The combination of a DNA hypomethylating agent with a histone deacetylase inhibitor has synergistic antileukemia activity and may restore sensitivity to all-trans retinoic acid (ATRA). We conducted a phase 1/2 study of the combination of 5-azacitidine (5-AZA), valproic acid (VPA), and ATRA in patients with acute myeloid leukemia or high-risk myelodysplastic syndrome. 5-AZA was administered subcutaneously at a fixed dose of 75 mg/m2 daily for 7 days. VPA was dose-escalated and given orally daily for 7 days concomitantly with 5-AZA. ATRA was given at 45 mg/m2 orally daily for 5 days, starting on day 3. A total of 53 patients were treated. Their median age was 69 years (range, 5-84 years). The maximum tolerated dose of VPA in this combination was 50 mg/kg daily for 7 days. Dose-limiting toxicity was reversible neurotoxicity. The overall response rate was 42%. In previously untreated older patients, the response rate was 52%. Median number of courses to response was 1 (range, 1-3 courses). Median remission duration was 26 weeks, and median survival has not been reached. A significant decrease in global DNA methylation and induction of histone acetylation were achieved. VPA blood levels were higher in responders (P < .005). In conclusion, the combination studied is safe and has significant clinical activity. This clinical trial was registered at www.clinicaltrials.gov as no. NCT00326170.
Introduction
5-azacytidine (5-AZA) is a cytidine analog with clinical activity in myelodysplastic syndromes (MDSs)1 and in acute myeloid leukemia (AML).2,3 5-AZA is a DNA methyltransferase inhibitor that induces DNA hypomethylation4 both in vitro5 and in vivo. DNA methylation is a mechanism of epigenetic regulation of gene transcription.6 It involves the addition of a methyl group to a cytosine when it is followed by guanine in the so-called cytosine-guanine pairs (CpGs). CpGs are underrepresented in the human genome, except in areas known as CpG islands that are located near gene promoters and repetitive elements such as long interspersed nucleotide elements (LINEs).7 DNA methylation is a physiologic process involved in imprinting and other mechanisms of gene dosage control.6 Aberrant DNA methylation of promoter-associated CpG islands is frequently detected in cancer.7 Because methylation of promoter-associated CpG islands is frequently associated with gene silencing,6 it is considered a functional inactivating mechanism of tumor suppressor genes.7 In contrast to deletions or mutations of tumor suppressor genes, aberrant DNA methylation is reversible both in vitro and in vivo with the use of hypomethylating agents such as 5-AZA or 5-aza-2′deoxycytidine (decitabine).4 The understanding of the role of 5-AZA as a modulator of DNA methylation has led to rational combination therapies exploiting mechanisms of epigenetic regulation of gene expression. One such mechanism is the biochemical modification of chromatin-associated histone proteins, such as histone H3 or H4 acetylation.4 This process is controlled by histone acetyl transferases and histone deacetylases (HDACs).4,8 Inhibition of HDAC activity results in histone acetylation that is associated with a permissive gene expression state.8 A number of HDAC inhibitors have shown activity in leukemia and lymphoma.8 The combination of a hypomethylating agent with an HDAC inhibitor has synergistic effects on gene reactivation and leukemia cell kill.9,10 Valproic acid (VPA), a short-chain fatty acid used clinically for the treatment of epilepsy and mood disorders, is an HDAC inhibitor.11,–13 As a single agent, VPA has modest clinical activity in MDS14 and AML.15 In combination with decitabine, VPA has synergistic antileukemic activity in vitro,9 and in a recent clinical study, it was shown to be active in patients with poor-risk leukemia.16
All-trans retinoic acid (ATRA) is a cell differentiation agent with clinical activity in acute promyelocytic leukemia (APL)17 but not in other types of leukemias.18 In APL, ATRA releases corepressors and HDACs and induces the expression of target genes.19 In ATRA-resistant APL variants, the use of an HDAC inhibitor can restore sensitivity to ATRA.19 In APL, DNA methyltransferases are also recruited to target gene promoters.20 ATRA can induce hypomethylation and re-expression of the RAR-β2gene.20 This activity is enhanced by 5-AZA.20 Therefore, it is possible that the combination of a hypomethylating agent with an HDAC inhibitor may sensitize ATRA-resistant AML to the effects of ATRA.
Based on this information, we designed a study to test whether the combination of 5-AZA, VPA, and ATRA is safe and active in patients with high-risk MDS and AML.
Patients, materials, and methods
Study group eligibility
Patients with refractory or relapsed AML or high-risk MDS, defined as having 10% or more bone marrow blasts, were included. Untreated patients older than 60 years with AML or high-risk MDS who refused or were not candidates for front-line chemotherapy were also eligible. Whether or not a patient was a candidate for intensive chemotherapy was determined by the treating physician after discussion with the patient. Other inclusion criteria included Eastern Cooperative Oncology (ECOG) performance status score of 2 or less, aged 2 years or older, and adequate hepatic and renal functions. Patients must have been off chemotherapy for at least 2 weeks prior to entering the study. Patients with previously untreated core binding-factor leukemias, nursing or pregnant women, or patients with active or uncontrolled illnesses were excluded. The protocol was approved by the M. D. Anderson Cancer Center (MDACC) ethics committee. All patients signed informed consent in accordance with the Declaration of Helsinki following institutional guidelines.
Treatment
Treatment consisted of 5-AZA, VPA, and ATRA. 5-AZA was given at a fixed dose of 75 mg/m2 subcutaneous daily for 7 days (days 1-7). VPA was administered orally daily for 7 days (days 1-7) concomitantly with 5-AZA administration. ATRA was administered at a dose of 45 mg/m2 orally daily for 5 days, starting on day 3 of 5-AZA and VPA (days 3-7). Courses of therapy were repeated not earlier than every 3 weeks. Patients were allowed to receive supportive care measures including antibiotics, antiemetics, and growth factors as clinically indicated and according to institutional guidelines. In the phase 1 portion, 3 different dose levels of VPA were studied: 50, 62.5, and 75 mg/kg orally daily for 7 days. In phase 2, VPA was administered at its maximum tolerated dose (MTD) in combination with 5-AZA and ATRA.
Response criteria and statistical methods
A complete response (CR) required the disappearance of all signs and symptoms related to the disease, a bone marrow with 5% or fewer blasts and peripheral blood count with an absolute neutrophil count of 109/L or more and platelet count of 100 × 109/L or more. A CRp was defined as a CR except for a platelet count increase by 50% to more than 30 × 109/L but less than 100 × 109/L. A bone marrow (BM) response was defined as bone marrow blast of 5% or less but without meeting the peripheral blood count criteria for CR or CRp. For a response to be considered, it had to last for at least 4 weeks. Remission duration was calculated from the date of first response until relapse, and survival from start of therapy until death from any cause. Bone marrow aspiration procedures were performed uniformly during course no. 1 of therapy pretreatment and on days 21 and 28 (± 3 days). On subsequent courses, they were performed on day 28 of each cycle until documentation of response, and thereafter as clinically indicated.
In phase 1 of the study, cohorts of 3 patients were entered at each dose level. If no grade 3 or higher nonhematologic toxicity was observed, VPA was escalated to the next dose level. If 1 patient developed such toxicity, 3 more patients were accrued at that dose level. If 2 or more patients developed grade 3 or higher nonhematologic toxicity, that dose of VPA was considered too toxic. The MTD was defined as the dose below the 1 producing dose-limiting toxicity (DLT) in 2 or more patients. A total of 10 patients were to be treated at the MTD to confirm the rate of toxicity. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria (NCI CTC version 3).
The phase 2 portion was designed to evaluate the activity of the combination. The study would stop early if the probability was less than 10% that the response rate (CR + CRp) was at least 30%. The probability of a patient response was assessed in cohorts of 10 patients. The study was to stop if there were 0 responses in the first 10 patients, 3 or fewer responses in 20 patients, or 5 or fewer in 30 patients. Otherwise, the maximum number of patients that could be treated during phase 2 was 40. Patients treated during the phase 1 portion of the study at the MTD of VPA could be included in the phase 2 analysis.
Groups were compared using the 2-sample t test. Survival and relapse-free survival were reported using Kaplan-Meier curves. All P values were 2-sided; statistical significance was declared if P was less than .05. The Statistica 6.1 software (Stat Soft, Tulsa, OK) was used to perform these calculations.
Cytogenetic analysis
Cytogenetic analysis was performed in the clinical cytogenetics laboratory at MDACC. Baseline cytogenetic analysis was performed prior to therapy, and subsequently with each course of therapy when possible in patients with abnormal cytogenetics as previously described.16
Detection of VPA levels
Free and bound VPA levels were measured using commercially available assays on days 0, 5, and 7 of each course of therapy as previously described.16
Isolation of human mononuclear cells
Peripheral blood human mononuclear cells were used to perform DNA methylation and mRNA studies. Peripheral blood specimens from patients were drawn into heparin (30 U/mL)–containing tubes. Mononuclear cells were separated using Ficoll-Paque PLUS gradient centrifugation (Amersham Biosciences AB, Uppsala, Sweden). The monocyte-enriched fraction was collected and washed twice with calcium and magnesium-free phosphate-buffered saline (PBS). After centrifugation, PBS was removed, and the samples were immediately frozen at −80°C.
Analysis of DNA methylation
Analysis of histone acetylation
Histone H3 and H4 acetylation was analyzed by Western blot using antibodies directed against acetylated histone H3 and H4 as previously described.16 In brief, proteins were isolated from peripheral blood mononuclear cells by sonication using a lysis buffer (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 1 mM PMSF) and posteriorly quantitated. A total of 30 μg protein from each cell lysate were separated in 12% SDS–polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to Immobilion-P Transfer Membranes (Millipore, Billerica, MA). Membranes were blocked in 3% nonfat milk and probed overnight at 4°C with 1:2000 dilution of acetylated histone H3 antibody (Upstate Biotechnology, Waltham, MA). Membranes were washed 3 times with PBS containing 0.1% Tween 20 (PBS-T) and incubated with an anti-rabbit peroxidase-conjugated secondary antibody (1:2000; Amersham Biosciences, Piscataway, NJ) for 1 hour at room temperature. Membranes were washed 3 times in PBS-T, and the film was developed by using enhanced chemuluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). β-actin (1:5000; Sigma, St Louis, MO) was used as internal control. HL-60 cells treated with VPA were used as positive controls.
Real-time PCR analysis of p21CIP1 and p15 mRNA gene expression
P21CIP1 and p15 mRNA expression was conducted as previously described.16 In summary, total cellular RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Total RNA (2-3 μg) was used for reverse transcription (RT) reactions using the Moloney murine leukemia virus RT enzyme (Invitrogen). Levels of p21CIP1 and p15 mRNA expression were analyzed using real-time polymerase chain reaction (PCR). Primers and probes were purchased from Applied Biosystems and analyzed using an Applied Biosystems Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). PCR reactions were performed using 20 × Assays-On-Demand Gene Expression Assay Mix (contained unlabeled PCR primers and TaqMan probes) and TaqMan Universal PCR Master mix (Applied Biosystems). PCR conditions were 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute, repeated for 40 cycles. Experiments were performed in triplicate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Quantitative values were obtained from the cycle number (CT value) at which the increase in fluorescent signal associated with an exponential accumulation of PCR products was detected. The amount of target gene was normalized to the endogenous reference GAPDH to obtain the relative threshold cycle (ΔCT) and then related to the CT of the control to obtain the relative expression level (2−ΔΔCT) of the target gene.
Results
Study group
A total of 53 patients were treated. Patient characteristics are shown in Table 1. A total of 19 patients were treated during the phase 1 portion of the study, and 34 during the phase 2 portion, which included 10 patients that were an expansion cohort treated at the MTD. Of these 33 (62%) patients were older than 60 years and had previously untreated disease. These patients refused or were not considered candidates for frontline intensive chemotherapy. A total of 34 (64%) patients had poor-risk cytogenetics.
Patient characteristics (n = 53)
| Characteristic . | Total . | Untreated . |
|---|---|---|
| No. patients | 53 | 33 |
| Median age, y (range) | 69 (5-84) | 74 (61-84) |
| Age older than 60 y, no. (%) | 44 (83) | 33 (100) |
| Diagnosis, no. (%) | ||
| AML | 49 (92) | 30 (90) |
| MDS | 4 (8) | 3 (10) |
| Median no. prior therapies (range) | 0 (0-6) | 0 |
| Untreated, no. (%) | 33 (62) | 33 (100) |
| First salvage, no. (%) | 9 (17) | 0 |
| More than first salvage, no. (%) | 11 (21) | 0 |
| Cytogenetics, no. (%) | ||
| Diploid | 18 (34) | 14 (42) |
| CBF | 1 (2) | 0 |
| Other | 34 (64) | 19 (57) |
| Median WBC count (×109/L) | 2.6 (0.5-116) | 4.3 (0.7-116) |
| Characteristic . | Total . | Untreated . |
|---|---|---|
| No. patients | 53 | 33 |
| Median age, y (range) | 69 (5-84) | 74 (61-84) |
| Age older than 60 y, no. (%) | 44 (83) | 33 (100) |
| Diagnosis, no. (%) | ||
| AML | 49 (92) | 30 (90) |
| MDS | 4 (8) | 3 (10) |
| Median no. prior therapies (range) | 0 (0-6) | 0 |
| Untreated, no. (%) | 33 (62) | 33 (100) |
| First salvage, no. (%) | 9 (17) | 0 |
| More than first salvage, no. (%) | 11 (21) | 0 |
| Cytogenetics, no. (%) | ||
| Diploid | 18 (34) | 14 (42) |
| CBF | 1 (2) | 0 |
| Other | 34 (64) | 19 (57) |
| Median WBC count (×109/L) | 2.6 (0.5-116) | 4.3 (0.7-116) |
CBF indicates core-binding factor; WBC, white blood cell.
VPA dose escalation and toxicities
A total of 3 dose levels of VPA (50, 62.5, and 75 mg/kg orally daily for 7 days) were explored in combination with 5-AZA and ATRA. No grades 3 to 4 nonhematologic toxicity attributable to 5-AZA or ATRA was observed at any dose level. The number of patients who experienced grades 3 to 4 toxicities related to therapy during the first course of therapy is shown in Table 2. We assessed toxicities in cohorts of 3 patients. The starting dose of VPA was 50 mg/kg. One of the first 3 patients experienced a DLT (grade 3 neurotoxicity), and 3 more patients were enrolled in that cohort. Subsequently, the dose was escalated to 62.5 mg/kg orally daily for 7 days. The first 3 patients enrolled in this cohort did not experienced DLTs, and the dose of VPA was further increased to 75 mg/kg orally daily for 7 days. One of the first 3 patients at this dose level experienced a DLT. An additional 3 patients were enrolled at this dose level, with 1 additional patient developing toxicity. Subsequently, VPA was decreased to 62.5 mg/kg orally daily for 7 days. DLTs were observed in 2 additional patients at this dose level, and VPA was decreased again to 50 mg/kg, which was declared as the MTD after treating 10 patients at this dose level. Subsequently, a total of 34 patients were treated in phase 2 at the MTD, including 10 patients in the expanded phase 1 cohort at the MTD. Table 3 summarizes the most frequent toxicities during course 1 and thereafter. Most frequent events included neurotoxicities, mainly somnolence and confusion, followed by fatigue. Toxicities were transient and reversible. Only 3 (5%) of 53 patients died during induction; none of them was previously untreated. Overall, there were no differences in terms of toxicities between the previously untreated group and those patients that had received prior therapy, nor between responding patients and those that did not achieve a response.
Frequency of nonhematologic toxicities (drug-related, first course)
| VPA dose* . | No. with toxicity . | ||||
|---|---|---|---|---|---|
| No. . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| 50 mg/kg | 6 | 1 | 4 | 1 | 0 |
| 62.5 mg/kg | 7 | 0 | 5 | 2 | 0 |
| 75 mg/kg | 6 | 1 | 3 | 2 | 0 |
| Phase 2, 50 mg/kg | 34 | 5 | 14 | 6 | 2 |
| VPA dose* . | No. with toxicity . | ||||
|---|---|---|---|---|---|
| No. . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| 50 mg/kg | 6 | 1 | 4 | 1 | 0 |
| 62.5 mg/kg | 7 | 0 | 5 | 2 | 0 |
| 75 mg/kg | 6 | 1 | 3 | 2 | 0 |
| Phase 2, 50 mg/kg | 34 | 5 | 14 | 6 | 2 |
Daily for 7 days.
Most common nonhematologic side effects (53 patients; 166 courses)
| . | No. patients . | |
|---|---|---|
| Toxicity, grades 3/4 | Course 1 | More than course 1 |
| Confusion | 4 | 2 |
| Somnolence | 9 | 0 |
| Nausea/vomiting | 1 | 2 |
| Fatigue | 1 | 2 |
| Diarrhea | 0 | 1 |
| Hepatic | 1 | 1 |
| . | No. patients . | |
|---|---|---|
| Toxicity, grades 3/4 | Course 1 | More than course 1 |
| Confusion | 4 | 2 |
| Somnolence | 9 | 0 |
| Nausea/vomiting | 1 | 2 |
| Fatigue | 1 | 2 |
| Diarrhea | 0 | 1 |
| Hepatic | 1 | 1 |
Because the entry criteria for the study allowed patients to be off prior chemotherapy for just 2 weeks, we analyzed the impact of prior therapy on this group of patients. Of the 20 previously treated patients, only 3 were treated less than 1 month after their prior third, fourth, and fifth salvage attempts. All were taken off because of no response. No toxicity was observed. The median time after last therapy was 2 months (range, 0.5 to 72 months). Therefore, prior therapy did not seem to have an impact on response or toxicity.
Clinical activity
Responses are summarized in Table 4. An overall response rate of 42% was observed. This included 12 (22%) CRs, 3 (5%) CRp's, and 7 (13%) BM responses. In the subgroup of 33 previously untreated patients older than 60 years, 11 (33%) CRs, 3 (9%) CRp's, and 3 (9%) BM responses were demonstrated, for an overall response rate of 52%. In this subgroup, 9 CRs occurred at the VPA dose of 50 mg/kg, 1 CR at 62.5 mg/kg, and 1 CR at 75 mg/kg. The 3 BM responses in this subgroup were observed at 50 mg/kg. In the subgroup of previously treated patients, 1 CR was seen at the 50 mg/kg dose. A total of 3 BM responses were observed at the VPA dose level of 50 mg/kg, and 1 BM response was observed at 75 mg/kg.
Clinical responses
| VPA dose . | No. . | CR . | CRp . | BM response . | Overall no. response (%) . | No. courses to response (range) . |
|---|---|---|---|---|---|---|
| 50 mg/kg | 40 | 10 | 3 | 6 | 19 (47) | 1 (1-3) |
| 62.5 mg/kg | 7 | 1 | 0 | 0 | 1 (14) | 2 |
| 75 mg/kg | 6 | 1 | 0 | 1 | 2 (33) | 1 |
| Total | 53 | 12 | 3 | 7 | 22 (42) | 1 (1-3) |
| Previously untreated | 33 | 11 | 3 | 3 | 17 (52) | 1 (1-3) |
| Previously treated | 20 | 1 | 0 | 4 | 5 (25) | 1 (1-2) |
| VPA dose . | No. . | CR . | CRp . | BM response . | Overall no. response (%) . | No. courses to response (range) . |
|---|---|---|---|---|---|---|
| 50 mg/kg | 40 | 10 | 3 | 6 | 19 (47) | 1 (1-3) |
| 62.5 mg/kg | 7 | 1 | 0 | 0 | 1 (14) | 2 |
| 75 mg/kg | 6 | 1 | 0 | 1 | 2 (33) | 1 |
| Total | 53 | 12 | 3 | 7 | 22 (42) | 1 (1-3) |
| Previously untreated | 33 | 11 | 3 | 3 | 17 (52) | 1 (1-3) |
| Previously treated | 20 | 1 | 0 | 4 | 5 (25) | 1 (1-2) |
With a median follow up of 21 weeks, the median survival in patients achieving CR or CRp has not been reached (Figure 1). Median remission duration was 26 weeks (Figure 2).
Overall, the median number of courses of therapy administered was 2 (range, 1-10+ courses). In the untreated patients older than 60 years, it was 3 (range, 1-10+ courses). The median number of courses to first response was 1 (range, 1-3 courses). Among 4 patients with high-risk MDS, 2 achieved CR, and 2 achieved a BM response.
Cytogenetic responses
Pretreatment cytogenetic characteristics are shown in Table 5. A total of 5 (62%) of 8 patients with poor-risk cytogenetics had a response. A total of 4 (36%) of 11 responders with informative cytogenetics achieved a cytogenetic response. Of these, 2 had a complete cytogenetic response, and 2 had a partial cytogenetic response.
Pretreatment cytogenetics and clinical responses
| Cytogenetic characteristic . | No. (%) . | Response no. (%) . |
|---|---|---|
| +8 | 1 (2) | 0 (0) |
| −5/−7 | 8 (15) | 5 (62) |
| Diploid | 18 (34) | 7 (39) |
| Insufficient metaphases | 2 (4) | 1 (50) |
| Miscellaneous | 21 (40) | 7 (33) |
| t(8;21) | 1 (2) | 0 (0) |
| −y | 2 (4) | 0 (0) |
| Cytogenetic response* | 11 | 4 (36) |
| Cytogenetic characteristic . | No. (%) . | Response no. (%) . |
|---|---|---|
| +8 | 1 (2) | 0 (0) |
| −5/−7 | 8 (15) | 5 (62) |
| Diploid | 18 (34) | 7 (39) |
| Insufficient metaphases | 2 (4) | 1 (50) |
| Miscellaneous | 21 (40) | 7 (33) |
| t(8;21) | 1 (2) | 0 (0) |
| −y | 2 (4) | 0 (0) |
| Cytogenetic response* | 11 | 4 (36) |
Patients with evaluable metaphases.
VPA levels
VPA levels were measured sequentially pretreatment on day 0, and prior to drug administration on days 5 and 7. Free (milligrams per liter) and bound (micrograms per milliliter) VPA levels during cycle 1 are shown in Table 6. The median free and bound VPA levels on days 5 and 7 were significantly higher in patients achieving a response. Median free VPA level on day 5 in responders was 36.8 mg/L (range, 29.4-61.6 mg/L), whereas it was 21.6 mg/L (range, 4.2-46.4 mg/L) in nonresponders (P < .005). Median bound VPA level on day 5 was 132 μg/mL (range, 69-202 μg/mL) in responders and 104 μg/mL (range, 26-165 μg/mL) in nonresponders (P < .005). A similar pattern was observed on day 7 (Table 6). Despite the association between VPA blood levels and response, there was no association between dose level and response.
VPA levels and response
| . | Median value (range) . | ||||
|---|---|---|---|---|---|
| FVPA day 5, mg/L | FVPA day 7, mg/L | BVPA day 5, μg/mL | BVPA day 7, μg/mL | P | |
| Responders | 36.8 (29.4-61.6) | 35.1 (17.6-96.4) | 132 (69-202) | 124 (63-177) | .005 |
| Nonresponders | 21.6 (4.2-46.4) | 25.05 (7-56.8) | 104 (26-165) | 106.5 (15-161) | — |
| . | Median value (range) . | ||||
|---|---|---|---|---|---|
| FVPA day 5, mg/L | FVPA day 7, mg/L | BVPA day 5, μg/mL | BVPA day 7, μg/mL | P | |
| Responders | 36.8 (29.4-61.6) | 35.1 (17.6-96.4) | 132 (69-202) | 124 (63-177) | .005 |
| Nonresponders | 21.6 (4.2-46.4) | 25.05 (7-56.8) | 104 (26-165) | 106.5 (15-161) | — |
FVPA indicates free VPA; BVPA, bound VPA; and —, not applicable.
Induction of histone acetylation
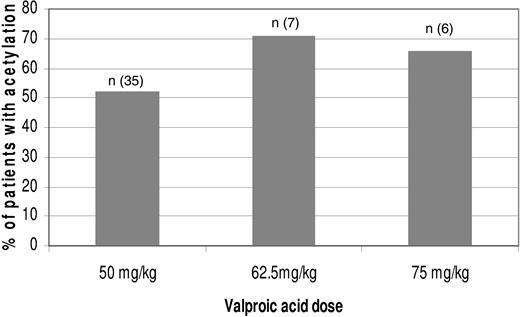
Histone H3 and H4 acetylation was evaluated using western blots on days 0, 2, 7, and 21 in 48 patients. Histone acetylation was detected in 26 (54%) patients. The frequency of histone acetylation per dose level is shown in Figure 3. There was no correlation between VPA dose level and histone acetylation or histone acetylation and response.
Frequency of histone acetylation by VPA dose. Histone acetylation was measured using Western blots.16 Bars represent the percentage of patients with histone acetylation; n, the number of patients evaluated.
Frequency of histone acetylation by VPA dose. Histone acetylation was measured using Western blots.16 Bars represent the percentage of patients with histone acetylation; n, the number of patients evaluated.
DNA methylation analysis
To study the DNA methylation dynamics during treatment, global DNA methylation was analyzed on days 0, 2, 7, and 21. We used the LINE pyrosequencing assay, which is a surrogate marker of global methylation.21 We found a significant decrease in global methylation by day 7, the last day of treatment, with a return toward baseline by day 0 of the next cycle as shown in Figure 4. The degree of global DNA hypomethylation achieved during treatment was not different between responders and nonresponders.
Dynamics of global DNA methylation with 5-AZA therapy. Global DNA methylation was measured using the LINE bisulfite pyrosequencing assay.16 It was measured pretreatment and on days 2, 7, and 21 of the first course and prior to initiation of the second course. There was a significant difference in terms of DNA methylation comparing day 0 with day 7. No differences were observed between responders and nonresponders. C indicates course; D, day. Error bars indicate standard deviation of the mean.
Dynamics of global DNA methylation with 5-AZA therapy. Global DNA methylation was measured using the LINE bisulfite pyrosequencing assay.16 It was measured pretreatment and on days 2, 7, and 21 of the first course and prior to initiation of the second course. There was a significant difference in terms of DNA methylation comparing day 0 with day 7. No differences were observed between responders and nonresponders. C indicates course; D, day. Error bars indicate standard deviation of the mean.
Induction of p21 and p15 mRNA expression
We measured the expression of p21 and p15 mRNA pretreatment and sequentially on days 7 and 21 in 43 patients with available samples. Results are shown in Figure 5. Although a modest but significant increment in p15 and p21 mRNA expression was detected, this was not associated with clinical response.
Induction of p21 and p15 mRNA expression. p21 and p15 mRNA expression was measured using real-time PCR on days 0, 7, and 21. Horizontal bars indicate mean.
Induction of p21 and p15 mRNA expression. p21 and p15 mRNA expression was measured using real-time PCR on days 0, 7, and 21. Horizontal bars indicate mean.
Subset analysis of pediatric patients
A total of 3 patients aged 5, 15, and 18 years were treated in this study. All of them had recurring or refractory AML. A 15-year-old patient achieved a BM response during therapy but died after 9 weeks. No significant toxicities were observed in this age group. Of the 3 patients, 2 had evidence of histone acetylation. No difference in the dynamics of DNA hypomethylation was observed between children and adults (data not shown).
Discussion
In this study, we demonstrated that the combination of 5-AZA, VPA at 50 mg/kg orally daily for 7 days, and ATRA was safe. This combination was also active and associated with induction of global DNA hypomethylation and histone acetylation. We defined the MTD of VPA to be 50 mg/kg orally for 7 days. This is consistent with our prior experience with decitabine and VPA, where a dose of 50 mg/kg orally for 10 days could be tolerated.16 VPA administration at these doses was associated with reversible neurotoxicity, which resolved shortly after drug interruption. No significant toxicities related to 5-AZA or ATRA administration were observed. In particular, no evidence of acute cell differentiation syndrome (ie, ATRA syndrome) was observed in any patient. The overall treatment mortality was 5%: 3 patients, all of them with recurring/refractory disease, died during induction. The combination of 5-AZA, VPA, and ATRA was clinically active: the overall response rate was 42%, including 12 (22%) CRs. In the subset of patients older than 60 years with previously untreated disease, the response rate was 52% with no induction deaths. Their median survival has not been reached. Therefore, the overall response and toxicity profile observed in this trial for this group of patients with advanced age and poor prognosis are encouraging. The responses observed in our trial are robust. With a median follow up of 21 weeks, the median survival in responding patients has not been reached. The median relapse-free survival was 26 weeks. Clinical activity was observed in patients with poor-risk cytogenetics, as 62% of them achieved a response. A total of 4 patients had high-risk MDS. All of them were older than 60 years (range, 65-83 years), and 2 CRs and 2 BM responses were noted, which support the activity of this combination in high-risk MDS. That said, it should be noted that a fraction of these responses (13%) included complete bone marrow responses, in particular in patients with recurring/refractory disease. The clinical significance of that type of response assessment is unclear at the present time but may benefit patients being prepared, for instance, for allogeneic stem cell transplantation.
Patients aged 2 years and older were allowed to be enrolled in our study. The reason for the age restriction was the reported hepatotoxicity of VPA when used in patients less than 2 years old.22,–24 In our study, the combination of 5-AZA, VPA, and ATRA was safe in the 3 pediatric patients with recurring/refractory AML, and 1 BM response was observed. These findings are similar to the results with the combination of VPA and decitabine.16 Combinations of VPA with 5-AZA or decitabine and ATRA need to be further explored in specific pediatric phase 1 studies in leukemia.
In this study, we evaluated 4 potential biomarkers: VPA levels, histone acetylation, induction of global DNA hypomethylation, and induction of p21 and p15 mRNA expression. First, higher VPA levels were demonstrated in patients who responded versus those that did not. This is consistent with our in vitro data that indicate who the antileukemic effect of VPA is directly related to its concentration9 and with our previous study of decitabine and VPA,16 in which untreated patients with higher VPA levels had a higher response rate. The implications of these findings are important in that replacement of VPA with a more potent HDAC inhibitor with a better toxicity profile may improve the activity and tolerability of this type of combination. Another potential implication is the specific targeting of plasma levels of VPA in future clinical trials, instead of using doses based on weight. Second, HDAC inhibition resulted in histone acetylation. Consistent with the in vitro HDAC inhibitory effect, we observed that 54% of the patients had evidence of histone acetylation. Induction of histone acetylation was not associated with response. A similar phenomenon has been observed in patients treated with single-agent vorinostat25 and with the combination of 5-AZA and phenylbutyrate26 and other studies.16 The lack of association between histone acetylation and response could have different explanations. One explanation may relate to the sensitivity of the assays used to measure acetylation. It is also possible that clinical responses are related to other molecular effects unrelated or downstream of HDAC inhibition. It should be noted that VPA levels were not associated with histone acetylation. Third, similar to the experience of Gore et al27 , we confirmed that 5-AZA had in vivo hypomethylating activity. We have tested this using the LINE bisulfite pyrosequencing assay. As with decitabine, we have documented that global methylation is a transient phenomenon that peaks shortly after 5-AZA exposure to return gradually to baseline. As with decitabine, there was no correlation between response and induction of hypomethylation. Because LINE methylation occurs in both normal and neoplastic cells, the analysis of global methylation dynamics could be considered as a marker of the biological activity of the drug, but is not necessarily related to its clinical activity. It is possible that specific gene methylation studies may identify markers of response. Those studies are ongoing. It is also possible that the clinical activities of both 5-AZA and VPA are only partially related to the induction of DNA methylation or histone acetylation. Finally, the combination therapy used here resulted in modest induction of p21 and p15 mRNA expression, a phenomenon not associated with clinical response.
While the regimen was safe and active, we did not prove that the combination is superior to single-agent 5-AZA, as this study was not designed to test that hypothesis. This should be tested in a randomized study. Our results suggest that the combination may be superior. An analysis by Silverman et al3 reported a complete response rate of 10% to 17% and a median number of 3 courses to response in patients with AML treated in different Cancer and Leukemia Group B (CALGB) studies. Similarly, Sudan et al2 reported a complete response in 4 (20%) of 20 patients older than 55 years with AML, also with a median time to response of 3 months (range, 2-5 months). Because of the heterogeneous characteristics of the study groups, it is difficult to compare our results with those reported by Silverman et al3 and Sudan et al.2 Acknowledging this limitation, the response rate in patients older than 60 years was 52%, the complete response rate was 33%, and the median number of courses to response was 1 (range, 1-3 months), which may support the contribution of VPA and ATRA to the single-agent activity of 5-AZA. Furthermore, VPA levels were higher in patients achieving a response
VPA has been used as a single agent or in combination with ATRA in patients with MDS14,28 and AML.15,28,29 Kuendgen et al15 reported 58 patients with AML treated with single-agent VPA or in combination with ATRA who were not considered candidates for intensive chemotherapy. The response rate was 5%. In that study, VPA levels of 50 to 100 μg/mL were targeted. HDAC inhibition is observed at concentrations of VPA greater than 1 mM9 . This is greater than the concentration achieved with targeted VPA levels of 50 to 100 μg/mL used in the treatment of seizures. These low levels of VPA may explain the low number of responses observed in that study.15 The higher levels of VPA achieved in our study, particularly in responders, support a role of VPA in this combination.
There are several limitations in our study. First, 5-AZA was used at 75 mg/m2 subcutaneously daily for 7 days, although this is the same 5-AZA dose used in the CALGB studies in patients with high-risk MDS1,3 ; it is unknown if this is the most effective dose, and it is possible that other schedules and doses may be better. Second, VPA, the HDAC inhibitor used in this study, is 1 of the less effective HDAC inhibitors, and its use at high concentrations is associated with potential serious toxicities. The combination of 5-AZA with more effective and less toxic HDAC inhibitors may improve the results observed in this trial. A study combining 5-AZA with MGCD0103, an oral isotype-selective HDAC inhibitor, is currently ongoing with promising interim results.30 Third, so far we have not found a molecular marker predictive of response, as both global DNA methylation and histone acetylation were not different in responders. Further ongoing molecular analysis of gene-specific methylation, gene expression, and effects downstream of histone acetylation may unravel such molecular predictors. Fourth, the role of ATRA in this combination has not been defined. We had based the decision to add it to the combination based on data from Kuendgen et al described in this section,14 and by the observation that the addition of decitabine to ATRA could result in apoptosis in ATRA-refractory leukemia cell lines.31 Also, ATRA can induce cell differentiation and has clinical activity in APL17 but not in non-APL leukemias.18 In ATRA-resistant APL variants, the use of a HDAC inhibitor like VPA can restore sensitivity to ATRA.19 ATRA can induce hypomethylation and re-expression of the RAR-β2gene.20 This activity is enhanced by 5-AZA.20 VPA and 5-AZA have been proposed to sensitize AML cells to the effects of ATRA.20,32,33 Based on this, and on the experience of Kuendgen et al14,15 with VPA in combination with ATRA in MDS and AML, we added ATRA to the combination of 5-AZA and VPA. That said, in this study we have not determined the contribution of ATRA in this combination. Fifth, in this study we have not systematically studied the effects of therapy on cell differentiation. In vitro, the combination had no effect on cell differentiation,9 nor have we observed any case of ATRA-syndrome or morphologic evidence of differentiation. That being said, detailed flow cytometry studies could clarify this issue.
Finally, 1 remaining issue pertains to the schedule used here. We elected to administer these agents concomitantly. This was based on our prior experience with decitabine and VPA16 and in vitro models that indicated no advantage to a sequential administration.9 That being said, in vitro data looking at gene reactivation have suggested a benefit to the sequential administration of these agents.34 Therefore, it is possible that other schedules may result in enhance clinical activity.
In summary, this study demonstrated that the combination of 5-AZA, VPA, and ATRA is safe and active. This therapy was associated with significant DNA hypomethylation and induction of histone acetylation. Despite the clinical activity observed, in particular in the older untreated patients, the number of patients treated was small, and this combination should be considered investigational and should not be recommended outside the setting of a clinical trial.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Jonathan Clavell for technical assistance and to Mark Brandt for data analysis.
This work was supported by Pharmion (Boulder, CO) and by the University of Texas M. D. Anderson Cancer Center's Physician-Scientist Program funded by the Commonwealth Cancer Foundation for Research (both to G.G.-M.).
Authorship
Contribution: A.O.S. and H.M.K. performed research, analyzed data, and wrote the manuscript. H.Y., S.F., Z.E., F.G., F.R., J.C., W.G.W., S. O., A.Q., and J.-P.J.I. performed research. S.P. and E.E. analyzed data. G.G.-M. designed the study, analyzed data, performed research, wrote the manuscript, and funded the studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillermo Garcia-Manero, Department of Leukemia, Box 428, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:ggarciam@mdanderson.org.