figure 3B,C). A representative flow-cytometric analysis for Foxp3 expression on day 6 is shown in Figure 3C. Treg cells isolated from RAPA- containing cultures displayed increased suppressor activity compared with Tregs cultured in the absence of RAPA when used as suppressor cells against allo-Ag driven T-cell proliferation (Figure 3C).
RAPA inhibited Treg expansion less than Tconv and preserved suppressive activity and Foxp3 expression. (A) In vitro expansion of luciferase transgenic CD4+CD25high or CD4+CD25− T cells (C57BL/6) in the presence of CD3/CD28 stimulation, IL-2 (100 IU/mL), for 48 hours in complete media (cRPMI) and the indicated RAPA (R) doses. Percentages represent the relative reduction compared with the absence of RAPA (100%). One representative experiment of 3 independent experiments is shown. (B) Mean fluorescence intensity (MFI) for Foxp3 surface expression of CD4+CD25high cells at different timepoints during stimulation as described in panel A is quantified in the presence or absence of RAPA (*P < .05). (C) A representative histogram on day 6 of culture for the 3 different culture conditions described in panel A is shown. (D) Suppressive activity of Tregs recovered from the respective cultures. Thymidine incorporation of conventional T cells is significantly reduced when Tregs are included in the culture. Tregs derived from RAPA-containing cultures are more suppressive in vitro (*P < .05). One representative proliferation analysis of 3 independent experiments is shown.
RAPA inhibited Treg expansion less than Tconv and preserved suppressive activity and Foxp3 expression. (A) In vitro expansion of luciferase transgenic CD4+CD25high or CD4+CD25− T cells (C57BL/6) in the presence of CD3/CD28 stimulation, IL-2 (100 IU/mL), for 48 hours in complete media (cRPMI) and the indicated RAPA (R) doses. Percentages represent the relative reduction compared with the absence of RAPA (100%). One representative experiment of 3 independent experiments is shown. (B) Mean fluorescence intensity (MFI) for Foxp3 surface expression of CD4+CD25high cells at different timepoints during stimulation as described in panel A is quantified in the presence or absence of RAPA (*P < .05). (C) A representative histogram on day 6 of culture for the 3 different culture conditions described in panel A is shown. (D) Suppressive activity of Tregs recovered from the respective cultures. Thymidine incorporation of conventional T cells is significantly reduced when Tregs are included in the culture. Tregs derived from RAPA-containing cultures are more suppressive in vitro (*P < .05). One representative proliferation analysis of 3 independent experiments is shown.
In vivo expansion of Tconv and Tregs with and without RAPA
To evaluate the impact of RAPA on Tconv and Tregs in vivo we used a dosage of 1.5 mg/kg of body weight that has previously been demonstrated to reduce T-cell expansion after aHCT.3 We transferred luc+ Tconv or luc+ Tregs (H-2kb) with TCD-BM into irradiated BALB/c recipients. The signal derived from proliferating luc+ Tconv projecting over secondary lymphoid and GVHD target organs was reduced by addition of RAPA compared with PBS (Figure 4A, first versus second group of 5 animals from left). Conversely, expansion of Tregs was only minimally affected, with no significant overall reduction when measuring the amount of emitted photons/mouse/total body area (Figure 4B). Consistent with comparable expansion kinetics assessed by in vivo bioluminescence, equal numbers of donor type CD4+Foxp3+ cells were detected in peripheral lymph nodes and spleen. A representative FACS analysis is shown for CD4+Foxp3+ cells isolated from the spleen on day 22 after transplantation (Figure 4C). These data are consistent with our observation that in vitro mTOR inhibition during activation is compatible with high Foxp3 expression.
RAPA allows for polyclonal Treg expansion in vivo. (A) Expansion of luc+ Tconv (CD4+CD25−) or luc+ Tregs as shown for 5 representative animals of each group in the presence or absence of RAPA (1.5 mg/kg) on days 4 and 16 after BMT (C57BL/6 → BALB/c). Addition of RAPA reduces the expansion of luc+ Tconv (first vs second column of 5 animals from left), whereas expansion of luc+ Tregs is not significantly affected (third vs fourth column of 5 animals from left). (B) Expansion of luciferase-labeled T cells as quantified in emitted photons over total body area at serial timepoints after BMT. BLI signal intensity of mice receiving TCD-BM (△, n = 10), with luc+ T cells with PBS (□, n = 10) or RAPA 1.5 mg/kg (■, n = 10), luc+ Treg with PBS (○, n = 10) or RAPA 1.5 mg/kg (●, n = 10). Signal intensity is significantly higher in animals receiving T cells + PBS compared with T cells + RAPA (□ vs ■, * P < .05). (C) Frequency of splenic CD4+FoxP+ T cells on day 5 after transplantation is not different when PBS compared with RAPA is given in vivo. One representative FACS analysis of 3 independent experiments is shown. (D) BALB/c mice were given 5 × 106 TCD-BM together with 5 × 105 Tregs (C57BL/6) after lethal irradiation with 800 cGy and were injected with PBS or RAPA (1.5 mg/kg) daily. Expression of the indicated markers on splenic Thy1.1+CD4+CD25+ on day 5 after transplantation is shown. Black histogram, recipient received Treg + PBS. Light gray histogram, Treg + RAPA. Dark gray histogram, Isotype control Ab. (E) TCR Vβ usage of Thy1.1+CD4+CD25+ on day 30 after transplantation in BALB/c recipients is shown.
RAPA allows for polyclonal Treg expansion in vivo. (A) Expansion of luc+ Tconv (CD4+CD25−) or luc+ Tregs as shown for 5 representative animals of each group in the presence or absence of RAPA (1.5 mg/kg) on days 4 and 16 after BMT (C57BL/6 → BALB/c). Addition of RAPA reduces the expansion of luc+ Tconv (first vs second column of 5 animals from left), whereas expansion of luc+ Tregs is not significantly affected (third vs fourth column of 5 animals from left). (B) Expansion of luciferase-labeled T cells as quantified in emitted photons over total body area at serial timepoints after BMT. BLI signal intensity of mice receiving TCD-BM (△, n = 10), with luc+ T cells with PBS (□, n = 10) or RAPA 1.5 mg/kg (■, n = 10), luc+ Treg with PBS (○, n = 10) or RAPA 1.5 mg/kg (●, n = 10). Signal intensity is significantly higher in animals receiving T cells + PBS compared with T cells + RAPA (□ vs ■, * P < .05). (C) Frequency of splenic CD4+FoxP+ T cells on day 5 after transplantation is not different when PBS compared with RAPA is given in vivo. One representative FACS analysis of 3 independent experiments is shown. (D) BALB/c mice were given 5 × 106 TCD-BM together with 5 × 105 Tregs (C57BL/6) after lethal irradiation with 800 cGy and were injected with PBS or RAPA (1.5 mg/kg) daily. Expression of the indicated markers on splenic Thy1.1+CD4+CD25+ on day 5 after transplantation is shown. Black histogram, recipient received Treg + PBS. Light gray histogram, Treg + RAPA. Dark gray histogram, Isotype control Ab. (E) TCR Vβ usage of Thy1.1+CD4+CD25+ on day 30 after transplantation in BALB/c recipients is shown.
To evaluate the impact of RAPA on surface marker expression relevant for regulatory activity, we investigated expression of CTLA-4,19,20 GITR,21,22 and CD2723,24 as well as the homing molecules CD62L, CD103, CCR4, CCR5, and CCR7, several of which have been described to be relevant for Treg+mediated aGVHD protection.25,–27 Comparison of the MFI for these surface markers demonstrated that in vivo administration of RAPA did not interfere with a regulatory phenotype or homing molecule expression (Figure 4D). We found an increased expression of GITR and CTLA-4 on the surface of donor type Tregs as measured in MFI on day 5 after BMT when additional Tconv cells were given on day 2 compared with the absence of Tconv (data not shown). This increase was independent of the presence or absence of 1.5 mg/kg RAPA.
To further analyze the impact of RAPA on the T-cell receptor (TCR) Vβ usage by Tregs that expand in allogeneic recipients, donor-derived Tregs were isolated from secondary lymphoid organs on day 30 after aHCT. Vβ usage was quantified by FACS analysis (Figure 4E). Daily administration of RAPA did not interfere with the polyclonal expansion of donor-type Tregs after transplantation.
Differential AKT/mTOR pathway activity in Tregs and Tconv
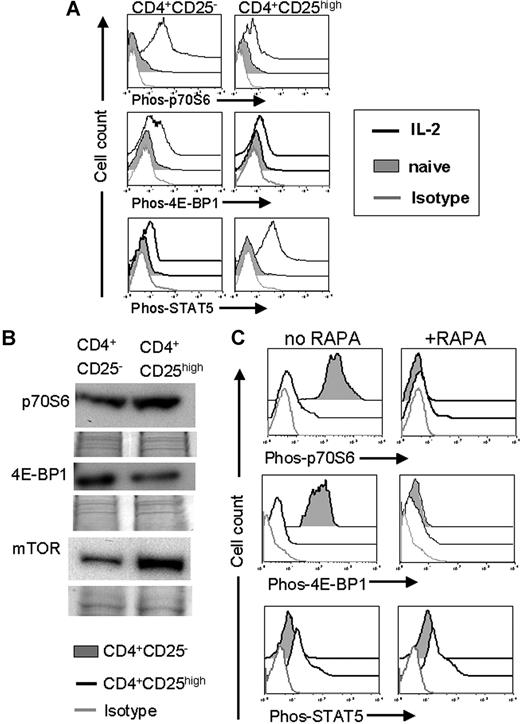
To investigate the relevance of the mTOR pathway for Tconv and Treg activation after allogeneic stimulation, cells were exposed to γ-irradiated (30 Gy) allogeneic APC and/or IL-2 and the phosphorylation of mTOR downstream products and STAT5 were quantified. STAT5 was chosen because signaling through the IL-2 receptor activates the STAT5 pathway and based on its previously reported relevance for Treg development.12 We used flow cytometry to analyze phosphorylation events relevant to intracellular signaling cascades as described previously.14,28 p70S6 and 4-EBP1 are direct targets of mTOR and their phosphorylation status is a parameter for mTOR activity.29 Phosphorylation of both mTOR downstream products p70S6 and 4E-BP1 after 48-h stimulation with IL-2 increased significantly in Tconv but less so in Treg cells (Figure 5A), indicating a minimal usage of the mTOR pathway by Treg. Conversely, Treg preferentially responded to the activation via STAT5 phosphorylation (Figure 5A). Naive Tregs expressed higher amounts of mTOR and p70S6 protein and slightly less 4-EBP1 protein compared with their CD4+CD25− counterparts (Figure 5B). Addition of RAPA to the allo-Ag/IL-2 activation culture led to an abrogation of mTOR downstream product phosphorylation in Tconv, whereas the low baseline phosphorylation of these products in Tregs was minimally affected (Figure 5C). It is noteworthy that phosphorylation of STAT5 was equally high in the presence or absence of RAPA in Tregs (Figure 5C) suggesting a preferential usage of this pathway and possibly referring a relative resistance to mTOR inhibition and increasing activity of the IL-2R/STAT5/Foxp3 pathway.
Preferential STAT-5 pathway usage in Tregs predicted resistance to mTOR inhibition. (A) The amount of phosphorylated p70S6, 4E-BP1, and STAT-5 was quantified by phospho-flow analysis within Tconv cells (CD4CD25−, left panel) and Tregs (CD4CD25high, right panel). T cells were isolated after 48 hours of IL-2 (100 IU/mL) stimulation. The amount of phosphorylated protein as measured in MFI increased significantly for p70S6, 4E-BP1 in Tconv (4 vs 172, and 11 vs 94, P < .001), but not in Tregs. MFI for phospho-STAT5 increases in Tregs in response to IL-2 (9 vs 103, P < .001). (B) Total protein expression in naive CD4+CD25high or CD4+CD25− T cells isolated from C57BL/6 mice. Expression of p70S6 and mTOR qA increased in Tregs compared with CD4+CD25− T cells. (C) The amount of phosphorylated p70S6, 4E-BP1, and STAT-5 is quantified by phospho-flow analysis within Tconv cells (CD4CD25−, filled gray histogram) and Treg cells (CD4CD25high, black solid line) both on C57BL/6 background. T cells were isolated after 48 hours of IL-2 (100 IU/mL) and irradiated (30 Gy, γ-irradiation) allogeneic CD11c+ APC (BALB/c) stimulation. The amount of phosphorylated protein as measured in MFI increases significantly more in Tconv compared with Treg for p70S6, 4E-BP1 (343 vs 16, and 1035 vs 10, P < .001) in response to alloantigen and IL-2. Addition of RAPA (10 ng/mL) abrogates phosphorylation of p70S6 and 4E-BP1 (right panel). MFI for phospho-STAT5 remains constant in Tregs when RAPA is added (29 vs 32, NS).
Preferential STAT-5 pathway usage in Tregs predicted resistance to mTOR inhibition. (A) The amount of phosphorylated p70S6, 4E-BP1, and STAT-5 was quantified by phospho-flow analysis within Tconv cells (CD4CD25−, left panel) and Tregs (CD4CD25high, right panel). T cells were isolated after 48 hours of IL-2 (100 IU/mL) stimulation. The amount of phosphorylated protein as measured in MFI increased significantly for p70S6, 4E-BP1 in Tconv (4 vs 172, and 11 vs 94, P < .001), but not in Tregs. MFI for phospho-STAT5 increases in Tregs in response to IL-2 (9 vs 103, P < .001). (B) Total protein expression in naive CD4+CD25high or CD4+CD25− T cells isolated from C57BL/6 mice. Expression of p70S6 and mTOR qA increased in Tregs compared with CD4+CD25− T cells. (C) The amount of phosphorylated p70S6, 4E-BP1, and STAT-5 is quantified by phospho-flow analysis within Tconv cells (CD4CD25−, filled gray histogram) and Treg cells (CD4CD25high, black solid line) both on C57BL/6 background. T cells were isolated after 48 hours of IL-2 (100 IU/mL) and irradiated (30 Gy, γ-irradiation) allogeneic CD11c+ APC (BALB/c) stimulation. The amount of phosphorylated protein as measured in MFI increases significantly more in Tconv compared with Treg for p70S6, 4E-BP1 (343 vs 16, and 1035 vs 10, P < .001) in response to alloantigen and IL-2. Addition of RAPA (10 ng/mL) abrogates phosphorylation of p70S6 and 4E-BP1 (right panel). MFI for phospho-STAT5 remains constant in Tregs when RAPA is added (29 vs 32, NS).
PTEN expression predicted mTOR susceptibility
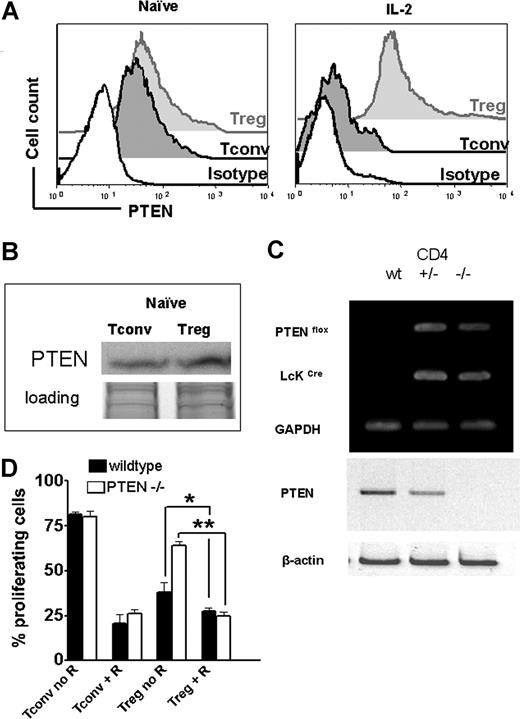
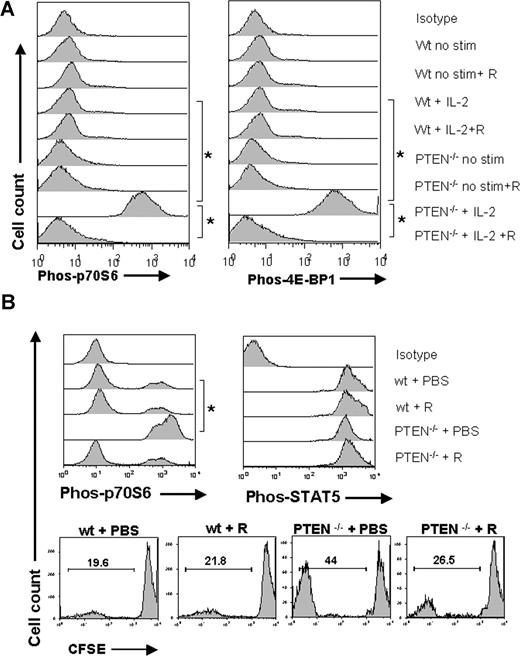
PTEN, a phosphoinositol 3,4,5-triphosphatase, catalyzes the reverse reaction of PI3K, thereby negatively regulating the activation of the mTOR downstream signaling pathway.11 PTEN expression was equal in naive Tregs and Tconv as assessed by flow cytometry (Figure 6A) and Western blot analysis (Figure 6B). It is noteworthy that PTEN expression decreased when Tconv were stimulated with IL-2, whereas Tregs maintained a constant level of PTEN expression (Figure 6A). To investigate the role of PTEN in Tregs, we generated mice with thymocytes that constitutively lack PTEN by crossing transgenic mouse lines that express the Cre recombinase under the control of the distal promoter of the mouse Lck gene30 with mice homozygous for the Ptenflox allele both on the C57BL/6 background. CD4+ T cells isolated from these mice displayed no expression of PTEN compared with wt or heterozygous control animals (Figure 6C). To investigate the relevance of PTEN on the expansion kinetics of CFSE-labeled Treg, wild-type or PTEN−/− animals were used as Treg donors based on previous studies indicating a critical role for PTEN in regulating Treg proliferation in response to IL-2.31 The proliferation advantage of Tregs in the presence of RAPA was abrogated when PTEN−/− donors were used (Figure 6D), indicating that PTEN was involved in the altered PI3K signaling in Tregs compared with Tconv. These data indicate that intact PTEN expression in wild-type Tregs accounted, at least in part, for the reduced signaling through the PI3K/mTOR pathway and thereby decreased susceptibility to RAPA. When comparing the amount of Phos-p70S6 and Phos-4E-BP1 in response to IL-2, we found that PTEN−/− Treg displayed significantly more phosphorylated mTOR downstream products (*P < .01) than their wild-type counterparts (Figure 7A). Conversely, when RAPA was present in the culture, the amount of Phos-p70S6 and Phos-4E-BP1 decreased significantly in PTEN−/− Tregs (*P < .01) and was not different from the level of wt Tregs (Figure 7A).
Tregs display high PTEN expression levels during stimulation and PTEN deficiency partially antagonizes resistance of Tregs toward RAPA. (A) Levels of PTEN protein expression are analyzed by FACS for naive (left histogram) or activated (right histogram) Tconv (dark gray-filled histogram) and Tregs (light gray-filled histogram) cells. Activation was by allo-Ag (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL) for 48 hours. The black open histogram represents the isotype control. MFI for PTEN is higher in Tregs compared with Tconv after activation (241 vs 82, P < .001). (B) Western blot analysis demonstrates that Treg cells display slightly higher amounts of PTEN protein compared with Tconv cells while both were in the naive state. (C) Specific recombination at the PTEN locus in the presence of Cre is shown by PCR amplification of genomic DNA isolated from CD4 + T cells from Crenegative (+/+), Ptenflox/+Cre+ (+/−), and Ptenflox/floxCre+ (−/−) littermates. Western blot analysis demonstrated absence of PTEN in Ptenflox/floxCre+ (−/−) mice. (D) Percentage of proliferating wild-type ( ) or PTEN-deficient (
) or PTEN-deficient ( ) Tconv or Treg after 48-hour stimulation with (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL), in the presence or absence of RAPA (R, 10 ng/mL) as indicated (*P < .05; **P < .01). Proliferation was assessed by serial CFSE dilution.
) Tconv or Treg after 48-hour stimulation with (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL), in the presence or absence of RAPA (R, 10 ng/mL) as indicated (*P < .05; **P < .01). Proliferation was assessed by serial CFSE dilution.
Tregs display high PTEN expression levels during stimulation and PTEN deficiency partially antagonizes resistance of Tregs toward RAPA. (A) Levels of PTEN protein expression are analyzed by FACS for naive (left histogram) or activated (right histogram) Tconv (dark gray-filled histogram) and Tregs (light gray-filled histogram) cells. Activation was by allo-Ag (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL) for 48 hours. The black open histogram represents the isotype control. MFI for PTEN is higher in Tregs compared with Tconv after activation (241 vs 82, P < .001). (B) Western blot analysis demonstrates that Treg cells display slightly higher amounts of PTEN protein compared with Tconv cells while both were in the naive state. (C) Specific recombination at the PTEN locus in the presence of Cre is shown by PCR amplification of genomic DNA isolated from CD4 + T cells from Crenegative (+/+), Ptenflox/+Cre+ (+/−), and Ptenflox/floxCre+ (−/−) littermates. Western blot analysis demonstrated absence of PTEN in Ptenflox/floxCre+ (−/−) mice. (D) Percentage of proliferating wild-type ( ) or PTEN-deficient (
) or PTEN-deficient ( ) Tconv or Treg after 48-hour stimulation with (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL), in the presence or absence of RAPA (R, 10 ng/mL) as indicated (*P < .05; **P < .01). Proliferation was assessed by serial CFSE dilution.
) Tconv or Treg after 48-hour stimulation with (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL), in the presence or absence of RAPA (R, 10 ng/mL) as indicated (*P < .05; **P < .01). Proliferation was assessed by serial CFSE dilution.
PTEN deficiency reversed low mTOR pathway usage and renders Tregs sensitive to RAPA. (A) Treg cells were isolated after 48 hours of culture. Where indicated, IL-2 (100 IU/mL) and/or RAPA (10 ng/mL) was present in the culture. The amount of phosphorylated p70S6 and 4E-BP1 was quantified by phospho-flow analysis within wt or PTEN−/− Tregs. The amount of phosphorylated protein as measured in MFI was significantly higher for phospho-p70S6 (left panel) phospho-4E-BP1 (right panel) in PTEN−/− compared with wt Treg cells (* P < .01). Addition of RAPA (10 ng/mL) to the culture reduced the amount of phospho-p70S6 and phospho-4E-BP1 in PTEN−/− Treg cells. (B) BALB/c mice were given 5 × 106 TCD-BM together with 5 × 105 wt or PTEN−/− Treg (C57BL/6) after lethal irradiation with 800 cGy and were injected with PBS or RAPA (1.5 mg/kg) daily. The amount of phospho-p70S6, phospho-STAT5 in wt, or PTEN−/− donor type Treg cells (H-2kb) is displayed (*P < .01). Percentage of dividing donor type (H-2kb) CFSE labeled Tregs was higher in PTEN−/− donors compared with wt donors (44% vs 19.6%, P < .05). Increased expansion of PTEN−/− Tregs was antagonized when the recipients were treated with RAPA (44% vs 26.5%, P < .05).
PTEN deficiency reversed low mTOR pathway usage and renders Tregs sensitive to RAPA. (A) Treg cells were isolated after 48 hours of culture. Where indicated, IL-2 (100 IU/mL) and/or RAPA (10 ng/mL) was present in the culture. The amount of phosphorylated p70S6 and 4E-BP1 was quantified by phospho-flow analysis within wt or PTEN−/− Tregs. The amount of phosphorylated protein as measured in MFI was significantly higher for phospho-p70S6 (left panel) phospho-4E-BP1 (right panel) in PTEN−/− compared with wt Treg cells (* P < .01). Addition of RAPA (10 ng/mL) to the culture reduced the amount of phospho-p70S6 and phospho-4E-BP1 in PTEN−/− Treg cells. (B) BALB/c mice were given 5 × 106 TCD-BM together with 5 × 105 wt or PTEN−/− Treg (C57BL/6) after lethal irradiation with 800 cGy and were injected with PBS or RAPA (1.5 mg/kg) daily. The amount of phospho-p70S6, phospho-STAT5 in wt, or PTEN−/− donor type Treg cells (H-2kb) is displayed (*P < .01). Percentage of dividing donor type (H-2kb) CFSE labeled Tregs was higher in PTEN−/− donors compared with wt donors (44% vs 19.6%, P < .05). Increased expansion of PTEN−/− Tregs was antagonized when the recipients were treated with RAPA (44% vs 26.5%, P < .05).
To further study the relevance of the observed in vitro effects of PTEN deficiency on Treg expansion in an in vivo situation, we used CFSE-labeled wt or PTEN−/− Tregs (C57BL/6) that were given on day 0 of BMT. Interestingly, the percentage of dividing donor type (H-2kb+) Tregs was higher in PTEN−/− donors compared with wt donors (44% vs 19.6%, P < .05) (Figure 7B bottom panel). Treatment of the recipients with RAPA antagonized increased expansion of PTEN−/− Tregs, which demonstrated the central role of PTEN for the susceptibility to RAPA (Figure 7B bottom panel). This was paralleled by increased p70S6 phosphorylation in PTEN−/− Tregs that expanded in vivo (Figure 7B top panel). Addition of RAPA did reduce p70S6 phosphorylation in PTEN−/− Tregs but did not affect STAT5 phosphorylation (Figure 7B top panel). STAT-5 phosphorylation was higher in wt compared with PTEN−/− Tregs after adoptive transfer, possibly because of the preferential usage of the mTOR pathway (Figure 7B top panel).
Discussion
In this report, we characterized the impact of RAPA on Foxp3+CD4+CD25+ Treg biology after aHCT as well as on a molecular level and found important differences compared with its CD4+CD25− Tconv counterparts. We chose RAPA because it was shown to promote TCR-induced T-cell anergy even in the presence of costimulation32 and allows for induction of operational tolerance.33,34 Furthermore, we have described previously that in vivo RAPA administration was favorable with respect to Treg function compared with other immunosuppressants such as CSA, which inhibited Treg function.3
A major finding of the present study is that Tregs and RAPA have a synergistic protective effect on aGvHD as a pathologic immune response. We have shown that the combined approach is more potent at reducing the expansion of alloreactive Tconv cells, which translates into reduced GvHD target organ damage as shown by histologic evaluation. We have shown previously that graft-versus-tumor effects are preserved when Tregs are combined with Tconv35 and maintained in the presence of RAPA.3 A comparable synergism between Tregs and RAPA has been demonstrated in a model of bone marrow graft rejection.36 Furthermore, recent data indicate that the combination of RAPA with an agonistic CD28 antibody down-modulates GvHD.37 Based on these findings, we hypothesized that mTOR inhibition has a differential effect on Tregs compared with Tconv. In support of this hypothesis, we observed that high Foxp3 protein expression was different in the presence and absence of RAPA in cultures containing CD4+CD25high cells and in vivo expanding Tregs. These observations can be explained by 2 different mechanisms that are not mutually exclusive.
First, RAPA may prevent overgrowth of Foxp3− T cells, which is consistent with the observation that Tregs isolated from RAPA-containing cultures were more suppressive than Tregs cultured in the absence of RAPA. These observations are compatible with data, indicating the enrichment of Tregs in a system with OT transgenic CD4 T cells expanded in the presence of RAPA.38
Second, the in vitro data can be explained by an increased usage of the STAT5 pathway over the PI3K pathway in response to IL-2 and the presence of mTOR inhibition that may skew the differentiation of CD4+ T cells toward a Foxp3-expressing phenotype. This is in line with recent data indicating that STAT5 activation is sufficient to promote the IL-2Rβ-dependent development of CD4+Foxp3+ Tregs12 and that Stat5a/b directly regulate Foxp3.39
Because in vitro and in vivo kinetics of Tregs were shown to follow different dynamics,40 we investigated expansion of luciferase transgenic Tregs in comparison to Tconv in the irradiated host. The expansion kinetics of Tregs in this model are based on proinflammatory cytokines, up-regulation of CD153 on dendritic cells,41 and lymphopenia developing within the first 7 days after transplantation. Our observation that in vivo expansion was inhibited significantly more in Tconv compared with Tregs when RAPA was injected is compatible with previous reports demonstrating the preferential expansion of human Tregs over Tconv in the presence of RAPA in vitro42 and more potent apoptosis induction by RAPA in human conventional T cells.43 Of clinical importance is the observation that Tregs exposed to RAPA after aHCT maintained a polyclonal Vβ TCR usage and lymph node homing molecules that have been demonstrated to be critical for their in vivo function of Tregs.25,–27,44 The observation that the transfer of Tconv in addition to TCD-BM leads to an increase of GITR and CTLA-4 on the surface of donor-type Tregs may be due to cytokine release during the alloantigen-driven immune response.
Our previous studies demonstrated a critical role for calcineurin-dependent IL-2 production for Treg function in protecting against aGvHD during aHCT3 ; therefore, we aimed to study further the effects of RAPA on conventional CD4+ T cells and Treg cells. IL-2R signaling is primarily mediated through activation of JAK1 and JAK3 with subsequent phosphorylation and activation of STAT5.45 IL-2R triggering also leads to activation of other signaling pathways, including MAPK and PI3K. Because previous studies have shown that IL-2 primarily induces JAK/STAT signaling rather than PI3K signaling in Tregs,46,47 we aimed to investigate the impact of the differential pathway response on the sensitivity to mTOR inhibition.
It is noteworthy that we found that Treg activation by alloantigen and IL-2 led to a preferential activity of the STAT5 pathway and only very little mTOR activity. These findings are compatible with an altered AKT/mTOR signaling in Tregs compared with Tconv.48 Our data indicate that mTOR inhibition in the presence of IL-2 allowed Tregs to be constantly activated through the STAT5 pathway and promoted their preferential expansion and Foxp3 expression in the presence of RAPA, whereas conventional CD4 T cells were much more affected by RAPA. This notion is consistent with previous data indicating that RAPA-resistant proliferation of CD8+ T-cell clones could be blocked by anti-IL-2 Abs, suggesting that some of the pathways parallel to the PI3K/mTOR pathway, in this case the STAT5 pathway, triggered by IL-2R signaling account for the Ag-driven RAPA resistance.49
Furthermore, recent data indicate that expression of PTEN is critical for the hyporesponsive phenotype of Treg cells and that Tregs develop normally in mice with a specific deletion of PTEN in the T-cell compartment.31
These findings prompted us to analyze the relevance of PTEN for the relative resistance of Tregs toward mTOR inhibition. Although expression levels of PTEN were equal in naive cells, activation induced a rapid down-regulation of PTEN in Tconv but not in Treg cells. The physiologic relevance of this finding may be that Tconv down-regulate PTEN, as a negative regulator of T-cell expansion, when they are activated. By negatively regulating TCR signals, PTEN has been shown to impose a requirement for CD28 costimulation, defining an important mechanism for its role in self-tolerance.50 Our finding that PTEN deficiency was associated with a reversal of the relative resistance of Tregs toward mTOR inhibition is compatible with data from different tumor models in which PTEN knockdown sensitizes established tumors to RAPA in vitro and in vivo.51
In conclusion, our data indicate that Tregs and Tconv display differential expansion kinetics when exposed to RAPA. We identified a relative resistance toward RAPA in Tregs based on reduced usage of the mTOR pathway and on functional PTEN. The finding that mTOR inhibition has differential effects on Tregs and Tconv may help to understand the synergistic effect of RAPA and Tregs in aGvHD protection, which has important implications when using Tregs in clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH; R01-CA0800065 and P01-HL075462) and in part through the National Cancer Institute's (NCI) Small Animal Imaging Resource Program (SAIRP grant number R24-CA92862) and the NCI In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50-CA114747. This study was also supported in part by the Deutsche Krebshilfe (Dr Mildred-Scheel-Stiftung), Bonn, Germany (grant 108034 to R.Z.).
We are grateful to Ruby M. Wong for expert assistance with statistical analysis and to the members of the Negrin laboratory for helpful discussion.
National Institutes of Health
Authorship
Contribution: R.Z. designed and performed research, analyzed data, and wrote the manuscript. D.B.L.-G. helped to design experiments, performed research, and analyzed data. E.Z., J.Z.H., J.L. performed research and analyzed data. A.B. contributed new reagents and performed research. N.K. analyzed GVHD target- organ tissue samples and scored histologic slides. R.S.N. designed research and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, MD, Center for Clinical Science Research, 269 W Campus Drive, Rm 2205, Division of Blood and Marrow Transplantation, Stanford University School of Medicine, Stanford, CA 94305; e-mail: negrs@stanford.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal