Abstract
Asthma is a pulmonary inflammatory disease dependent on eosinophil and mast cell infiltration into the lung. CD34 is a sialomucin expressed by both of these cell types, and we have used CD34−/− mice and a standard mouse model of asthma to evaluate the importance of CD34 expression on disease development. In comparison with wild-type (wt) mice, CD34−/− mice exhibited a dramatic reduction in all hallmarks of allergic asthma, including lowered airway inflammatory cell infiltration, airway hyperresponsiveness, and mast-cell recruitment. Bone marrow transplantation experiments confirmed that these defects are due to CD34 expression by bone marrow–derived cells. This was not, however, due to an inability to respond to antigen as, on a per cell basis, wt and CD34−/− inflammatory cells exhibit identical responses in cytokine production. We found a striking reduction in mobility of CD34−/− eosinophils in vitro, the major component of inflammatory infiltrates, which was consistent with proposed models for CD34 as an inhibitor of cell-cell adhesion. In summary, our data suggest that CD34 enhances mast-cell and eosinophil invasiveness and that its expression by these cells is a prerequisite for development of allergic asthma.
Introduction
Asthma is a pulmonary inflammatory disease characterized in humans by airway inflammation, airway hyperresponsiveness (AHR), and tissue remodeling.1 In mice, induction of asthma leads to a Th2-polarized cytokine response, development of antigen-specific IgEs, a dramatic infiltration of inflammatory cells (eosinophils, lymphocytes, and neutrophils) into the lung tissue and alveolar space, and increased airway resistance (AR).2 The importance of individual hematopoietic cell types such as eosinophils and mast cells in asthma pathogenesis is still debated. Although some studies using mast cell– and/or eosinophil-deficient mice have shown that these cell types are required for AHR and airway inflammation,3,4 alternative reports (using a different strain of eosinophil-deficient mice on a different genetic background) suggest development of AHR and airway inflammation is eosinophil-independent but that these cells play a role in collagen deposition and remodeling.5 It is known that many of the inflammatory factors released by eosinophils and mast cells in the lung, such as IL-5, TNFα, monocyte chemotactic protein-1 (MCP-1), and IL-6,1,6–8 play an important role in perpetuating lung inflammation. In addition, degranulation by eosinophils and mast cells provokes the release of bronchoconstrictive agents such as leukotrienes and bradykinins, which play a key role in development of AHR.9,10 In summary, although mast cells, eosinophils, and their products are known to have potent immunomodulatory functions, their precise role in allergic asthma remains controversial
Many pathways are involved in the recruitment of mast cells and eosinophils to the site of inflammation. Human eosinophils in vitro respond to a wide variety of chemotactic agents including eotaxin, RANTES, and 5-oxo-6,8,11,14-eicosatetraneoic acid (5-oxo-ETE).11,12 Migration of human eosinophils through basement membrane components is dependent on intracellular calcium fluxes,13 release of matrix metalloproteinases (MMPs),14 and activation of the plasminogen/plasmin pathway.11 Migration of murine eosinophils from the circulation to the alveolar space is known to require adhesion via P-selectin glycoprotein ligand-1 (PSGL-1)/P-selectin interactions15 and α4β1 integrin/vascular cell adhesion molecule-1 (VCAM-1) interactions.16 Although the importance of mast-cell recruitment to the lung versus local proliferation in situ has been debated, recent experiments suggest that mast cells are recruited to lung via α4β1 or α4β7 integrin and VCAM-1–dependent adhesion.17
CD34 is a cell-surface sialomucin originally identified as a marker of hematopoietic stem cells (HSCs), early hematopoietic progenitors, and vascular endothelia18 and was more recently identified on mast cells and eosinophils.19,20 In fact, it was shown that in a mouse model of asthma, 50% of the recruited eosinophils were CD34+.20 Surprisingly, despite the wide attention CD34 has garnered as a marker of hematopoietic precursors (> 14 000 citations), little is know of its function on these cells. On high endothelial venules (HEVs), a specialized type of lymph node endothelium, CD34 is modified with a rare glycan structure, sialyl Lewis-X, which allows it to function as an adhesive ligand for L-selectin.21 However, the essential modifications to make CD34 a ligand for selectins have not been observed on CD34 expressed by the vast majority of vascular endothelial cells or hematopoietic cells, and therefore its function on most cell types has remained obscure.
Attempts to clarify the role of CD34 on hematopoietic precursors through 2 independent gene deletion studies have yielded cryptic and inconsistent phenotypes. One line of CD34−/− mice exhibited essentially normal hematopoietic-cell development and function in vivo, but a modest reduction in colony number and size from early hematopoietic precursors, suggesting a possible defect in proliferation or differentiation.22 A second line of CD34−/− mice showed no defects in hematopoietic cell development but a cryptic defect in eosinophil migration to the lung in response to allergens, possibly the result of CD34 loss as a selectin ligand from lung endothelium (although this was never clearly resolved).23
By examining cultured mast cells from CD34−/− mice, we recently found that loss of CD34 leads to a clear enhancement of mast cell adhesion and aggregation that can be fully reversed by ectopic re-expression of the protein.24 These data suggest that CD34 serves to reduce adhesion, potentially enhancing mobility of cells. Here, we have exploited these observations to examine the relevance of CD34 expression in mast cell– and eosinophil-dependent allergic responses. We find that CD34 deficiency leads to highly attenuated allergic inflammation and airway restriction in vivo and impaired eosinophil trafficking through extracellular matrix in vitro. The results suggest an essential role for this antiadhesin in normal mast cell and eosinophil function in vivo and in asthma pathogenesis.
Materials and methods
Mice
Mice were maintained in specific pathogen-free conditions at The Biomedical Research Center and the local animal care committee approved all procedures. Six- to 8-week-old females C57Bl/6 (wt) and CD34−/− (kindly provided by Dr T. W. Mak23 and bred at The Biomedical Research Center) were used throughout, unless otherwise stated. CD34−/− mice have been backcrossed more than 5 generations onto a C57Bl/6 background and show no evidence of rejection in transplantation or parabiosis experiments with C57Bl/6 mice.
Induction of asthma and assessment of alveolar inflammation
Asthma was induced in wt and CD34−/− mice as previously described2 with minor modifications. Briefly, mice were sensitized intraperitoneally with 0.2% chicken ovalbumin (OVA) in Al(OH)3 (both from Sigma, St Louis, MO) on days 1 and 8. Mice were subsequently intranasally challenged on days 22, 23, 24, 26, and 28 with 50 μL 2% OVA. On day 29, mice were anaesthetized with 200 mg/kg ketamine/10 mg/kg xylazine and blood was collected by cardiac puncture for analysis of blood eosinophil frequency. Bronchoalveolar lavages (BALs) were performed by 3 subsequent introductions and aspirations of 1.0 mL sterile PBS. Total BAL cells were counted and differential counts obtained from cytospin preparations stained with modified May-Grünwald Giemsa stain (HemaStain Set; Fisher Scientific, Kalamazoo, MI). Analysis of total and differential counts was also repeated with CD34−/− mice and wt littermates.
A section of the left lung was fixed in 10% formalin for histologic studies. The remainder of each lung was digested in 20 U/mL collagenase IV (Sigma) for 30 minutes, and total inflammatory cells were used for the OVA recall assay and eosinophil sorting. In a separate experiment, lung hematopoietic cells were separated by gradient Percoll centrifugation, counted, and used for eosinophil frequency analysis.
For eosinophil morphology, CCR3+CD3−B220− cells were sorted from total BAL cells, cytospun onto microscope slides and stained with modified Mya-Grunwald Giemsa stain. All images were captured on a Zeiss Axioplan2 microscope (Zeiss, Toronto, ON) using 40×/0.75 dry or 100×/1.30 oil objectives. Images were captured using a Qimaging Retiga EX CCD camera (Minneapolis, MN) and Openlab 4.0.4 software.
Histology
Formalin-fixed lung tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) for evaluation of lung inflammation or toluidine blue for evaluation of mast cell infiltration. Histologic score was obtained from H&E-stained slides. A score from 0 to 5 was blindly attributed (0=no sign of disease, 5=profound inflammation) for each of the following: perivascular infiltration, peribronchial infiltration, and parenchymal infiltration for a potential maximum score of 12. Mast-cell infiltration was blindly evaluated by counting numbers of mast cells in each section from the toluidine blue–stained slides.
OVA recall assay and cytokine production
Total lung cells were obtained from OVA-sensitized and challenged wt and CD34−/− mice. Cells (500 000) were plated in RPMI media with 10% fetal bovine serum and 1% penicillin/streptomycin and stimulated with increasing doses of OVA. Supernatants were collected and cytokine production was evaluated using a cytometric bead essay (Mouse Inflammatory Cytokine Kit; BD Biosciences, San Diego, CA) according to the manufacturer's instructions.
Assessment of airway hyperresponsiveness
On day 29, wt and CD34−/− mice were anaesthetized with a 200-μL intraperitoneal injection of a 10 mg/mL ketamine/1 mg/mL xylazine solution, tracheotomized, and intubated with an 18-G catheter. Airway resistance (AR) was measured with a Flexivent apparatus (SCIREQ, Montreal, QC). Respiratory frequency was set at 160 breaths/min with a tidal volume of 0.2 mL, and a positive end-expiratory pressure of 2 to 4 mL H2O was applied. Increasing concentrations of metacholine (MCh; 0 to 125 mg/kg) were administered via the jugular vein. AR was recorded every 15 seconds by a snapshot measure. Baseline AR was reached prior to administrating subsequent doses of MCh. The percentage increase in AR was calculated for each MCh dose.
Flow cytometric analysis of BM progenitors, BAL, blood, and tissue eosinophils
Cells were blocked with 10% mouse serum prior to staining. The following antibodies were used: rat antimouse B220-biotin (clone RA-6B2), CD3-biotin (clone 2C11), CD34-biotin (BD Pharmingen, San Jose, CA), CD4-biotin, and Gr-1-biotin (clone Ly-6G) followed by streptavidin-APC (BD Pharmingen); rat antimouse CCR3-PE (R&D Systems, Minneapolis, MN); Sca-1-APC (eBioSciences, San Diego, CA); antimouse CD45.2-PerCP, IL5R-alpha-PE (clone T21.2), Mac-1-PE, and Ter119-PE (all from BD Pharmingen). CCR3+/B220−/CD3− BAL cells were sorted by FACSAria (BD Biosciences). Sorted cells were cytospun and Giemsa-stained to verify eosinophil purity. Alternately, BAL cells were stained for CCR3 and CD34 to assess eosinophil surface expression. Frequency of CD45+/CCR3+/B220−/CD3− (eosinophils) in blood and collagenase-treated individual lungs was analyzed. For analysis of bone marrow (BM) progenitors, BM was flushed from femurs of OVA-induced mice with 10 mL PBS–0.1% BSA and populations of B220+, CD3+, CD4+, Gr-1+, Sca-1+, IL5Ralpha+, Mac-1+, and Ter119+cells were assessed. BAL CD34 staining, blood, lung tissue, and BM data were collected on a BD FACSCalibur (BD Biosciences). Data were analyzed with FlowJo software (Treestar, Ashland, OR).
Eosinophil migration assay
Sorted BAL CCR3+ eosinophils were placed in the upper chamber of 24-well Biocoat Matrigel Invasion Chambers (BD Biosciences). Eotaxin (30 ng/mL) (synthesized at the Biomedical Research Center) was added in the lower chamber as a chemoattractant. Chambers were incubated at 37°C + 5% CO2 for 18 hours. At the end of the incubation period, cells in both the upper and lower chambers were removed by aspiration and 10 000 microbeads were added to each sample to count by fluorescence-activated cell sorting (FACS). For each sample, beads were gated and samples collected until 5000 beads had been counted. For both wt and CD34−/− cells, the migration index was calculated as follows: the number of cells in the lower chamber of the Matrigel Invasion Chamber was divided by the total number of cells in both lower and upper chamber and multiplied by 100. That migration percentage was then divided by the percentage of migration in a control chamber lacking Matrigel or eotaxin (spontaneous migration), and multiplied by 100.
Bone marrow reconstitutions
BM was isolated from both wt (Ly 5.2) and CD34−/− (Ly 5.2) mice. Cells (107) were injected intravenously into C57Bl/6-Ly 5.1 lethally irradiated recipients, and 12 weeks were allowed for complete BM reconstitution. Reconstitution was evaluated by Ly 5.2 versus Ly 5.1 expression on peripheral blood, and mice were considered reconstituted when more than 80% of hematopoietic cells (in Ly 5.1 recipients) originated from the Ly 5.2 donors. Reconstituted mice were then sensitized and challenged with OVA and inflammatory parameters evaluated as previously described.
Statistics
For analysis of total cells, hematopoietic cell subtypes in BAL and AHR, statistics were made using an ANOVA table followed by a Fisher posthoc test. For analysis of histology, cytokine release, hematopoietic progenitors, blood and lung eosinophil frequency, and eosinophil migration, an unpaired t test was used.
Results
Allergic airway inflammation is attenuated in CD34−/− mice
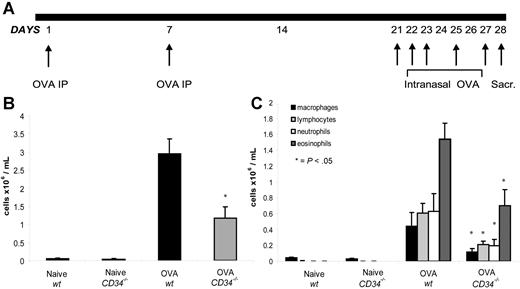
To examine the role of CD34 expression in mast cell and eosinophil responses, wt and CD34−/− mice were sensitized with OVA twice intraperitoneally and subsequently challenged intranasally (Figure 1A). As shown in Figure 1B, OVA sensitization and challenge in wt mice caused an inflammatory cell infiltration in the BAL when compared with control mice (2.94 ± 0.42 × 106 cells/mL in OVA-sensitized mice compared with 0.05 ± 0.01 × 106 cells/mL in naives). Interestingly, CD34−/− mice exhibited a more attenuated response, with less than half the number of infiltrating cells in the BAL (1.16 ± 0.32 × 106 cells/mL, Figure 1B) compared with challenged wt animals. To clarify which cell types were present in the infiltrates, differential counts were performed (Figure 1C). In wt mice, the majority of infiltrating cells (> 50%) were eosinophils followed by an equal frequency (approximately 20%) of neutrophils, lymphocytes, and macrophages. Interestingly, CD34−/− mice showed a similar profile of inflammatory cell infiltration with a clear reduction in overall cell numbers, suggesting a general decrease in the degree of inflammation (Figure 1C). Similar results were obtained in CD34−/− and wt littermate comparisons (data not shown).
Protocol for OVA sensitization and BAL analysis. (A) Schematic of the sensitization and challenges protocol used to induce asthma in wt and CD34−/− mice. (B) Total cell counts in BAL from naive and challenged (OVA) wt and CD34−/− mice (n=6; error bars=SEM on mean total cell counts for each group). (C) Differential count results from naive and challenged (OVA) wt and CD34−/− mice (n=6; error bars=SEM on mean cell number for each population of each group).
Protocol for OVA sensitization and BAL analysis. (A) Schematic of the sensitization and challenges protocol used to induce asthma in wt and CD34−/− mice. (B) Total cell counts in BAL from naive and challenged (OVA) wt and CD34−/− mice (n=6; error bars=SEM on mean total cell counts for each group). (C) Differential count results from naive and challenged (OVA) wt and CD34−/− mice (n=6; error bars=SEM on mean cell number for each population of each group).
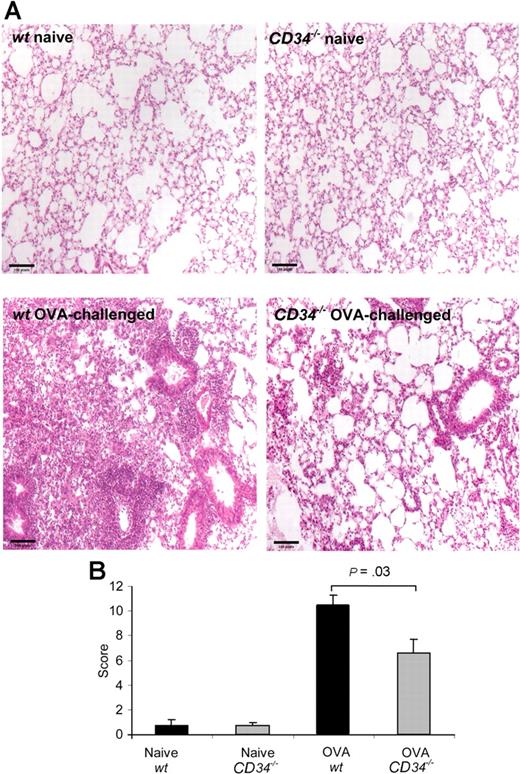
Attenuation of inflammatory responses in CD34−/− mice was further confirmed by histologic analyses. H&E- and toluidine blue–stained slides were blindly evaluated and an inflammation score was awarded (0=no sign of disease, 5=profound inflammation) for the following: perivascular infiltration, peribronchial infiltration, epithelium damage, and parenchymal infiltration for a potential score of 20. As shown in Figure 2A, OVA sensitization and challenge induced potent perivascular, peribronchial, and parenchymal inflammatory cell infiltration. Scoring of histologic inflammation (Figure 2B) revealed a significantly milder tissue inflammation in CD34−/− mice compared with wt (score=10.3 ± 0.9 in wt vs 6.6 ± 1.2 in CD34−/−, P=.03, n=6). The main differences between the 2 groups were observed in peribronchial and parenchymal infiltration, which were lower in CD34−/− mice. One can note the obvious differences in lung tissue inflammation when challenged wt and CD34−/− lung sections are compared (Figure 2A). Although CD34−/− mice still show signs of inflammatory cell infiltration around bronchi and vessels, infiltration in wt mice is more severe and extends deeper to the parenchyma.
H&E-stained lung sections from naive and challenged mice. Lung sections were fixed in 10% formalin 24 hours after the last OVA challenge. (A) naive wt mouse, naive CD34−/− mouse, OVA-challenged wt mouse, and OVA-challenged CD34−/− mouse. (B) Mean histologic scoring of peribronchial, perivascular, and parenchymal infiltration and epithelium damage (n=6). A score of 1 to 5 (0=no sign of disease, 5=profound inflammation) for each parameter was blindly attributed after analysis of 5 fields from each sample. Error bars represent SEM on mean score for each group.
H&E-stained lung sections from naive and challenged mice. Lung sections were fixed in 10% formalin 24 hours after the last OVA challenge. (A) naive wt mouse, naive CD34−/− mouse, OVA-challenged wt mouse, and OVA-challenged CD34−/− mouse. (B) Mean histologic scoring of peribronchial, perivascular, and parenchymal infiltration and epithelium damage (n=6). A score of 1 to 5 (0=no sign of disease, 5=profound inflammation) for each parameter was blindly attributed after analysis of 5 fields from each sample. Error bars represent SEM on mean score for each group.
CD34−/− mice do not develop airway hyperresponsiveness (AHR) in response to OVA challenges
In addition to inflammatory cell infiltration, a second major hallmark of allergic asthma is the development of AHR. We assessed airway resistance (AR; a measure of AHR) in naive and OVA-sensitized mice through a metacholine (MCh) challenge. As shown in Figure 3, naive wt and CD34−/− mice showed similar responses to MCh prior to OVA sensitization. As expected, in wt mice OVA sensitization significantly increased AR in response to MCh. Strikingly, OVA-sensitized CD34−/− mice exhibited no increase in AR even at the highest doses of MCh tested on this strain (500 μg/kg, Figure 3; data not shown). CD34−/− mice demonstrated a significantly decreased response to MCh compared with wt starting at the 3.9-μg/kg dose and throughout all higher dosages for the rest of the challenge (P < .05 for every dose, n=6). Surprisingly, the MCh response in CD34−/− OVA-challenged mice was statistically significantly lower than in CD34−/− naive mice starting at the 15.6-μg/kg dose (P < .05 for 15.6-μg/kg dose and following doses; n=6). Thus, CD34−/− mice show a complete failure in developing this second phenotypic hallmark of asthma.
Airway resistance (AR) in naive and OVA-challenged mice. Naive and OVA-challenged mice were administrated increasing doses of MCh (intravenously) and the increase in airway resistance from baseline (R [% increase]) was measured for each MCh dose. (*P < .05 when OVA challenged mice are compared with their respective naive controls; n=6 for each dose; error bars=SEM.)
Airway resistance (AR) in naive and OVA-challenged mice. Naive and OVA-challenged mice were administrated increasing doses of MCh (intravenously) and the increase in airway resistance from baseline (R [% increase]) was measured for each MCh dose. (*P < .05 when OVA challenged mice are compared with their respective naive controls; n=6 for each dose; error bars=SEM.)
Normal antigen-specific recall responses in OVA-challenged CD34−/− mice
To test whether the attenuated allergic responses in CD34−/− mice were due to an inability to effectively present antigen or produce the appropriate cytokines, total lung cells from wt and CD34−/− mice were isolated and stimulated ex vivo with OVA to evaluate antigen-specific cytokine responses. Results from 3 independent cytokine production experiments are compiled and presented in Figure 4. No statistically significant difference was found between wt and CD34−/− cells in their ability to release TNF, IL-6, MCP-1, or IL-5 (on a per cell basis) in response to OVA sensitization. We conclude that there are no major defects in antigen presentation or cytokine production in CD34−/− mice.
Cytokine recall essay. Isolated total lung tissue cells were cultured for 24 hours with the indicated doses of OVA and cytokine release was measured. (A) TNF, (B) IL-6, (C) MCP-1, and (D) IL-5 release (on a per-cell basis) in response to OVA-stimulation of isolated lung tissue inflammatory cells. There is no statistically significant difference in OVA-specific cytokine release between wt and CD34−/− mice (n=3 independent experiments).
Cytokine recall essay. Isolated total lung tissue cells were cultured for 24 hours with the indicated doses of OVA and cytokine release was measured. (A) TNF, (B) IL-6, (C) MCP-1, and (D) IL-5 release (on a per-cell basis) in response to OVA-stimulation of isolated lung tissue inflammatory cells. There is no statistically significant difference in OVA-specific cytokine release between wt and CD34−/− mice (n=3 independent experiments).
Normal frequency of hematopoietic precursors, increased eosinophil frequency in blood, and decreased numbers of lung eosinophils in OVA-challenged CD34−/− mice
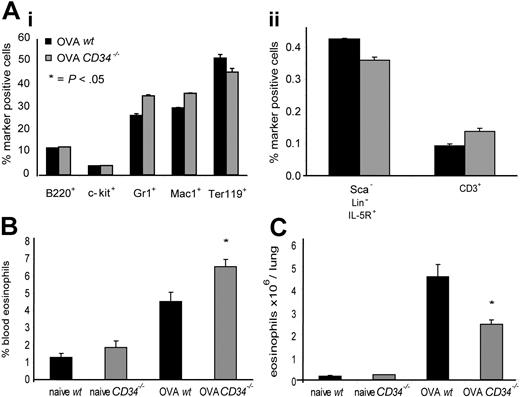
To elucidate which steps in eosinophil recruitment to the lung in vivo were affected by loss of CD34, we first examined the profile of hematopoietic precursor cells in the BM of wt and CD34−/− mice following allergen challenge. No statistically significant differences were observed in the profile of eosinophil progenitors, granulocyte, macrophage, B-lineage, T-lineage, or erythroid lineage cells in either naive animals (not shown) or allergic animals (Figure 5Ai,ii). This suggests that differences in the asthmatic response are not a result of deficiencies in progenitor-cell development or differentiation in the BM. We also tested the frequency of blood and lung tissue eosinophils (CD45+/CCR3+ cells) and noted a statistically significant increase in blood CD45+/CCR3+ cells in CD34−/−/OVA-challenged mice (6.55% ± 0.44%) compared with wt (4.53% ± 0.55%; P=.02, n=6) (Figure 5B). This was complemented by a decrease in total CD45+/CCR3+ cells/lung in OVA-challenged CD34−/− mice (2.43 × 106 ± 0.22 eosinophils/lung) compared with wt (4.54 × 106 ± 0.53 eosinophils/lung; P=.01, n=4) (Figure 5C).
Bone marrow progenitor populations, frequency of blood eosinophils, and lung tissue eosinophil numbers in wt and CD34−/− mice. (A) Frequency of bone marrow hematopoietic progenitor markers in OVA-challenged mice. (Ai) B220+ indicates B lymphocytes; c-kit+, immature progenitors; Gr1+, granulocytes; Mac-1+, monocytes; and Ter-119+, erythroid; (Aii) Lin−Sca−IL5R+ indicates eosinophil progenitors; and CD3+, T lymphocytes. All cells were analyzed by FACS (n=4; error bars represent SEM for each marker). (B) Frequency of blood CD45+/CCR3+ cells (eosinophils) in naive and OVA-challenged wt and CD34−/− mice (n=6; *P=.02; error bars=SEM). (C) Frequency of CD45+/CCR3+ cells in hematopoietic-cell preparations from individual lungs of naive and OVA-challenged wt and CD34−/− mice was analyzed by FACS. Total hematopoietic cells/lung counts were obtained and used to determine total eosinophils/lung. (n=4; *P=.01; error bars=SEM).
Bone marrow progenitor populations, frequency of blood eosinophils, and lung tissue eosinophil numbers in wt and CD34−/− mice. (A) Frequency of bone marrow hematopoietic progenitor markers in OVA-challenged mice. (Ai) B220+ indicates B lymphocytes; c-kit+, immature progenitors; Gr1+, granulocytes; Mac-1+, monocytes; and Ter-119+, erythroid; (Aii) Lin−Sca−IL5R+ indicates eosinophil progenitors; and CD3+, T lymphocytes. All cells were analyzed by FACS (n=4; error bars represent SEM for each marker). (B) Frequency of blood CD45+/CCR3+ cells (eosinophils) in naive and OVA-challenged wt and CD34−/− mice (n=6; *P=.02; error bars=SEM). (C) Frequency of CD45+/CCR3+ cells in hematopoietic-cell preparations from individual lungs of naive and OVA-challenged wt and CD34−/− mice was analyzed by FACS. Total hematopoietic cells/lung counts were obtained and used to determine total eosinophils/lung. (n=4; *P=.01; error bars=SEM).
Defective mast-cell trafficking and eosinophil migration in CD34−/− mice
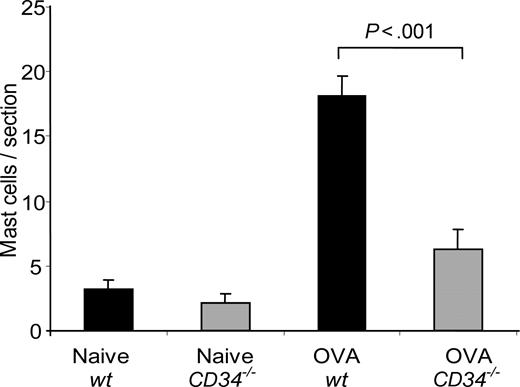
The failure of CD34−/− mice to develop a robust allergic response despite normal production of inflammatory cells in BM and response to antigen in cytokine recall assays suggests that other defects underlie the attenuated inflammatory response in these animals. Previously, we have shown that, on mast cells, CD34 can act as a potent inhibitor of cell adhesion and that mice lacking CD34 exhibit defects in mast-cell trafficking. Since AHR has previously been reported to be defective in mice lacking mast cells, we analyzed the number of mast cells in wt and CD34−/− lungs before and after OVA challenge. Although wt and CD34−/− mice showed similar numbers of lung mast cells prior to challenge (as presented in Figure 6), wt mice exhibited a potent recruitment of mast cells to the lungs after OVA challenge (18.1 ± 1.5 mast cells/section versus 3.3 ± 0.6 mast cells/section in naives; P < .001, n=6), while CD34−/− mice exhibited a clear impairment in the recruitment of mast cells after OVA challenge (6.3 ± 1.5 mast cells/section; P=.001 compared with wt; n=6). We conclude that impaired mast-cell recruitment is likely one of the causes for the reduced AHR in CD34−/− mice.
Mast-cell lung infiltration in response to OVA challenge. The number of mast cells/lung section was obtained from toluidine blue–stained lung sections of naive and challenged mice (n=6; error bars=SEM for each group).
Mast-cell lung infiltration in response to OVA challenge. The number of mast cells/lung section was obtained from toluidine blue–stained lung sections of naive and challenged mice (n=6; error bars=SEM for each group).
Additionally, studies have suggested that the trafficking of eosinophils to the lung during an allergic response can occur in the absence of mast cells.4 Thus, although the lack of AHR in CD34−/− mice is likely, in part, due to impaired mast-cell recruitment, the failure of eosinophils to infiltrate the lung may be due to a separate defect. Interestingly, recent reports have suggested that eosinophils can also express CD34.20 To confirm this observation and to test whether CD34 loss has a role in eosinophil trafficking, we compared BAL eosinophils from wt and CD34−/− mice for expression of CD34 and for their ability to migrate in vitro. To identify eosinophils, BAL fluid and dissociated lung tissue were costained for CD34 and the eotaxin receptor CCR3, a well-known marker of eosinophils that is required for their efficient homing to the lung during allergic inflammation.20 Figure 7A,B shows identical levels of CCR3 expression by wt and CD34−/− BAL eosinophils, suggesting no inherent defect in the ability of these cells to respond to eotaxin. Differential analysis of Giemsa-stained cytospin preparations from CCR3+/B220−/CD3− sorted cells (Figure 7C) revealed 98% eosinophil purity, and no obvious visible difference in cell morphology between purified wt and CD34−/− eosinophils. Wt CCR3+ cells exhibited a low but reproducible level of staining with CD34 antibody, while CCR3+ cells from CD34−/− mice showed no such staining, confirming the presence of this molecule on eosinophils. CD34 staining levels on CD34−/− eosinophils were equivalent to unstained controls from both wt and CD34−/− cells (data not shown) (Figure 7D,E).
FACS profiles of CCR3 and CD34 expression on BAL and parenchymal cells, and migration assay results. (A,B) FACS profiles showing granularity (as measured by side light scatter [SSC]) versus CCR3 fluorescence staining on purified cells. (C) Giemsa staining of cytospin preparations from CCR3+ sorted cells. Eosinophils (CCR3+CD3−B220−) were sorted from total BAL from OVA-challenged mice. (D,E) Single-color histograms showing CD34 staining intensity on SSChi CCR3+ gated populations from panels A and B. Gray-filled histogram represents staining intensity from CD34−/− cells, while the black line represents staining of wt cells. (F) Matrigel migration assay of wt and CD34−/− sorted eosinophils. The migration index (in response to 30 nM eotaxin) was compared between wt (normalized to 100% migration) and CD34−/− mice (n=4 independent experiments using pooled BALs cells from 4-6 mice for each experiment; error bar=SEM).
FACS profiles of CCR3 and CD34 expression on BAL and parenchymal cells, and migration assay results. (A,B) FACS profiles showing granularity (as measured by side light scatter [SSC]) versus CCR3 fluorescence staining on purified cells. (C) Giemsa staining of cytospin preparations from CCR3+ sorted cells. Eosinophils (CCR3+CD3−B220−) were sorted from total BAL from OVA-challenged mice. (D,E) Single-color histograms showing CD34 staining intensity on SSChi CCR3+ gated populations from panels A and B. Gray-filled histogram represents staining intensity from CD34−/− cells, while the black line represents staining of wt cells. (F) Matrigel migration assay of wt and CD34−/− sorted eosinophils. The migration index (in response to 30 nM eotaxin) was compared between wt (normalized to 100% migration) and CD34−/− mice (n=4 independent experiments using pooled BALs cells from 4-6 mice for each experiment; error bar=SEM).
Interestingly, CCR3+ cells in the BAL fluid showed higher levels of CD34 than CCR3+ cells in the underlying lung tissue, suggesting a possible correlation between CD34+ cells and cell invasiveness. To test this notion, CCR3+/B220−/CD3− sorted cells from BAL of wt and CD34−/− mice were compared in a side-by-side chemotaxis assay for their ability to migrate through Matrigel in response to eotaxin. CD34−/− eosinophils exhibited 44.9% (± 8.6%) lower ability to migrate through Matrigel when compared with wt cells (Figure 7F; n=4; P=.002). Since differential selectin binding by CD34−/− and wt eosinophils is also a potential explanation for poorer eosinophil trafficking in the lung, we used FACS to determine the ability of wt and CD34−/− sorted BAL eosinophils to bind L-, P-, and E-selectin. Binding was identical between the 2 populations (data not shown). We therefore conclude that CD34−/− eosinophils have an intrinsic defect in their ability to migrate through matrix in vitro.
Phenotype of CD34−/− mice is due to CD34 expression on hematopoietic cells
Although all these data are consistent with an intrinsic defect in the ability of CD34−/− eosinophils and mast cells to chemotax, CD34 is also expressed by most vascular endothelial cells including those in the lung. Thus, the possibility remained that some defects in the allergic homing of CD34−/− cells were due to loss of CD34 from the vascular endothelia. To address this issue, we generated hematopoietic chimeras by reconstituting Ly5.1 C57BL/6 mice (CD45.1 allotype) with wt or CD34−/− (CD45.2) BM. In each case, peripheral blood cells were analyzed 12 weeks after transplantation to ensure that more than 80% of the white blood cells of these chimeras were derived from donor BM. These mice were then sensitized and challenged with OVA as in previous protocols. Total and differential cell counts in the BAL are shown in Figure 8. As expected, mice reconstituted with wt BM showed an increase in total BAL inflammatory cells in response to OVA compared with naive controls. Interestingly, the number of inflammatory cells was again approximately 50% lower in mice that were reconstituted with CD34−/− BM. Moreover, differential count analysis shows that the differential profile of each hematopoietic population is similar between the 2 groups, but the number of eosinophils and lymphocytes was significantly lower in CD34−/− reconstituted mice compared with wt reconstituted mice. As these results match results in nonreconstituted animals, we conclude that the allergic phenotypes observed in CD34−/− mice is due to a loss of CD34 expression in cells of hematopoietic origin (ie, eosinophils and mast cells) rather than a loss of this molecule from the nonhematopoietic microenvironment.
BAL analysis of wt and CD34−/− bone marrow– reconstituted mice. (A) Total cells in the BAL of wt bone marrow–reconstituted mice (wt in Ly5.1) or CD34−/− bone marrow–reconstituted mice (CD34−/− in Ly5.1) following OVA sensitization and challenge. (B) Differential counts of BAL cells from wt bone marrow–reconstituted mice (wt in Ly5.1) or CD34−/− bone marrow–reconstituted mice (CD34−/− in Ly5.1). (*P < .05 when wt are compared with CD34−/−; n=6; error bars = SEM).
BAL analysis of wt and CD34−/− bone marrow– reconstituted mice. (A) Total cells in the BAL of wt bone marrow–reconstituted mice (wt in Ly5.1) or CD34−/− bone marrow–reconstituted mice (CD34−/− in Ly5.1) following OVA sensitization and challenge. (B) Differential counts of BAL cells from wt bone marrow–reconstituted mice (wt in Ly5.1) or CD34−/− bone marrow–reconstituted mice (CD34−/− in Ly5.1). (*P < .05 when wt are compared with CD34−/−; n=6; error bars = SEM).
Discussion
Although CD34 is best known as a marker of HSC and early hematopoietic precursors, recent studies have shown that it is also expressed by eosinophils and mast cells.19,20 Here we have addressed its role on these mature hematopoietic cells using a well-defined, eosinophil- and mast cell–dependent asthma model. CD34−/− mice exhibited a complete absence of AHR and a striking attenuation in the degree of inflammatory cell infiltration, 2 major hallmarks of allergic asthma. Since (1) these defects are known to be eosinophil and mast cell dependent, (2) these cells types are rapidly recruited to the lung in response to allergic challenge, and (3) both cell types have recently been shown to express CD34, we focused our attention on these cells in the mutant mice. We noted a clear defect in the recruitment of both cell types in CD34−/− mice.
This suggests that lack of CD34 expression impairs the capacity of hematopoietic cells to cross from the circulation into the lung tissue and then to leave the tissue toward the alveolar space. Each of these steps involves movement through the basement membrane and extracellular matrix components. This migration process can be mimicked by using Matrigel-coated transwell migration chambers. Since eosinophils that lack expression of CD34 showed an impaired capacity to migrate through Matrigel and CD34−/− OVA-challenged mice had higher frequency of blood eosinophils and lower tissue eosinophils, it is likely that CD34 is required for optimal transmigration from the circulation into the lung tissue and alveolar space, and that suboptimal migration underlies the reduced number of eosinophils in the BAL of CD34−/− mice.
How does CD34 loss impair allergic responses? We observed no defects in the ability of CD34−/− mice to generate hematopoietic subsets in the BM, nor did we observe any defects in the ability of lung-infiltrating hematopoietic cells to produce inflammatory cytokines in response to specific antigen challenge. Thus, CD34−/− mice appear to be fully competent to mount an appropriate immune response. The best evidence to date suggests CD34 serves a proadhesive or an antiadhesive function depending on its cellular context. On HEVs in lymph nodes, CD34 has previously been shown to act as a glycosylation-dependent ligand for L-selectin on naive lymphocytes and to facilitate their trafficking into the lymph nodes.18 Although CD34 is normally expressed on lung endothelium, several experiments would argue against a proadhesive function for CD34 on these cells as the cause of defects in CD34-deficient mice. First, CD34 binding to L-selectin is known to be highly dependent on HEV-specific glycosylation of CD34 and the appropriate modifications are not present on CD34 expressed by lung endothelia or eosinophils. Second, we observed defective migration of CD34−/− eosinophils in in vitro chemotaxis assays in the absence of vascular endothelial cells or L-selectin, suggesting an intrinsic hematopoietic defect rather than a vascular defect. Finally, and most importantly, all observed defects in allergic responses in CD34−/− mice could be recapitulated in chimeric wt mice that had received a transplant of CD34−/− BM. Since these mice have wt endothelium and nonhematopoietic cells, the data would argue that the failure to develop allergic asthma is due to intrinsic defects in the hematopoietic cells.
Particularly relevant to the present study, we have previously shown that CD34 is a marker of mast cells and their precursors and that loss of the CD34 leads to a clear increase in their adhesiveness.19 We have also shown that enhanced adhesion can be reversed by the ectopic re-expression of CD34 in mast cells. Finally, we have shown that loss of CD34 in mice results in no difference in kinetics of mast-cell proliferation or survival but striking defects in HSC and mast-cell trafficking, presumably due to enhanced adhesion.24,25 Recent studies, using a similar model to the one described here, have suggested that the rapid increase in the frequency of lung mast cells during allergic challenge is the result of VCAM-1– and α4β1 integrin–dependent mast cell recruitment, rather then local mast-cell proliferation in situ.17 Thus, the decreased mast-cell migration into the lung of allergic CD34−/− mice likely reflects impaired trafficking of these cells.
Previous reports have suggested that approximately 50% of eosinophils recruited to the lung during allergic lung inflammation express CD34.20 We have re-examined this issue by comparing CD34−/− and wt eosinophils. Our data suggest that CD34 is expressed at low levels by all eosinophils, but the highest level by those that have migrated to the BAL. We have also shown that CD34−/− eosinophils have a clear defect in the ability to migrate in chemotaxis assays in vitro and to infiltrate lungs in vivo. It is unlikely that this was due to differences in cell maturation, shape, or chemokine signaling, as CCR3 expression was equivalent in both strains of mice and analysis of Giemsa-stained eosinophil preparations suggests no obvious difference in shape or maturation status between CD34−/− and wt eosinophils, but could be explained by differences in cell flexibility or impaired intracellular signaling in CD34−/− cells. Moreover, the absence of L-selectin or vascular endothelial cells to act as an adhesive substrate in in vitro assays, again, would argue against a proadhesive role for CD34 on eosinophils and would instead suggest that the defect in migration is the result of enhanced adhesiveness of cells lacking this highly charged sialomucin.
Using mast cell–deficient4 and eosinophil-deficient3 mice, other groups have shown that development of full AHR in mice models of asthma is dependent on the presence of mast cells and eosinophils. Therefore, the lack of AHR in CD34−/− mice could be due to the decreased recruitment of both of these cell populations to the lung. On the other hand, although the recruitment of eosinophils to the lung is reduced 2- to 3-fold in CD34−/− mice, it is noteworthy that it is not completely eliminated and is still substantially higher than that of naive mice, while AHR is completely abrogated, which could argue against a direct link between eosinophil trafficking and development of AHR. This interpretation would be supported by previous reports showing that an independent strain of eosinophil-deficient mice (Δdbl GATA mice) develop AHR in the complete absence of eosinophils,5 and by several studies in humans that failed to show a correlation between eosinophil infiltration and AHR.26 Finally, the fact that CD34−/− OVA-challenged mice exhibit even less AHR than CD34−/− naive mice would again argue against a direct link between eosinophil trafficking to the lungs and AHR. One of many possible explanations for the complete lack of AHR in CD34−/− mice could be that CD34 is required for airway smooth muscle contraction or for airway smooth muscle cell progenitor recruitment. In this regard, it is intriguing that smooth muscle cells have been reported to express CD34 in humans.27 Further studies of smooth muscle progenitor recruitment in a more chronic model of asthma may shed light on this possibility.
Mast cells and eosinophils are associated with a wide variety of pathological inflammatory responses including allergy, arthritis, multiple sclerosis–like syndromes, and lung fibrosis. Our data suggest that loss of CD34 from mast cells and eosinophils could lead to attenuation of much of the inflammatory damage associated with these relatively common and severe diseases and that it may be an ideal target molecule for therapy. The fact that CD34−/− mice show no major defects in normal development would suggest that therapies based on transiently impairing its expression should be well tolerated. Further experiments will be required to evaluate the importance of CD34 in these additional diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by operating grants from the Canadian Institutes for Health Research/Allergen Network Center of Excellence and fellowships through the Canadian Institutes for Health Research (CIHR)/Heart & Stroke Foundation of Canada (HSFC) Strategic Training Program in Transfusion Sciences through the Center for Blood Research (CBR) and through the Canadian Institutes for Health Research (CIHR)/Canadian Asthma, Allergy and Immunology Foundation (CAAIF) Training Program. K.M.M. is a Michael Smith Foundation for Health Research Scholar.
The authors would like to thank Dr Yvon Cormier for helpful discussions and generously allowing the use of the FlexiVent Apparatus in the airway hyperresponsiveness studies.
Authorship
Contribution: M.-R.B. designed and performed research, and wrote the paper; S.M. and D.J.H. performed some of the research and helped in writing the paper; L.Z. and H.M. performed a part of the research; and K.M.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kelly M. McNagny, The Biomedical Research Centre, University of British Columbia, 2222 Health Sciences Mall, Vancouver, British Columbia, V6T 1Z3 Canada; e-mail: kelly@brc.ubc.ca.

![Figure 3. Airway resistance (AR) in naive and OVA-challenged mice. Naive and OVA-challenged mice were administrated increasing doses of MCh (intravenously) and the increase in airway resistance from baseline (R [% increase]) was measured for each MCh dose. (*P < .05 when OVA challenged mice are compared with their respective naive controls; n=6 for each dose; error bars=SEM.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2006-12-062448/7/m_zh80190707400003.jpeg?Expires=1767712520&Signature=g6eOD3FgfZG8hBn5xG8q0erAmQbSz--SOcBFyxllcezD6D98uuZcR3iAbyZOzJ0dMMY0e78QxmhRAzvydZifPe76K6jJKDrmpqF4Nd6fCFX0AP1GhPtJ4m2zqspAYbvEO1J-8Cti1Ykp~F-7wq-dTIsbhFWEGkb6bldqqmcBtxBKwqxIQmCmFcKlT~Q2czkd8tvygDJmLcjGtqQnxsk2AarnF2vx0F9YCdC9Y~6pNONwXMzzdfMy~pkjmAJ9M1xvmVsTKqQXQxGsxfmVR5w~NHJ4pS3b-D8KezMM5okfPywgj8FoINXUFi5CSKIcsy6p3VtN6wZmgLTmtxotHyoIsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. FACS profiles of CCR3 and CD34 expression on BAL and parenchymal cells, and migration assay results. (A,B) FACS profiles showing granularity (as measured by side light scatter [SSC]) versus CCR3 fluorescence staining on purified cells. (C) Giemsa staining of cytospin preparations from CCR3+ sorted cells. Eosinophils (CCR3+CD3−B220−) were sorted from total BAL from OVA-challenged mice. (D,E) Single-color histograms showing CD34 staining intensity on SSChi CCR3+ gated populations from panels A and B. Gray-filled histogram represents staining intensity from CD34−/− cells, while the black line represents staining of wt cells. (F) Matrigel migration assay of wt and CD34−/− sorted eosinophils. The migration index (in response to 30 nM eotaxin) was compared between wt (normalized to 100% migration) and CD34−/− mice (n=4 independent experiments using pooled BALs cells from 4-6 mice for each experiment; error bar=SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2006-12-062448/7/m_zh80190707400007.jpeg?Expires=1767712520&Signature=Xg7EAwaflAQjbGQ~QqqjKsRBoGGkWSjuVDBw93eFVb4~jHEm9-PdgaQZbislnRitVvDTLxKwBLt7Qm8f7khlNpBadK63hBGmBlKiQKlorUARQyW4xTMKONC-MXf5d38L-ViEjpYevmKjrYf7qNMkfIrm5GqYRfB7kfCPHKxYXy0A3toygC9uueqMtDidS-VAhKgtpK5QipQBCqDAyFQfcm2mc9l45zPZdJdPrbWn9qjp6Fg5FN8y00NJNLWKi2VNJVXhpm0w1hilVVpR3uwhKyWkrByf-EgiuCBBlOoFy8-XzazAZL1ysCuz~2HflwDlhRv2V2ooxjsJ-r-PFnbVxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal