Allogeneic conventional hematopoietic cell transplantation (HCT) can be curative treatment for lymphoid malignancies, but it has been characterized by high nonrelapse mortality (NRM). Here, we compared outcomes among patients with lymphoma or chronic lymphocytic leukemia given either nonmyeloablative (n = 152) or myeloablative (n = 68) conditioning. Outcomes were stratified by the HCT-specific comorbidity index. Patients in the nonmyeloablative group were older, had more previous treatment and more comorbidities, more frequently had unrelated donors, and more often had malignancy in remission compared with patients in the myeloablative group. Patients with indolent versus aggressive malignancies were equally distributed among both cohorts. After HCT, patients without comorbidities both in the nonmyeloablative and myeloablative cohorts had comparable NRM (P = .74), overall survival (P = .75), and progression-free survival (P = .40). No significant differences were observed (P = .91, P = .89, and P = .40, respectively) after adjustment for pretransplantation variables. Patients with comorbidities experienced lower NRM (P = .009) and better survival (P = .04) after nonmyeloablative conditioning. These differences became more significant (P < .001 and .007, respectively) after adjustment for other variables. Further, nonmyeloablative patients with comorbidities had favorable adjusted progression-free survival (P = .01). Patients without comorbidities could be enrolled in prospective randomized studies comparing different conditioning intensities. Younger patients with comorbidities might benefit from reduced conditioning intensity.
Introduction
Only limited options for curative treatment are available for patients with relapsed or refractory lymphoid malignancies who either failed autologous hematopoietic cell transplantation (HCT) or lacked stem cells for autologous HCT.1,–3 In some patients, prolonged remissions have been achieved with allogeneic HCT after myeloablative conditioning, presumably resulting from tumor cell kill from both high-intensity conditioning and graft-versus-malignancy effects and because of the use of tumor-free grafts.4,–6 However, up to 60% nonrelapse mortality (NRM) has been observed even among patients younger than 50 years of age with acceptable performance status.7,,,–11 Unfortunately, most patients with lymphoma or chronic lymphocytic leukemia (CLL) are older than 50 and have comorbidities.12 Reduced-intensity conditioning regimens have been developed to decrease NRM and to extend the use of allogeneic HCT to these older patients and to those with comorbidities.13,,,,,,,,,,,,,,,–29 Systematic comparisons between reduced-intensity and conventional HCT have been underreported in the literature, particularly those balanced by the impact of important prognostic factors. Accurate risk assessment for the 2 approaches would be useful for patient counseling and stratification in prospective studies. In the present study, we assessed the role of conditioning intensity on outcomes among patients with lymphoma and CLL given allogeneic HCT following either nonmyeloablative or myeloablative conditioning regimens.
Methods
The retrospective analysis was approved by the institutional review board of the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA). Informed consent was obtained from all patients at time of transplantation in accordance with the Declaration of Helsinki.
Patients
Patients and disease characteristics are described in Table 1. Consecutive patients (n = 220) with lymphoma or CLL given allogeneic HCT at the FHCRC between December 1997 and June 2005 were included in this analysis. December 1997 was chosen as the starting point because it coincided with the initiation of nonmyeloablative conditioning at FHCRC. Most patients (n = 152) received nonmyeloablative conditioning regimens (nonmyeloablative patients), which consisted of 2 Gy total-body irradiation (TBI) alone or preceded by fludarabine, 90 mg/m2; all received postgrafting immunosuppression with mycophenolate mofetil and cyclosporine (CSP).30,–32 Sixty-eight patients received myeloablative conditioning regimens (myeloablative patients), which included cyclophosphamide (CY) combined with either at least 12 Gy TBI or busulfan (levels targeted to plasma mean steady-state concentrations of 800-900 ng/mL)33 ; most were given methotrexate/CSP for graft-versus-host disease (GVHD) prophylaxis.34 Overall, nonmyeloablative protocols were offered to patients who were either 50 or older or, if younger than 50, had significant pre-existing medical problems or had failed high-dose autologous HCT. Only 8 patients, 38 to 48 years old, who did not meet these criteria were enrolled in nonmyeloablative protocols based on their own preference (n = 4) or on consensus decisions from the Patient Care Conference of the FHCRC Faculty (n = 4), which was based on the chemosensitive nature of their disease. Nonmyeloablative patients were treated in the outpatient clinic during the first 100 days before returning to their referring physicians and were admitted to the hospital only as required for treatment of complications. All myeloablative patients were hospitalized for 3 to 4 weeks before being discharged to the outpatient clinic.
Patients and donors were matched for HLA-A, -B, and -C antigens by either intermediate resolution DNA typing (to a level at least as sensitive as serology) or by high-resolution techniques. Patients and donors were matched for HLA-DRB1 and DQB1 alleles.35 All patients received infection prophylaxis according to standard institutional guidelines.36,,,–40 Aggressive diseases included CLL with Richter transformation, Hodgkin disease (HD) with the exception of nodular lymphocyte-predominant subtype, and aggressive lymphoma as defined by the Physician Data Query Modification of the Revised European American Lymphoma Classification of Lymphoproliferative Diseases (see the link http://www.cancer.gov/cancertopics/pdq/treatment/adult-non-hodgkins/HealthProfessional/page2#Section_17), whereas indolent diseases included all other histologic subtypes.
Assessment of pretransplantation comorbidities
Comorbidities were assessed by comprehensive review of medical records and computer database systems. Scores were assigned by a single investigator (M.L.S.) using definitions of the 17 comorbidities included in the HCT-specific comorbidity index (HCT-CI).41 The HCT-CI included thresholds for pulmonary, hepatic, and renal function tests to allow for sensitive detection of organ impairments that might compromise the ability of patients to tolerate a given conditioning regimen.
Half of the current patients were included in the original training set used to develop the HCT-CI. Because no direct comparisons of outcomes on the basis of comorbidity scores were made, inclusion of these patients was not thought to bias the comparison of outcomes on the basis of conditioning regimen intensity.
Statistical methods
Survival curves and probabilities were estimated according to the Kaplan-Meier method, whereas cumulative incidence curves and probabilities for NRM were estimated as described.42 Multivariate hazard ratios (HRs) for NRM and survival outcomes were estimated from Cox regression models, treating NRM and relapse or progression of malignancy as competing risks where appropriate. Adjusted survival curves were estimated based on methods described by Makuch.43 Briefly, the adjusted survival curve for the nonmyeloablative group represented a model-based projection of survival for a group of patients with the same baseline hazard function as estimated for those patients, but with the covariate characteristics of the myeloablative group. These estimates were derived from Cox regression models incorporating the adjustment factors as covariates and stratified on conditioning group. Adjusted cumulative incidence curves were estimated by applying the same adjustment procedure to the component cause-specific survival functions and then computing the cumulative incidence curve in the usual way.
Results
Pretransplantation characteristics
In part because of protocol inclusion and exclusion criteria, the following differences were found among pretransplantation characteristics of the 2 groups (Table 1).
Patients and disease characteristics
| Characteristics . | Nonmyeloablative patients, n = 152 . | Myeloablative patients, n = 68 . |
|---|---|---|
| Median age, y (range) | 60 (18-70) | 46 (10-59) |
| Median follow-up, mo (range) | 44 (7-90) | 61 (21-97) |
| Median number of previous regimens (range) | 5 (1-13) | 3 (1-7) |
| Median interval between diagnosis and HCT, mo (range) | 7.0 (0.2-254) | 6.6 (0.2-157) |
| Previous autologous high-dose HCT, % | ||
| Overall | 52 | 10 |
| Planned/failed | 11/41 | 0/10 |
| Conditioning regimens, % | ||
| 2 Gy TBI | 18 | 0 |
| Flu + 2 Gy TBI | 82 | 0 |
| Cy + ≥12 Gy TBI | 0 | 87 |
| tBu + Cy | 0 | 13 |
| Diagnosis, % | ||
| NHL | 54 | 78 |
| Low-grade | 14 | 27 |
| High-grade | 40 | 51 |
| CLL | 26 | 15 |
| HD | 20 | 7 |
| Disease nature, %* | ||
| Indolent | 37 | 41 |
| Aggressive | 63 | 59 |
| Disease status at the time of HCT, % | ||
| Sensitive† | 46 | 25 |
| Refractory/untested relapse | 40/14 | 53/21 |
| Hematopoietic cell source, % | ||
| G-PBMC | 99 | 69 |
| Marrow | 1 | 31 |
| Donor type, % | ||
| HLA-matched sibling | 55 | 68 |
| HLA- antigen mismatched related | 1 | 4 |
| HLA-matched unrelated‡ | 39 | 25 |
| HLA- antigen mismatched unrelated | 5 | 3 |
| Patient CMV sero-status, % | ||
| Negative | 43 | 44 |
| Positive | 57 | 56 |
| Characteristics . | Nonmyeloablative patients, n = 152 . | Myeloablative patients, n = 68 . |
|---|---|---|
| Median age, y (range) | 60 (18-70) | 46 (10-59) |
| Median follow-up, mo (range) | 44 (7-90) | 61 (21-97) |
| Median number of previous regimens (range) | 5 (1-13) | 3 (1-7) |
| Median interval between diagnosis and HCT, mo (range) | 7.0 (0.2-254) | 6.6 (0.2-157) |
| Previous autologous high-dose HCT, % | ||
| Overall | 52 | 10 |
| Planned/failed | 11/41 | 0/10 |
| Conditioning regimens, % | ||
| 2 Gy TBI | 18 | 0 |
| Flu + 2 Gy TBI | 82 | 0 |
| Cy + ≥12 Gy TBI | 0 | 87 |
| tBu + Cy | 0 | 13 |
| Diagnosis, % | ||
| NHL | 54 | 78 |
| Low-grade | 14 | 27 |
| High-grade | 40 | 51 |
| CLL | 26 | 15 |
| HD | 20 | 7 |
| Disease nature, %* | ||
| Indolent | 37 | 41 |
| Aggressive | 63 | 59 |
| Disease status at the time of HCT, % | ||
| Sensitive† | 46 | 25 |
| Refractory/untested relapse | 40/14 | 53/21 |
| Hematopoietic cell source, % | ||
| G-PBMC | 99 | 69 |
| Marrow | 1 | 31 |
| Donor type, % | ||
| HLA-matched sibling | 55 | 68 |
| HLA- antigen mismatched related | 1 | 4 |
| HLA-matched unrelated‡ | 39 | 25 |
| HLA- antigen mismatched unrelated | 5 | 3 |
| Patient CMV sero-status, % | ||
| Negative | 43 | 44 |
| Positive | 57 | 56 |
Flu indicates fludarabine; and tBu, targeted busulfan.
Aggressive diseases included CLL with Richter's transformation, HD with the exception of nodular lymphocyte–predominant subtype, and aggressive lymphoma as defined by the Physician Data Query Modification of the Revised European American Lymphoma Classification of Lymphoproliferative Diseases (see http://www.cancer.gov/cancertopics/pdq/treatment/adult-non-hodgkins/HealthProfessional/page2#Section_17), whereas indolent diseases included all other histological subtypes.
Complete or partial remission.
Included 6 nonmyeloablative and 2 myeloablative patient/donor pairs with single allele mismatch.
Nonmyeloablative patients were older (median age 60 vs 46 years), with 59% versus 18% 50 or older and 21% versus 0% 60 or older, had received more preceding chemotherapy regimens (median of 5 vs 3) and more often received previous high-dose HCT (52% vs 10%) than myeloablative patients. In addition, nonmyeloablative patients more often received grafts from unrelated donors (44% vs 28%) and granulocyte-colony stimulating factor-mobilized peripheral blood mononuclear cell (G-PBMC) grafts (99% versus 69%) compared with myeloablative patients. Diagnoses included non-Hodgkin lymphoma (NHL), HD, and CLL in 53%, 20%, and 27% of nonmyeloablative patients and in 78%, 7%, and 15% of myeloablative patients. Although no differences were found in the distribution of indolent versus aggressive lymphoproliferative malignancies between nonmyeloablative and myeloablative patients, more nonmyeloablative patients had complete or partial remissions at the time of HCT compared with their myeloablative counterparts (46% vs 25%). The median follow-up times of surviving nonmyeloablative and myeloablative patients were 44 and 61 months, respectively.
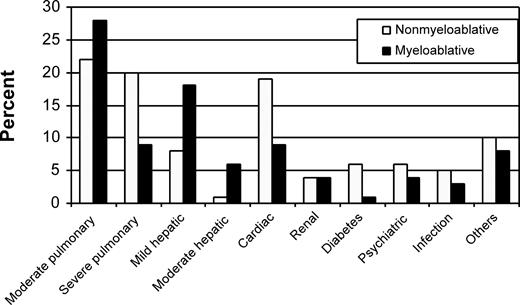
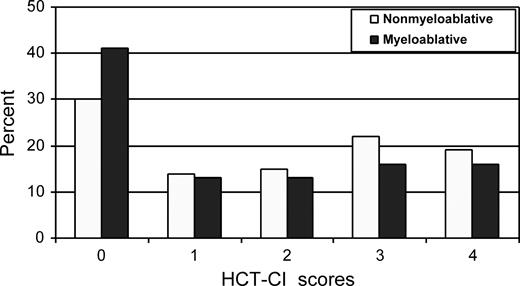
Lungs, liver, and heart were the organs most frequently affected by comorbidity in both patient cohorts (Figure 1). HCT-CI scores of 0, 1-2, and 3 or more were assigned to 30%, 30%, and 41% of nonmyeloablative patients, respectively, compared with 41%, 26%, and 32% of myeloablative patients (Figure 2; P = .01). The distributions of these comorbidity scores among nonmyeloablative (31%, 26%, and 43%) and myeloablative (46%, 27%, and 27%) patients remained comparable after exclusion of patients whose data were used to develop the HCT-CI.
Distribution of individual comorbidities among nonmyeloablative and myeloablative patients as assessed by the HCT-CI. Other comorbidities included rheumatologic, gastrointestinal, previous malignancy, and obesity. Of note, scores of 2 and 3 were assigned for moderate and severe pulmonary comorbidities, whereas scores of 1 and 3 were assigned for mild and moderate hepatic comorbidities.
Distribution of individual comorbidities among nonmyeloablative and myeloablative patients as assessed by the HCT-CI. Other comorbidities included rheumatologic, gastrointestinal, previous malignancy, and obesity. Of note, scores of 2 and 3 were assigned for moderate and severe pulmonary comorbidities, whereas scores of 1 and 3 were assigned for mild and moderate hepatic comorbidities.
Distribution of scores as assigned by the HCT-CI to nonmyeloablative compared with myeloablative patients with lymphoma or CLL.
Distribution of scores as assigned by the HCT-CI to nonmyeloablative compared with myeloablative patients with lymphoma or CLL.
Risk factors for HCT outcomes
The 3-year NRM and overall survival rates for all patients were 28% and 51%, respectively. These percentages were 25% and 53% for nonmyeloablative patients versus 35% and 45% for myeloablative patients. Cox regression multivariate models were constructed to define risk factors for NRM and overall mortality for all patients (Table 2). Conditioning type, HCT-CI scores, age, disease diagnosis and status, previous regimens, donor type, stem-cell source type, and cytomegalovirus (CMV) serology results were tested in these analyses as potential risk factors. The 2 most influential risk factors associated with increased HRs for both NRM and overall mortality were HCT-CI scores of 1 or more (P < .001 and .002) and myeloablative conditioning (P < .001 and .007), respectively. Less significant associations were found for age 50 or older for NRM (P = .006), diagnosis of NHL versus HD, versus CLL (P = .05) and previous CMV infection (P = .03) for overall mortality, and use of G-PBMC versus marrow for both NRM and overall mortality (P = .01 and .05, respectively). Effects of other factors did not reach statistical significance.
Multivariate analyses of risk factors for NRM and mortality among all patients with lymphoma or CLL treated with allogeneic HCT
| Risk factors . | NRM . | Mortality . | ||
|---|---|---|---|---|
| HR* (95% CI) . | P . | HR* (95% CI) . | P . | |
| Conditioning | <.001 | .007 | ||
| Nonmyeloablative | 1.0 | 1.0 | ||
| Myeloablative | 3.70 (2.0-10.0) | 2.04 (1.25-3.33) | ||
| HCT-CI scores | <.001 | .002 | ||
| 0 | 1.0 | 1.0 | ||
| 1-2 | 2.48 (1.2-5.3) | 1.71 (1.0-2.09) | ||
| ≥3 | 3.82 (1.9-7.8) | 2.41 (1.5-4.0) | ||
| Age | .006 | .11 | ||
| <50 years | 1.0 | 1.0 | ||
| ≥50 years | 2.58 (1.3-5.1) | 1.49 (0.9-2.4) | ||
| Diagnosis | .19 | .05 | ||
| CLL | 1.0 | 1.0 | ||
| HD | 1.11 (0.45-2.5) | 1.16 (0.63-2.0) | ||
| NHL | 1.89 (0.91-3.33) | 1.89 (1.11-3.33) | ||
| Previous regimens | .12 | .11 | ||
| 0-2 | 1.0 | 1.0 | ||
| 3-4 | 1.15 (0.7-2.0) | 1.38 (0.9-2.1) | ||
| ≥5 | 0.38 (0.1-1.3) | 0.70 (0.3-1.6) | ||
| Disease status | .83 | .17 | ||
| Remission | 1.0 | 1.0 | ||
| Refractory/relapse | 1.06 (0.6-1.8) | 1.32 (0.9-2.0) | ||
| Donor | .12 | .64 | ||
| Matched siblings | 1.0 | 1.0 | ||
| Others† | 1.55 (0.9-2.7) | 1.10 (0.7-1.6) | ||
| Stem-cell source | .01 | .05 | ||
| Marrow | 1.0 | 1.0 | ||
| G-PBMC | 3.12 (1.11-10.0) | 2.0 (1.0-5.0) | ||
| Patient CMV sero-status | .09 | .03 | ||
| Negative | 1.0 | 1.0 | ||
| Positive | 1.45 (0.8-2.5) | 1.43 (1.0-2.1) | ||
| Risk factors . | NRM . | Mortality . | ||
|---|---|---|---|---|
| HR* (95% CI) . | P . | HR* (95% CI) . | P . | |
| Conditioning | <.001 | .007 | ||
| Nonmyeloablative | 1.0 | 1.0 | ||
| Myeloablative | 3.70 (2.0-10.0) | 2.04 (1.25-3.33) | ||
| HCT-CI scores | <.001 | .002 | ||
| 0 | 1.0 | 1.0 | ||
| 1-2 | 2.48 (1.2-5.3) | 1.71 (1.0-2.09) | ||
| ≥3 | 3.82 (1.9-7.8) | 2.41 (1.5-4.0) | ||
| Age | .006 | .11 | ||
| <50 years | 1.0 | 1.0 | ||
| ≥50 years | 2.58 (1.3-5.1) | 1.49 (0.9-2.4) | ||
| Diagnosis | .19 | .05 | ||
| CLL | 1.0 | 1.0 | ||
| HD | 1.11 (0.45-2.5) | 1.16 (0.63-2.0) | ||
| NHL | 1.89 (0.91-3.33) | 1.89 (1.11-3.33) | ||
| Previous regimens | .12 | .11 | ||
| 0-2 | 1.0 | 1.0 | ||
| 3-4 | 1.15 (0.7-2.0) | 1.38 (0.9-2.1) | ||
| ≥5 | 0.38 (0.1-1.3) | 0.70 (0.3-1.6) | ||
| Disease status | .83 | .17 | ||
| Remission | 1.0 | 1.0 | ||
| Refractory/relapse | 1.06 (0.6-1.8) | 1.32 (0.9-2.0) | ||
| Donor | .12 | .64 | ||
| Matched siblings | 1.0 | 1.0 | ||
| Others† | 1.55 (0.9-2.7) | 1.10 (0.7-1.6) | ||
| Stem-cell source | .01 | .05 | ||
| Marrow | 1.0 | 1.0 | ||
| G-PBMC | 3.12 (1.11-10.0) | 2.0 (1.0-5.0) | ||
| Patient CMV sero-status | .09 | .03 | ||
| Negative | 1.0 | 1.0 | ||
| Positive | 1.45 (0.8-2.5) | 1.43 (1.0-2.1) | ||
CI indicates confidence interval.
Higher HR indicates worse outcomes.
Includes antigen-mismatched related and all unrelated donors.
Risk stratification of HCT outcomes
Since HCT-CI scores and conditioning intensity were the two most influential risk factors, we stratified patients into 4 groups based on nonmyeloablative versus myeloablative conditioning and HCT-CI scores of 0 versus 1 or more.
Patients with HCT-CI score of 0
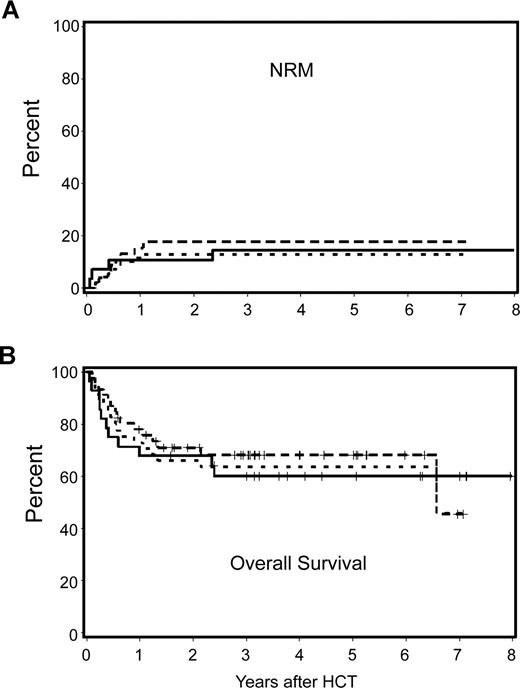
Nonmyeloablative and myeloablative patients with an HCT-CI score of 0 had 3-year NRM rates of 18% and 15% and overall survival rates of 68% and 60%, respectively. Differences in these outcomes were not statistically significant (the unadjusted HR for NRM and survival were 1.23 [P = .74] and .88 [P = .75], respectively). Likewise, there was no statistically significant difference in risk for relapse (HR: 1.41; P = .43). After adjustment for other risk factors, differences in NRM, relapse, and overall survival among nonmyeloablative and myeloablative patients remained statistically not significant (HR = 0.90, P = .91; HR = 1.94, P = .27; and HR = 0.94, P = .89, respectively). Figure 3A,B shows the observed NRM and overall survivals for nonmyeloablative and myeloablative patients, together with the hypothetical outcome for nonmyeloablative patients after adjustment for pretransplantation differences. Further, the HR for progression-free survival among nonmyeloablative and myeloablative patients with an HCT-CI score of 0 was not statistically significant (1.44; P = .40).
Cumulative incidence estimates of NRM and Kaplan–Meier survival estimates among nonmyeloablative compared with myeloablative patients with lymphoma or CLL and HCT-CI score of 0. No statistically significant differences were found between outcomes among nonmyeloablative and myeloablative patients. Further, there were no statistically significant differences between the observed outcomes among myeloablative patients and the hypothetical outcomes among nonmyeloablative patients after adjustment for pretransplantation variables including age, previous HCT, previous regimens, previous CMV infection, type and stage of malignancy, donor type, and stem-cell source. — indicates observed outcomes for myeloablative patients;  , observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
, observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
Cumulative incidence estimates of NRM and Kaplan–Meier survival estimates among nonmyeloablative compared with myeloablative patients with lymphoma or CLL and HCT-CI score of 0. No statistically significant differences were found between outcomes among nonmyeloablative and myeloablative patients. Further, there were no statistically significant differences between the observed outcomes among myeloablative patients and the hypothetical outcomes among nonmyeloablative patients after adjustment for pretransplantation variables including age, previous HCT, previous regimens, previous CMV infection, type and stage of malignancy, donor type, and stem-cell source. — indicates observed outcomes for myeloablative patients;  , observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
, observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
We also investigated inpatient hospitalization days, outpatient follow-up days, and outpatient clinic visits among the 2 patient cohorts (Table 3). No differences were observed in the mean number of hospitalization (P = .53) and outpatient follow-up days (P = .06). Nonmyeloablative patients had a statistically significant lower median number of inpatient days (P = .004) and, conversely, a higher median number of total outpatient follow-up days (P < .001). Mean and median numbers of outpatient days associated with clinic visits (P = .02 and .03, respectively) were statistically significantly higher among nonmyeloablative patients. When we calculated the mean and median percentages of outpatient days associated with clinic visits among the total outpatient days for each patient, no statistically significant differences were found between the 2 cohorts (P = .46 and .41, respectively). Overall, 9 nonmyeloablative patients had inpatient hospital stays longer than 30 days because of severe acute GVHD (n = 5), infections (n = 3), and pathologic femur fracture (n = 1).
Inpatient and outpatient (OP) days and actual clinic visits among nonmyeloablative compared with myeloablative patients with lymphoma or CLL and HCT-CI scores of 0
| Parameters . | Nonmyeloablative patients, mean days plus or minus SD . | Myeloablative patients, mean days plus or minus SD . | P* . | Nonmyeloablative patients, median no. days (range) . | Myeloablative patients, median no. days (range) . | P* . |
|---|---|---|---|---|---|---|
| Inpatient hospitalization | 19.0 ± 43.2 | 23.4 ± 15.7) | .53 | 3.5 (0-232) | 22 (0-61) | .004 |
| OP follow-up | 106 ± 46.6 | 73.6 ± 82.0 | .06 | 97 (19-241) | 64 (0-470) | <.001 |
| OP actual clinic visits | 42.8 ± 21.0 | 27.9 ± 13.3 | .02 | 45 (7-94) | 31 (2-50) | .03 |
| Clinic visits per OP follow-up days | 44.4 ± 16.5 | 38.9 ± 20.8 | .46 | 43.9 (14.8-71.4) | 39.5 (7.2-80.6) | .41 |
| Parameters . | Nonmyeloablative patients, mean days plus or minus SD . | Myeloablative patients, mean days plus or minus SD . | P* . | Nonmyeloablative patients, median no. days (range) . | Myeloablative patients, median no. days (range) . | P* . |
|---|---|---|---|---|---|---|
| Inpatient hospitalization | 19.0 ± 43.2 | 23.4 ± 15.7) | .53 | 3.5 (0-232) | 22 (0-61) | .004 |
| OP follow-up | 106 ± 46.6 | 73.6 ± 82.0 | .06 | 97 (19-241) | 64 (0-470) | <.001 |
| OP actual clinic visits | 42.8 ± 21.0 | 27.9 ± 13.3 | .02 | 45 (7-94) | 31 (2-50) | .03 |
| Clinic visits per OP follow-up days | 44.4 ± 16.5 | 38.9 ± 20.8 | .46 | 43.9 (14.8-71.4) | 39.5 (7.2-80.6) | .41 |
Student t test or Wilcoxon rank sum test.
Patients with HCT-CI scores of 1 or more
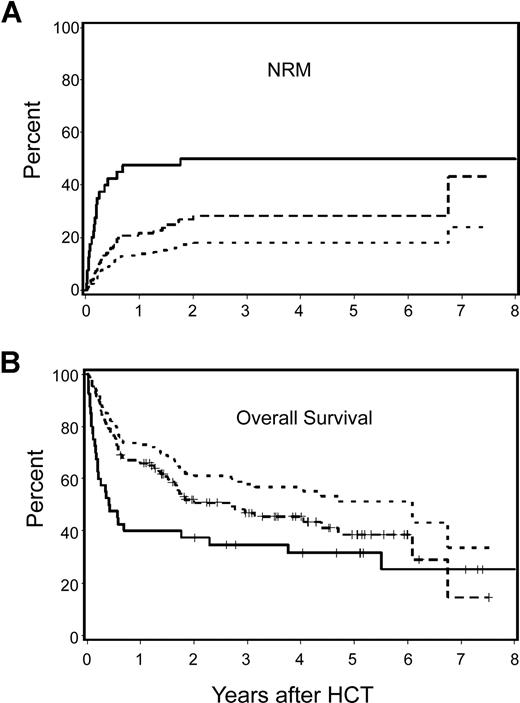
Among patients with HCT-CI scores of 1 or more, those receiving nonmyeloablative conditioning had a statistically significantly lower incidence of NRM (28% versus 50%; HR: .47, P = .009) and a better overall survival rate (47% versus 35%; HR: 0.63; P = .04) compared with myeloablative patients (Figure 4A,B). After adjustment for other pretransplantation risk factors, differences became more significant for NRM (HR: 0.19; P < .001) and overall survival (HR: 0.33; P = .007). Relapse risks were not statistically significantly different among the 2 cohorts before (HR: 1.54; P = .22) or after adjustment for other risk factors (HR: 1.96; P = .26). As a result, HR for progression-free survival was better among nonmyeloablative patients when we adjusted for other risk factors (unadjusted HR: 0.78, P = .26 and adjusted HR: 0.47, P = .01).
Cumulative incidence estimates of NRM and Kaplan–Meier survival estimates among nonmyeloablative compared with myeloablative patients with lymphoma or CLL and HCT-CI score of 1 or more. Nonmyeloablative patients had statistically significantly better outcomes compared with those of myeloablative patients. Differences between groups increased after adjustment for pretransplantation variables including age, previous HCT, previous regimens, previous CMV infection, type and stage of malignancy, donor type, and stem-cell source. — indicates observed outcomes for myeloablative patients;  , observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
, observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
Cumulative incidence estimates of NRM and Kaplan–Meier survival estimates among nonmyeloablative compared with myeloablative patients with lymphoma or CLL and HCT-CI score of 1 or more. Nonmyeloablative patients had statistically significantly better outcomes compared with those of myeloablative patients. Differences between groups increased after adjustment for pretransplantation variables including age, previous HCT, previous regimens, previous CMV infection, type and stage of malignancy, donor type, and stem-cell source. — indicates observed outcomes for myeloablative patients;  , observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
, observed outcomes for nonmyeloablative patients; and …, adjusted outcomes for nonmyeloablative patients.
Outcomes of patients with indolent versus aggressive diseases
We adjusted the previous comparisons for indolent versus aggressive diseases and found no change in the association between conditioning intensity and any of the outcomes. We also evaluated the interaction between indolent versus aggressive diseases and conditioning intensity. Patients with indolent diseases had higher NRM (HR: 3.16; P = .02) and higher mortality (HR: 2.02; P = .07) after myeloablative compared with nonmyeloablative conditioning. Similarly, patients with aggressive diseases had higher NRM (HR: 4.46; P = .007) and mortality (HR: 2.21; P = .01) after myeloablative conditioning. Conversely, relapse risks were lower after myeloablative conditioning among patients with indolent (HR: 0.56; P = .33) or aggressive diseases (HR: 0.51; P = .11), although these differences were not statistically significant. There were no statistically significant interactions between disease histology and conditioning intensity for NRM (P = .56), mortality (P = .84), or relapse (P = .88)
We also assessed the impact of grade of NHL on outcomes after myeloablative and nonmyeloablative HCT. Relapse risks were not statistically significantly different between the 2 cohorts among patients with indolent (HR: 1.97; P = .37) or aggressive NHL (HR: 0.72; P = .50). Conversely, statistically significant higher NRM was observed after myeloablative conditioning among patients with both indolent (HR: 3.35; P = .07) and aggressive NHL (HR: 6.95; P = .006). Consequently, patients with both indolent and aggressive NHL had statistically significant higher mortality after myeloablative than nonmyeloablative conditioning (HR: 3.34, P = .02 and HR: 3.02, P = .003), respectively.
Discussion
Patients with CLL or lymphoma with poor prognostic features44 or resistance to salvage chemotherapy and patients with recurrent or progressive malignancy after autologous HCT1,2 have no potentially curative treatment except allogeneic HCT. Conventional allogeneic HCT with high-dose conditioning has been restricted to younger and medically fit patients out of concern for high NRM. This restriction has limited therapeutic options because patients with lymphoid malignancies have median ages of 65 to 70 years45 at diagnoses, and the prevalence of comorbidity often exceeds 50%.46 The advent of reduced-intensity conditioning regimens, which promised less NRM, has expanded the pool of patients with lymphoid malignancies offered allogeneic HCT. Efforts are needed to risk-stratify patient care and determine the optimal conditioning regimens for individual patients with lymphoid malignancies. Our results of this retrospective study suggest that increased conditioning dose intensity does not lower relapse rates but does result in higher NRM, particularly among patients with comorbidities.
The finding of comparable relapse rates is complicated by differences in pretransplantation characteristics, including more complete or partial remissions among nonmyeloablative patients compared with their myeloablative counterparts (46% vs 25%). However, relapse risks among the 2 cohorts were not statistically significantly different even after adjustment for differences in disease status and other variables. These findings persisted even after stratifying patients into indolent versus aggressive lymphoma/CLL. This suggests that graft-versus-leukemia effects are the most important in controlling disease relapse among patients with CLL or lymphoma. However, although relapse rates were comparable, patients with high comorbidity scores given high conditioning intensity had increased NRM and worse overall survival.
Patients with an HCT-CI score of 0 tolerated either conditioning regimen equally well and had comparable outcomes. Further, they did not differ with respect to median and mean percentages of clinic visits in relation to their total outpatient stays. Nonmyeloablative patients were generally older and more intensively pretreated than myeloablative patients. It was unknown whether there would be less frequent clinic visits and possible survival benefits if young, less frequently treated patients were given nonmyeloablative and not myeloablative conditioning. Within the limitations of this retrospective analysis, adjustment for pretransplantation differences did not demonstrate a statistically significant survival benefit for nonmyeloablative conditioning among patients with an HCT-CI score of 0. Results suggested that younger patients (< 60 years of age) with an HCT-CI score of 0 could be enrolled in prospective randomized trials designed to test which conditioning regimen would be appropriate.
In our series, myeloablative patients with CLL or lymphoma who had an HCT-CI score of 0 experienced relatively low NRM (15% at 3 years) compared with the overall historical experience, which showed NRM ranging from 40% to 61%.7,,,–11 Conversely, a 3-year NRM of 50% was observed among myeloablative patients with HCT-CI scores of 1 or more, which was consistent with the reported experience after conventional HCT for lymphoid malignancies.7,,,–11 The high NRM in these reports might have been partly attributable to the effects of unidentified comorbidities. Nonmyeloablative patients with similar HCT-CI scores had a 3-year NRM of 28%, which agreed with published reports by us13,,,–17 and others.18,,,,,,,,,,–29 The survival benefits for nonmyeloablative compared with myeloablative patients with HCT-CI scores of 1 or more gained additional statistical significance after adjustment for pretransplantation differences, in particular age, suggesting that young patients with comorbidities could further benefit from nonmyeloablative conditioning.
The HCT-CI was designed to reliably identify even minimal degrees of organ impairment.41 HCT-CI scores of 1 or more were assigned to 70% and 59% of nonmyeloablative and myeloablative patients, respectively, and this difference persisted even after excluding those patients who had contributed to the development of the index (69% vs 54%, respectively). More than half of myeloablative patients had comorbidities because subclinical organ impairments detected by the HCT-CI (eg, pulmonary function impairments in the range of 66%-80% of predicted values) were not considered as exclusion criteria when these patients were enrolled. Thus, future use of the HCT-CI might allow for more appropriate assignment of patients to different intensity conditioning regimens
In conclusion, patients without comorbidities (34% of our total population) tolerated nonmyeloablative and myeloablative conditioning regimens equally well, whereas those with comorbidities (whether young or old) had better survival after reduced-intensity conditioning. Our results from this retrospective study suggested that conditioning regimen intensity played a minor role in disease control while contributing to excessive NRM for patients with CLL or lymphoma who have comorbidities. For patients with no comorbidities and indolent or aggressive diseases, a randomized study might answer the question whether nonmyeloablative or myeloablative conditioning would be the most appropriate regimen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank data coordinators Gary Schoch, Chris Davis, Jennifer Freese, and Heather Hildebrant and study nurses Mary Hinds, John Sedgwick, Michelle Bouvier, and Joanne Greene for their invaluable help in making the study possible. Bonnie Larson, Helen Crawford, Karen Carbonneau, and Sue Carbonneau provided assistance with manuscript preparation. We also thank all the transplantation teams of the Seattle Cancer Care Alliance.
This work was supported in part by grants CA78902, CA18029, CA15704, and HL088021 from the National Institutes of Health, Bethesda, MD, and in part by the Paros Family Fund.
National Institutes of Health
Authorship
Contribution: M.L.S. designed the overall study, collected data, analyzed and interpreted the data, and wrote the manuscript. B.E.S. performed the statistical analysis. D.G.M. contributed study patients, contributed to data interpretation, and edited the manuscript. B.M.S. contributed study patients and contributed to study design. P.J.M. contributed to study design and edited the manuscript. R.S. contributed to study design and data interpretation and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohamed Sorror, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: msorror@fhcrc.org.