Previous studies have documented that, in malignant B cells, rituximab elicits a complex and not yet totally understood signaling network contributing to its antitumor effect. In this context, we investigated the role of protein kinase C ζ (PKCζ), an atypical PKC isoform, in the cellular response to rituximab. We found that follicular lymphoma cells displayed an increase in PKCζ expression and activity levels, compared with nonmalignant B cells, and that this enzyme was a critical regulator of the classical MAPK module by stimulating Raf-1 kinase activity. PKCζ appeared to be a significant contributor of abnormal mTOR regulation in follicular lymphoma cells through a MAPK-dependent mechanism. Rituximab was found to inhibit the PKCζ/MAPK/mTOR module in these cells but not in other B-cell lymphomas. Importantly, the expression of a constitutively active form of PKCζ resulted in an efficient protection of these cells toward rituximab. Altogether, our study describes a new regulatory component of mTOR pathway in follicular cell lymphoma and demonstrates that PKCζ is a target for rituximab. Therefore, PKCζ could represent an important parameter for rituximab efficacy and a promising target for future targeted therapy in follicular lymphoma.
Introduction
Rituximab (RTX) is an anti-CD20 mouse/human chimeric IgG1-κ antibody that targets the CD20 antigen found on the surface of malignant and normal B cells. RTX used as a single agent has demonstrated efficacy in patients with various lymphoid malignancies, including indolent and aggressive forms of non-Hodgkin lymphoma (NHL), as well as in chronic lymphocytic leukemia (CLL). Moreover, it has some therapeutic activity in antibody-based autoimmune diseases.1 However, the most significant contribution of this new agent is that it greatly improves the efficacy of chemotherapy in the treatment of various forms of lymphoid neoplasias, including follicular lymphoma (FL), mantle cell lymphoma (MCL), or diffuse large B-cell lymphoma (DLBCL).2,–4
The mechanism by which RTX facilitates tumor eradication by chemotherapy remains unknown. Several hypotheses have been raised, including a simple additive effect or a true synergistic mechanism mediated by RTX-induced activation of proapoptotic signals. Indeed, as documented by Bonavida and coworkers, RTX-mediated CD20 engagement resulted in reduced expression of Bcl-2 or Bcl-xL, enhanced expression of Fas death receptor, as well as in the negative regulation of NF-κB or MAPK signaling pathways, depending on the cellular models (Jazirehi et al5 and Vega et al6 ). In parallel, our group has described that RTX can also activate the sphingomyelin cycle, a well-defined metabolic process resulting in the accumulation of ceramide, a lipid second messenger that primes cell to inhibition of proliferation and/or apoptosis.7
Based on these findings, we raised the possibility that, in the context of RTX-treated FL cells, ceramide targets a common regulator of one or several of these prosurvival signaling pathways. This question presents an evident practical interest since, if this is the case, expression or function of this presumed regulator might greatly influence the clinical benefit of RTX in FL. In this perspective, we speculated that this regulator could be the protein kinase C zeta (PKCζ), an atypical form of PKC. Indeed, 2 lines of evidence argue for this hypothesis. First, PKCζ is indeed a target for ceramide.8,9 Second, at least in other cellular systems, PKCζ is a positive regulator of classical MAPK module by influencing MEK activity,10 NF-κB pathway by interacting directly with IKK-β,11 and Bcl-2 expression9 through AP-1.12,13
Therefore, we hypothesized that, in FL cells, PKCζ is a target for RTX, and that reduced PKC activity plays an important role in RTX inhibitory effect through inhibition of prosurvival signaling pathways. We show herein for the first time that, at least in FL cells, treatment with RTX results in the disruption of a PKCζ-MAPK module and the subsequent inhibition of mTOR, an essential component of FL cell survival, as we have documented recently.14 Moreover, the present study provides direct evidence that PKCζ exerts a potent protective effect against RTX. Finally, it appears that, in FL cells, PKCζ is both a target for RTX and a regulator of its antileukemic effect.
Methods
Cell lines and reagents
RL and Karpas-422 are FL cells carrying the t(14;18) and were obtained from the ATCC (American Type Culture Collection, Rockville, MD) and DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, respectively. MEC-2 and JVM-3 are B-CLL cell lines obtained from DSMZ. Granta 519 and Rec-1 are MCL cells carrying t(11;14), and were a gift of Dr Pelletier and Dr Hermine (Necker, Paris, France). These cell lines, except Granta 519 and MEC-2, were cultured at 37°C in 5% CO2 in RPMI supplemented with 10% fetal calf serum (FCS), glutamine (2 mM), streptomycin (10 μg/mL), and penicillin (200 U/mL) (Invitrogen, Cergy Pontoise, France). DEAU and LIB derived from DLBCL were kindly provided by Prof Delsol (INSERM U563, Toulouse, France). These cell lines as well as Granta 519 and MEC-2 were cultured at 37°C in 5% CO2 in IMDM supplemented with 20% FCS, glutamine (2 mM), streptomycin (10 μg/mL), and penicillin (200 U/mL). Cells were treated at exponential phase. Fresh CLL cells were collected from peripheral blood of B-CLL patients after informed consent was obtained in accordance with the Declaration of Helsinki (Service d'hématologie, CHU Purpan, Toulouse, France) and separated by Ficoll-Hypaque density gradient (GE Healthcare, Orsay, France). For each sample, flow cytometric analysis revealed that CLL cells displayed a common CD19+ CD5+ B-CLL phenotype. Myelin basic protein (MBP) was purchased from Sigma Aldrich (St Quentin Fallavier, France). PKCζ-pseudosubstrate was obtained from Millegen (Labège, France). RTX was kindly provided by Roche (Hertfordshire, United Kingdom). Nonimmune human IgG fraction used for control was purchased from Sigma Aldrich.
PKCζ transfection
Karpas-422 or RL cells (107) were stably transfected by Amaxa nucleofactor technology (Cologne, Germany) with 9 μg plasmids pcDNA3, DN-PKCζ, or CA-PKCζ. Cells were then cultured in complete medium, and after 5 days, positive cells were treated with 0.5 mg/mL G418 (Invitrogen) for 7 days. Then, clonage was performed by plating cells in 96 wells at 0.3 cell/well. For Karpas-422 cells, we analyzed 2 clones for pcDNA3 (2E10, 3G7), 2 clones for DN-PKCζ (5E9 and 6E5), and 2 clones for CA-PKCζ (7B2, 8C6). For RL cells, we analyzed 1 clone for pcDNA3 (1G5) and 2 clones for CA-PKCζ (6F9, 7E5). The effect of loss or gain of PKCζ was analyzed on MAPK pathway and mTOR activity as described in “Western blot analysis” and “Immunokinase assays.” DN-PKCζ and CA-PKCζ plasmids were kindly provided by Dr Lehoux (Sherbrooke, QC).
Isolation of normal B lymphocytes
After isolation of peripheral blood mononuclear cells from healthy donors by Ficoll-Hypaque density-gradient centrifugation, negative selection of B lymphocytes was performed with the B-cell isolation kit II human according to the manufacturer's instructions (Miltenyi Biotec, Paris, France). The purity of the enriched B cells (around 90%) was evaluated by flow cytometry using a CD19-PE antibody (BD Biosciences, le Pont de Claix, France).
Western blot analysis
Cells or tissue sections (1 × 106) of FL specimens were washed in PBS before addition of Laemmli sample buffer (2% SDS; 10% glycerol; 5% β-mercaptoethanol; 60 mM Tris, pH 6.8; 0.001% bromophenol blue). Samples were sonicated for 15 to 20 seconds and boiled for 5 minutes at 95°C. Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane (Hybond C-extra; Amersham Biosciences, Cergy Pontoise, France). Nonspecific binding sites were blocked in PBS 0.1% Tween-20 and 3% BSA. Membranes were then incubated with primary antibody overnight at 4°C, and antigen-antibody complexes were detected by enhanced chemiluminescence system ECL Kit (Amersham Pharmacia Biotech, Saclay, France). All experiments were carried out independently at least 3 times. The different antibodies used in this study were as follows: anti-p70S6K, anti–phospho-p70S6K (Thr389), anti–phospho-PKCζ, anti-MEK, anti–phospho-MEK (Ser217/221), and anti–phospho-ERK (Thr202/Tyr204) (all from Ozyme, St Quentin Yvelines, France). Anti-PKCζ and anti-ERK antibodies were purchased from Tebu (Le Perray en Yvelines, France). Horseradish peroxidase–conjugated secondary antibodies against mouse and rabbit immunoglobulins were obtained from Jackson Immunoresearch Laboratories (Immunotech, Marseille, France).
Immunokinase assays
Cells were lysed in buffer containing 20 mM HEPES (pH 7.4), 12 mM EDTA, 250 mM NaCl, 1% NP-40, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1 mM PMSF, 0.5 μg/mL benzamidine, and 1 mM DTT. Proteins (500 μg) were immunoprecipitated with 3 μg anti-PKCζ, anti–Raf-1 (C-12), or anti-ERK1 (C16) antibodies (Tebu) overnight at 4°C. Immune complexes were collected by incubation with protein A/G Sepharose beads (Amersham Biosciences, Orsay, France) for 2 hours at 4°C. The beads were extensively washed with lysis buffer and kinase buffer (20 mM HEPES [pH 7.4], 1 mM DTT, 25 mM NaCl). Kinase assays were performed 15 minutes at 30°C using MBP as substrate in 20 mM HEPES (pH 7.4), 10 mM MgCl2, 1 mM DTT, and 0.37 MBq (10 μCi) (γ32P) ATP (ICN, Orsay, France). Reaction was stopped with the addition of 15 μL 2 × SDS sample buffer and boiled for 5 minutes. Proteins were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membrane. Phosphorylated MBP was visualized by a phosphorimager (Molecular Dynamics, GE Healthcare, Orsay, France).
Clonogenic assays
Cells (5 × 103) were incubated with increased doses of RTX in 35-mm Petri dishes and grown in H4230 Stem Cell Technologies methyl cellulose medium (Stem Cell Technologies, Vancouver, BC) in a humidified CO2 incubator (37°C). After 7 days, the B-lymphoma colonies (more than 20 cells) were scored under an inverted microscope. Clonogenic efficiency was measured by dividing clone number per number of cells plated.
Results
PKCζ is constitutively activated in follicular lymphoma cells
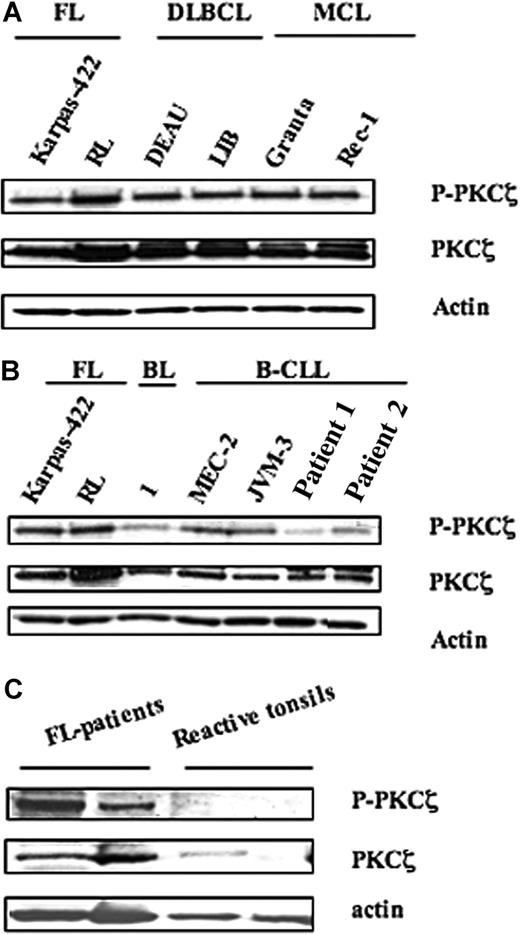
PKCζ activity was measured by Western blot analysis using an antibody directed against the phosphorylated form of PKCζ (Thr410/403), based on previous studies, including ours, which established the reliability of this method compared with immunokinase assay.15,–17 As shown in Figure 1, these experiments revealed important variations in both expression and activity of PKCζ among the different B-cell populations studied. Thus, PKCζ appeared to be highly expressed in B-cell lymphomas, including FL (Karpas-422 and RL), DLBCL (DEAU and LIB), and MCL (Granta and Rec-1) (Figure 1A). However, PKCζ expression was much lower in chronic lymphocytic leukemia (CLL) cells from patients (patient 1 and patient 2) or CLL cell lines (MEC-2 and JVM-3) and in normal B cells (BL) (Figure 1B). In malignant B cells, PKCζ activity level correlated with PKCζ expression. Thus, PKCζ activity was much higher in FL cells than in normal B cells. Moreover, Western blot analysis revealed an increase in both PKCζ expression and activity in FL tissue samples (n = 2), compared with tonsil reactive tissue (n = 2). These results suggest that PKCζ is abnormally regulated not only in FL cells but also in other malignant B cells.
Expression and activity of PKCζ in NHL cell lines. PKCζ expression and activity were evaluated by Western blot using anti-PKCζ and anti–phospho-PKCζ (Thr410/403) in (A) FL (Karpas-422 and RL), DLBCL (DEAU and LIB), and MCL (Granta and Rec-1) cell lines; (B) FL cell lines compared with B lymphocytes from healthy donor (BL), B-CLL cell lines (MEC-2 and JVM-3), and B-CLL patients (patient 1 and patient 2); and (C) tissue extracts from either FL specimen or tonsils. For panels A,B, results are representative of 3 independent experiments.
Expression and activity of PKCζ in NHL cell lines. PKCζ expression and activity were evaluated by Western blot using anti-PKCζ and anti–phospho-PKCζ (Thr410/403) in (A) FL (Karpas-422 and RL), DLBCL (DEAU and LIB), and MCL (Granta and Rec-1) cell lines; (B) FL cell lines compared with B lymphocytes from healthy donor (BL), B-CLL cell lines (MEC-2 and JVM-3), and B-CLL patients (patient 1 and patient 2); and (C) tissue extracts from either FL specimen or tonsils. For panels A,B, results are representative of 3 independent experiments.
Rituximab inhibited PKCζ activity
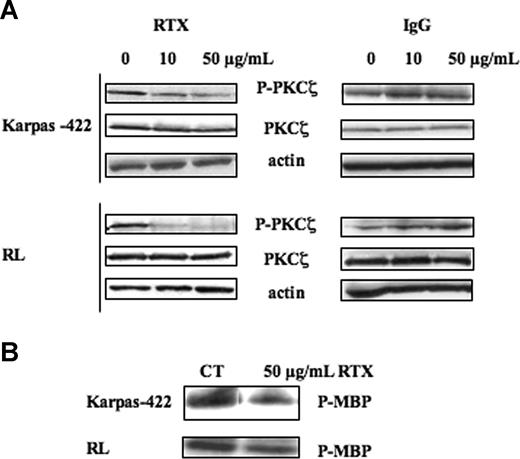
As shown in Figure 2, in FL cells, treatment with RTX for 24 hours resulted in a reduction of PKCζ activity in a dose-dependent manner as attested not only by Western blot using phospho-PKCζ antibody (Figure 2A left panel) but also by immunokinase assay (Figure 2B). The latter revealed that, at the maximum concentration of RTX (50 μg/mL), PKCζ inhibition was as potent as 40% reduction. Importantly, following treatment with RTX, PKCζ expression level remained unchanged, suggesting that the antibody acted specifically against enzymatic activity. RTX-induced PKCζ inhibition was more pronounced in RL cells, compared with Karpas-422. In RL cells, PKCζ inhibition was effective for RTX dose as low as 10 μg/mL. This concentration is relevant with the clinical use of RTX in humans. To test the specificity of RTX, we treated FL cells with human IgG. In these conditions, we found no inhibition of phospho-PKCζ or PKCζ expression even at 50 μg/mL (Figure 2A right panel). Interestingly, RTX had no impact on PKCζ activity in other malignant B cells. Indeed, in repeated experiments (n = 5), we were unable to detect any reduction in PKCζ activity in MCL and DLBCL treated with RTX although used at higher concentrations (data not shown). These results suggest that rituximab inhibits PKCζ activity, and that this effect is restricted to FL cells.
Effect of RTX on PKCζ activity. (A) FL cell lines were treated or not with RTX or IgG at 10 and 50 μg/mL for 24 hours. PKCζ activity was then evaluated by Western blot using anti–phospho-PKCζ antibody. PKCζ was used as control of protein expression. Results are representative of 3 independent experiments. (B) FL cell lines were treated or not with RTX at 50 μg/mL for 24 hours. PKCζ activity was then evaluated by immunokinase assay as described in “Immunokinase assays.” Results are representative of 3 independent experiments.
Effect of RTX on PKCζ activity. (A) FL cell lines were treated or not with RTX or IgG at 10 and 50 μg/mL for 24 hours. PKCζ activity was then evaluated by Western blot using anti–phospho-PKCζ antibody. PKCζ was used as control of protein expression. Results are representative of 3 independent experiments. (B) FL cell lines were treated or not with RTX at 50 μg/mL for 24 hours. PKCζ activity was then evaluated by immunokinase assay as described in “Immunokinase assays.” Results are representative of 3 independent experiments.
PKCζ inhibition mediates the antagonistic effect of rituximab on MAPK pathway
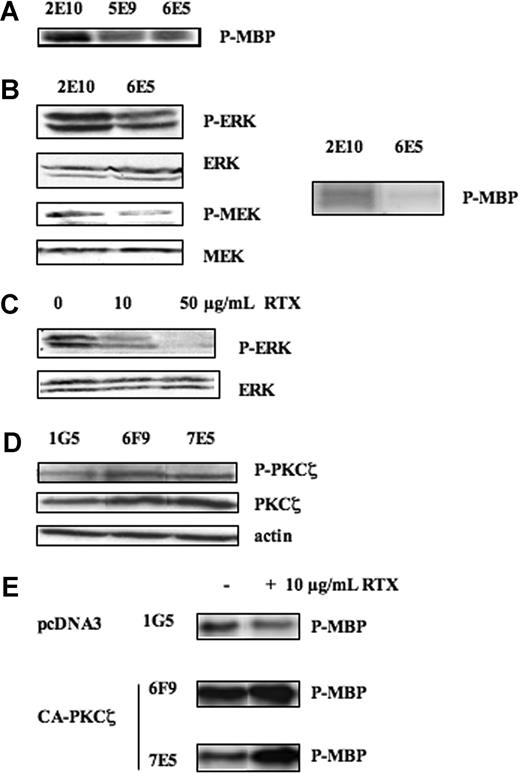
Previous studies have documented that PKCζ may regulate ERK by operating at a different step of the classical MAPK module (ie, upstream Raf-1 through RKIP,18 Raf-1,19 or even MEK10 ). The mechanism by which PKCζ interferes with MAPK pathway appears to be highly dependent on the cellular model, but the final inhibitory effect on ERK phosphorylation has been documented so far in mouse fibroblasts,20 Cos-7,21 and normal murine B cells.22 Therefore, we first evaluated the role of PKCζ as a significant regulator of ERK phosphorylation in FL cells. As a matter of fact, the stable transfection of DN-PKCζ in Karpas-422 cells (clone 6E5), which resulted in a decrease of PKCζ activity (Figure 3A), induced an inhibition of Raf-1 activity (Figure 3B right panel) as well as MEK and ERK phosphorylation (Figure 3B left panel), compared with the empty vector (clone 2E10). Bonavida and coworkers have described that in the Ramos Burkitt lymphoma cells, treatment with RTX resulted in a significant reduction in the phosphorylation level of MAPK components, including Raf-1, MEK, and ERK (Jazirehi et al23 ). As shown in Figure 3C, this effect was also observed in FL cells. Therefore, it was tempting to speculate that, in RTX-treated cells, ERK inhibition was due to reduced PKCζ activity. To address this question, we evaluated the influence of constitutively active mutant form of PKCζ (CA-PKCζ) in RL cells (clones 6F9 and 7E5) on RTX-mediated ERK inhibition. As expected, expression of CA-PKCζ resulted in increased PKCζ activity (Figure 3D). Moreover, as shown in Figure 3E, in these cells, RTX was no more able to repress ERK activity as revealed by immunokinase assay. These results suggest that PKCζ inhibition governs the inhibitory effect of rituximab on the MAPK pathway.
Effect of RTX on MAPK pathway. (A) PKCζ activity was evaluated on Karpas-422 stably transfected with pcDNA3 (2E10) or DN-PKCζ (6E5) by immunokinase assay as described in “Immunokinase assays.” (B) (Left panel) The effect of PKCζ inhibition on ERK and MEK activities was evaluated on Karpas-422–transfected clones (2E10 or 6E5) by Western blot using anti–phospho-ERK (Thr202/Tyr204) and anti–P-MEK (Ser217/221) antibodies. MEK and ERK were used as control of protein expression. (Right panel) The effect of PKCζ inhibition on Raf-1 activity was evaluated on 2E10 and 6E5 clones by immunokinase assay as described in “Immunokinase assays.” Results are representative of 3 independent experiments. (C) RL cell lines were treated or not with RTX at 10 and 50 μg/mL for 24 hours, and then ERK activity was evaluated as described in panel B. ERK was used as control of protein expression. Results are representative of 3 independent experiments. (D) PKCζ activity and expression were evaluated on RL stably transfected with pcDNA3 (1G5) and CA-PKCζ (6F9, 7E5) by Western blot using anti–phospho-PKCζ antibody and anti-PKCζ antibodies, respectively. Actin was used as control of protein expression. (E) ERK activity was evaluated by immunokinase assay on RL stably transfected with pcDNA3 (1G5) and CA-PKCζ (6F9, 7E5) treated or not with RTX at 10 μg/mL.
Effect of RTX on MAPK pathway. (A) PKCζ activity was evaluated on Karpas-422 stably transfected with pcDNA3 (2E10) or DN-PKCζ (6E5) by immunokinase assay as described in “Immunokinase assays.” (B) (Left panel) The effect of PKCζ inhibition on ERK and MEK activities was evaluated on Karpas-422–transfected clones (2E10 or 6E5) by Western blot using anti–phospho-ERK (Thr202/Tyr204) and anti–P-MEK (Ser217/221) antibodies. MEK and ERK were used as control of protein expression. (Right panel) The effect of PKCζ inhibition on Raf-1 activity was evaluated on 2E10 and 6E5 clones by immunokinase assay as described in “Immunokinase assays.” Results are representative of 3 independent experiments. (C) RL cell lines were treated or not with RTX at 10 and 50 μg/mL for 24 hours, and then ERK activity was evaluated as described in panel B. ERK was used as control of protein expression. Results are representative of 3 independent experiments. (D) PKCζ activity and expression were evaluated on RL stably transfected with pcDNA3 (1G5) and CA-PKCζ (6F9, 7E5) by Western blot using anti–phospho-PKCζ antibody and anti-PKCζ antibodies, respectively. Actin was used as control of protein expression. (E) ERK activity was evaluated by immunokinase assay on RL stably transfected with pcDNA3 (1G5) and CA-PKCζ (6F9, 7E5) treated or not with RTX at 10 μg/mL.
PKCζ is a regulator of mTOR in FL cells
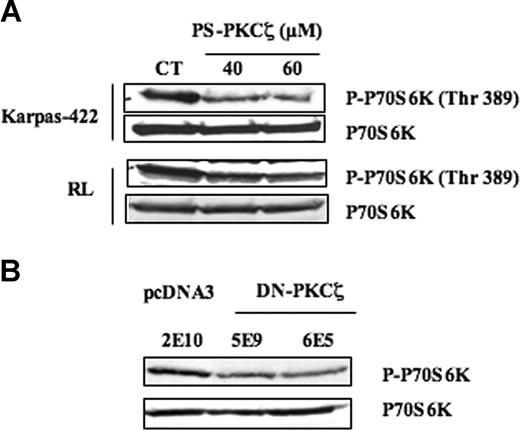
In a recent study, we described that mTOR pathway is constitutively activated in FL cells such as Karpas-422 and RL, as well as in FL lymph node biopsy specimens.14 We also showed that FL cells were sensitive to rapamycin to the same extent as MCL.24 In the same study, we propose that, in these cells, at least 4 distinct signaling components, including PI3K/Akt, PLD/phosphatidic acid (PA), Syk, and MAPK, exert a redundant stimulating function converging toward mTOR and its main target, the p70 S6 kinase (p70S6K). Since PKCζ appeared to regulate MAPK, we speculated that PKCζ could also regulate mTOR through a MAPK-dependent mechanism. Indeed, treatment with PKCζ pseudosubstrate inhibitory peptide (PS-PKCζ) resulted in a decrease in p70S6K phosphorylation level, the magnitude of this effect being more pronounced in Karpas-422 than in RL cells (Figure 4A). At the concentrations used (40 and 60 μM), we have previously found that PS-PKCζ induced a dramatic and specific reduction in basal PKCζ activity as demonstrated with purified PKCζ in a cell-free system.25 Importantly, p70S6K phosphorylation level has been evaluated using an antibody directed against the phosphorylated site specifically targeted by mTOR (Thr389). To strengthen the role of PKCζ as a mTOR regulator, we evaluated the influence of DN-PKCζ on mTOR pathway. In DN-PKCζ cells (clones 5E9 and 6E5), we found that p70S6K phosphorylation level was much lower, compared with cells transfected with the empty vector (clone 2E10) (Figure 4B). These results suggest that PKCζ is indeed a regulator of mTOR in FL cells.
Effect of PKCζ on mTOR. (A) The effect of PKCζ inhibition on mTOR activity was evaluated by Western blot using anti–phospho-p70S6K (Thr389) antibody in FL cells treated with PS-PKCζ at 40 to 60 μM for 30 minutes. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments. (B) The effect of PKCζ inhibition on mTOR activity was evaluated in Karpas-422 cell lines stably transfected with pcDNA3 (2E10) or DN-PKCζ (5E9, 6E5). p70S6K was used as control of protein expression. Results are representative of 3 independent experiments.
Effect of PKCζ on mTOR. (A) The effect of PKCζ inhibition on mTOR activity was evaluated by Western blot using anti–phospho-p70S6K (Thr389) antibody in FL cells treated with PS-PKCζ at 40 to 60 μM for 30 minutes. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments. (B) The effect of PKCζ inhibition on mTOR activity was evaluated in Karpas-422 cell lines stably transfected with pcDNA3 (2E10) or DN-PKCζ (5E9, 6E5). p70S6K was used as control of protein expression. Results are representative of 3 independent experiments.
PKCζ inhibition mediates the antagonistic effect of rituximab on mTOR pathway
Therefore, since RTX acts as a PKCζ inhibitor, we hypothesized that RTX could also interfere with mTOR pathway. Indeed, treatment with RTX for 24 hours resulted in a dose-dependent decrease of mTOR activity in both Karpas-422 and RL cells (Figure 5A). Moreover, the expression of CA-PKCζ in RL (Figure 5B) or in Karpas-422 (data not shown) cells prevented RTX-induced decrease of p70S6K phosphorylation. These results showed that RTX inhibited mTOR pathway in FL cells, and that this effect was efficiently regulated by PKCζ. Based on these findings, we investigated whether RTX could negatively regulate mTOR pathway in other human B-cell lymphomas. We found that RTX had no effect on p70S6K phosphorylation level in MCL (Granta and Rec-1) or in DLBCL (DEAU and LIB) (data not shown). This result was expected since, as described above, in these cells, RTX was unable to inhibit PKCζ. Based on our previous study, we also investigated whether RTX could interfere with the other components of mTOR regulation in FL cells. However, we found that treatment with RTX induced no significant decrease in the activities of PLD, Syk, and PI3K (data not shown). Altogether, these results suggest that, in FL cells, rituximab inhibits mTOR pathway through PKCζ inhibition.
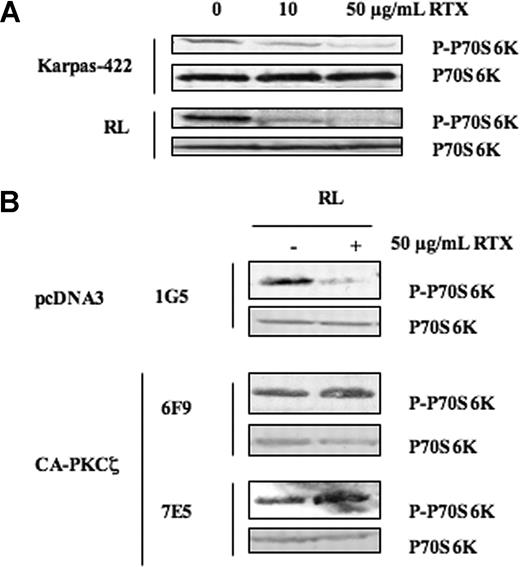
Role of PKCζ on mTOR inhibition induced by RTX in FL cell lines. (A) FL cell lines were treated or not with RTX at 10 and 50 μg/mL for 24 hours, and then mTOR activity was evaluated by visualizing p70S6K phosphorylation on Thr389. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments. (B) The effect of PKCζ on mTOR inhibition induced by RTX was evaluated by Western blot using anti–phospho-p70S6K antibody in RL cells stably transfected with pcDNA3 (1G5) or CA-PKCζ (6F9, 7E5) treated with RTX at 50 μg/mL. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments.
Role of PKCζ on mTOR inhibition induced by RTX in FL cell lines. (A) FL cell lines were treated or not with RTX at 10 and 50 μg/mL for 24 hours, and then mTOR activity was evaluated by visualizing p70S6K phosphorylation on Thr389. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments. (B) The effect of PKCζ on mTOR inhibition induced by RTX was evaluated by Western blot using anti–phospho-p70S6K antibody in RL cells stably transfected with pcDNA3 (1G5) or CA-PKCζ (6F9, 7E5) treated with RTX at 50 μg/mL. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments.
Influence of PKCζ on FL cell response to rituximab
In our previous work, we have shown that mTOR plays an important role in FL cell proliferation.14 The present study established that RTX inhibited mTOR and that this effect was efficiently counteracted by CA-PKCζ transfection. Therefore, we speculated that PKCζ could confer a significant protection toward RTX in FL cells. For this reason, we evaluated the influence of RTX used at various doses on the clonogenicity of FL cells transfected or not with CA-PKCζ. Interestingly, we found that CA-PKCζ induced a 5-fold higher RL cell clonogenicity (data not shown), suggesting that PKCζ is an important contributor for cell proliferation in FL. Moreover, as depicted in Figure 6A, CA-PKCζ transfection (clones 6F9 and 7E5) conferred total protection of FL cells toward RTX, whatever the dose used. These results suggest that PKCζ not only is a target for rituximab signaling, but also represents an important factor of resistance to rituximab's antileukemic effect.
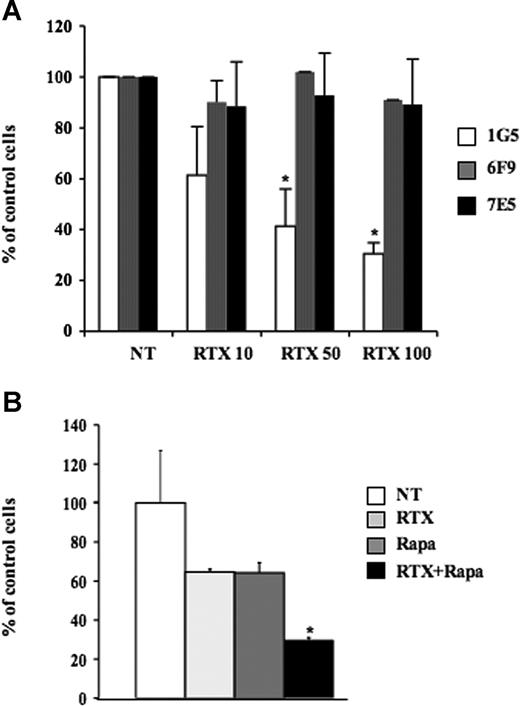
Role of PKCζ on FL response to RTX. (A) Clonogenicity was evaluated on RL cells stably transfected with pcDNA3 (1G5) or CA-PKCζ (6F9, 7E5) treated with RTX at 10, 50, or 100 μg/mL. Clonogenicity was observed after 7 days in methylcellulose medium as described in “Clonogenic assays.” Results are the mean (± SD) of 3 independent experiments. *P < .05. (B) Effect of RTX and rapamycin association was evaluated on RL cell proliferation by trypan blue exclusion assay 7 days after treatment with RTX at 10 μg/mL, rapamycin at 5 nM, or both. Results are the mean (± SD) of 3 independents experiments. *P < .05.
Role of PKCζ on FL response to RTX. (A) Clonogenicity was evaluated on RL cells stably transfected with pcDNA3 (1G5) or CA-PKCζ (6F9, 7E5) treated with RTX at 10, 50, or 100 μg/mL. Clonogenicity was observed after 7 days in methylcellulose medium as described in “Clonogenic assays.” Results are the mean (± SD) of 3 independent experiments. *P < .05. (B) Effect of RTX and rapamycin association was evaluated on RL cell proliferation by trypan blue exclusion assay 7 days after treatment with RTX at 10 μg/mL, rapamycin at 5 nM, or both. Results are the mean (± SD) of 3 independents experiments. *P < .05.
Discussion
Our study shows for the first time that FL cells and other lymphoma cells display high level of PKCζ expression, compared with normal B cells, and that the enzyme is present under its active form. PKCζ distribution in human normal or malignant B cells has received very little attention, compared with other PKC isoforms.26 In mice, previous studies have shown that, whereas normal lymphoid organs (spleen, thymus, and lymph nodes) express barely detectable amounts of PKCζ, the expression of the enzyme increases in most murine B-cell lymphomas and plasma tumor cells tested.27 This provocative result was not extended to human B cells until our study. The lack of information about PKCζ distribution in human tissue samples is likely because that there is no antibody from a commercial source available for immunohistochemistry. As a matter of fact, using either Cell Signaling (Ozyme) or Santa Cruz (Tebu) reagents, we failed to achieve immunostaining on FL or tonsil cryostat sections. The fact that PKCζ expression increases in lymphoma cells suggests that PKCζ contributes to the malignant transformation of lymphocytes. This finding is not totally surprising since PKCζ plays an important role in the development of secondary lymphoid tissue and B-cell function by participating in BCR signaling as suggested by PKCζ knockout experiments.22 Altogether, these findings support the idea that PKCζ exerts a survival function in B cells and that its expression is abnormally regulated in B-cell neoplasms through yet-undefined mechanisms. The reason for which PKCζ is not only overexpressed but also activated remains also unknown. In other cellular models, it has been shown that this enzyme can be activated by lipid second messengers such as PA, Src kinases, oncogenic products, or growth factors.9 Thus, it is possible that FL cells possess intrinsically abnormal regulation of one or several of these pathways, resulting in amplification of PKCζ function. Since we have previously described that PLD activity was remarkably high in FL cells,14 a plausible candidate for PKCζ activity regulation is PA. Moreover, based on our previous study that shows that Syk, a nonreceptor tyrosine kinase, is overexpressed in FL cells and plays an important role in FL survival, we also investigated whether Syk could regulate PKCζ in these cells. However, we found that pharmacological inhibition of Syk by piceatannol or Syk depletion by siRNA had no influence on PKCζ expression or activity (data not shown).
Our study shows for the first time that RTX inhibits PKCζ in FL cells, but not in DLBCL and MCL cells. In a previous study, we described that RTX-mediated CD20 activation resulted in the induction of SM cycle and subsequent accumulation of ceramide, a plausible candidate for PKCζ inhibition. Indeed, previous studies have established that ceramide specifically binds to isolated PKCζ and regulates its kinase activity in vitro. Although low doses of ceramide may exert a stimulatory effect, ceramide is a potent inhibitor of the enzyme when used at high doses.8 In intact cells, recent studies have indicated that ceramide could also act indirectly by facilitating the binding of the enzyme to its inhibitor protein, Par-4.28 The effect of ceramide on PKCζ could be even more complex since it can also alter its intracellular redistribution.29 Based on these findings, we propose a model in which, in FL cells, ceramide accumulated during RTX-induced SM cycle activation is responsible for PKCζ inhibition. The fact that, in FL cells, we found that exogenous ceramide could inhibit PKCζ (data not shown), thus mimicking RTX effect, argued for this hypothesis. However, it remains to be determined why treatment with RTX has no effect on PKCζ in DLBCL or MCL. This result raised several possibilities, including abnormal regulation of SM cycle, enhanced ceramide metabolism, or reduced Par-4 expression. At present, we find no difference in terms of ceramide production or Par-4 expression among the different cell lines (data not shown).
Our study shows for the first time that PKCζ is an important component for regulating mTOR in FL cells. In a recent study, we have reported that, in FL cells, mTOR is constitutively activated, and that mTOR is regulated by at least 3 distinct pathways: a PI3K-Akt module, PLD/PA, and a Syk, PI3K- and PLD-independent, autonomous pathway.14 In the present study, we describe another pathway consisting of a PKCζ-Raf-MEK-ERK module. The functional link between PKCζ and the classical MAPK module was not unexpected because we and others have established in other conditions that PKCζ may indeed interact with and regulates Raf-1.19 Moreover, the role of ERK as a regulator of mTOR pathway has been also established.30 The identification of another mTOR-regulating component illustrates the complexity of mTOR network in FL cells. The fact that mTOR appears to be regulated by several distinct intracellular signaling pathways, designates this kinase as an important molecular target for FL therapy. It is important to note that the inhibitory effect of RTX on mTOR is selectively related to the inhibition of the PKCζ-MAPK module. Indeed, we found that, at least in FL cells, RTX had no effect on Syk activity and inhibited neither PLD activity nor PI3K-Akt pathway (data not shown). The latter result conflicts with recent findings coming from Bonavida's group, which described that RTX inhibits Akt phosphorylation at both Ser473 and Thr308 sites in Ramos and Daudi cells (Suzuki et al31 ). These discrepancies illustrate the cell specificity of RTX molecular effects.
Our results suggest that RTX inhibits the Raf/MEK/ERK signaling pathway by inhibiting PKCζ. The inhibitory effect of RTX on MAPK has been largely documented by Bonavida and coworkers (Jazirehi et al23 ). This group has shown that RTX acts by increasing the expression of RKIP, a negative regulator of Raf-1, and that the subsequent adverse regulation of MAPK resulted in Bcl-xL down-regulation. As far as FL cells are concerned, we were unable to detect any variation of RKIP, even after prolonged RTX exposure, as well as modification of Bcl-2 family protein expression (data not shown). Again, these discrepancies confirm that RTX operates differently depending on lymphoma cell subtype.
Our study may have potentially important clinical implications. First, we show that combined therapy with RTX and rapamycin resulted in an additive antileukemic effect. This result should encourage investigators to design in FL clinical studies based on the association between RTX and new derivatives directed against mTOR such as RAD001. Moreover, the impact of RTX on PKCζ could also explain the synergistic effect between RTX and chemotherapy. Indeed, we and others have previously documented that this enzyme plays an important role not only in the regulation of post-DNA damage response,12,17,26 but also in drug-induced DNA damage or DNA repair. Thus, we have previously described that PKCζ inhibits the formation of DNA double-strand break generated by topoisomerase II poisons through topoisomerase II activity inhibition, resulting in a significant protection against anthracyclines or podophyllotoxins.17 In another study, we have also reported that PKCζ is efficient for regulating nucleotide excision repair, an important contributor for protecting cells against cisplatin derivatives.32 Finally, we have also reported that PKCζ is a potent regulator of radical oxygen species (ROS) detoxication.33 Since ROSs are important mediators of anthracycline-induced cytotoxicity, it is also conceivable that RTX-induced PKCζ inhibition contributes to sensitize cells to doxorubicin contained in rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen by reducing antioxidant defenses.
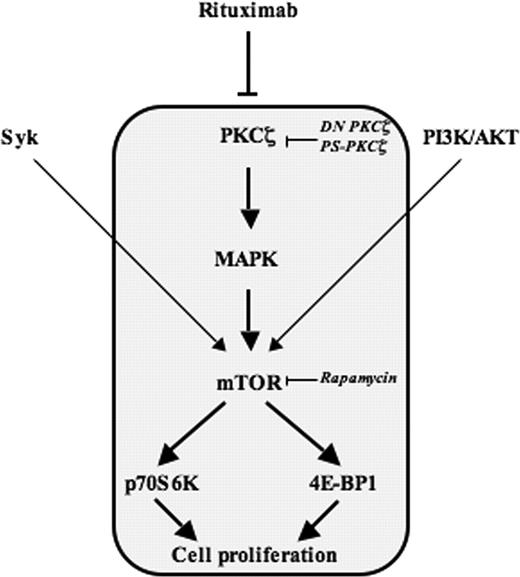
To conclude, our study shows that, in FL cells, RTX inhibits a PKCζ-MAPK-mTOR module (Figure 7), and that PKCζ is not only a target for this antibody but also a potent regulator of its intrinsic antileukemic effect.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Roche for providing RTX; Catherine Trichard for technical assistance; Dr Fournié's team (INSERM U563, Toulouse, France) for B-lymphocyte isolation; and Dr Al Saati and Florence Capilla (plateau technique d'histopathologie expérimentale, IFR30, Toulouse, France) for immunochemistry experiments.
We also thank Dr Lehoux (Sherbrooke University) for PKCζ plasmid constructs; Drs Pelletier and Hermine (Necker) for MCL cell lines; and Prof Delsol (INSERM U563) for DLBCL cell lines.
This work was supported by la Fondation de France (C.B.), l'Association pour la Recherche sur le Cancer (G.L.), and la Ligue régionale contre le cancer. L.L. is the recipient of a grant from leMinistère délégué à l'enseignement supérieur et à la recherche.
Authorship
Contribution: L.L. performed the main corpus of the research; M.R. and A.B. performed experiments; C.L. obtained the tissues section from FL patients; D.O. reviewed the manuscript; and L.L., G.L., and C.B. designed the research, and drafted and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine Bezombes, INSERM U563-CPTP, Bât B, pavillon Lefebvre, Département d'Oncogenèse, Signalisation et Innovation thérapeutique, CHU Purpan-BP3028, 31024 Toulouse cedex 3, France; e-mail: christine.bezombes-cagnac@toulouse.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal