In Ph+ chronic myeloid leukemia (CML), the constitutively active Bcr-Abl kinase leads to the up-regulation and activation of multiple genes, which may subsequently result in the expression of leukemia-associated antigens. In this study, we investigated the immunogenicity of Bcr-Abl–regulated antigens by stimulating CD8+ T lymphocytes with autologous dendritic cells transfected with RNA coding for Bcr-Abl wild-type or a kinase-deficient mutant. Significant HLA class I–restricted T-cell responses were detected against antigens regulated by the Bcr-Abl kinase, but not toward the Bcr-Abl protein itself. The T-cell repertoire of a patient with CML in major molecular remission due to imatinib mesylate was also dominated by T cells directed against Bcr-Abl–regulated antigens. These results encourage the development of immunotherapeutic approaches against Bcr-Abl–regulated antigens for the treatment of CML patients with residual disease following therapy with Bcr-Abl kinase inhibitors.
Introduction
The initiating oncogenic event leading to the development of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) is the t(9;22) translocation, in which c-ABL gene sequences from chromosome 9 are fused to breakpoint-cluster-region (BCR) gene sequences on chromosome 22. The product of this chimeric gene is the constitutively active tyrosine kinase Bcr-Abl, which leads to the activation and enhanced expression of multiple genes and consecutively results in the malignant phenotype of a Ph+ cell. The gene-expression pattern of the CML bone marrow is not static, but constantly undergoes changes according to the phase of disease.1
Hence, the leukemia-specific Bcr-Abl oncogenic protein is a favored target for the development of new therapeutics for the treatment of CML, including the small molecule kinase inhibitors. Impressive response rates are achieved treating chronic myeloid leukemia in chronic phase with the Bcr-Abl kinase inhibitor imatinib mesylate (IM, Gleevec; Novartis, Basel, Switzerland).2 However, resistance to IM frequently evolves in advanced-phase CML due to Bcr-Abl gene amplification and mutations.3 The second-generation kinase inhibitors may be also associated with clinical resistance due to a spectrum of point mutations.4 In addition, the kinase inhibitors may have differential effects on CML cells depending on their state of differentiation. It has been shown that IM is highly toxic to differentiated CML progenitors, but CML stem cells are relatively or even completely resistant to the drug.5 The insensitivity of quiescent leukemic stem cells toward kinase inhibitors may lead to the selective outgrowth of these cells and finally to disease relapse even after years of continuous treatment.
Cytotoxic T lymphocytes have the potential to eliminate CML stem cells. Proof of principle has been demonstrated in an exceptional clinical situation where donor lymphocyte infusions can induce complete cytogenetic remissions of CML relapsed after allogeneic stem cell transplantation.6 The donor's T lymphocytes include allorestricted T cells, which may ideally combine antigen specificity, high avidity, and a superior leukemia-lytic function. However, most of the allorestricted T cells display broad peptide specificity or even a peptide-independent HLA-dominant binding, both characteristics leading to a wide reactivity and potentially to graft-versus-host disease. Therefore, the current immunotherapeutic concepts focus on targeting those antigens that are preferentially or even exclusively expressed by CML cells, including the CML stem cell.
The research on developing CML-directed immunotherapies has focused on the Bcr-Abl protein, because first, Bcr-Abl contributes to the malignant phenotype and, therefore, is exclusively expressed by the malignant cells, and second, the joining region segment of p210 Bcr-Abl may serve as a neoantigen being recognized by high-avidity cytolytic T cells with an excellent tumor recognition efficiency.7 In humans, it has been demonstrated that a nonapeptide derived from the Bcr-Abl b3a2 fusion region is naturally presented in context with HLA-A*0301 and can be recognized by preexisting T cells.8 However, the most recent research on immunogenic peptides spanning the fusion region uncovered some limitations of this approach: first, the number of breakpoint peptides potentially being presented in context with HLA class I molecules is limited9 ; second, cytotoxic T-cell responses against junctional peptides were rarely detected and, in particular, the presence of high-avidity T cells could not to be demonstrated in CML patients with or without immunotherapy.10,11 These observations indicate that the Bcr-Abl breakpoint peptides may not be highly immunogenic and that other antigens than Bcr-Abl seem to be responsible for the immunogenicity of CML cells. This leads to the hypothesis that not Bcr-Abl, itself, but genes that are up-regulated by the Bcr-Abl kinase activity may represent the crucial antigens for the induction of a cytotoxic T-cell response against Ph+ CML cells.12,13 For example, the tumor-associated antigens PRAME and PCNA have been identified as Bcr-Abl inducible genes, which are further expressed as proteins and presented as peptides with HLA class I.14,15 However, the dominance of these Bcr-Abl–regulated antigens in a CML-directed T-cell response remains unclear.
In this study, we demonstrate that the constitutively active kinase domain of Bcr-Abl has a key role in enhancing the immunogenicity of Bcr-Abl+ cells as the HLA class I–restricted T-cell responses were dominated by Bcr-Abl–regulated antigens, and not by Bcr-Abl itself. The inhibition of Bcr-Abl kinase activity by IM significantly diminished the immunogenicity of Bcr-Abl+ cells. These results will contribute to the design of immunotherapeutic approaches for the treatment of CML patients with minimal residual disease, in particular following successful induction therapy with Bcr-Abl kinase inhibitors.
Patients, materials, and methods
Site-directed mutagenesis of Bcr-Abl
To knock down the Bcr-Abl kinase domain, the lysine of codon 1172 of the p210 Bcr-Abl wild type (WT) was changed to an arginine by an adenine-to-guanine mutation (AAG to AGG). The introduction of the mutation was performed in a 2-step polymerase chain reaction (PCR) approach using mutated primers. The resultant construct Bcr-Abl kinase-deficient (KD) variant carried the previously described mutation K1172R.16 Inactivation of the kinase domain in the new construct Bcr-Abl KD was confirmed in Western blot analysis (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Production of in vitro–transcribed mRNA
The in vitro transcription reaction to create mRNA of the Bcr-Abl WT and KD constructs was performed at CureVac (Tuebingen, Germany). The constructs were cloned into a T7 vector containing a poly-A sequence and a stabilizing sequence derived from the untranslated region of the Xenopus laevis β-globin gene17 between the Bcr-Abl gene and the poly-A tail.
Stimulation of T cells toward Bcr-Abl–regulated antigens
Approval for this study was obtained from the Institutional Ethics Committee of the Klinikum rechts der Isar, Technical University of Munich. Informed consent was obtained in accordance with the Declaration of Helsinki. The peripheral blood mononuclear cells (PBMCs) were derived from healthy donors and CML patients, and the CD8+ T cells were isolated using magnetic-activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) after indirect antibody staining. The isolated CD8+ T cells were stimulated with dendritic cells (DCs) that had been transfected with the mRNA coding for Bcr-Abl WT or KD. CD8+ T cells (105) and transfected DCs (5 × 103) were cocultured in 200 μL AIM-V medium/well (Gibco, Carlsbad, CA) of a 96-well round-bottom plate with 5% human AB-Serum (Milan Analytica, LaRoche Switzerland), 1000 U/mL IL6, and 10 ng/mL IL12. Second and third stimulations were set up as described above in this paragraph except that the T-cell cultures were split 1:2 and 50 U/mL IL2 and 5 ng/mL IL7 were added instead of IL6 and IL12. The term “T-cell population” refers to the CD8+ T cells that had been stimulated with autologous DCs together in one well of a 96-well plate.
Specificity analysis of stimulated T cells
Following 3 stimulations of CD8+ T cells with autologous Bcr-Abl WT– and Bcr-Abl KD–expressing DCs, the production of IFN-γ or granzyme B by the stimulated T cells was determined in an enzyme-linked immunosorbent spot (ELISpot) assay (for details, see Document S1). Median spot values and their interquartile range were calculated for each set of T-cell populations using Delta Graph software (Delta Graph 5.5.1; Red Rock Software, Salt Lake City, UT). For the comparison of different groups, the P value was calculated in a Mann-Whitney test. IFN-γ spot indices (SIs) were calculated to compare the responses in the healthy donors and the CML patient as follows: mean spot value against Bcr-Abl WT+ DCs/mean spot value against control DCs.
Results and discussion
Leukemia-associated antigens can be up-regulated in DCs expressing kinase-active Bcr-Abl
In this study, we have imitated the physiological interaction of Bcr-Abl+ DCs and autologous CD8+ T lymphocytes in an in vitro priming model to analyze the T-cell repertoire toward Bcr-Abl–expressing cells. For this purpose, we generated DCs expressing Bcr-Abl as a WT or KD variant following transfection with the respective RNA (Figure S1A). To confirm the tyrosine kinase activity of Bcr-Abl WT in the RNA-transfected DCs, we documented the enhanced expression of PRAME, an antigenic protein that is known to be up-regulated by Bcr-Abl (Figure S1B).14 Vice versa, the expression of PRAME was unchanged in DCs that had been transfected with the inactive KD variant of Bcr-Abl. Using this experimental setting, we could identify 2 additional antigens that are induced by the Bcr-Abl kinase activity: first, the up-regulation of PR3—an antigen known to be overexpressed in CML cells and to be immunogenic in CML patients18,19 ; second, the de novo expression of HAGE—a cancer-testis antigen frequently expressed in CML.20 The close relationship between Bcr-Abl activity and expression of PR3 and HAGE further supports the significance of these CML-associated antigens for the development of immunotherapeutic approaches against CML.
Stimulation of CD8+ T lymphocytes with Bcr-Abl WT+ DCs results in T-cell activation toward Bcr-Abl–regulated antigens
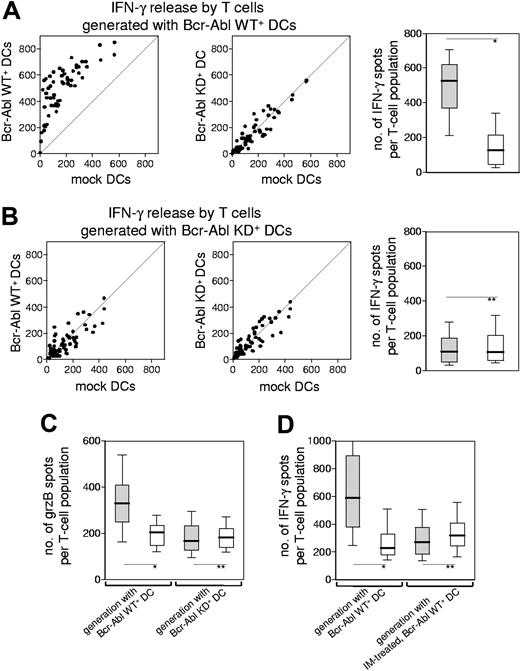
Based on our findings that Bcr-Abl–expressing DCs were able to up-regulate potentially immunogenic antigens, we were led to question whether these Bcr-Abl+ DCs were also capable of inducing a primary CTL response against the Bcr-Abl–regulated antigens. To answer this question, CD8+ T lymphocytes from healthy donors were stimulated with autologous DCs that had been transfected with Bcr-Abl WT. Specificity and functionality of the proliferating T-cell populations were documented by analyzing their IFN-γ release in the presence of autologous DCs that had been transfected either with the functionally active Bcr-Abl WT or the kinase-inactive variant Bcr-Abl KD (Figure 1A). Using this experimental setting, we were able to compare the immunogenic potential of those antigens, which had been up-regulated by Bcr-Abl, with the immunogenicity of the Bcr-Abl protein itself. The pattern of T-cell–derived IFN-γ release strongly suggests that the Bcr-Abl molecule is not an immunodominant antigen, but that the enhanced immunogenicity of Bcr-Abl WT+ DCs is due to antigens up-regulated by Bcr-Abl. This hypothesis was confirmed by the fact that DCs transfected with Bcr-Abl KD were unable to generate T-cell populations with superior activity toward dendritic cells expressing the wild type of Bcr-Abl (Figure 1B). The cytolytic potential of these T cells was documented by measuring their release of granzyme B (Figure 1C). Of note, the pattern of granzyme B (grzB) release upon stimulation with Bcr-Abl WT+ DCs or with Bcr-Abl KD+ DCs was identical to the results obtained by measuring IFN-γ production confirming the immunogenicity of Bcr-Abl WT+ DCs. To validate the significance of Bcr-Abl–regulated antigens for the immunogenicity of Ph+ leukemic cells, we performed T-cell stimulations with Bcr-Abl WT+ DCs or Bcr-Abl KD+ DCs derived from an HLA-A2+ donor and screened the responding autologous T cells for their reactivity against T2 cells loaded with the PRAME-derived peptide p300-309 known to be naturally presented with HLA-A2.21 Indeed, the T cells stimulated with Bcr-Abl WT+ DCs, but not with Bcr-Abl KD+ DCs, released specifically IFN-γ against the PRAME-derived peptide p300-309, but not against the HLA-A2–binding irrelevant peptide p476-484 derived from HIVpol (data not shown).
The immunogenicity of Bcr-Abl–expressing dendritic cells is dependent on the Bcr-Abl kinase activity. (A) CD8+ T-cell populations from a healthy donor (HD no. 1, Table S1) were primed with autologous Bcr-Abl WT+ DCs. IFN-γ production was tested 1 week after the third stimulation in an ELISpot assay. Every single T-cell population was divided into 3 aliquots and coincubated with autologous Bcr-Abl WT+ DCs, Bcr-Abl KD+ DCs, or mock-transfected DCs to provoke IFN-γ release by the T cells. The scatterplots show the number of IFN-γ spots produced by every T-cell population (n = 60) after incubation with the respective DCs (left and middle). Incubation with Bcr-Abl WT+ DCs resulted in higher IFN-γ production compared with Bcr-Abl KD+ DCs or mock-transfected DCs. The magnitudes of responses against Bcr-Abl WT+ (▒) or KD+ DCs (░) were compared in a box plot showing the median distribution of IFN-γ–producing T-cell populations (right). Black lines in boxes indicate median spot value; boxes represent interquartile range; whiskers extend from the 10th percentile at the bottom and the 90th percentile at the top. (B) CD8+ T-cell populations from the same donor were primed with autologous Bcr-Abl KD+ DCs. IFN-γ production of T cells was assessed 1 week after the third stimulation in an ELISpot assay (left and middle). Incubation with Bcr-Abl WT+ or Bcr-Abl KD+ DCs as stimulator cells did not result in an increased IFN-γ production compared with the mock control. Statistical evaluation of the magnitudes of responses against Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░) (right). (A-B) Similar results have been obtained from additional 2 healthy donors (HD no. 2 and HD no. 4). (C) GrzB release by CD8+ T cells after 3 stimulations with Bcr-Abl WT+ DCs (left) or Bcr-Abl KD+ DCs (right) derived from HD no. 3 in the presence of autologous Bcr-Abl WT+ DCs (▒) and Bcr-Abl KD+ DCs (░) (*P < .001; **P = ns; Mann-Whitney test). (D) IFN-γ release of CD8+ T-cell populations (n = 60) that had been generated by repetitive stimulation with Bcr-Abl WT+ DCs derived from HD no. 5, either untreated (left) or IM treated (right). To provoke IFN-γ release, the autologous Bcr-Abl WT+ DCs were pretreated with IM or left untreated prior to coculture with the established T-cell populations. The box plot shows the median distribution of IFN-γ–producing T-cell populations after incubation with untreated (▒) or IM pretreated (░) Bcr-Abl WT+ DCs (*P < .001; **P = ns; Mann-Whitney test).
The immunogenicity of Bcr-Abl–expressing dendritic cells is dependent on the Bcr-Abl kinase activity. (A) CD8+ T-cell populations from a healthy donor (HD no. 1, Table S1) were primed with autologous Bcr-Abl WT+ DCs. IFN-γ production was tested 1 week after the third stimulation in an ELISpot assay. Every single T-cell population was divided into 3 aliquots and coincubated with autologous Bcr-Abl WT+ DCs, Bcr-Abl KD+ DCs, or mock-transfected DCs to provoke IFN-γ release by the T cells. The scatterplots show the number of IFN-γ spots produced by every T-cell population (n = 60) after incubation with the respective DCs (left and middle). Incubation with Bcr-Abl WT+ DCs resulted in higher IFN-γ production compared with Bcr-Abl KD+ DCs or mock-transfected DCs. The magnitudes of responses against Bcr-Abl WT+ (▒) or KD+ DCs (░) were compared in a box plot showing the median distribution of IFN-γ–producing T-cell populations (right). Black lines in boxes indicate median spot value; boxes represent interquartile range; whiskers extend from the 10th percentile at the bottom and the 90th percentile at the top. (B) CD8+ T-cell populations from the same donor were primed with autologous Bcr-Abl KD+ DCs. IFN-γ production of T cells was assessed 1 week after the third stimulation in an ELISpot assay (left and middle). Incubation with Bcr-Abl WT+ or Bcr-Abl KD+ DCs as stimulator cells did not result in an increased IFN-γ production compared with the mock control. Statistical evaluation of the magnitudes of responses against Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░) (right). (A-B) Similar results have been obtained from additional 2 healthy donors (HD no. 2 and HD no. 4). (C) GrzB release by CD8+ T cells after 3 stimulations with Bcr-Abl WT+ DCs (left) or Bcr-Abl KD+ DCs (right) derived from HD no. 3 in the presence of autologous Bcr-Abl WT+ DCs (▒) and Bcr-Abl KD+ DCs (░) (*P < .001; **P = ns; Mann-Whitney test). (D) IFN-γ release of CD8+ T-cell populations (n = 60) that had been generated by repetitive stimulation with Bcr-Abl WT+ DCs derived from HD no. 5, either untreated (left) or IM treated (right). To provoke IFN-γ release, the autologous Bcr-Abl WT+ DCs were pretreated with IM or left untreated prior to coculture with the established T-cell populations. The box plot shows the median distribution of IFN-γ–producing T-cell populations after incubation with untreated (▒) or IM pretreated (░) Bcr-Abl WT+ DCs (*P < .001; **P = ns; Mann-Whitney test).
IM reduces the immunogenicity of Bcr-Abl WT+ DCs
We next addressed the question of whether the use of Bcr-Abl WT+ DCs pretreated with 3 μM of the tyrosine kinase inhibitor IM results in a reduced T-cell activation pattern as found after stimulation with Bcr-Abl KD+ DCs. The concentration of 3 μM IM corresponds to the average serum level of patients on the standard therapeutic dose of 400 mg by mouth per day and was shown to completely inhibit the Bcr-Abl kinase activity in Ph+ cell lines (data not shown). The treatment of Bcr-Abl WT+ DCs with IM resulted in significantly decreased T-cell responses, confirming that the active kinase domain of Bcr-Abl is a pivotal element for the immunogenicity of Bcr-Abl+ DCs (Figure 1D). Of note, a suppressive effect of IM itself on DC maturation and function, which had been previously observed in a different model,22 was not apparent in our experimental setting (Figure S2 and data not shown).
T cells stimulated with Bcr-Abl WT+ DCs recognize Ph+ leukemia cells in an HLA-dependent manner
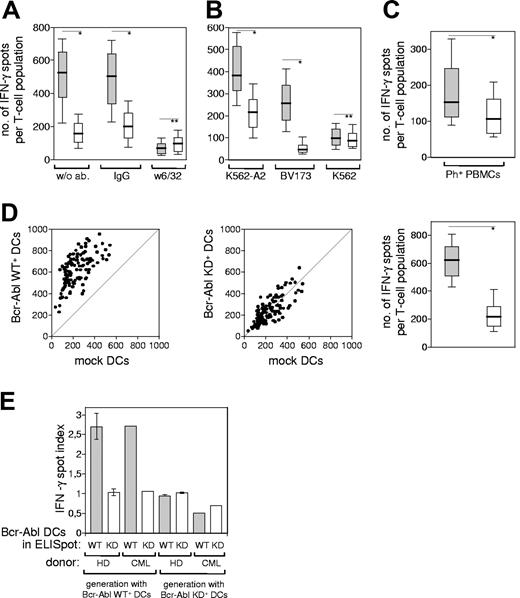
It has been shown that the Bcr-Abl oncogene controls the expression of MHC class I chain-related molecule A (MICA), one of the ligands for the NK cell–activating receptor NKG2D.23 As not only NK cells but also CD8+ T cells can express NKG2D,24 the herein shown T-cell activation via Bcr-Abl WT+ DCs could potentially be independent of the antigen/TCR interaction. We excluded this possibility by showing that the CD8+ T cells recognized the Bcr-Abl–related antigens in an HLA-dependent manner, because the blockade with an inhibiting antibody against HLA class I abrogated the IFN-γ release (Figure 2A). In addition, the CD8+ T-cell populations generated with Bcr-Abl WT+ DCs recognized the Bcr-Abl–regulated antigens also on HLA-A2–matched Ph+ leukemia cell lines (Figure 2B) as well as Ph+ primary leukemic PBMCs derived from a patient in chronic-phase CML (Figure 2C). These results confirm, first, the HLA-dependent antigen recognition and, second, the relevance of the T-cell–defined antigens as CML antigens.
The T-cell response toward Bcr-Abl+ leukemia cells is HLA dependent and detectable in a CML patient. CD8+ T cells from HD no. 3 were stimulated with autologous Bcr-Abl WT+ DCs, and the IFN-γ production by the activated T-cell populations (n = 96) was measured by ELISpot after the third stimulation. (A) Box plot of the IFN-γ spots in the presence of following DCs: Bcr-Abl WT+ DCs (▒) or mock-transfected DCs (░) treated either with mAb W6/32, IgG control antibody, or without antibody. (B) CD8+ T lymphocytes derived from the same HLA-A2+ healthy donor were stimulated 3 times with autologous Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░). IFN-γ release of the activated T-cell populations (n = 60) was measured in the presence of Bcr-Abl+ leukemia cell lines. The box plots show the number of IFN-γ–releasing T cells upon restimulation with the HLA-A2+, Bcr-Abl+ cell lines K562-A2 and BV-173, and the HLA-A2−, Bcr-Abl+ cell line K562. (C) CD8+ T lymphocytes derived from an HLA-A2+ healthy donor (HD no. 3) were stimulated 3 times with the autologous Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░). The IFN-γ release by the activated T-cell populations (n = 96) was measured in the presence of HLA-A2–matched, allogeneic PBMCs derived from an HLA-A2+ CML patient in chronic phase (CML patient no. 1). (D) IFN-γ production by CD8+ T lymphocytes derived from a CML patient (CML patient no. 2) that had been stimulated 3 times with autologous DCs transfected with Bcr-Abl WT or Bcr-Abl KD. Incubation of these preactivated T-cell populations (n = 120) with Bcr-Abl WT–transfected DCs resulted in a higher amount of IFN-γ spots than the coculture with mock-transfected DCs (left). Incubation with Bcr-Abl KD+ DCs did not result in a specific IFN-γ production compared with the mock control (middle). The magnitudes of responses against Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░) were statistically evaluated in a box plot showing the median distribution of IFN-γ–producing T-cell populations (right, *P < .001; **P = ns; Mann-Whitney test, whiskers as in Figure 1). (E) Bar graph comparing IFN-γ spot indices between 3 healthy donors (HD no. 1, HD no. 2, HD no. 4) and CML patient no. 2. Error bars are SE.
The T-cell response toward Bcr-Abl+ leukemia cells is HLA dependent and detectable in a CML patient. CD8+ T cells from HD no. 3 were stimulated with autologous Bcr-Abl WT+ DCs, and the IFN-γ production by the activated T-cell populations (n = 96) was measured by ELISpot after the third stimulation. (A) Box plot of the IFN-γ spots in the presence of following DCs: Bcr-Abl WT+ DCs (▒) or mock-transfected DCs (░) treated either with mAb W6/32, IgG control antibody, or without antibody. (B) CD8+ T lymphocytes derived from the same HLA-A2+ healthy donor were stimulated 3 times with autologous Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░). IFN-γ release of the activated T-cell populations (n = 60) was measured in the presence of Bcr-Abl+ leukemia cell lines. The box plots show the number of IFN-γ–releasing T cells upon restimulation with the HLA-A2+, Bcr-Abl+ cell lines K562-A2 and BV-173, and the HLA-A2−, Bcr-Abl+ cell line K562. (C) CD8+ T lymphocytes derived from an HLA-A2+ healthy donor (HD no. 3) were stimulated 3 times with the autologous Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░). The IFN-γ release by the activated T-cell populations (n = 96) was measured in the presence of HLA-A2–matched, allogeneic PBMCs derived from an HLA-A2+ CML patient in chronic phase (CML patient no. 1). (D) IFN-γ production by CD8+ T lymphocytes derived from a CML patient (CML patient no. 2) that had been stimulated 3 times with autologous DCs transfected with Bcr-Abl WT or Bcr-Abl KD. Incubation of these preactivated T-cell populations (n = 120) with Bcr-Abl WT–transfected DCs resulted in a higher amount of IFN-γ spots than the coculture with mock-transfected DCs (left). Incubation with Bcr-Abl KD+ DCs did not result in a specific IFN-γ production compared with the mock control (middle). The magnitudes of responses against Bcr-Abl WT+ DCs (▒) or Bcr-Abl KD+ DCs (░) were statistically evaluated in a box plot showing the median distribution of IFN-γ–producing T-cell populations (right, *P < .001; **P = ns; Mann-Whitney test, whiskers as in Figure 1). (E) Bar graph comparing IFN-γ spot indices between 3 healthy donors (HD no. 1, HD no. 2, HD no. 4) and CML patient no. 2. Error bars are SE.
Bcr-Abl+ DCs from a CML patient induce T-cell activity against Bcr-Abl–regulated antigens
The clinical goal of our studies is the immunotherapy of CML patients. Therefore, we addressed the question of whether the T-cell response of a CML patient is also directed predominantly against Bcr-Abl–regulated antigens rather than against Bcr-Abl itself. As CML patients with stable residual disease seem to be the most promising patient population for successful immunologic therapies,11 we determined the immune response of a CML patient in major molecular remission under IM treatment. The patient's T-cell repertoire was comparable with those of healthy donors and was also dominated by T cells directed against Bcr-Abl–regulated antigens and not against Bcr-Abl itself (Figure 2D). The comparison of IFN-γ spot indices detected in healthy donors and the above-described CML patient confirmed these results (Figure 2E).
Our findings suggest that immunotherapeutic interventions directed against multiple immunogenic Bcr-Abl–associated antigens may be superior to vaccinations based solely on Bcr-Abl breakpoint peptides.11,25 Targeting Bcr-Abl directly by small inhibitors and indirectly by vaccine- or T-cell–based immunotherapies combines 2 different treatment modalities with a potentially synergistic effect: first, Bcr-Abl kinase inhibitors induce major molecular remissions in a high percentage of CML patients, a situation that is favorable for the success of immunotherapeutic approaches; second, immunotherapies have the potential to overcome resistance toward kinase inhibitors by eliminating the Ph+ CML stem cell.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the German José Carreras Leukemia Foundation and the German Research Council (SFB 456).
We thank Julia Neudorfer for excellent technical assistance and Dirk H. Busch for HLA/peptide multimer production. We also thank Alois Wölpl (Laboratory for Immunogenetics, Ludwig Maximilian University, Munich) for HLA typing.
Authorship
Contribution: F.S. performed research, collected and analyzed data, and wrote the paper; J.D. provided vital tools and designed research; C.P. supervised research; H.B. designed and supervised research, reviewed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helga Bernhard, 3rd Department of Internal Medicine, Hematology/Oncology, Klinikum rechts der Isar, Technical University of Munich, Ismaninger Straße 22, 81675 Munich, Germany; e-mail:helga.bernhard@lrz.tum.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal