Monocyte recruitment from the vasculature involves sequential engagement of multiple receptors, culminating in transendothelial migration and extravasation. Junctional adhesion molecule-C (JAM-C) is localized at endothelial intercellular junctions and plays a role in monocyte transmigration. Here, we show that blockade of JAM-B/-C interaction reduced monocyte numbers in the extravascular compartment through increased reverse transmigration rather than by reduced transmigration. This was confirmed in vivo, showing that an anti–JAM-C antibody reduced the number of monocytes in inflammatory tissue and increased the number of monocytes with a reverse-transmigratory phenotype in the peripheral blood. All together, our results suggest a novel mechanism of reducing accumulation of monocytes at inflammation sites by disruption of JAM-C–mediated monocyte retention.
Introduction
Targeting leukocyte migration from the vasculature to sites of inflammation requires a series of coordinated adhesive interactions.1 Of particular interest are molecules distributed at endothelial junctions, where cis- and trans-interactions allow endothelial cells (ECs) to interact with and regulate leukocyte migration into underlying tissues.
Junctional adhesion molecules (JAMs) encompass a family of 6 immunoglobulin-like proteins: CAR, ESAM, JAM4, JAM-A, JAM-B, and JAM-C.2,3 Differential expression and redistribution of JAM-B and JAM-C at the endothelial junction contribute to leukocyte interactions and trafficking.4 Endothelial cell JAM-C preferentially interacts with JAM-B, forming a 2-dimensional network of both molecules localized at endothelial junctions.4,–6 Complex hierarchies dictate how JAM-B/-C multimers interact with integrin counterreceptors. JAM-B can bind the integrin VLA-4 (α4β1; CD29a/CD49d), but requires prior engagement with JAM-C,7 whereas JAM-C can act as a counterreceptor for the integrin Mac-1 (αMβ2; CD11b/CD18) independently of JAM-B.8
Although JAM-C is expressed by fibroblasts9 and epithelial cells,10,–12 the role of endothelial JAM-C in leukocyte accumulation during inflammation13 has received particular attention. In humans, JAM-C is expressed on circulating platelets, natural killer (NK) cells, dendritic cells, and subsets of T and B cells,4,7,14,–16 but not on circulating mouse leukocytes.5 Preliminary studies with functional blocking antibodies to JAM-C showed reduced transmigration of peripheral blood lymphocytes across cultured human umbilical vein endothelial cells (HUVECs),15 and later studies identified Mac-1 as a ligand partner that can mediate adhesion and transmigration for neutrophils and monocytes and transepithelial migration of neutrophils.8,10,17,18
Numerous studies have addressed this dual functionality of JAM-C as an adhesion and transmigration regulatory molecule. Experimental animal models of inflammation support a role for JAM-C in regulating leukocyte accumulation within inflammatory lesions. Soluble JAM-C (solJAM-C) and antibodies to JAM-C inhibit leukocyte emigration in mouse models of inflammation.5,17 In addition, in mouse models of allergic contact dermatitis, the regulatory role of JAM-C in inflammation has also been associated with modulation of JAM-B/-C interactions, where combined blocking antibodies to JAM-B and JAM-C had an additive effect on blocking leukocyte recruitment.19 Furthermore, recent reports have identified additional steps requiring the disruption/engagement of the JAM-B/-C complex, before the respective integrin counterreceptor is engaged.6,7
In this study, we have developed a flow assay that allows detailed analysis of individual monocyte interactions with ECs. This analysis can begin at the initial phase of capture from free-flow under physiologic conditions and continue to posttransmigrational events. Using this model, we elucidated a novel role for endothelial JAM-C in regulating monocyte retention in the abluminal compartment after primary transmigration. Specifically, JAM-B/-C blockade appeared to decrease the number of monocytes in the ablumen, the result of an increased number of multiple reverse-transmigration events, leading to a net reduction. Analysis of monocyte accumulation in mouse models of inflammation confirmed a reduction in monocyte numbers when treated with a blocking antibody to JAM-C. However, further in vivo studies identified an expanded population of peripheral blood inflammatory monocytes lacking L-selectin expression after JAM-B/-C blockade, a phenotype consistent with monocytes having undergone transmigration. Collectively, these findings provide a novel mechanism in which JAM-C mediates a regulated and polarized monocyte transmigration response at inflammatory sites.
Materials and methods
Mice
Male CX3CR1GFP/GFP mice (> 20 g), used for tracking monocytes in vivo,20 were bred within the animal facilities of Imperial College London. For thioglycollate-induced peritonitis, 8- to 10-week-old C57BL/6 mice (25-30 g) were provided by Iffa-Credo (Saint-Germain-sur-l'Arbresle, France). All mice were maintained according to Veterinary Swiss National Law.
Reagents and antibodies
All reagents were purchased from Sigma-Aldrich (Buchs, Switzerland) unless otherwise stated. For flow cytometry, the following monoclonal antibodies (mAbs) were used: anti-CD3, anti-CD19, anti-CD62L, and anti-CD14 (FITC conjugated), and CD41A (Cychrome-3–conjugated) (BD Pharmingen, San Diego, CA). Rabbit serum prior to immunization was used as a control (day 0) for labeling with polyclonal Ab to murine JAM-C (day 74) (Covalab, Lyon, France). Reagents used in the peritonitis model were mAbs Gr1-APC, M1/70-PE, F4/80 FITC (Caltag, Burlingame, CA), and Mel-14-biotin (Southern Biotech, Birmingham, AL), streptavidin-Cychrome (BD Pharmingen) and Texas-Red goat anti-rabbit antibody (Jackson Immunoresearch, Westgrove, PA). For functional blocking studies, human JAM-C–Fc (R&D Systems, Minneapolis, MN) and a PE-conjugated (fab)′2 goat anti–human IgG-Fc γ-fragment (Jackson ImmunoResearch) were used. All anti–JAM-C mAbs used were produced in house. We previously described the blocking functions of JAM-B/-C interactions of our mAbs in biochemical pull-downs.6 All Abs competed with each other for binding to JAM-C, indicating overlapping epitopes (data not shown) and that H33 did not affect JAM-C/Mac-1 interactions.6 Other mAbs used were M1/70, Mel14, and mcb-64 (American Type Culture Collection, Manassas, VA).
ECs
HUVECs were isolated by collagenase treatment of umbilical veins21 and maintained (passages 2-4) in M199 containing 10% fetal calf serum (FCS), 15 μg/mL endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, NY), 100 μg/mL heparin, and 50 μM hydrocortisone.
Transfection of JAM-C siRNAs
HUVECs were transfected with 300 nM human JAM-C siRNAs (JAM3-HSS130321 and JAM3-HSS130322; Stealth; Invitrogen, Carlsbad, CA) using the Amaxa Nucleofector (Amaxa, Koln, Germany) and cultured for 48 hours before coculture under flow or analysis for JAM-C expression by real-time polymerase chain reaction (PCR; data not shown), and flow cytometry.
Flow cytometry
Monocytes suspended in wash buffer (PBS/0.15% BSA) were preincubated with human serum prior to all immunostaining with primary antibodies. Monocytes were analyzed directly or resuspended for secondary immunostaining before analysis by flow cytometry (Becton Dickinson, Mountain View, CA).
Functional blocking of human JAM-B/-C interactions
Human JAM-C/Fc was preclustered with PE-conjugated goat anti-human IgG-Fc (100 μg/mL) for 30 minutes on ice. This mixture was incubated with Chinese hamster ovary (CHO) cells transfected with human JAM-B in the presence of H33 (20 μg/mL) or isotype control Ab 9B5 (20 μg/mL) for 30 minutes before analysis by flow cytometry.
Intravital microscopy
Intravital microscopy on the mouse cremaster muscle was performed as previously described.22 Observations were made using an upright fixed-stage microscope (Axioscope FS; Zeiss, Welwyn Garden City, United Kingdom) equipped with water-dipping objective (20×/0.5 NA PhZ, Zeiss). Video recordings were made with a color chilled 3 CCD video camera (C5810-01; Hamamatsu Photonics, Enfield, United Kingdom) and video cassette recorder (AG-6730; Panasonic, Bracknell, United Kingdom). Male mice were injected intravenously with H33 or an isotype control (3 mg/kg) 15 minutes prior to TNFα intrascrotal injection. Transmigrated leukocytes were counted in the extravasculature within 50 μm of the vessel/200-μm vessel segments at 2 to 4 hours after TNFα stimulation. Observations were made using an LSM 5 Pascal confocal microscope (Zeiss) equipped with water-dipping objective (40×/0.75 NA; Zeiss). Image acquisition and analysis were conducted using Zeiss LSM Pascal software (v3.2). The cremaster muscle tissue was then fixed (4% paraformaldehyde) and immunostained with a Cy3-labeled anti–α-actin mAb for confocal microscopy.23 Immunostaining with H33 on fixed cremaster muscle sections confirmed JAM-C expression and mAb binding on cremaster endothelial vessels (data not shown).
Immunostaining of HUVEC-monocyte cocultures
Slides containing HUVEC-monocyte cocultures recovered from the flow assay were fixed in methanol (−20°C) for 5 minutes and washed in wash buffer. Preincubation with human serum was conducted before secondary immunostaining (anti-rabbit Texas-Red) and fixation (4% paraformaldehyde). Slides were mounted in Mowiol/DABCO for confocal microscopy (LSM510; Carl Zeiss, Feldbach, Switzerland). JAM-C localization was unaffected by TNFα stimulation in the time scales used in all flow assays (data not shown).
Thioglycollate-induced peritonitis
Analysis of peripheral blood monocytes in mice injected intraperitoneally with thioglycollate was performed as previously described.5 Mice were injected intravenously with H33 or an isotype control 1 hour prior to thioglycollate treatment. Peripheral blood leukocytes were immunostained for Mac-1, Gr1, F4/80, and L-selectin. Inflammatory monocytes were identified as Mac-1+ F4/80+/Gr1+ and analyzed for L-selectin expression. L-selectin− inflammatory monocytes were expressed as a percentage of total inflammatory monocytes.
Primary monocytes
Platelet-free monocytes (85%-95%) were purified from citrated blood collected from healthy donors using a monocyte isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Platelet-decorated monocytes (70%-80%), were purified from blood (7.5 mM EDTA) using NycoPrep 1.068 (Axis-Shield, Oslo, Norway)
Electron microscopy
Monocytes were sequentially fixed in 0.1 M cocadylate buffer containg 1% and 2% glutaraldehyde before postfixation treatment with 2% osmium tetroxide and staining en bloc with 2% uranyl acetate. The samples were dehydrated with a graded series of ethanol and embedded in EPON 812 (Sigma). Ultrathin sections were prepared using an Ultracut E microtome (Leica Mikrosysteme, Austria) and treated with 5% uranyl acetate and 2% lead citrate before examination using a Phillips EM400 electron microscope (Eindhoven, the Netherlands) at 80 kV.
Flow assays
Glass slides (22 × 50 mm) were treated with 3-aminopropyltriethoxysilane.24 Slides were coated with PBS/0.2% gelatin/4% FCS/10% collagen stock (Vitrogen100; AngioTech, Palo Alto, CA). HUVECs were cultured at 4 to 5 × 105 cells/slide for 2 to 3 days and treated for 4 hours with TNFα (500 U/mL). The slides were attached to a flow chamber at 37°C (CAF10; Immunetics, Cambridge, MA) and flow-generated over the HUVEC monolayer by perfusing wash buffer (M199/0.15% BSA) or a monocyte suspension (2.5 × 106 cells/mL) using a calibrated pump (74 900; Cole Parmer, Vernon Hills, IL). The flow rate was representative of shear rates in small venules (0.05 Pa). Observations made using phase-contrast microscopy (Model: Axiovert 35; Objective: 20×/0.3 NA air; Carl Zeiss) were recorded using a high-resolution camera (D70; Nikon, Zurich, Switzerland). Individual images were recorded every 30 seconds with Nikon capture software (v4.2) and compiled into movie sequences using Adobe Photoshop (v7.0) and Image J (v1.33u), allowing analysis of individual monocytes over large areas. mAb concentrations, with the exception of M1/70, were maintained at 50 μg/mL due to the low affinity of anti–JAM-C mAbs. mAb preincubations were done with HUVECs and monocytes 20 minutes prior to coculture. Monocytes were perfused over activated HUVECs for 5 minutes (referred to as a bolus) followed by 20- to 60-minute wash buffer. Monocytes adhered to the surface of the HUVECs have a phase-white appearance, whereas cells that have transmigrated have a phase-black appearance.25 For individual cell tracking, the phase appearance of each monocyte was marked at 1-minute intervals. Reverse transmigration was defined as continued migration in the abluminal-to-luminal direction for 1 minute or more after primary transmigration. Repeat transmigration was defined as luminal-to-abluminal migration for 1 minute or more after reverse transmigration. Transmigration events are presented as a percentage of total monocytes captured from flow per unit field. All experiments were carried out using triplicate fields and presented as a mean value (± SEM).
Monocyte rescue from HUVEC cocultures under flow
Slides containing monocyte HUVEC cocultures were removed from the flow apparatus after monocyte primary transmigration had exceeded 80% (10-15 minutes). The slides were washed in PBS and incubated with trypsin for 1 to 2 minutes, and cells were recovered in M199/10% FCS before immunostaining. Controls included purified monocytes treated with trypsin and processed in parallel with coculture suspensions.
Results
JAM-C regulates accumulation of monocytes in vivo
Previous studies have demonstrated that Antibodies against JAM-C block inflammatory pathologies such as pancreatitis and lung inflammatory diseases.5,26 Because these effects could be in part the result of leukocyte firm adhesion and/or transmigration, we used the cremaster muscle tissue as an in vivo model for investigating TNFα-induced leukocyte transmigration in real time by intravital microscopy. In this model, mice treated with a control mAb exhibited a time-dependent increase in leukocyte transmigration 2 to 4 hours following local injection of TNFα. Mice treated with a blocking anti–JAM-C mAb H33 showed a trend toward a reduced number of leukocytes in the tissue at every time point analyzed, with the 4-hour time point showing a significant reduction (Figure 1A). In the same studies, JAM-C blockade did not show an effect on leukocyte firm adhesion (data not shown). Because the mice used expressed GFP-tagged monocytes (CX3CR1GFP/GFP),27 and transmigrated monocytes could clearly be visualized in fixed tissues using higher-resolution confocal microscopy (Figure 1B), the effect of the anti–JAM-C mAb on monocyte transmigration was quantified by analysis of tissues on these still images. This quantification procedure indicated that mice treated with H33 exhibited a significantly reduced number of monocytes in the extravascular tissue (Figure 1C).
Anti–JAM-C mAb suppresses TNFα-stimulated monocyte accumulation in the mouse cremaster muscle tissue. (A) CX3CR1 GFP/GFP mice were injected intravenously with an isotype control mAb or the anti–JAM-C mAb H33 (both at 3 mg/kg), and 15 minutes later, mice were injected with intrascrotal TNFα (300 ng/mouse). After 2 hours, the cremaster muscle was exteriorized, and leukocyte transmigration was quantified for 2 hours at different time points by intravital microscopy. Error bars represent SEM. Results are from n = 3 mice/group, and statistical comparisons are shown by lines (*P < .05). (B) A representative micrograph of a TNFα-stimulated cremasteric venule. The fixed section was immunostained with an anti–α-SMA (SMC/pericyte marker) to visualize the venular wall (red) and contained GFP-labeled monocytes (green), reconstructed in 3D and captured by still confocal microscopy (“Intravital microscopy” and “Flow assays”) in order to optimize resolution. The image shows monocytes both in the luminal compartment and in the extravascular tissue. Scale bar equals 10 μm. (C) There was a reduction in monocytes entering the extravasculature of mice treated with blocking antibody to JAM-C. At the end of the intravital microscopy studies detailed, cremasteric tissues were dissected away from the mice and analyzed for monocyte numbers (visualized by their GFP label) in the extravascular tissue by confocal microscopy. Data show that mice treated with the anti–JAM-C mAb exhibited a significant reduction in abluminal monocyte numbers (*P < .05). Error bars represent SEM.
Anti–JAM-C mAb suppresses TNFα-stimulated monocyte accumulation in the mouse cremaster muscle tissue. (A) CX3CR1 GFP/GFP mice were injected intravenously with an isotype control mAb or the anti–JAM-C mAb H33 (both at 3 mg/kg), and 15 minutes later, mice were injected with intrascrotal TNFα (300 ng/mouse). After 2 hours, the cremaster muscle was exteriorized, and leukocyte transmigration was quantified for 2 hours at different time points by intravital microscopy. Error bars represent SEM. Results are from n = 3 mice/group, and statistical comparisons are shown by lines (*P < .05). (B) A representative micrograph of a TNFα-stimulated cremasteric venule. The fixed section was immunostained with an anti–α-SMA (SMC/pericyte marker) to visualize the venular wall (red) and contained GFP-labeled monocytes (green), reconstructed in 3D and captured by still confocal microscopy (“Intravital microscopy” and “Flow assays”) in order to optimize resolution. The image shows monocytes both in the luminal compartment and in the extravascular tissue. Scale bar equals 10 μm. (C) There was a reduction in monocytes entering the extravasculature of mice treated with blocking antibody to JAM-C. At the end of the intravital microscopy studies detailed, cremasteric tissues were dissected away from the mice and analyzed for monocyte numbers (visualized by their GFP label) in the extravascular tissue by confocal microscopy. Data show that mice treated with the anti–JAM-C mAb exhibited a significant reduction in abluminal monocyte numbers (*P < .05). Error bars represent SEM.
JAM-C regulates accumulation of abluminal monocytes on cultured HUVECs under flow
The mAb H33 is known to disrupt murine JAM-B/-C interactions.6 Extension of this cross-reactivity to human JAM-B/-C interactions was confirmed by competition studies using CHO cells transfected with human JAM-B that were labeled with solJAM-C–Fc construct (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) in competition with the mAb H33 (Figure S1B) or an isotype control (Figure S1C). The disruption of JAM-B/-C interactions by H33 prompted us to further investigate the role of JAM-C in human leukocyte interactions with vascular endothelium.
Previous reports have shown that solJAM-C can block adhesion of a monocytic cell line to vascular endothelium under shear stress.18 To address the precise stage in the transmigration response mediated by JAM-C, we established a flow assay system using a high-resolution camera to record the different phases of adhesion of individual monocytes with cultured endothelial monolayers. Monocytes captured from free flow by TNFα-activated HUVECs had a round phase-white appearance that rapidly changed to phase-gray upon activation as the monocyte migrated on the luminal surfaces. Monocytes that transmigrated beneath the HUVEC monolayer changed to a phase-black appearance. This change from phase-white/gray (luminal) to phase-black (abluminal) generally occurs within a few minutes (Figure 2A).25 To mimic the interstitial space, HUVECs were cultured on slides precoated with a semisolid collagen/gelatin gel.
Transmigration of a single human monocyte on activated HUVECs under flow. (A) Human monocytes that adhere out of free flow typically activate within 1 minute, changing from a phase-white (0 minutes) to a phase-gray appearance (2 minutes) before migrating on luminal surfaces (5 minutes) to an endothelial cell junction (white arrows). Transmigration between the lumen and ablumen (7 minutes) leads to a change in phase appearance (black) as the monocyte migrates under the HUVEC monolayer (11-12 minutes). (B) There was a reduction in adherent monocytes occupying the ablumen on HUVECs with blocking mAbs to JAM-C. A 5-minute bolus of monocytes was flowed over activated HUVECs at a shear stress of 0.05 Pa. Adherent monocytes were cocultured under continued flow with the HUVECs for 20 minutes before monocytes occupying the lumen (phase-gray) and ablumen (phase-black) were counted. Abluminal monocyte counts were presented as a percentage of total adherent monocyte per field. Each group is expressed as the mean of triplicate cell counts (± SEM; **P < .01).
Transmigration of a single human monocyte on activated HUVECs under flow. (A) Human monocytes that adhere out of free flow typically activate within 1 minute, changing from a phase-white (0 minutes) to a phase-gray appearance (2 minutes) before migrating on luminal surfaces (5 minutes) to an endothelial cell junction (white arrows). Transmigration between the lumen and ablumen (7 minutes) leads to a change in phase appearance (black) as the monocyte migrates under the HUVEC monolayer (11-12 minutes). (B) There was a reduction in adherent monocytes occupying the ablumen on HUVECs with blocking mAbs to JAM-C. A 5-minute bolus of monocytes was flowed over activated HUVECs at a shear stress of 0.05 Pa. Adherent monocytes were cocultured under continued flow with the HUVECs for 20 minutes before monocytes occupying the lumen (phase-gray) and ablumen (phase-black) were counted. Abluminal monocyte counts were presented as a percentage of total adherent monocyte per field. Each group is expressed as the mean of triplicate cell counts (± SEM; **P < .01).
In the initial study, monocytes occupying the different compartments were analyzed at a single time point of 20 minutes, where the presence of blocking anti–JAM-C mAbs H33 and D33 led to a reduction in the percentage of abluminal monocytes, whereas D22 did not (Figure 2B). These mAbs have previously been shown to block JAM-B/-C interactions, but not JAM-C/Mac-1 interactions.6 Interestingly, anti–JAM-C mAb D22 did not have any effect and is consistent with previous findings identifying this mAb as a low-affinity/nonfunctional blocker.6 Furthermore, a potential role for Fc receptor ligation mediating monocyte activation through anti–JAM-C mAbs decorating endothelial surfaces was excluded by the inclusion of mAb D22 in this study.
Taken together, the reduction in the percentage of abluminal monocytes and the reduced number of monocytes in the TNFα-driven in vivo model detailed here suggest that JAM-C plays a role in regulating trafficking of leukocytes from the vasculature to underlying tissue, but the mechanisms involved were unclear, and were thus further explored.
Role of JAM-C expression on platelets
To fully determine a role for JAM-C in monocyte-HUVEC cocultures, we investigated JAM-C expression potentially associated with purified monocytes. Previous studies have shown that human platelets express JAM-C and that they adhere to monocytes during purification from peripheral blood.8,28,–30
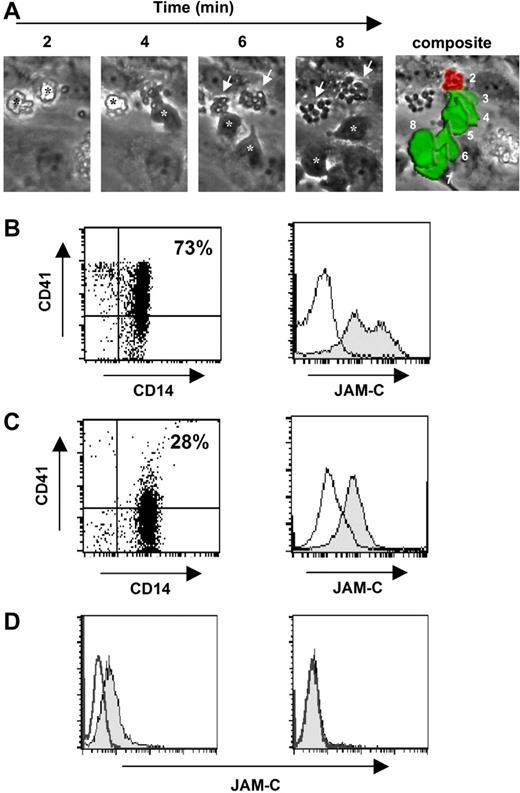
To characterize a potential role for bound platelets in our flow assay, we used monocytes prepared by gradient centrifugation (NycoPrep). These monocytes were heavily decorated with platelets that were easily visualized by electron microscopy (Figure S2A) and the high-resolution camera used in the flow assay (Figure 3A). Furthermore, flow cytometry analysis confirmed a higher and more heterogeneous expression level of the platelet marker CD41 and JAM-C on these monocytes (Figure 3B). During the flow assay, we observed that these monocytes deposited adherent platelets onto the luminal surface of the HUVEC monolayer during transmigration (Figure 3A; Video S1). These stripped platelets remained grouped at the site of transmigration during the course of the flow assay, indicating that platelets remained excluded from the abluminal compartment. However, we could not avoid platelet contamination, and we therefore switched to a purification procedure that used cell sorting by negative selection. Indeed, monocytes prepared in this way appeared to be devoid of adherent platelets (Figure S2B), but flow cytometry analysis still showed a low level of CD41 and JAM-C expression most likely resulting from monocyte-associated platelet microparticles (Figure 3C). When these monocytes underwent transmigration under flow, they no longer displayed JAM-C on cell surfaces, confirming that JAM-C expression was in fact associated with adherent platelet microparticles (Figure 3D). Therefore, transmigrated monocytes in the ablumen were JAM-C−, restricting any role for monocyte-associated JAM-C to events preceding transmigration. This gives concordance with posttransmigrational events observed in the mouse and human models used in this study, with transmigrated monocytes in both systems being JAM-C−.
Transmigration of adherent human monocytes decorated with platelets under flow. (A) Human monocytes decorated with platelets (NycoPrep preparation) were captured onto HUVEC luminal surfaces under flow (separate panels; black stars). The attached platelets switched adhesion from monocyte to endothelial luminal surfaces (white arrows) as the monocyte underwent transmigration into the abluminal compartment (white stars). The composite image demonstrates that the platelets remained adherent under flow at the original site of transmigration on the endothelium (red area) during the course of monocyte abluminal migration (2-8 minutes; green area). (B) Monocytes purified by NycoPrep displayed high levels of the platelet marker CD41 and of JAM-C. Monocytes (CD14+) purified using NycoPrep displayed higher, more heterogeneous levels of CD41 (top right quadrant; 28% total monocyte population). Histogram of JAM-C expression on gated CD14+ cells also showed high heterogeneous levels of JAM-C on membrane surfaces (▩). (C) Monocytes purified by negative selection displayed markers associated with adherent platelets. Monocytes (CD14+) purified by negative selection and devoid of visible platelets still displayed lower levels of CD41 (CD41+ population designated in top right quadrant; 73% total monocyte population). Histogram of JAM-C expression confirmed expression of JAM-C but at lower levels (▩) to monocytes prepared by NycoPrep. (D) Monocyte-associated JAM-C expression was lost after transmigration. Transmigrated monocytes (95%+) in HUVEC coculture under flow were rescued by trypsinisation and labeled with an antibody to JAM-C. Analysis by flow cytometry of monocyte suspensions treated with trypsin remained JAM-C+ (▩; left panel), but rescued monocytes from trypsinized HUVEC–monocyte suspensions were JAM-C− (▩; right panel).
Transmigration of adherent human monocytes decorated with platelets under flow. (A) Human monocytes decorated with platelets (NycoPrep preparation) were captured onto HUVEC luminal surfaces under flow (separate panels; black stars). The attached platelets switched adhesion from monocyte to endothelial luminal surfaces (white arrows) as the monocyte underwent transmigration into the abluminal compartment (white stars). The composite image demonstrates that the platelets remained adherent under flow at the original site of transmigration on the endothelium (red area) during the course of monocyte abluminal migration (2-8 minutes; green area). (B) Monocytes purified by NycoPrep displayed high levels of the platelet marker CD41 and of JAM-C. Monocytes (CD14+) purified using NycoPrep displayed higher, more heterogeneous levels of CD41 (top right quadrant; 28% total monocyte population). Histogram of JAM-C expression on gated CD14+ cells also showed high heterogeneous levels of JAM-C on membrane surfaces (▩). (C) Monocytes purified by negative selection displayed markers associated with adherent platelets. Monocytes (CD14+) purified by negative selection and devoid of visible platelets still displayed lower levels of CD41 (CD41+ population designated in top right quadrant; 73% total monocyte population). Histogram of JAM-C expression confirmed expression of JAM-C but at lower levels (▩) to monocytes prepared by NycoPrep. (D) Monocyte-associated JAM-C expression was lost after transmigration. Transmigrated monocytes (95%+) in HUVEC coculture under flow were rescued by trypsinisation and labeled with an antibody to JAM-C. Analysis by flow cytometry of monocyte suspensions treated with trypsin remained JAM-C+ (▩; left panel), but rescued monocytes from trypsinized HUVEC–monocyte suspensions were JAM-C− (▩; right panel).
Blocking mAbs to JAM-C induce reverse transmigration of monocytes but do not inhibit adhesion or transmigration
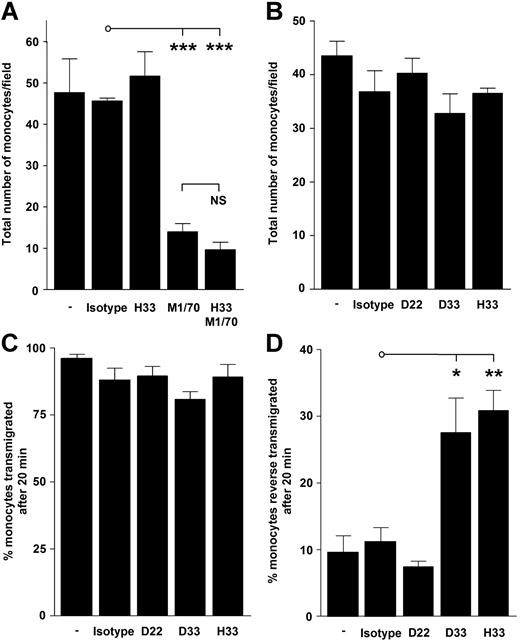
To further investigate the mechanism by which JAM-C may mediate monocyte transmigration, we monitored the interactions of individual monocytes with activated HUVECs under fluid shear-stress conditions. We found that blocking JAM-B/-C function with the mAb H33 did not affect monocyte capture from flow (Figure 4A). This finding contrasted completely with that achieved using a mAb that blocked Mac-1 interactions, a potential ligand for JAM-C. Consistent with a previous report, this Mac-1–blocking antibody dramatically decreased monocyte capture from flow.18 However, combining the 2 mAbs had no cumulative effect on blocking monocyte capture, indicating H33 did not interfere with anti–Mac-1 blocking function. Finally, we used solJAM-C and did not detect a significant drop in monocyte capture (Figure S3). The results of this experiment exclude a role for JAM-C in monocyte capture onto endothelial surfaces, but show that Mac-1 interactions are involved.
Capture and transmigration of human monocytes on activated HUVECs unaffected by a blocking antibody to JAM-C. (A) A 5-minute bolus of monocytes was flowed over activated HUVECs at a shear stress of 0.05 Pa. Adherent monocytes were then counted and expressed as a mean of triplicate cell counts (± SEM). Decreased monocyte capture was observed with Mac-1–blocking antibody (M1/70; ***P < .005), but not with a blocking antibody to JAM-C. NS indicates not significant. (B) A separate flow assay conducted with a different donor confirmed monocyte capture was unaffected by mAbs to JAM-C. Data represents mean number of cells counted per unit field (± SEM). (C) Adherent monocytes were then cocultured for a further 15 minutes, and the number of monocytes that exhibited migration in the luminal-to-abluminal direction over 20 minutes was recorded and expressed as a mean percentage of total adherent monocytes that remained in field (± SEM). (D) There was increased reverse transmigration with functional blocking mAbs to JAM-C under flow. Cocultured adherent monocytes that underwent primary transmigration that exhibited migration in the abluminal-to-luminal direction over 20 minutes were also recorded. Data presented are the mean percentage of total adherent monocytes per field (± SEM). Increased reverse transmigration was observed for mAbs D33 (*P < .05) and H33 (**P < .01). Percentage of transmigration and reverse transmigration for monocytes treated with M1/70 was not included because of the low number of monocytes captured. Transmigration and reverse-transmigration profiles for flow assays treated under control conditions and with the mAb H33 in panel A were representative of panels B-D (data not shown).
Capture and transmigration of human monocytes on activated HUVECs unaffected by a blocking antibody to JAM-C. (A) A 5-minute bolus of monocytes was flowed over activated HUVECs at a shear stress of 0.05 Pa. Adherent monocytes were then counted and expressed as a mean of triplicate cell counts (± SEM). Decreased monocyte capture was observed with Mac-1–blocking antibody (M1/70; ***P < .005), but not with a blocking antibody to JAM-C. NS indicates not significant. (B) A separate flow assay conducted with a different donor confirmed monocyte capture was unaffected by mAbs to JAM-C. Data represents mean number of cells counted per unit field (± SEM). (C) Adherent monocytes were then cocultured for a further 15 minutes, and the number of monocytes that exhibited migration in the luminal-to-abluminal direction over 20 minutes was recorded and expressed as a mean percentage of total adherent monocytes that remained in field (± SEM). (D) There was increased reverse transmigration with functional blocking mAbs to JAM-C under flow. Cocultured adherent monocytes that underwent primary transmigration that exhibited migration in the abluminal-to-luminal direction over 20 minutes were also recorded. Data presented are the mean percentage of total adherent monocytes per field (± SEM). Increased reverse transmigration was observed for mAbs D33 (*P < .05) and H33 (**P < .01). Percentage of transmigration and reverse transmigration for monocytes treated with M1/70 was not included because of the low number of monocytes captured. Transmigration and reverse-transmigration profiles for flow assays treated under control conditions and with the mAb H33 in panel A were representative of panels B-D (data not shown).
The inability of solJAM-C and the mAb H33 to block monocyte capture under shear stress led us to further investigate the effect of blocking JAM-C function in monocyte HUVEC cocultures. Analysis of individual monocyte transmigration confirmed the inability of the anti–JAM-C mAbs to block either monocyte adhesion (Figure 4B), the number of transmigrated monocytes (Figure 4C), or the time taken to transmigrate (data not shown). Because this outcome appears at odds with data obtained from analysis of the final position of the monocytes at the end point (Figure 1C), we analyzed the behavior of individual cells in more detail. The findings showed that abluminal transmigrated monocytes exhibited a significant increase in reverse transmigration in the presence of anti–JAM-C mAb by migrating back to the luminal side of the endothelium (Figure 4D). This phenomenon of reverse transmigration was only seen with mAbs H33 and D33, both of which block JAM-B/-C interactions. Increased reverse transmigration induced by these mAbs indicates that they disrupt JAM-C function exclusively on the endothelium, as monocytes that have transmigrated into the ablumen were JAM-C−. Therefore, the major role of JAM-C may be maintaining monocyte position in the abluminal compartment after transmigration.
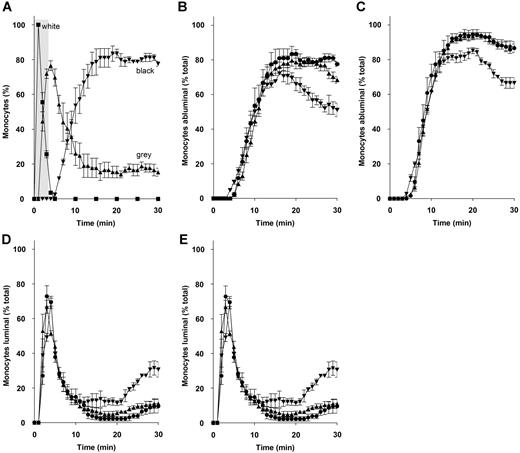
To investigate further the kinetics of reverse monocyte transmigration, cells were tracked at 1-minute intervals to give a more precise measurement of monocyte position and movement between different endothelial compartments (Figure 5A). As with the previous experiments, we confirmed JAM-C mAbs and solJAM-C did not affect the time required for monocyte activation upon capture and tethering, as all monocytes switched from a phase-white to a phase-gray phenotype within 1 minute (data not shown). This outcome indicates that rolling of monocytes led to rapid activation and firm adhesion on luminal surfaces within this time frame for all experimental conditions (data not shown). There was no significant effect with solJAM-C or H33 in the time frames required for an adherent monocyte to migrate toward an endothelial junction and transmigrate (data not shown). The rate of increase in monocytes entering the ablumen during the first 15 minutes was unaffected by the mAb H33 (Figure 5B) and solJAM-C (Figure 5C). However, these monocytes treated with H33 (Figure 5B,D) or solJAM-C (Figure 5C,E) were observed to reverse transmigrate at significantly higher rates than monocytes treated with control mAbs within the observed 30-minute time period.
Migration pattern of human monocytes in HUVEC cocultures under flow. (A) Monocytes were flowed over activated HUVECs for 5 minutes (area marked in gray). Monocytes were individually tracked, and their positions were marked at 1-minute intervals. Monocytes captured from free flow become rapidly activated by rolling on the luminal surface, changing phase from white (■) to gray (▴) before transmigrating into the ablumen (phase-black; ▾). (B) A functional blocking antibody to JAM-C and solJAM-C induced reverse transmigration. The abluminal (B) and luminal positions (D) of monocytes in HUVEC cocultures are shown untreated with antibody (●) or treated with D22 (▴) or H33 (▾). The abluminal (C) and luminal positions (E) of monocytes in HUVEC cocultures are shown untreated (●) or treated with flag peptide control (▴) or solJAM-C (▾). A decrease in abluminal monocytes was paralleled by an increase in abluminal monocytes for H33 (B,D) and solJAM-C (C,E), indicating an increased rate of reverse transmigration. Data are presented as the means of 3 fields (± SEM).
Migration pattern of human monocytes in HUVEC cocultures under flow. (A) Monocytes were flowed over activated HUVECs for 5 minutes (area marked in gray). Monocytes were individually tracked, and their positions were marked at 1-minute intervals. Monocytes captured from free flow become rapidly activated by rolling on the luminal surface, changing phase from white (■) to gray (▴) before transmigrating into the ablumen (phase-black; ▾). (B) A functional blocking antibody to JAM-C and solJAM-C induced reverse transmigration. The abluminal (B) and luminal positions (D) of monocytes in HUVEC cocultures are shown untreated with antibody (●) or treated with D22 (▴) or H33 (▾). The abluminal (C) and luminal positions (E) of monocytes in HUVEC cocultures are shown untreated (●) or treated with flag peptide control (▴) or solJAM-C (▾). A decrease in abluminal monocytes was paralleled by an increase in abluminal monocytes for H33 (B,D) and solJAM-C (C,E), indicating an increased rate of reverse transmigration. Data are presented as the means of 3 fields (± SEM).
Blocking antibody to JAM-C and human solJAM-C increases monocyte movement between luminal and abluminal surfaces
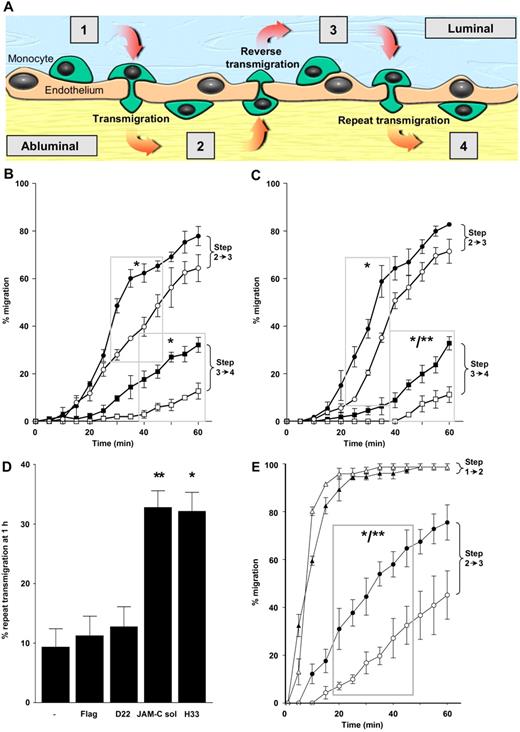
The increased rate of reverse transmigration observed in the presence of mAb H33 and solJAM-C prompted us to look at how monocyte position may vary at later time points. Individual cell tracking was performed under different conditions, and movement between luminal and abluminal sides at separate time points recorded (Figure 6A; Video S2). A significant increase in antibody-induced reverse transmigration was observed at time points between 30 and 45 minutes (Figure 6B; steps 2-3). At later time points, the rate of reverse transmigration decreased and was no longer significantly different from control groups. However, this drop in significance was associated with additional monocyte transmigration events. Monocytes that had undergone reverse transmigration onto the luminal surface could repeat the transmigration in a luminal-to-abluminal direction (Figure 6A; Video S2). This reversal was observed using the blocking mAb H33, with significantly higher rates observed at time points between 40 and 60 minutes (Figure 6B,D; steps 3-4).
Increased multiple transmigration events of human monocytes with blocking antibody to JAM-C and solJAM-C in flow. (A) Monocytes captured from free flow became activated and firmly adhered to HUVEC luminal surfaces (step 1). Monocytes migrating on luminal surfaces could move into the ablumen by migrating between junctions of adjacent endothelial cells (transmigration; step 2), which could be followed by transmigration in the abluminal-to-luminal direction (reverse transmigration) back onto luminal surfaces (step 3). A further transendothelial migration event (repeat transmigration) led to a return to the ablumen (step 4). (B) Adherent monocytes in coculture with HUVECs were individually tracked and monitored for transmigration between different compartments over 60 minutes. Increased reverse transmigration was observed for cocultures treated with functional blocking mAbs to JAM-C (●) compared with nonfunctional blocking antibody D22 (○). Monocytes with a reverse-transmigratory phenotype treated with H33 repeat-transmigrate back into the ablumen at higher levels (■) compared with D22 (□). (C) This increases in reverse, and repeat transmigration was also seen with solJAM-C (■, ●) compared with flag peptide control (□, ○). Increased repeat transmigration was observed for mAb H33 and solJAM-C (*P < .05; **P < .01) at time points marked with gray boxes. (D) A summary of the number of monocytes that had repeat-transmigrated in 60 minutes demonstrates that mAb H33 and solJAM-C induced similar levels of repeat transmigration. Reverse and repeat transmigration are presented as a percentage of total monocytes captured from flow per unit field. Data are presented as the mean of 3 fields with SEM and represent a single representative experiment (N = 3). (E) Monocytes cocultured on HUVECs under flow transfected with siRNAs against JAM-C (▴) or a sham-transfected control (▵) exhibited similar transmigration profiles (steps 1-2), but increased reverse transmigration (steps 3-4) was associated with reduced JAM-C expression (●) compared with the control (○) at time points marked by the gray box (*P < .05; **P < .01). Error bars in all figures represent SEM.
Increased multiple transmigration events of human monocytes with blocking antibody to JAM-C and solJAM-C in flow. (A) Monocytes captured from free flow became activated and firmly adhered to HUVEC luminal surfaces (step 1). Monocytes migrating on luminal surfaces could move into the ablumen by migrating between junctions of adjacent endothelial cells (transmigration; step 2), which could be followed by transmigration in the abluminal-to-luminal direction (reverse transmigration) back onto luminal surfaces (step 3). A further transendothelial migration event (repeat transmigration) led to a return to the ablumen (step 4). (B) Adherent monocytes in coculture with HUVECs were individually tracked and monitored for transmigration between different compartments over 60 minutes. Increased reverse transmigration was observed for cocultures treated with functional blocking mAbs to JAM-C (●) compared with nonfunctional blocking antibody D22 (○). Monocytes with a reverse-transmigratory phenotype treated with H33 repeat-transmigrate back into the ablumen at higher levels (■) compared with D22 (□). (C) This increases in reverse, and repeat transmigration was also seen with solJAM-C (■, ●) compared with flag peptide control (□, ○). Increased repeat transmigration was observed for mAb H33 and solJAM-C (*P < .05; **P < .01) at time points marked with gray boxes. (D) A summary of the number of monocytes that had repeat-transmigrated in 60 minutes demonstrates that mAb H33 and solJAM-C induced similar levels of repeat transmigration. Reverse and repeat transmigration are presented as a percentage of total monocytes captured from flow per unit field. Data are presented as the mean of 3 fields with SEM and represent a single representative experiment (N = 3). (E) Monocytes cocultured on HUVECs under flow transfected with siRNAs against JAM-C (▴) or a sham-transfected control (▵) exhibited similar transmigration profiles (steps 1-2), but increased reverse transmigration (steps 3-4) was associated with reduced JAM-C expression (●) compared with the control (○) at time points marked by the gray box (*P < .05; **P < .01). Error bars in all figures represent SEM.
A similar effect was seen with solJAM-C, with increased reverse transmigration observed between 25 and 35 minutes (Figure 6C; steps 2,3) and a significant increase in repeat transmigration between 40 and 60 minutes (Figure 6C,D; steps 3,4). Thus, the higher rate of repeat transmigration seen in the presence of the blocking reagents was the result of the higher number of monocytes that had undergone reverse transmigration. Interestingly, this association with a reduction in the rate of reverse transmigration and increased repeat transmigration may reflect the experimental protocol involving a single bolus delivery of monocytes to the flow chamber containing the HUVEC monolayer. This approach led to an observed phasic shift in the direction of monocyte migration and the significance of reverse and repeat transmigration being restricted to certain times. However, prolonged observations of monocytes using this in vitro system were not possible because the monocyte HUVECs cocultures deteriorated over periods exceeding 1 hour. A role for JAM-C in regulating reverse transmigration was confirmed by observations in monocyte cocultures with HUVECs that exhibited reduced JAM-C surface expression (Figure S4). HUVECs transfected with siRNAs against JAM-C supported normal monocyte capture (data not shown) and transmigration under flow, but demonstrated increased levels of reverse transmigration (Figure 6E).
As these studies were conducted in vitro, we wished to address the same phenomenon in vivo. Although the use of GFP-labeled CX3CR1 monocytes seems optimal for characterizing such observations, our intravital microscope equipment did not provide sufficient resolution or cover a wide enough field to visualize large numbers of transmigratory events at the thin endothelial interface. Therefore, we applied an alternative strategy to characterize this phenomenon in vivo.
Blocking antibody to JAM-C increases the number of peripheral blood monocytes with a reverse-transmigratory phenotype in vivo
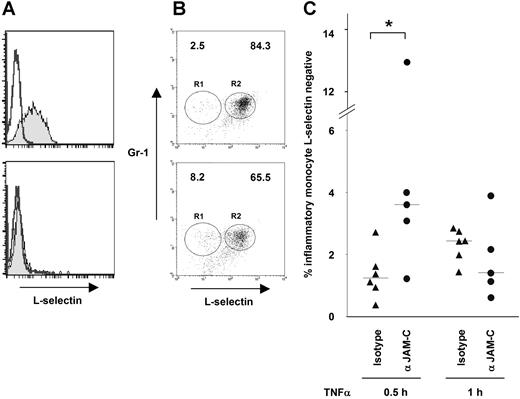
A series of studies have confirmed peripheral blood leukocytes that have undergone reverse transmigration do not express L-selectin.31,–33 A preliminary study with neutrophils had shown that L-selectin expression is lost by cell-surface shedding during transmigration.34 We confirmed this finding for monocytes in our model: human monocytes recovered from the ablumen of HUVECs lacked L-selectin expression, even though all monocytes expressed L-selectin before transmigration.
We have established a protocol for recovery of monocytes from endothelial coculture by incubation with trypsin. This short time period (1-2 minutes) did not lead to complete cleavage of L-selectin from monocyte surfaces. Transmigrated monocytes recovered by trypsinisation of the endothelial monolayer were shown to be L-selectin−, in contrast to nontransmigrated monocytes treated with trypsin as a control, which remained L-selectin+ (Figure 7A). Thus, the loss of L-selectin expression occurs during transmigration,35 and the absence of L-selectin expression was expected to identify monocytes that have reverse-transmigrated and returned to circulation.
Blocking antibody to JAM-C increases number of L-selectin− inflammatory monocytes in the blood of mice with peritonitis. (A) L-selectin expression on purified human monocytes was lost after transmigration. Transmigrated monocytes were rescued by trypsinisation and labeled with anti–L-selectin FITC. As a control, analysis by flow cytometry of monocyte suspensions treated with trypsin at the same concentration for the same time remained L-selectin+ (top panel), but rescued monocytes from trypsinized HUVEC-monocyte suspensions were L-selectin− (bottom panel). (B) L-selectin expression on peripheral blood mouse inflammatory monocytes at 30 minutes after thioglycollate-induced peritonitis is shown. Example profiles are shown of L-selectin expression analysis on peripheral blood inflammatory monocytes (Mac-1+/F4/80+/Gr1+) from an individual mouse treated with either a control antibody (top panel) or a blocking antibody to JAM-C (bottom panel). L-selectin− monocytes are gated on R1 and L-selectin+ monocytes are gated on R2. Numbers represent percentage of total gated population. (C) Mice treated with the JAM-C–blocking mAb H33 (n = 5) showed a significant increase in L-selectin− inflammatory monocytes in the blood (median, 3.61%) at 30 minutes compared with an isotype control antibody (median, 1.24%; n = 6). The median value for each experimental group is marked with a horizontal bar. Statistical analysis was conducted using the Mann-Whitney U test (*P = .03).
Blocking antibody to JAM-C increases number of L-selectin− inflammatory monocytes in the blood of mice with peritonitis. (A) L-selectin expression on purified human monocytes was lost after transmigration. Transmigrated monocytes were rescued by trypsinisation and labeled with anti–L-selectin FITC. As a control, analysis by flow cytometry of monocyte suspensions treated with trypsin at the same concentration for the same time remained L-selectin+ (top panel), but rescued monocytes from trypsinized HUVEC-monocyte suspensions were L-selectin− (bottom panel). (B) L-selectin expression on peripheral blood mouse inflammatory monocytes at 30 minutes after thioglycollate-induced peritonitis is shown. Example profiles are shown of L-selectin expression analysis on peripheral blood inflammatory monocytes (Mac-1+/F4/80+/Gr1+) from an individual mouse treated with either a control antibody (top panel) or a blocking antibody to JAM-C (bottom panel). L-selectin− monocytes are gated on R1 and L-selectin+ monocytes are gated on R2. Numbers represent percentage of total gated population. (C) Mice treated with the JAM-C–blocking mAb H33 (n = 5) showed a significant increase in L-selectin− inflammatory monocytes in the blood (median, 3.61%) at 30 minutes compared with an isotype control antibody (median, 1.24%; n = 6). The median value for each experimental group is marked with a horizontal bar. Statistical analysis was conducted using the Mann-Whitney U test (*P = .03).
To test whether blocking JAM-C function in vivo results in increased reverse transmigration of monocytes, we followed L-selectin expression on circulating inflammatory monocytes identified by Mac-1, Gr1, and F4/80 expression during the course of thioglycollate-induced peritonitis.27,36 The blood of mice pretreated with an isotype control or an anti–JAM-C antibody was analyzed at 30 minutes after induction of peritonitis, since the maximal rate of monocyte reverse transmigration was observed at 15 to 30 minutes in vitro. Indeed, we found increased numbers of L-selectin− inflammatory monocytes from mice treated with blocking antibody to JAM-C at 30 minutes, but not at a later time point (Figure 7B,C). This significant increase in monocytes with an L-selectin− inflammatory phenotype in the vascular compartment did not reflect any increase in peripheral blood monocyte numbers, a phenomenon associated with later time points (data not shown). Such a rapid increase in L-selectin− inflammatory monocytes in the blood suggests that JAM-C forms an endothelial barrier that confines recently recruited monocytes to inflammatory sites.
Discussion
In this study, we investigated the involvement of JAM-C in the accumulation of monocytes at inflammation sites. Surprisingly, blocking reagents to JAM-C did not abrogate monocyte recruitment from the vasculature, but led to increased monocyte reverse transmigration and return to the circulation. We analyzed the role of JAM-C from the initial phase of monocyte interaction with activated endothelium under shear stress to transmigration and retention of the monocytes within the ablumen. Importantly, stimulating HUVECs did not alter JAM-C expression in the 4-hour time scale used for the flow assays. Therefore, endothelial function during an early inflammatory reaction appears to be facilitated by the constitutive expression of JAM-C.
The concept of reverse transmigration has proven intriguing.37 Previous reports have described this phenomenon in vitro for monocytes38,39 and neutrophils,32 but characterizing this phenomenon in vivo has been technically challenging and controversial. However, a recent report has visualized bidirectional transmigration of neutrophils across vascular endothelium in zebrafish, implicating reverse transmigration of leukocytes as a viable mechanism in regulating inflammation.40 Our in vivo experiments have further expanded the understanding of this phenomenon by showing that blocking JAM-C led to an expanded population of inflammatory monocytes with a reverse-transmigratory phenotype in the blood (Mac-1+, Gr1+, F4/80+, and L-selectin−), confirming JAM-C as a player in this molecular process.
In the current study, substantial levels of monocyte reverse transmigration were observed under control conditions, suggesting that ECs have an intrinsic capacity to regulate monocyte retention. Furthermore, multiple transmigration events of leukocytes at the endothelial junction may add another level of control to the complex decision-making processes that regulate extravasation or a return to circulation. Together, the results of these studies suggest that ECs can regulate accumulation of infiltrating leukocytes by a mechanism distinct from recruitment and can counteract the excessive inflammatory stimuli that can lead to a disproportionate recruitment of leukocytes.
Our findings are consistent with a previous study using neutrophils, which demonstrated adhesion and transmigration on cultured HUVECs under flow was JAM-C independent.41 However, experimental models of inflammation in mice demonstrated a reduced extravasation of leukocytes using blocking reagents to JAM-C.5,17,19 This discrepancy prompted us to investigate the role of JAM-C after transmigration of monocytes and their passage into the ablumen. We showed that JAM-C–blocking reagents disrupted the retention of transmigrated monocytes in the ablumen, allowing them to return to the luminal surface of the endothelium. Thus, in vivo ECs may be able to modulate the number of infiltrating monocytes entering the adjacent mesenchyme by a novel mechanism of retention; indeed, previous in vivo studies using transgenic mice that overexpress JAM-C on the vascular endothelium showed more leukocytes accumulated at sites of inflammation.5,32
As part of this study, we also investigated an extended role for JAM-C in monocyte recirculation beyond the vasculature and the abluminal compartment. We postulated that blocking JAM-C function within the mesenchyme might suffice to increase cell permeability across multiple tissue barriers to allow monocyte-derived cells to re-enter the vasculature. However, adoptive transfer experiments using labeled peritoneal monocyte infiltrates in the presence of a JAM-C–blocking antibody did not lead to a return to circulation (data not shown). Previous studies have shown that inflammatory macrophages are rapidly cleared from the peritoneal cavity by emigration through the lymphatics.42,43 Further studies also demonstrated that macrophage efflux from the inflammatory mesenchyme is a highly regulated process mediated by multiple adhesive interactions on mesothelial cells that line the draining lymphatics.44 Therefore, the regulatory role of JAM-C in monocyte countertrafficking only occurs from the abluminal compartment, and monocyte-derived cells that have entered the mesenchyme are committed to recirculation through the lymphatics.
We have shown that JAM-C displayed on monocytes is exclusively associated with adherent platelets and platelet microparticles. This in vitro manifestation may lead to activation of integrins on monocytes by adherent platelets, a phenomenon previously described.28,–30 The existence of leukocyte-platelet complexes in the vasculature has been described in numerous inflammatory disorders, although the physiologic relevance of this manifestation is still unclear.29,45 Previous studies have shown that expression of P-selectin on immobilized platelet cell surfaces can augment leukocyte recruitment at sites of recent injury by mediating capture of neutrophils from flow.46,47 Therefore, deposition of platelets at sites of transmigration by monocyte-platelet aggregates facilitates capture and transmigration of circulating leukocytes to sites of inflammation, but platelets do not play a major role in transmigration.
Previous studies have shown that JAM-A is capable of forming cis-interactions with the integrin αvβ3 on ECs regulating migration through the mitogen-activated protein kinase (MAPK) pathway.48,49 Similarly, JAM-C expressed on epithelial cells has been shown to modulate cell migration by regulating β3 integrin activation.50 We have shown that transmigrated monocytes are JAM-C−, excluding a potential role for either of these mechanisms in directly regulating monocyte migration. An extended role for endothelial JAM-C in regulating migration of monocytes by trans-interactions with unknown ligands is still not known. For example, JAM-C could activate endothelial αvβ3 integrin as a ligand for a so-far uncharacterised binding partner on monocytes. This proof of concept has recently been described.51 However, it has been shown that the blocking antibody to JAM-C used in this study specifically blocked JAM-B /-C and not JAM-C/Mac-1 interactions,6 restricting any potential role of blocking JAM-C to ECs.
Recent studies have identified a specific role for ECs in monocyte recruitment during the very early stages of inflammation.52,–54 It has also been shown that early recruitment of Gr1hi monocytes is critical in efficient crosspriming of CD8+ cytotoxic lymphocytes with soluble antigen within mucocutaneous tissues.55 This function is distinct from major histocompatibility complex (MHC) class II antigen presentation by resident Langerhans cells and is mediated by newly recruited monocytes from the vasculature at the very onset of inflammation. Since the effect of blocking JAM-C occurs within 1 hour after the onset of inflammation, we suggest that JAM-C plays a role in initiating the adaptive immune response. Indeed, our in vitro and in vivo experiments have shown that the role of JAM-C decreases during later phases of inflammation.
In vivo studies have shown that JAM-C–blocking reagents reduced leukocyte numbers in inflammatory infiltrates.5,17,18 Based on our observations, we suggest that this reduction does not arise from events preceding transmigration, but is a consequence of increased reverse transmigration and a “leakage” of recruited monocytes from the ablumen. In our in vitro model, we have established that blocking reagents to JAM-C led to a shift in equilibrium of leukocyte ingress/egress at the endothelial junction, resulting in a net decrease of the number of abluminal monocytes. We have shown in vivo that there is an increase in monocytes with a reverse-transmigratory phenotype in circulation during early inflammation, although the precise mechanism of this event is unknown. Observations made in this study have confirmed that monocytes do not spontaneously “de-adhere” and return to flow within the time frame of the flow assay. However, we have established that blocking JAM-C function leads to an increased monocyte presence on the lumen by reverse transmigration, a clear prerequisite to detachment. Because targeting JAM-C expression on vascular ECs may induce increased reverse transmigration of recruited monocytes occupying the ablumen, JAM-C may prove to be a suitable target for reversing existing inflammation. In conclusion, we have defined a novel mechanism and model by identifying a further checkpoint for regulating monocyte accumulation at sites of inflammation. At this checkpoint, endothelial JAM-B/-C can play a pivotal role in modulating the immune response by controlling monocyte retention within the ablumen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Philip Hammel and Stephane Jemelin for their expert technical assistance.
This work was supported by Krebsforschung Schweiz (OCS-01653-02-2005), Swiss National Science Foundation (3100AO-100697/2), and the National Institutes of Health grants HL53993 and HL36028.
National Institutes of Health
Authorship
Contribution: P.F.B. designed the project and developed the flow assay and specialist analysis. C.S. and S.N. conducted the cremaster muscle ex vivo analysis and intravital microscopy. Specialist flow assay equipment was developed in collaboration with G.B.N. and E.G.R. Analysis of platelet attachment and JAM-C expression on monocytes was conducted in collaboration with C.O. Development of flow assay using HUVECs transfected with JAM-C siRNAs done in collaboration with M.M.L. Design of protocols for monocyte purification and utilization done in collaboration with F.W.L. Thioglycollate-induced peritonitis models and adaptive transfer experiments conducted in collaboration with M.A.L. Project development and interpretation of results done in collaboration with M.A.L and B.A.I. Project supported and conducted in the laboratory of B.A.I.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beat A. Imhof, Department of Pathology and Immunology, University Medical Centre, 1 Rue Michel-Servet, CH-1211, Geneva 4, Switzerland; e-mail:beat.imhof@medecine.unige.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal