FTY720 is an immunosuppressant developed to prevent organ transplant rejection. Recent studies indicate an additional role for FTY720 in inducing cell apoptosis. We demonstrate here that FTY720 mediates toxic effects in cell lines representing different B-cell malignancies and primary B cells from patients with chronic lymphocytic leukemia (CLL). In contrast to previous reports in T-cell lines, FTY720-induced toxicity in the Raji cell line and primary CLL B cells is independent of activation of caspases or poly(ADP-ribose) polymerase processing. Further, pancaspase inhibitor Z-VAD-fmk failed to rescue these cells from apoptosis mediated by FTY720. FTY720 induced down-regulation of Mcl-1 but not Bcl-2 in CLL B cells. Overexpression of Bcl-2 failed to protect transformed B cells from FTY720-induced apoptosis, suggesting a Bcl-2–independent mechanism. Interestingly, FTY720 induced protein phosphatase 2a (PP2a) activation and downstream dephosphorylation of ERK1/2, whereas okadaic acid at concentrations that inhibited the FTY720-induced PP2a activation also resulted in inhibition of FTY720-mediated apoptosis and restoration of baseline ERK1/2 phosphorylation in primary CLL cells, indicating a role for PP2a activation in FTY720-induced cytotoxicity. Further, FTY720 treatment resulted in significant prolonged survival in a xenograft severe combined immunodeficiency (SCID) mouse model of disseminated B-cell lymphoma/leukemia. These results provide the first evidence for the potential use of FTY720 as a therapeutic agent in a variety of B-cell malignancies, including CLL.
Introduction
Despite the progress that has been made in the treatment of acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL), most patients with these diseases eventually develop resistance and ultimately die from their leukemia. Mechanisms of resistance in ALL and CLL have been shown to involve alteration in Bcl-2 family member expression that prevents apoptosis and aberrant signaling through the PKC, PI3 kinase/AKT, and ERK pathways that is in part mediated through stromal cell interactions.1,2 New therapies that act through novel mechanisms of action that either are independent of Bcl-2 family member expression and/or antagonize aberrant signal transduction pathways are therefore needed for both CLL and ALL. Normal lymphocytes use serine/threonine phosphatases such as PP1, PP2a, and PP2b to both inactivate signal transduction pathways and antagonize the action of Bcl-2 family members, including Bcl-2 and Bad.3,,–6 In B-cell lymphoproliferative disorders, these same phosphatases are often silenced, a process that may contribute further to the drug resistance observed in these diseases. Therapeutic agents that activate serine/threonine phosphatases such as PP2a are not clinically available at the present time and therefore have not been tested in the clinic for CLL and ALL.
FTY720 ((2-amino-2-[2-(4-octylphenyl) ethyl] propane 1, 3-diol hydrochloride) is a synthetic compound produced by modification of a natural immunosuppressant, ISP-1.7 FTY720 was noted to interfere with T-cell trafficking and was demonstrated to prolong survival of transplanted allograft organs without noticeable toxicity to the host in preclinical studies.8,–10 Early phase 1/2 clinical studies of FTY720 to treat and prevent organ rejection demonstrated promise.11,,,–15 As a consequence, FTY720 is currently in phase 3 clinical trials as an immunosuppressant for renal transplant rejection.14,16,–18 FTY720 elicits lymphopenia resulting from a reversible redistribution of lymphocytes from circulation to secondary lymphoid tissues.19 FTY720 is phosphorylated by sphingosine kinase 2, and the phosphorylated compound is a potent agonist at 4 sphingosine-1-phosphate (S1P) receptors, which modulate chemotactic responses and lymphocyte trafficking. Previous studies have also suggested that FTY720 might also promote activation of the serine/threonine phosphatase PP2a.20,21 Based upon the potential of FTY720 to activate PP2a and recent studies demonstrating FTY720 induced apoptosis in T lymphocytes20,22,23 and multiple myeloma cell lines,24 we sought to investigate the in vitro and in vivo activity of this agent in ALL and CLL. Herein, we report that FTY720 has potent in vitro and in vivo activity in a variety of B-cell malignancies and mediates apoptosis in a Bcl-2– and caspase-independent manner through activation of the serine/threonine phosphatase PP2a. Further evidence for FTY720 activity independent of S1P receptor signaling is also presented, suggesting a novel mechanism of FTY720-induced apoptosis in CLL cells.
Methods
Cells
Blood was obtained from patients with CLL under a protocol approved by The Ohio State University hospital internal review board. Informed consent was obtained in accordance with the Declaration of Helsinki. All patients examined in this series had immunophenotypically defined CLL as outlined by the modified 1996 National Cancer Institute criteria.26,27 CLL B cells were isolated from freshly donated blood using ficoll density gradient centrifugation (Ficoll-Paque Plus; Amershan Biosciences, Piscataway, NJ). Isolated mononuclear cells were incubated in RPMI 1640 media (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), 2 mM l-glutamine (Invitrogen, Carlsbad, CA), and penicillin (100 U/mL)/streptomycin (100 μg/mL) (Sigma-Aldrich, St Louis, MO) at 37°C in an atmosphere of 5% CO2. Freshly isolated CLL cells were used in all experiments described herein. Enriched B-lymphocyte fractions were prepared by using a magnetic-activated cell sorter (MACS) negative selection kit from Miltenyi Biotech (Auburn, CA) or by the “Rosette-Sep” kit from Stem Cell Technologies (Vancouver, BC, Canada) according to the manufacturer's instructions. Human B-lymphocyte cell lines Raji and Ramos were obtained from American Type Culture Collection (ATCC, Manassas, VA) and the MEC-1 cells were obtained from the German cell line bank (Braunschweig, Germany). The 697-neo and 697-Bcl-2 cell lines were a kind gift from Dr John Reed (Burnham Institute, La Jolla, CA).
Chemical reagents
FTY720 was synthesized according to a reported procedure with subsequent high-performance liquid chromatography (HPLC) purification.25 The identity and purity were confirmed by nuclear magnetic resonance and mass spectrometry. Z-VAD-fmk (Sigma-Aldrich), calyculin A, and okadaic acid (OA; Upstate Biotechnology, Lake Placid, NY) were used at indicated concentrations.
Analysis of cell viability and apoptosis
The cell viability was carried out by dual staining with annexin V conjugated to flourescein isothiocyanate (FITC) and propidium iodide (PI) as described previously.28 Briefly, 1 × 106 cells were stained with annexin V–FITC (BD Pharmingen, San Diego, CA) and propidium iodide (BD Pharmingen) for 15 minutes in the dark and analyzed by flow cytometry using a Beckman-Coulter EPICS XL cytometer (Beckman-Coulter, Miami, FL). Apoptotic cells were identified as annexin V+ and/or PI+ cells. Cells excluding both FITC and PI were considered viable. The annexin V−/PI− cells normalized to untreated controls are represented as a percentage of live cells.
MTT assay
Cell growth was assessed using the MTT assay. The cells (1 × 106) were placed in 200 μL of media with or without indicated concentrations of FTY720 in each well of a 96-well flat-bottomed microtiter plates in triplicate cultures and incubated overnight at 37°C in an incubator at 5% CO2 atmosphere. MTT was prepared at 5 mg/mL in phosphate-buffered saline (PBS) and added to each well at 12 or 36 hours. The cell cultures were continued for another 12 hours at 37°C. The color development solution was added to each well, and the absorbance was measured at a 570-nm wavelength using a microculture plate reader. The cell viability was expressed as a percentage of absorbance in cells with indicated treatments to that in cells with vehicle control treatment.
Western blotting
Cell lysates were prepared and quantified by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Lysates with 50 μg of total protein were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to 0.2-μm nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The blots were probed with indicated primary antibodies followed by horseradish peroxidase (HRP)–conjugated goat anti-rabbit or goat anti-mouse IgG (Bio-Rad Laboratories, Richmond, CA). Detection was made with chemiluminescent substrate (SuperSignal; Pierce). The poly-ADP-ribose polymerase (PARP; Ab-2) and caspase-9 antibodies were purchased from Oncogene/Calbiochem/EMD Biosciences (San Diego, CA). The Bcl-2 and Mcl-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-ERK1/2 and ERK1/2 were from Upstate (Charlottesville, CA). Caspase-3 (AR-14), caspase 3/cpp32, and caspase-8 (AR-18) antibodies were kind gift from Dr John Reed (Burnham Institute).
PP2a activity (nonradioactive assay)
The protein phosphatase activity of total cellular lysate was determined by measuring the generation of free phosphate from the threonine phosphopeptide using the malachite green–phosphate complex assay as described by the manufacturer (Upstate). Cell lysates were prepared in a low-detergent lysis buffer (1% Nonidet P-40, 10 mM HEPES, 150 mM NaCl, 10% glycerol, 1 mM PMSF, 5 mM benzamidine, and 10 μg/mL leupeptin). The phosphatase assay was performed in a PP2a-specific reaction buffer (Upstate) using 750 μM phosphopeptide substrate. After 10 minutes of incubation at 30°C malachite dye was added, and free phosphate was measured by optical density at 650 nM. To avoid variability among different immunoprecipitated samples, the phosphatase activities were normalized with the amount of PP2a immunoprecipitated, as detected and quantified by immunoblot analysis of each treatment group.
PP2a activity (radioactive assay)
Myelin basic protein (MBP) was phosphorylated by cAMP-dependent kinase (PKA) using 32P-ATP according to the protocol provided by New England Biolabs (Ipswich, MA) and used as substrate for PP2a. Cell lysates were prepared the same as for the PP2a nonradioactive assay. PP2a was immunoprecipitated from cell lysate and incubated with 32P-MBP at 30°C for 10 minutes. The reaction was stopped by adding TCA. The mixture was centrifuged, and supernant was collected and read in a multipurpose scintillation counter (Beckman Coulter, Fullerton, CA).
PP2a and PP1 cell-free inhibition assay
Purified PP2a (Upstate) and PP1 (New England Biolabs) enzymes were commercially purchased. PP2a was in ABC trimer form and dissolved in 20 mM MOPS (pH 7.4), 0.1 M NaCl, 60 mM 2-mercaptoethanol, 1 mM MgCl2, 1mM EGTA, 0.1 mM MnCl2, 1mM DTT, 10% glycerol, and 0.1 mg/mL serum albumin. PPI and monomer was dissolved in 50 mM Tris-HCl (pH 7.0), 200 mM NaCl, 0.1 mM MnCl2, 0.1 mM EGTA, 5 mM DTT, 0.01% Brij35, and 50% glycerol. Neither NP40 nor BSA was used. The buffers supplied by Upstate and New England Biolabs, respectively, for PP2a and PP1 were used at 0.5 U per reaction. Okadaic acid was added to each at the respective concentrations and measured using the radioactive and nonradioactive assays described.
In vivo therapeutic efficacy evaluation in a xenograft model
The in vivo evaluation of FTY720 was carried out using the disseminated lymphoma bearing the severe combined immunodeficiency (SCID) mouse xenograft model. This model was generated using the human Raji B-cell line injected into SCID mice as previously described.30 Raji cells were cultured in RPMI 1640 media containing 10% fetal bovine serum. Confluent cultures with more than 95% viability were confirmed to express human CD19 by flow cytometry. Female 6- to 8-week-old C.B.-17 SCID mice (Taconic Farm, Germantown, NY) were injected with 2 × 106 cells intravenously by tail vein in 200 μL sterile PBS. At 72 hours after inoculation, the animals were divided into 4 equal treatment groups. The first 3 groups served as a control and received placebo (saline), trastuzumab, or rituximab dissolved in 1 mg/mL saline and injected via tail vein, and maintained every other day intravenously for 2 weeks (5 mg/kg per injection, 7 injections each mouse). The fourth group consisted of animals treated with FTY720 (5 mg/kg per injection) every day for 2 weeks intraperitoneally. Body weight was measured once every week. The mice in the placebo-control group developed symptomatic central nervous system (CNS) involvement resulting in progressive hind-limb paralysis associated with decreased mobility, loss of body weight, and death at 17 to 21 days after inoculation. All the animals were monitored daily for signs of illness and killed immediately if hind-limb paralysis, respiratory distress, or 30% body weight loss was noted. The endpoint of the study was survival defined as the time for the development of hind-limb paralysis. Animals that reached the endpoint or survived after 200 days of observation were killed. Histopathologic examination of liver, lung, and brain was performed to detect any residual disease. The histologic analyses were performed using the services of the mouse phenotyping core services at the Ohio State University Comprehensive Cancer Center. Histologic sections were examined using an Olympus BX41 (double-headed) microscope (Olympus America, Melville NY) and images were captured using a Nikon CoolPix 5400 digital camera (5.1 megapixel w/4× optical zoom, macro focus to 0.5 inch) and the images were transformed using Nikon View 6 image transfer program (Nikon, Tokyo, Japan). The images were processed using Windows Printing Wizard/Photoshop 2.0 (Microsoft, Redmond, WA). The presences of the CD19+ cells were evaluated in the bone marrow of these mice by flow cytometry.
Statistical analysis of data
All the analysis was performed by statisticians in Center for Biostatistics, The Ohio State University. SPSS software (version 9.0; SPSS, Chicago, IL) was used for all the statistical analysis. Significance was tested based on 2-sided P values. Comparison was made between different groups using the Wilcoxon signed-rank test and the paired t test for in vitro studies. The log-rank test was applied for analysis of animal survival study.
Results
FTY720-mediated toxicity in B-cell lines and purified B cells from patients with CLL
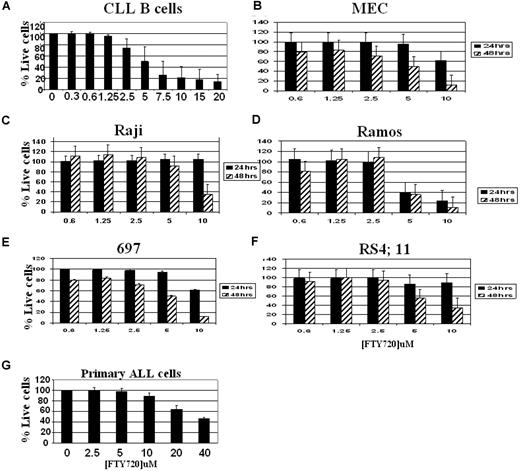
Several studies have demonstrated a role for FTY720 in the regulation of in vivo T- and B-cell homing and in vitro cytotoxicity in T-cell lines.19,22,23 However, systematic analysis of the effect of FTY720 in acute and B-cell chronic lymphocytic leukemia has not been performed. Incubation of CD19+ cells from patients with CLL with increasing concentrations of FTY720 ranging from 0, 0.5, 1, 1.5, 2.5, 5, or 10 μM resulted in a dose-dependent decrease in viable cells with a concomitant increase in annexin V+ and/or PI+ cells as measured by flow cytometry (Figure 1A; P < .001, untreated vs 10 μM–treated; n = 15). Consistent with increased cell death, the FTY720-treated cells also exhibited decreased viability as evidenced by MTT assays (data not shown).
FTY720-mediated toxicity in CLL B cells, MEC-1, Raji, Ramos,697 and RS4;11 B-cell lines: dose and time kinetic analysis. Purified CD19+ lymphocytes from patients with CLL (A), MEC (B), Raji (C), Ramos (D), 697 (E), and RS4;11 (F) cells or blasts from a patient with ALL (G) (105 cells/mL medium) were incubated with indicated concentrations of FTY720 or DMSO vehicle for 24 hours (■) or 48 hours ( ). The cells were stained with annexin V–FITC and PI, as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent percentages of annexin V−/PI− viable cells plus or minus SD that are normalized to media control. (CLL B cells, n = 6–15; ALL, n = 4; and cell lines, n = 3; *P < .001 when compared with media control.)
). The cells were stained with annexin V–FITC and PI, as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent percentages of annexin V−/PI− viable cells plus or minus SD that are normalized to media control. (CLL B cells, n = 6–15; ALL, n = 4; and cell lines, n = 3; *P < .001 when compared with media control.)
FTY720-mediated toxicity in CLL B cells, MEC-1, Raji, Ramos,697 and RS4;11 B-cell lines: dose and time kinetic analysis. Purified CD19+ lymphocytes from patients with CLL (A), MEC (B), Raji (C), Ramos (D), 697 (E), and RS4;11 (F) cells or blasts from a patient with ALL (G) (105 cells/mL medium) were incubated with indicated concentrations of FTY720 or DMSO vehicle for 24 hours (■) or 48 hours ( ). The cells were stained with annexin V–FITC and PI, as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent percentages of annexin V−/PI− viable cells plus or minus SD that are normalized to media control. (CLL B cells, n = 6–15; ALL, n = 4; and cell lines, n = 3; *P < .001 when compared with media control.)
). The cells were stained with annexin V–FITC and PI, as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent percentages of annexin V−/PI− viable cells plus or minus SD that are normalized to media control. (CLL B cells, n = 6–15; ALL, n = 4; and cell lines, n = 3; *P < .001 when compared with media control.)
Incubation of MEC-1, a B-cell line established from a patient with CLL, Ramos and Raji cell lines representing Burkitt leukemia/lymphoma, or acute lymphoblastic leukemia cell lines 697 and RS4;11 with 0.6, 1.25, 2.5, 5, or10 μM of FTY720 also resulted in a dose-dependent decrease of viable cells with a concomitant increase in annexin V+ and/or PI+ cells as measured by flow cytometry (Figure 1B-F). The FTY720-mediated toxicity in each of these cell lines was time dependent, with maximal effect seen as late as 48 hours after treatment. The IC50 value for each of these cell lines is shown in Table 1. The dose- and time-dependent toxic effect of FTY720 was further reflected in a parallel MTT reduction assay that is directly related to the number of viable cells (data not shown).
IC50 values
| Time, h . | CLL, μM IC50 . | MEC, μM IC50 . | Raji, μM IC50 . | Ramos, μM IC50 . | 697, μM IC50 . | RS4;11, μM IC50 . | Primary ALL cells, μM IC50 . |
|---|---|---|---|---|---|---|---|
| 24 | 4.01 | >10 | >10 | 4.03 | >10 | >10 | 35.42 |
| 48 | — | 5.82 | 7.84 | 4.67 | 4.56 | 5.82 | — |
| Time, h . | CLL, μM IC50 . | MEC, μM IC50 . | Raji, μM IC50 . | Ramos, μM IC50 . | 697, μM IC50 . | RS4;11, μM IC50 . | Primary ALL cells, μM IC50 . |
|---|---|---|---|---|---|---|---|
| 24 | 4.01 | >10 | >10 | 4.03 | >10 | >10 | 35.42 |
| 48 | — | 5.82 | 7.84 | 4.67 | 4.56 | 5.82 | — |
— indicates not detected.
IC50 values for each of these cell lines were calculated by Pharsight WinNolin 4.1 using an inhibitory sigmoidal model.
FTY720-induced apoptosis in CLL cells is not dependent on activation of caspases, the cysteine proteases of the CED3/ICE family
Several therapeutic agents such as glucocorticoids and chemotherapeutic agents such as fludarabine, chlorambucil, and 2-chloro-2-deoxyadenosine induce cytotoxicity in CLL cells through activating caspases, the cysteine proteases of CED3/ICE family.30,31 Inhibition of these caspases results in abrogation of apoptosis mediated by the cytotoxic stimuli. To determine if caspase activation is involved in the FTY720-induced cell death in primary CLL cells, we pretreated CD19+ CLL cells prior to treatment with FTY720 with a broad spectrum caspase inhibitor z-VAD-fmk. As shown in Figure 2A, concentrations of z-VAD-fmk (150 μM) that effectively rescued fludarabine-induced apoptosis failed to prevent FTY720-induced apoptosis (fludarabine vs fludarabine + z-VAD-fmk: P = .001, n = 5; FTY720 vs FTY720 + z-VAD-fmk: P = .99, n = 5). Activation of caspases results in cleavage of key cellular proteins, including PARP and caspase-3, caspase-8, and caspase-9. Consistent with the inability of z-VAD-fmk to rescue FTY720-induced apoptosis, Western blotting analysis of lysates prepared from CD19+ B cells from patients with CLL at 24 and 48 hours after treatment with FTY720 failed to reveal PARP cleavage. In addition, caspase-3, caspase-8, and caspase-9 also remained in precursor forms, demonstrating the lack of activation of caspases in FTY720-treated cells (Figure 2B; data not shown). Similar to the CLL cells, FTY720-induced toxicity in Raji B-cell line was not accompanied by processing of PARP. However, FTY720-induced cytotoxicity in the Ramos B-cell line was associated with processing of PARP (Figure 2C). Consistent with differential activation of caspase cascade, z-VAD-fmk rescued FTY720-induced cytotoxicity and associated activation of caspase-3, caspase-8, or caspase-9 in the Ramos B-cell line (data not shown).
FTY720-induced toxicity in CLL cells is independent on caspase activation. (A) Pancaspase inhibitor z-VAD-fmk failed to rescue CLL B cells from FTY720-induced cell death. Purified CD19+ cells from patients with CLL (106 cells/mL media) were incubated with 10 μM FTY720 in the presence or absence of z-VAD-fmk (150 μM) for 24 hours. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 3; *P = .001 when compared with FARA [2F-Adenine Arabinoside or fludarabine]-treated group; **P = .998 when compared with FTY720-treated group). (B) FTY720 failed to induce caspase-3, caspase-8, or PARP processing. Purified CD19+ cells from patients with CLL (106 cells/mL media) were incubated with DMSO (None) or 10 μM FTY720 for 24 and 48 hours. Western blot analysis of the lysates from each of the conditions were assessed for processed and unprocessed PARP, caspase-3, and caspase-8 as described in “Western blotting.” The bold arrow indicates the unprocessed form of each of the proteins; the dashed arrows indicate cleaved products. Untreated and UV-treated Jurkat cell lysates were used as positive controls. (C) FTY720 induced activation of PARP processing in the Ramos but not the Raji cell line. Ramos or Raji cells (105/mL media) were incubated with DMSO and 150 μM z-VAD-fmk with or without 10 μM FTY720 for 24 hours. Western blotting of PARP protein for each of the conditions for both cell lines is shown.
FTY720-induced toxicity in CLL cells is independent on caspase activation. (A) Pancaspase inhibitor z-VAD-fmk failed to rescue CLL B cells from FTY720-induced cell death. Purified CD19+ cells from patients with CLL (106 cells/mL media) were incubated with 10 μM FTY720 in the presence or absence of z-VAD-fmk (150 μM) for 24 hours. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 3; *P = .001 when compared with FARA [2F-Adenine Arabinoside or fludarabine]-treated group; **P = .998 when compared with FTY720-treated group). (B) FTY720 failed to induce caspase-3, caspase-8, or PARP processing. Purified CD19+ cells from patients with CLL (106 cells/mL media) were incubated with DMSO (None) or 10 μM FTY720 for 24 and 48 hours. Western blot analysis of the lysates from each of the conditions were assessed for processed and unprocessed PARP, caspase-3, and caspase-8 as described in “Western blotting.” The bold arrow indicates the unprocessed form of each of the proteins; the dashed arrows indicate cleaved products. Untreated and UV-treated Jurkat cell lysates were used as positive controls. (C) FTY720 induced activation of PARP processing in the Ramos but not the Raji cell line. Ramos or Raji cells (105/mL media) were incubated with DMSO and 150 μM z-VAD-fmk with or without 10 μM FTY720 for 24 hours. Western blotting of PARP protein for each of the conditions for both cell lines is shown.
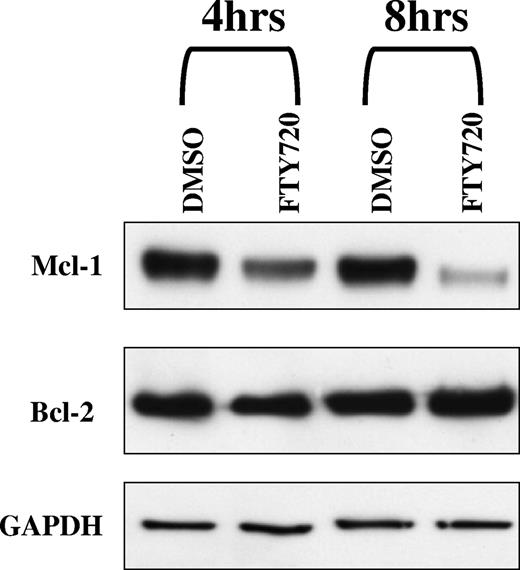
FTY720-induced downmodulation of Mcl-1 but not Bcl-2 in CLL B cells
Mcl-1 and Bcl-2 are 2 of the major antiapoptotic proteins overexpressed in CLL and ALL cells.32,33 siRNA-mediated down-regulation of Mcl-1 in CLL and ALL cells has been shown to result in apoptosis of these cells.34 The resistance to apoptosis has been implicated to the high levels of Bcl-2 expression in these cells.1,2 Decreased cell viability during in vitro culture conditions or in response to treatment with cytotoxic agents correlates with down-regulation of Bcl-2 expression.36,37 To determine if FTY720-induced apoptosis in CLL B cells was associated with modulation of Mcl-1 and/or Bcl-2, we investigated the effect of FTY720 on the levels of Mcl-1 and Bcl-2 by Western blotting analysis of lysates from CLL B cells treated with FTY720. FTY720 was found to down-regulate Mcl-1 as early as 4 hours, with minimal change in the levels of Bcl-2 (Figure 3).Constitutive overexpression of Bcl-2 in cell lines results in resistance to apoptosis. In order to determine if sustained expression of Bcl-2 will result in protection from FTY720-induced apoptosis, 697 cells stably transfected with neomycin-carrying vector or Bcl-2 expression vector were tested with increasing concentrations of FTY720. FTY720 induced comparable levels of cellular toxicity in both the control 697-neo cell line and the 697–Bcl-2 overexpressing cell line at 24, 48, and 72 hours, indicating a Bcl-2–independent mechanism of cytotoxicity (data not shown).
FTY720 induced down-modulation Mcl-1 but not Bcl-2 in CLL B cells. Primary CLL cells (1 × 106 cells/mL medium) were incubated with 5 μM FTY720 for 4 hours and 8 hours. Cell lysates from different time points were subjected to Western blot analysis using anti–Mcl-1, anti–Bcl-2, and GAPDH antibody.
FTY720 induced down-modulation Mcl-1 but not Bcl-2 in CLL B cells. Primary CLL cells (1 × 106 cells/mL medium) were incubated with 5 μM FTY720 for 4 hours and 8 hours. Cell lysates from different time points were subjected to Western blot analysis using anti–Mcl-1, anti–Bcl-2, and GAPDH antibody.
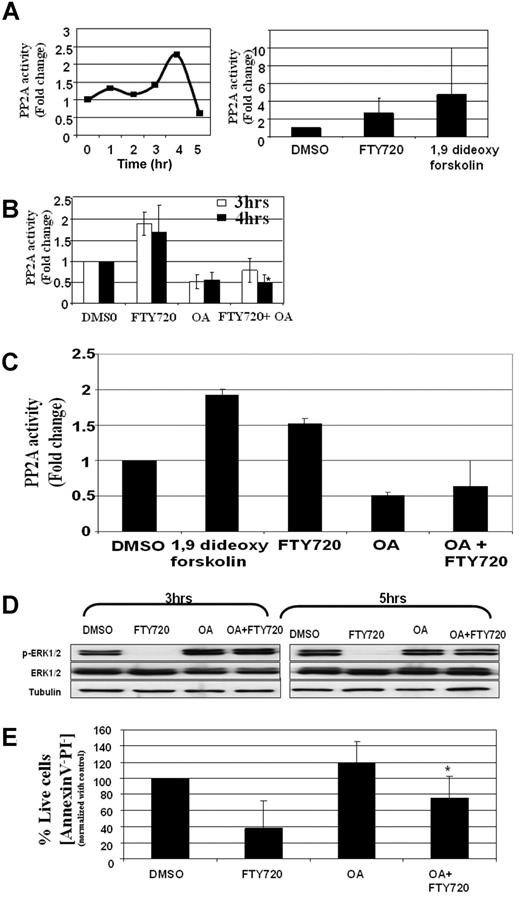
FTY720-mediated apoptosis is dependent on PP2a activation in CLL cells
FTY720 has been shown to induce activation of PP2a in several cell lines, including Jurkat T cells.20,21 To determine if FTY720 induced activation of the PP2a enzyme, CD19+ B cells from patients with CLL were treated with 10 μM FTY720 for 0, 1, 2, 3, 4, and 15 hours, and the PP2a activity in the lysates were quantified using a functional assay following immunoprecipitation of PP2a enzyme. As reported previously in T cells,20 FTY720 induced a consistent 1.5- to 3-fold increase in PP2a activity, with the peak response observed at 4 hours after treatment (Figure 4A). The increase in PP2a activity was not associated with alternation in the levels of PP2a protein (data not shown). In contrast, the FTY720-induced PP2a activity is associated with activation of PP2a enzyme, as pretreatment of CLL cells with 5 nM okadaic acid, a concentration that has been shown to inhibit protein phosphatase activity in cells, resulted in inhibition of the FTY720-induced PP2a activation as much as 3-fold (Figure 4B). Similar results were also obtained by a 32P/TCA assay, which validated the nonradioactive malachite green assay (Figure 4C). The OA at 5 nM was found to be highly selective to PP2a compared with PP1. Thus, using recombinant PP2a and PP1 enzymes in a radioactive 32P/TCA assay, the OA at 5 nM was found to be 100 times more potent at inhibiting PP2a compared with PP1. Consistent with the activation of PP2a enzyme, phospho-ERK1/2, a known target of PP2a, was dephosphorylated by FTY720 treatment (Figure 4D). Pretreatment of the cells with concentrations of okadaic acid that inhibited the FTY720-induced PP2a activity prevented the FTY720-induced dephosphorylation of ERK1/2 (Figure 4D). In contrast to ERK1/2, FTY720 treatment failed to dephosphorylate Bcl-2 (data not shown). In addition, okadaic acid at concentrations that resulted in inhibition of FTY720-induced PP2a activation also resulted in inhibition of FTY720-induced toxicity (Figure 4E). A similar phenomenon was also observed in the Ramos B-cell line. Thus, FTY720 induced activation of PP2a in the Ramos cell line as early as 3 hours, and this induced PP2a activity was inhibited by okadaic acid (Figure 5A,B). Pretreatment of Ramos cells with PP2a-inhibitory concentrations of okadaic acid resulted in inhibition of FTY720-induced apoptosis, indicating a role for FTY720-induced PP2a activation in toxicity (Figure 5C). Use of the less-specific phosphatase inhibitor calyculin A induced extensive cytotoxicity to CLL cells and failed to protect cells from FTY720 apoptosis (data not shown).
FTY720-induced toxicity in CLL cells is dependent on activation of PP2a. (A) FTY720-induced PP2A activity in CD19+ B cells from patients with CLL. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were incubated with DMSO or 10 μM FTY720 for 0, 1, 2, 3, or 15 hours. The PP2A activity in the cell lysates were measured as described in “Methods.” The left panel shows the time kinetics of a representative experiment. The right panel shows summary of PP2A activity at 4 hours in 5 independent samples in response to DMSO, 10 μM FTY720, or 1,9 di-deoxy-forskolin. (B) FTY720-induced PP2a activity in CD19+ B cells is inhibited by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for the indicated time periods. The PP2a activity in the cell lysates were measured by a nonradioactive assay as described in “PP2a activity (nonradioactive assay)” (n = 4; *P < .001 when compared with FTY720-treated group). (C) FTY720-induced PP2a activity in CD19+ B cells is inhibited by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for the indicated time periods. The PP2a activity in the cell lysates was measured using a radioactive assay as described in “PP2a activity (radioactive assay).” (D) FTY720-induced dephosphorylation of ERK1/2 in CD19+ B cells is inhibited by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for 3 hours. The ERK1/2 phosphorylation status was analyzed in cell lysates obtained under indicated treatment conditions using anti–phospho-ERK1/2 antibody. The levels of total ERK1/2 were analyzed in each lane by reprobing the blot with anti-ERK1/2 antibody. (E) FTY720-induced cellular toxicity is partially rescued by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720. The cells were stained with Annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and data were collected under list mode. The data shown represent the percentage of Annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 6; *P = .028 when comparing FTY720-treated vs OA plus FTY720-treated groups).
FTY720-induced toxicity in CLL cells is dependent on activation of PP2a. (A) FTY720-induced PP2A activity in CD19+ B cells from patients with CLL. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were incubated with DMSO or 10 μM FTY720 for 0, 1, 2, 3, or 15 hours. The PP2A activity in the cell lysates were measured as described in “Methods.” The left panel shows the time kinetics of a representative experiment. The right panel shows summary of PP2A activity at 4 hours in 5 independent samples in response to DMSO, 10 μM FTY720, or 1,9 di-deoxy-forskolin. (B) FTY720-induced PP2a activity in CD19+ B cells is inhibited by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for the indicated time periods. The PP2a activity in the cell lysates were measured by a nonradioactive assay as described in “PP2a activity (nonradioactive assay)” (n = 4; *P < .001 when compared with FTY720-treated group). (C) FTY720-induced PP2a activity in CD19+ B cells is inhibited by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for the indicated time periods. The PP2a activity in the cell lysates was measured using a radioactive assay as described in “PP2a activity (radioactive assay).” (D) FTY720-induced dephosphorylation of ERK1/2 in CD19+ B cells is inhibited by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for 3 hours. The ERK1/2 phosphorylation status was analyzed in cell lysates obtained under indicated treatment conditions using anti–phospho-ERK1/2 antibody. The levels of total ERK1/2 were analyzed in each lane by reprobing the blot with anti-ERK1/2 antibody. (E) FTY720-induced cellular toxicity is partially rescued by okadaic acid. Purified B-lymphocytes from patients with CLL (106 cells/mL media) were pretreated with media or okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720. The cells were stained with Annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and data were collected under list mode. The data shown represent the percentage of Annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 6; *P = .028 when comparing FTY720-treated vs OA plus FTY720-treated groups).
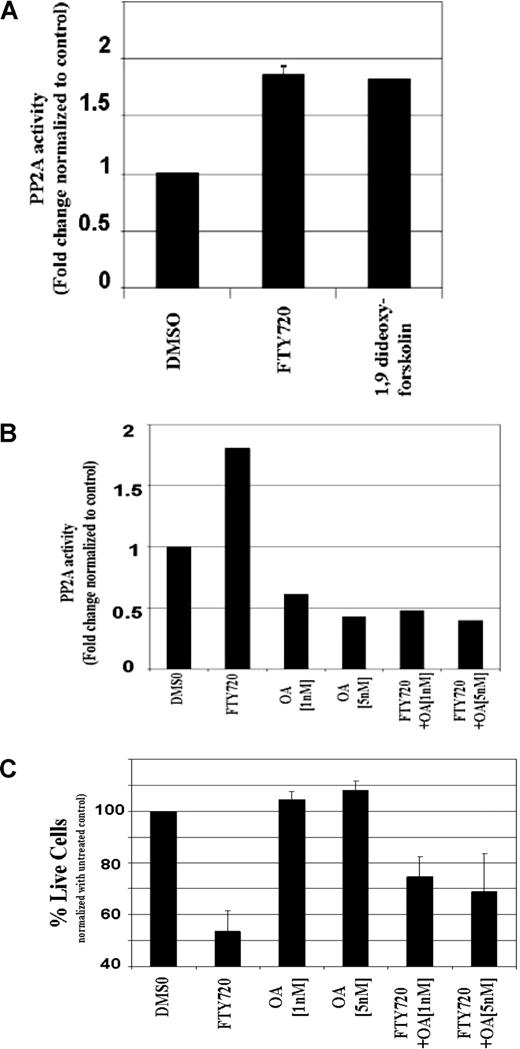
FTY720-induced toxicity in the Ramos B-cell line is dependent on activation of PP2a. (A) FTY720-induced PP2a activity in the Ramos B-cell line. Ramos B cells (105/mL media) were incubated with DMSO, 10 μM FTY720, or 100 μM 1,9 di-deoxy-forskolin for 4 hours. The PP2a activity in the cell lysates was measured as described “PP2a activity (nonradioactive assay).” The results shown are representative of 2 independent experiments. (B) FTY720-induced PP2a activity in the Ramos B-cell line is inhibited by okadaic acid. Ramos B cells (105/mL media) were pretreated with media or indicated concentrations of okadaic acid for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for 4 hours. The PP2a activity in the cell lysates was measured as described in “PP2a activity (nonradioactive assay).” The results shown are representative of 2 to 3 independent experiments. (C) FTY720-induced cellular toxicity is partially rescued by okadaic acid in Ramos B cells. Ramos B cells (105/mL media) were pretreated with media or indicated concentrations of okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry and the data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control. The results shown are the means (± SD) of 5 independent experiments.
FTY720-induced toxicity in the Ramos B-cell line is dependent on activation of PP2a. (A) FTY720-induced PP2a activity in the Ramos B-cell line. Ramos B cells (105/mL media) were incubated with DMSO, 10 μM FTY720, or 100 μM 1,9 di-deoxy-forskolin for 4 hours. The PP2a activity in the cell lysates was measured as described “PP2a activity (nonradioactive assay).” The results shown are representative of 2 independent experiments. (B) FTY720-induced PP2a activity in the Ramos B-cell line is inhibited by okadaic acid. Ramos B cells (105/mL media) were pretreated with media or indicated concentrations of okadaic acid for 2 hours, followed by incubation with DMSO or 10 μM FTY720 for 4 hours. The PP2a activity in the cell lysates was measured as described in “PP2a activity (nonradioactive assay).” The results shown are representative of 2 to 3 independent experiments. (C) FTY720-induced cellular toxicity is partially rescued by okadaic acid in Ramos B cells. Ramos B cells (105/mL media) were pretreated with media or indicated concentrations of okadaic acid (5 nM) for 2 hours, followed by incubation with DMSO or 10 μM FTY720. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry and the data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control. The results shown are the means (± SD) of 5 independent experiments.
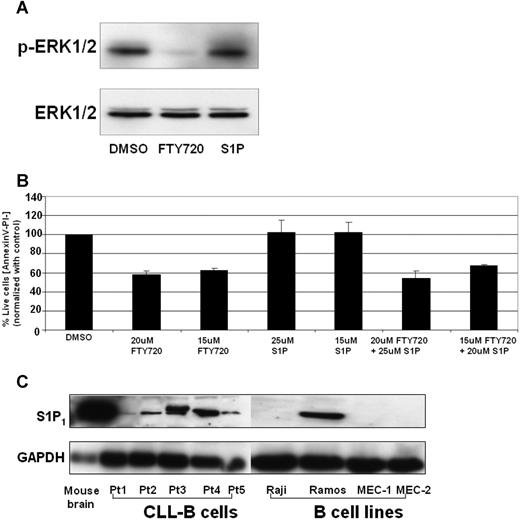
FTY720-mediated cell death is independent of spingosine-1-phosphate signaling in CLL B cells
FTY720 is phosphorylated by sphingosine kinase 2 and the phosphorylated compound is a potent agonist of 4 S1P receptors, which modulate chemotactic responses and lymphocyte trafficking. In order to investigate if the FTY720-mediated toxic effects are due to activation of S1P receptor1, we compared the effects of FTY720 or S1P in ERK1/2 dephosphorylation function. Treatment of CD19+ CLL B cells with FTY720 but not S1P induced dephosphorylation of ERK1/2 (Figure 6A). Furthermore, pretreatment of CLL B cells with S1P failed to either induce toxicity or rescue the toxic effects of FTY720 (Figure 6B), indicating FTY720 mediated its effects independent of S1P receptor 1 signaling. Consistent with this hypothesis, the Raji, MEC-1, and MEC-2 cell lines that lacked the predominant S1P receptor 1 expression still were susceptible to FTY720-induced toxicity (Figures 1, 6C; data not shown).
S1P receptor 1 (S1PR1) in FTY720-induced cell death. (A) FTY720 but not S1P induced ERK1/2 dephosphorylation in CLL B cells. CD19+ B cells (107) from patients with CLL were incubated with DMSO, FTY720 (5 μM), or S1P (1 μM) for 3 hours, and cell lysates were subjected to Western blotting as described in “Western blotting.” (B) S1P failed to prevent FTY720-induced cell death. CD19+ B cells (2 × 106) from patients with CLL were pretreated with indicated concentrations of S1P for 2 hours, followed by DMSO or FTY720 for 24 hours. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 3). (C) Expression of S1P1 in primary CLL B cells and B-cell lines. CD19+ B cells (107) from patients with CLL or Raji, Ramos, MEC-1, and MEC-2 B cells were lysed and subjected to Western blotting as described in “Western blotting.” Cell lysates from mouse brains were used as positive control (representative data from 2 experiments).
S1P receptor 1 (S1PR1) in FTY720-induced cell death. (A) FTY720 but not S1P induced ERK1/2 dephosphorylation in CLL B cells. CD19+ B cells (107) from patients with CLL were incubated with DMSO, FTY720 (5 μM), or S1P (1 μM) for 3 hours, and cell lysates were subjected to Western blotting as described in “Western blotting.” (B) S1P failed to prevent FTY720-induced cell death. CD19+ B cells (2 × 106) from patients with CLL were pretreated with indicated concentrations of S1P for 2 hours, followed by DMSO or FTY720 for 24 hours. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 3). (C) Expression of S1P1 in primary CLL B cells and B-cell lines. CD19+ B cells (107) from patients with CLL or Raji, Ramos, MEC-1, and MEC-2 B cells were lysed and subjected to Western blotting as described in “Western blotting.” Cell lysates from mouse brains were used as positive control (representative data from 2 experiments).
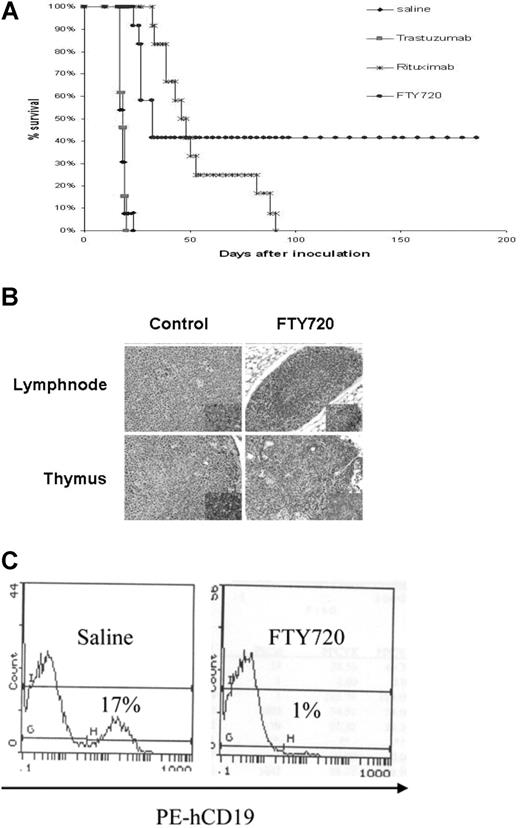
In vivo evaluation of FTY720 in a SCID xenograft mouse model of B-cell malignancy
In order to determine the in vivo effect of FTY720 in preventing B-cell tumor growth, we used a Raji cell–inoculated disseminated leukemia/lymphoma xenograft SCID mouse model. Intravenous injection of Raji cells in SCID mice resulted in infiltration of neoplastic cells in various organ systems, including bone marrow, lymph nodes, and CNS as revealed by histologic analysis of tissue sections. Multifocal neoplastic cell infiltration in the meninges associated with hind-limb paralysis between 17 and 21 days was noticed in all the control Raji cell–injected mice analyzed. Reproducible and reliable engraftment of Raji cells favored this model for investigating the in vivo therapeutic efficacy of FTY720, using hind-limb paralysis time/survival time as the primary endpoint for evaluation. The median survival time for FTY720-treated mice was 47 days (95% confidence interval [CI], 39-53). This is significantly prolonged compared with placebo controls (18 days; 95% CI, 17-19; FTY720 vs placebo P < .001). After a follow-up period of 200 days, 4 of the 12 mice treated with FTY720 were able to survive without signs of disease, including loss of body weight, lethargy, rough coat, or hind-limb paralysis (Figure 7A), which contrasts even with rituximab, a highly effective therapy for B-cell malignancies. Consistent with the elimination of the leukemic cells in the treated mice, immunohistochemical analysis using human CD45-specific antibody revealed elimination of injected Raji cells in the thymus and lymph node sections (Figure 7B). Flow cytometry analysis of bone marrow cells from these mice exhibited the presence of human CD19+ cells in the placebo-treated group (17%). In contrast, only 1% of human CD19+ cells were present in FTY720-treated mice (Figure 7C).
In vivo therapeutic evaluation of FTY720 in a SCID xenograft mouse model of disseminated lymphoma/leukemia. (A) Survival analysis of FTY720-treated mice. A total of 52 female 6- to 8-week-old C.B.-17 SCID mice were injected with 2 × 106 Raji cells intravenously by tail vein in 200 μL sterile PBS. At 74 hours after inoculation, the animals were divided into 4 equal treatment groups. The first 3 groups served as controls and received vehicle, trastuzumab, or rituximab injection (5 mg/kg) 3 times a week for 2 weeks; the fourth group consisted of animals treated with FTY720 (5 mg/kg) every day for 2 weeks intraperitoneally. All the animals were monitored daily for signs of illness and killed immediately if hind-limb paralysis, respiratory distress, or 30% body weight loss was noted. The endpoint of the study was survival defined as the time for the development of hind-limb paralysis. The median survival time for FTY720-treated mice was 47 days (95% CI, 39-53). This is significantly prolonged compared with placebo controls (18 days; 95% CI, 17-19; FTY720 vs placebo, P < .001). (B) Depletion of infiltrated human CD45+ cells in FTY720-treated mice, but not in control mice. Histologic (hematoxylin and eosin staining) analysis of lymph node (top panels) and thymus (bottom panels) from control untreated (left panels) or FTY720-treated (right panels) mice. Insert shows immunohistochemical analysis of human CD45+ cells in the respective sections from untreated and FTY720 treated mice. (C) Flow cytometric analysis of bone marrow cells from Raji cells engrafted mice at day 17 after inoculation. Mice were treated with placebo or FTY720, and the percentage of human CD19+ cells in the bone marrow were analyzed by flow cytometry using anti-human CD19 antibody that detects the human (Raji) but not mouse B cells.
In vivo therapeutic evaluation of FTY720 in a SCID xenograft mouse model of disseminated lymphoma/leukemia. (A) Survival analysis of FTY720-treated mice. A total of 52 female 6- to 8-week-old C.B.-17 SCID mice were injected with 2 × 106 Raji cells intravenously by tail vein in 200 μL sterile PBS. At 74 hours after inoculation, the animals were divided into 4 equal treatment groups. The first 3 groups served as controls and received vehicle, trastuzumab, or rituximab injection (5 mg/kg) 3 times a week for 2 weeks; the fourth group consisted of animals treated with FTY720 (5 mg/kg) every day for 2 weeks intraperitoneally. All the animals were monitored daily for signs of illness and killed immediately if hind-limb paralysis, respiratory distress, or 30% body weight loss was noted. The endpoint of the study was survival defined as the time for the development of hind-limb paralysis. The median survival time for FTY720-treated mice was 47 days (95% CI, 39-53). This is significantly prolonged compared with placebo controls (18 days; 95% CI, 17-19; FTY720 vs placebo, P < .001). (B) Depletion of infiltrated human CD45+ cells in FTY720-treated mice, but not in control mice. Histologic (hematoxylin and eosin staining) analysis of lymph node (top panels) and thymus (bottom panels) from control untreated (left panels) or FTY720-treated (right panels) mice. Insert shows immunohistochemical analysis of human CD45+ cells in the respective sections from untreated and FTY720 treated mice. (C) Flow cytometric analysis of bone marrow cells from Raji cells engrafted mice at day 17 after inoculation. Mice were treated with placebo or FTY720, and the percentage of human CD19+ cells in the bone marrow were analyzed by flow cytometry using anti-human CD19 antibody that detects the human (Raji) but not mouse B cells.
Discussion
The studies described herein demonstrate that FTY720 is a potent toxic agent for ALL and CLL. These studies are derived from a wide range of cell lines and primary leukemic cells from patients with CLL. Importantly, our data demonstrate that FTY720 is different from many therapeutic agents currently under study in lymphoid malignancies. First, FTY720 mediates cytotoxicity that is not dependent on activation of caspases, as demonstrated in both primary CLL cells and lymphoblastic cell lines. Second, FTY720 mediates its cytotoxic effect independent of Bcl-2 expression, a finding that is relatively uncommon among most therapeutics used to treat lymphoid malignancy. Finally, the biologic effect of FTY720 on ALL and CLL cells seems to be in part explained by activation of PP2a and potentially other phosphatases, distinguishing it from other therapeutics currently used in these diseases. These in vitro data demonstrating a novel mechanism of action and potential promising therapeutic efficacy are further supported by in vivo data in a xenograft-disseminated leukemia model in which FTY720 significantly prolongs survival and cures a subset of mice. FTY720 compares favorably to the highly effective therapeutic antibody rituximab in this in vivo model. Considered together, our data provide strong support for further clinical development of FTY720 for the treatment of B-cell ALL and CLL.
It is important to consider that our data explore a different target of FTY720 (PP2a activation), and requires higher concentrations of drug than identified for the S1P receptors when used in the setting of organ transplantation. Development of FTY720 will therefore require a classic phase 1 dose-escalation study in patients with leukemia to determine if the micromolar concentration of the drug is attainable which promotes apoptosis in CLL and ALL cells. As part of our studies, we did not perform pharmacokinetics with our murine model. The dose used in the current in vivo studies was determined by in vitro IC50 and in vivo pharmacokinetics data. Rat pharmacokinetics demonstrate that after a single dose of intravenous administration of 4 mg/kg, FTY720 achieved a 3.5-μM maximum plasma concentration.37 Therefore, our regimen of 5 mg/kg intraperitoneally every day for 2 weeks is likely to achieve micromolar steady-state concentration. The IC50 of FTY720 in Raji cells decreases with time, as shown in Table 1. At 48 hours, the IC50 of FTY720 is 7.84 μM. It is possible that the IC50 will be at a low micromolar range if incubation time increases, which can be very close to the IC50 observed with Raji cells in vitro. Indeed, therapeutic benefit in vivo was observed using the disseminated Raji leukemia model without any observed weight loss or toxicity in these animals. Given the detailed clinical data available with FTY720, we believe our data support the initiation of additional dose-escalation studies of this agent in patients with ALL and CLL.
Our data also showed that FTY720-mediated toxicity might be independent of S1P receptors. CLL cells express S1P1, which is the major receptor among all the known S1P receptors to play a role in lymphocyte sequestration in FTY720-treated organ transplantation patients. Neither S1P, native ligand for S1P receptors, nor the sphingosine rescued CLL B cells from FTY720-induced toxicity. On the contrary, sphingosine and FTY720 exhibited an additive effect on CLL B-cell apoptosis (data not shown). The mechanisms responsible for this additive effect remains to be examined.
The ability of FTY720 to mediate caspase-independent death in ALL and CLL cells is different from the previous reports on other cell types studied. Two separate studies examining both the Jurkat T lymphoblast cell line and multiple myeloma cell lines suggested that caspase activation was important.24,25 Although we found Raji cell lines and primary CLL cells undergo apoptosis through a caspase-independent mechanism as described herein, subsequent investigation of the Ramos cell line demonstrates caspase activation to be important to FTY720-mediated death, as observed in other cell types.24,25 It is very interesting that FTY720 can work through caspase-dependent and -independent mechanisms in different cell types. We speculate that it is related to the diversified specificity of PP2a. PP2a is a heterotrimer protein that contains a structural A subunit, a regulatory B subunit, and a catalytic C subunit. The A and C subunits are evolutionarily conserved and ubiquitously expressed, while the B subunit is expressed differently in tissues. Moreover, the B subunit is known to control the specificity of PP2a toward its substrates. Therefore, it is possible that different isoforms of B subunits are expressed in CLL B cells and Jurkat cells, which leads to their different actions on the substrates. In CLL B cells, we found that FTY720 dephosphorylates ERK1/2 but not Bcl-2 or Akt (data not shown). Phosphorylated Bcl-2 and Akt are tightly related with caspase pathway to regulate apoptosis. In those cells in which FTY720 works through a caspase-dependent mechanism, PP2a may be activated and dephosphorylate a crucial protein, such as Bcl-2, to activate the caspase pathway. The differences in caspase dependency in different cell types can also be attributed to intrinsic differences among the primary CLL cells freshly isolated from patients and transformed cell lines maintained in vitro, which have the propensity to accumulate mutations and selections in vitro. These differences (as evidenced by our microarray analysis of Raji and Ramos cell lines; data not shown) may include but not limited to differential expression of cytokine receptors such as IL-21 receptor and cytokines including but not limited to TGF-β, which are tightly involved in extrinsic caspase pathways. The changed expression of these proteins may result from the regulation of mRNA transcription, translation, and/or change of the proteins' stability that remains to be evaluated.
The FTY720-mediated cytotoxicity in CLL B cells appears to be distinct from that observed in other cell types, including multiple myeloma, Jurkat T cells, and Ramos cells. Although the differential effects could be attributed to intrinsic differences in expression and/or regulation of cell-growth molecules, the potential differences in FTY720 downstream targets remains to be tested. It can also be explained by the characteristic expression of some of the anti- or proapoptotic proteins in CLL B cells. For example, the antiapoptotic protein Mcl-1, which is found to be down-regulated by FTY720 in CLL cells (Figure 3), is overexpressed in CLL B cells. Although Mcl-1 downmodulation has been shown to be critical for CLL apoptosis,34 down-regulation of Mcl-1 failed to result in cell death in several cell lines, such as sarcoma cells.39 It is possible that modulation of Mcl-1 by FTY720 might be one of the unique mechanisms for apoptosis in CLL cells.
FTY720 has been shown to mediate activation of the PP2a enzyme in Jurkat T cells.20 Three lines of evidences indicate a role for PP2a induction by FTY720 in CLL B cells. First, consistent with the previous reports in T cells, FTY720 induced time-dependent activation of PP2a enzymatic activity in CLL and B-cell lines. Second, the FTY720-induced PP2a activity can be blocked by okadaic acid, an inhibitor of PP2a. The blocking of FTY720-induced PP2a activity with OA was further reflected in FTY720-induced dephosphorylation of the PP2a target phospho-ERK1/2. Third, consistent with a role for FTY720-induced PP2a activation in the induction of apoptosis, concentrations of okadaic acid that inhibited FTY720-induced PP2a activation also resulted in partial inhibition of FTY720-induced apoptosis. This suggests a possible role for activated PP2a in FTY720-induced apoptosis of CLL and lymphoblast cells. The precise role of PP2a, alternative phosphatases, and relevant downstream targets responsible for FTY720-induced apoptosis remain to be examined. Preliminary assessment of Akt, STAT3, Bcl-2, and Bad protein phosphorylation sites corresponding to those modified by PP2a at early time points prior to induction of death do not clearly demonstrate any change prior to cell death (data not shown). However, this is not contradictory to the findings in the literature. As mentioned previously, a regulatory B subunit of PP2a is differentially expressed in various organs and cells, which leads to its specificity toward different substrates in different cell types. The role of ERK1/2 dephosphorylation in FTY720-induced apoptosis in CLL cells remains to be examined. PP2a has many targets, and it is possible that a yet-unidentified target is being altered by the FTY720 target immediately after phosphatase activation. Alternatively, it is possible that PP2a activation is a relevant surrogate marker for FTY720-mediated cell death, but that either alternative phosphatases or upstream events are the primary events directly contributing to apoptosis induced by FTY720. In this context, future efforts should focus on ceramide generation upstream of activation of PP2a and potential alternative phosphatases activated by FTY720.
To this point, FTY720 has been solely developed as an immunosuppressive agent for the prevention of organ transplant rejection. Toxicity in the phases 1, 2, and 3 studies of FTY720 have been relatively unremarkable relative to the dose-limiting toxicities observed with many anticancer therapies.11,–13,15 Our in vitro and in vivo evaluation of FTY720 in acute and chronic lymphoid leukemia suggest this agent may have potential therapeutic benefit in these diseases. Recent investigation by others in prostate cancer,39 breast cancer,40,41 hepatocellular cancer,42,43 and multiple myeloma24 provide further support for development of FTY720 as an antineoplastic agent. It is quite possible that the therapeutic dose to activate PP2a and promote apoptosis will be different than the immunosuppressive dose used in the organ transplantation studies performed to date. Our PP2a activity induction data and ERK dephosphorylation occurring soon after treatment with FTY720 in primary CLL cells corresponding to the concentrations that apoptosis occurs provides a biomarker to accompany phase 1 dose escalation in this disease. Identification of the minimally effective dose of FTY720 that promotes PP2a activation should be part of FTY720 clinical development that hopefully will avoid excessive dose escalation in such studies. This approach has been used successfully with other molecularly targeted therapies.
In conclusion, our in vitro and in vivo data demonstrate that FTY720 exhibits potent antigrowth and proapoptotic properties in ALL and CLL cells. The novel caspase- and Bcl-2–independent mechanism of cell killing concurrent with identification of PP2a activation as a surrogate marker of cell killing provide further justification for clinical development of this agent in lymphoid leukemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John C. Reed for providing the 697 cell line that overexpresses Bcl-2.
This work was supported by grants from the Leukemia and Lymphoma Society (J.C.B.) and Specialized Center of Research from the Leukemia and Lymphoma Society (Q.L., X.Z., Y.M., C.S.C., J.C.B., and N.M.), The D. Warren Brown Foundation (J.C.B., N.M.), the National Cancer Institute (P01 CA95426 [J.C.B. and N.M.] and CA095512 [D.P.]), National Institutes of Health, Bethesda MD; and from the U.S. Army, Chronic Myelogenous Leukemia Research Program, DAMD17-03-1-0184 (D.P.); and the American-Italian Cancer Research Foundation (P.N.).
National Institutes of Health
Authorship
Contribution: Q.L. performed the majority of the in vitro research, analyzed the data, and wrote the initial draft of the paper. X.Z. performed the in vivo research and reviewed the drafts of the paper. F.F. assisted with the in vitro work and reviewed the drafts of the paper. Y.M. synthesized FTY720 and reviewed drafts of the paper. R.S. participated in the planning of experiments and reviewed drafts of the paper. D.J. particpicated in designing sample sizes used for each experiment, performed the statistical analysis, and reviewed drafts of the paper. A.L. particpicated in designing sample sizes used for each experiment, performed the statistical analysis, and reviewed drafts of the paper. D.P. participated in planning the studies and reviewing drafts of the paper. C.-S.C. supervised the synthesis of FTY720, assisted in planning the research, and reviewed drafts of the paper. J.T.D. assisted in planning these studies and reviewed drafts of the paper. N.M. and J.C.B. together designed the research, reviewed all of the data, participated in analysis of data, and modified the initial drafts of the paper.
N.M. and J.C.B. are senior authors who contributed equally to the work.
Conflict-of-interest disclosure: A provisional patent has been filed for the use of FTY720 to treat hematologic malignancies based on these data. The authors declare no additional competing financial interests.
Correspondence: John C. Byrd, B302 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu; or Natarajan Muthusamy, 455E OSUCCC Bldg, 410 West 12th Ave, Columbus, OH 43210; e-mail: raj.muthusamy@osumc.edu.

![Figure 2. FTY720-induced toxicity in CLL cells is independent on caspase activation. (A) Pancaspase inhibitor z-VAD-fmk failed to rescue CLL B cells from FTY720-induced cell death. Purified CD19+ cells from patients with CLL (106 cells/mL media) were incubated with 10 μM FTY720 in the presence or absence of z-VAD-fmk (150 μM) for 24 hours. The cells were stained with annexin V–FITC and PI as described in “Analysis of cell viability and apoptosis.” The cells were analyzed by flow cytometry, and the data were collected under list mode. The data shown represent the percentage of annexin V−/PI− viable cells plus or minus SD that are normalized to media control (n = 3; *P = .001 when compared with FARA [2F-Adenine Arabinoside or fludarabine]-treated group; **P = .998 when compared with FTY720-treated group). (B) FTY720 failed to induce caspase-3, caspase-8, or PARP processing. Purified CD19+ cells from patients with CLL (106 cells/mL media) were incubated with DMSO (None) or 10 μM FTY720 for 24 and 48 hours. Western blot analysis of the lysates from each of the conditions were assessed for processed and unprocessed PARP, caspase-3, and caspase-8 as described in “Western blotting.” The bold arrow indicates the unprocessed form of each of the proteins; the dashed arrows indicate cleaved products. Untreated and UV-treated Jurkat cell lysates were used as positive controls. (C) FTY720 induced activation of PARP processing in the Ramos but not the Raji cell line. Ramos or Raji cells (105/mL media) were incubated with DMSO and 150 μM z-VAD-fmk with or without 10 μM FTY720 for 24 hours. Western blotting of PARP protein for each of the conditions for both cell lines is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/1/10.1182_blood-2006-10-053884/3/m_zh80240710500002.jpeg?Expires=1769108742&Signature=S7ASgzlu00GhwDAxu8jQIeK4qoMHEXDR73Tp-rHmTXpUCp3x6p~JMKWEd41XYKrWy8~QHQf-ClLw-W9DPwINUuFdO~CtVni~T~AgHEmhGs2F5wrLtxqvC9HSwZ3ElJBi~5rEXGiqkQWDFf9Pn49BJZHgk4nQ43dhgfKFFbIXmTsq7eGZcBPq8Kl3KSgf-aFd8s6g9GnTmkOj0frNw~XxfRRfVRs6LdOrFEp5UMrs818lV9PhkscYOQmBHj-~RwUubw1Va7ncuir2mjdIIRJEOiIsq4sx9WZ8Y4gsP0QQHGJDwP6wdc9CXAxL6nGn8NhXDfs7WWQFF3IFvv7Yn5Bekw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal