Abstract
Relapses occurring in most patients with lymphoma after antibody or chemotherapy highlight a need for effective vaccination approaches. Autologous tumors are ideal sources of patient-specific tumor antigens for vaccines; however, their poor immunogenicity has been a major obstacle in practice. Natural killer T (NKT) cells have recently emerged as crucial regulators of autoimmunity and tumor immunosurveillance. Here, we show that an autologous lymphoma vaccine that activates NKT cells generated tumor-specific protective immunity in experimental mice. Single vaccination with α-galactosylceramide (αGC)-loaded A20 lymphoma cells elicited effective antitumor immunity against tumor challenge. This vaccination strategy also induced significant tumor regression in A20-bearing mice. Importantly, the survivors from primary tumor inoculation were all resistant to tumor rechallenge, indicative of established adaptive memory immunity. Depletion as well as adoptive transfer studies revealed an exclusive role of conventional CD4+ but not CD8+ T cells in mediating antitumor immunity. In addition, we found normal hematopoietic compartments in the vaccinated mice. Therefore, NKT ligand-loaded lymphoma elicits long-lasting and effective antitumor immunity, which can be further developed as patient- and tumor-specific immunotherapy against human lymphomas.
Introduction
Induction of tumor-specific adaptive immunity in individual patients with cancer is an ideal approach because its selectivity and memory for the same tumors eventually prevent the recurrence of residual tumors after conventional therapies. Among current strategies to elicit immunity against tumor-associated antigen (TAA), using autologous tumor as a source of TAAs is an attractive strategy since specific TAAs have not been identified for most tumors. Moreover, vaccinations with autologous tumors can provide a wide range of “polyvalent” TAAs, and thus defining TAAs in each individual is not necessary for immunotherapy. Because of their antigen-presenting capacity, vaccination with autologous tumors of B-cell origin may be particularly useful for treating B-cell lymphomas.1 Since these tumors are typically poorly immunogenic by themselves, many approaches have sought to improve immunogenicity, such as transducing cytokines or costimulators into tumor cells2–6 as well as pulsing dendritic cells (DCs) with tumors.7 These strategies have been shown to be successful at certain degrees to elicit antitumor activities and memory via activation of TAA-specific T cells. Nevertheless, technical and financial challenges, as well as safety issues for genetic modification or DC presentation, are major obstacles to their widespread use in clinical settings. Thus, development of a convenient and practical vaccine strategy with potent efficacy will be beneficial
Natural killer T (NKT) cells recognize glycolipid antigens on a nonclassical major histocompatibility complex (MHC) molecule, CD1d. Although they represent minor population in the immune system, NKT cells have become crucial regulators of tumor immunosurveillance as well as autoimmunity.8–12 By producing a large amount of proinflammatory cytokines and chemokines shortly after encountering their ligands, NKT cells regulate the activation of other immune cells including NK, T, and B cells, and DCs. Injection of α-galactosylceramide (αGC), a ligand of NKT cells, together with protein antigens, efficiently induces DC maturation and generates the antigen-specific CD4+ and CD8+ T-cell immunity in vivo.13 Moreover, coadministration of apoptotic tumors together with αGC induces antitumor T-cell immunity.14 These results demonstrate a potent adjuvant effect of soluble αGC. In addition, Fujii et al showed that αGC-loaded DCs were strong stimulators of NKT activation and their in vivo function, such as induction of antitumor immunity.15 In accordance, injection of αGC-associated DCs in patients with advanced cancer efficiently induces NKT-cell expansion.16 Thus, DCs coated with αGC may provide greater potency and specificity over soluble αGC in induction of cellular immunity against cancer.
Most lymphoma and leukemia cells in humans express CD1d on their surface and thus can induce the activation of NKT cells in vitro.17,18 Although immune cells other than DCs were originally thought to be poor stimulators of NKT cells,19 our recent work revealed that αGC-loaded B cells efficiently activate NKT cells in vivo.20 Based on these observations, we here tested whether loading lymphoma with αGC would render their recognition by the innate immune system to be “dangerous” and improve their immunogenicity, leading to adaptive tumor-specific immunity in vivo. Using the well-known nonimmunogenic murine lymphoma A20, we show here that vaccination with αGC-loaded lymphoma generates potent antitumor activity in tumor-bearing mice and establishes memory-type immunity. Interestingly, CD4+ T cells, rather than CD8+ T cells, are critical and sufficient in mediating antitumor response in our vaccine model.
Materials and methods
Tumor model and vaccination
αGC was prepared as described previously21 and dissolved in 0.5% polysorbate (vehicle). Cells from an A20 tumor, a B-cell lymphoma originating from a BALB/c mouse, were cocultured overnight with 1 μg/mL αGC (A20/αGC) or the same volume of solvent vehicle (A20/veh). These cells were extensively washed and irradiated (50 Gy) before intravenous injection (106 cells per mouse). To test protective antitumor effect, BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were vaccinated with either A20/αGC or A20/veh, or left untreated. At 7 days later, all mice were intravenously challenged with 106 live A20 cells, and then the survival of mice was regularly monitored for 70 days thereafter. To test antitumor effect in tumor-bearing mice, groups of BALB/c mice were intravenously challenged with 106 live A20 cells followed by vaccination with A20/αGC or A20/veh 5 or 10 days after tumor inoculation. To test recall antitumor response, survivors at 80 days after primary tumor inoculation were rechallenged with 106 live A20 cells, and survival was checked. To compare antitumor immunity generated by A20/αGC vaccination with that by other NKT-cell-mediated vaccine approaches, 5-day tumor-bearing mice were vaccinated with 2 μg αGC (intravenously), A20/veh together with 2 μg αGC (intravenously), or A20/αGC, and survival was monitored. All animal experiments were approved by our institutional committee on the use and care of laboratory animals (Institutional Animal Care and Use Committee; M. D. Anderson Cancer Center).
Antibody-mediated depletion and T-cell adoptive transfer
Monoclonal anti-CD4 (GK1.5) and anti-CD8 (2.43) antibodies (Abs) were affinity purified from ascites on a protein G-Sepharose column. To deplete CD4+, CD8+, or NK cells, 100 μg of anti-CD4 or anti-CD8 mAb, or 50 μg anti-asialo-GM1 polyclonal Abs (WAKO Pure Chemicals, Osaka, Japan) were injected intraperitoneally twice (on days −3 and −1) into the mice vaccinated with A20/αGC on day −7, respectively. On day 0, these mice were intravenously inoculated with 106 live A20 cells, and survival was monitored. For adoptive transfer, donor BALB/c mice were vaccinated as described, and CD4+ or CD8+ cells were separated from spleen and lymph node cells using anti-CD4 or anti-CD8 microbeads and AutoMacs (Miltenyi Biotech, Auburn, CA). In some experiments, the CD4+ cells were further stained with FITC-conjugated anti-CD4 Ab and APC-conjugated, PBS57-loaded CD1d tetramer22 (provided by National Institutes of Health [NIH] tetramer core facility; Atlanta, GA) and CD1d tetramer−CD4+ cells were sorted by FACSAria (BD Biosciences, San Jose, CA). Each population was transferred intravenously into syngeneic naive mice (2-3 × 107/mouse).
Flow cytometric analysis and cell sorting
Staining of NKT cells was performed by incubation with APC-conjugated PBS57-loaded CD1d tetramer in combination with FITC-conjugated anti-TCRβ Ab. FITC-conjugated anti-CD4 Ab was used to stain CD4+ T cells. For intracellular staining, cells were fixed and permeabilized before incubation with PE-conjugated anti-IFN-γ or anti-TNF-α Ab (1 μg/mL). For CD4+ T-cell intracellular staining, isolated CD4 T cells were cocultured with irradiated A20 cells (100 Gy) for 24 hours, and GolgiStop (BD Biosciences) was added for the final 6 hours before staining. To isolate NKT-cell-free conventional CD4+ T cells, lymphoid cells from vaccinated mice were stained with APC-conjugated CD1d tetramer, FITC-conjugated anti-CD4 Ab, and PE-conjugated antilineage markers (anti-B220, anti-CD11b, anti-CD11c, anti-CD8, and anti-NK1.1). APC−, PE−, and FITC+ population was purified by FACSAria. The sorted cells were TCRβ+CD4+ (> 99.7%) and CD1d tetramer− (< 0.02%).
ELISPOT
Anti-IFN-γ or anti-TNF-α Ab (10 μg/mL) was coated overnight on MultiScreen plates (Millipore, Bedford, MA). Highly purified CD4+ T cells (4 × 105/well) were cocultured with or without irradiated A20 (105/well). After 24 hours of culture, the plates were incubated with biotin-labeled anti-IFN-γ or anti-TNF-α Ab (2 μg/mL) for 2 hours, followed by horseradish peroxidase (HRP)-conjugated avidin for 30 minutes. Reactions were developed with AEC substrate (BD Biosciences). Analyses of the IFN-γ/TNF-α levels were performed using the Immunospot series Analyzer software (CTL, Cleveland, OH). The data indicate the number of spot-forming cells per 4 × 105 cells obtained with each experimental condition.
Statistics
The Kaplan-Meier method was used to determine the survival of in vivo tumor models, and the log-rank test was used for statistical analyses. The Student t test was used to assess all other statistical values. P values were determined, and error bars represent standard error of the mean (SEM).
Results
αGC-loaded A20 induces functional activation of NKT cells
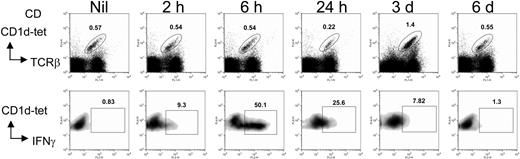
To test if αGC-loaded B-cell lymphoma could trigger NKT-cell activation, we used A20 lymphoma as a model tumor since it has been well characterized as nonimmunogenic, and the growth and localization of this tumor correlate well with diffuse large B lymphomas in humans.23 CD1d molecules are expressed on the surface of A20 at low levels.24 Loading of αGC on A20 cells (A20/αGC) was performed by culturing cells overnight in the presence of αGC (1 μg/mL), followed by irradiation (50 Gy) before application. A20 cells cocultured with vehicle (A20/veh) were used as control. Addition of αGC or vehicle did not change A20 surface expression level of costimulatory molecules such as CD40, CD80, CD86, and OX40 (not shown), ruling out endotoxin contamination in the reagents. When injected intravenously into BALB/c mice, A20/αGC triggered IFNγ production in CD1d tetramer+ cells, which peaked at 6 hours and lasted approximately 3 days after vaccination (Figure 1). The expansion of CD1d-tet+ cell population in the spleen was also observed at day 3 after vaccination, but subsequently declined to normal levels by day 6 (Figure 1). Therefore, αGC-loaded A20 efficiently induces the activation and expansion of NKT cells in vivo.
Activation and expansion of NKT cells in vivo after A20/αGC vaccination. A20 cells were cocultured overnight with 1 μg/mL αGC. After washing, these cells (A20/αGC) were irradiated (50 Gy) and intravenously injected into BALB/c mice. Lymphoid cells of the spleen from A20/αGC-vaccinated mice were isolated on indicated time points. The cells were stained with anti-TCRβ Ab plus CD1d tetramer and Ab for intracellular IFN-γ before being analyzed by flow cytometry. TCRβ+CD1d tetramer+ cells were gated and analyzed (bottom panels). Splenocytes from an untreated mouse (Nil) were used as control. Numbers indicate the percentage of each gate.
Activation and expansion of NKT cells in vivo after A20/αGC vaccination. A20 cells were cocultured overnight with 1 μg/mL αGC. After washing, these cells (A20/αGC) were irradiated (50 Gy) and intravenously injected into BALB/c mice. Lymphoid cells of the spleen from A20/αGC-vaccinated mice were isolated on indicated time points. The cells were stained with anti-TCRβ Ab plus CD1d tetramer and Ab for intracellular IFN-γ before being analyzed by flow cytometry. TCRβ+CD1d tetramer+ cells were gated and analyzed (bottom panels). Splenocytes from an untreated mouse (Nil) were used as control. Numbers indicate the percentage of each gate.
Vaccination with αGC-loaded A20 generates potent antitumor activity
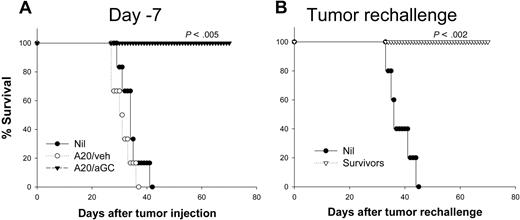
Since A20 coated with αGC activates NKT cells, we next examined if vaccination with A20/αGC could protect mice against tumor rechallenge. Syngeneic BALB/c mice were vaccinated once with A20/αGC or A20/veh 7 days (day −7) before they were challenged with live A20, and the survival of mice was analyzed. All nonvaccinated mice after receiving 1 × 106 live A20 cells died 4 to 6 weeks after tumor inoculation (Figure 2A), with massive tumor growth in liver, spleen, mesenteric lymph nodes, and bone marrow as reported.23 Mice vaccinated with A20/veh showed similar pattern of survival (Figure 2A), supporting the nonimmunogenic properties of this tumor.25 In sharp contrast, mice vaccinated with A20/αGC were all 100% resistant to live A20 (Figure 2A). This complete protective effect was confirmed in 3 independent experiments (Table 1). Importantly, all vaccinated mice were also resistant to secondary A20 rechallenge even 80 days after primary challenge (Figure 2B; Table 1), suggesting that they had developed immune memory against A20.
Preventive antitumor activity generated by A20/αGC vaccination. (A) A20 cells were cocultured overnight with 1 μg/mL αGC (A20/αGC) or vehicle (0.5% polysorbate; A20/veh) followed by irradiation (50 Gy). BALB/c mice (n=6-7 per group) were vaccinated with A20/veh, A20/αGC, or left untreated (Nil). After 7 days, all mice received 1 × 106 live A20 cells intravenously, and the survival was checked (day −7). (B) Survivors from panel A were rechallenged with live A20 cells 80 days after primary inoculation (Tumor rechallenge). Age-matched naive mice (n=5) were used as control (Nil). P values were calculated in comparison with the nonvaccinated group. Data shown represent at least 2 independent experiments.
Preventive antitumor activity generated by A20/αGC vaccination. (A) A20 cells were cocultured overnight with 1 μg/mL αGC (A20/αGC) or vehicle (0.5% polysorbate; A20/veh) followed by irradiation (50 Gy). BALB/c mice (n=6-7 per group) were vaccinated with A20/veh, A20/αGC, or left untreated (Nil). After 7 days, all mice received 1 × 106 live A20 cells intravenously, and the survival was checked (day −7). (B) Survivors from panel A were rechallenged with live A20 cells 80 days after primary inoculation (Tumor rechallenge). Age-matched naive mice (n=5) were used as control (Nil). P values were calculated in comparison with the nonvaccinated group. Data shown represent at least 2 independent experiments.
Summary of antitumor effect generated by αGC-loaded A20 vaccination
| Vaccination model . | Survival rate, no. (%) . | |
|---|---|---|
| First tumor challenge . | Second tumor challenge . | |
| Prophylactic | ||
| Nil | 0/20 (0) | 0/14 (0) |
| A20/veh | 0/19 (0) | — |
| A20/αGC | 19/19 (100) | 19/19 (100) |
| Therapeutic, day =5 | ||
| Nil | 0/15 (0) | 0/10 (0) |
| A20/veh | 0/16 (0) | — |
| A20/αGC | 7/16 (44) | 7/7 (100) |
| Therapeutic, day =10 | ||
| Nil | 0/10 (0) | ND |
| A20/veh | 0/10 (0) | ND |
| A20/αGC | 2/10 (20) | ND |
| Vaccination model . | Survival rate, no. (%) . | |
|---|---|---|
| First tumor challenge . | Second tumor challenge . | |
| Prophylactic | ||
| Nil | 0/20 (0) | 0/14 (0) |
| A20/veh | 0/19 (0) | — |
| A20/αGC | 19/19 (100) | 19/19 (100) |
| Therapeutic, day =5 | ||
| Nil | 0/15 (0) | 0/10 (0) |
| A20/veh | 0/16 (0) | — |
| A20/αGC | 7/16 (44) | 7/7 (100) |
| Therapeutic, day =10 | ||
| Nil | 0/10 (0) | ND |
| A20/veh | 0/10 (0) | ND |
| A20/αGC | 2/10 (20) | ND |
Data are pooled from 3 prophylactic and 3 therapeutic experiments.
ND indicates not determined; —, not applicable.
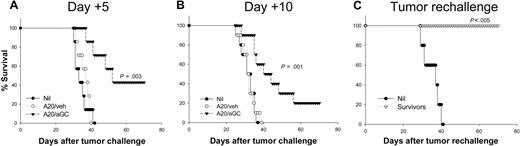
Since antitumor effect against established tumor is more clinically relevant, we assessed the therapeutic effect of our vaccine approach in tumor-bearing mice. A total of 106 live A20 cells were injected intravenously into BALB/c mice 5 days before vaccination (day +5). Mice vaccinated with A20/veh showed an almost identical pattern of survival rate to that of nonvaccinated mice (Figure 3A). Notably, mice receiving a single vaccination with A20/αGC showed substantially prolonged survival, and 44% of mice were cured of their tumor (Figure 3A; Table 1). Again, all survivors were completely resistant to tumor rechallenge, indicative of adaptive memory immunity against A20 (Figure 3C; Table 1). In another experiment, mice were vaccinated with A20/αGC or A20/veh 10 days after live A20 challenge (day +10). In this case, vaccination with A20/αGC still significantly prolonged the survival of the tumor-bearing mice, and 2 of 10 mice were cured of their tumor (Figure 3B). Therefore, a single vaccination with A20/αGC generated potent and long-lasting therapeutic adaptive immunity against A20.
Therapeutic antitumor activity generated by A20/αGC vaccination. (A,B) BALB/c mice were inoculated with 1 × 106 live A20 cells intravenously at day 0. On day 5 (day +5) or day 10 (day +10), mice were vaccinated with A20/veh, A20/αGC, or left untreated (Nil) (n=7-10 per group), and the survival was monitored. (C) Survivors from panel A were rechallenged with live A20 cells 80 days after primary tumor inoculation, and the survival was monitored. Age-matched naive mice were used as control (Nil; n=5). P values were calculated in comparison with the nonvaccinated group.
Therapeutic antitumor activity generated by A20/αGC vaccination. (A,B) BALB/c mice were inoculated with 1 × 106 live A20 cells intravenously at day 0. On day 5 (day +5) or day 10 (day +10), mice were vaccinated with A20/veh, A20/αGC, or left untreated (Nil) (n=7-10 per group), and the survival was monitored. (C) Survivors from panel A were rechallenged with live A20 cells 80 days after primary tumor inoculation, and the survival was monitored. Age-matched naive mice were used as control (Nil; n=5). P values were calculated in comparison with the nonvaccinated group.
Comparative analysis of A20/αGC vaccination with other NKT-cell–mediated vaccine approaches
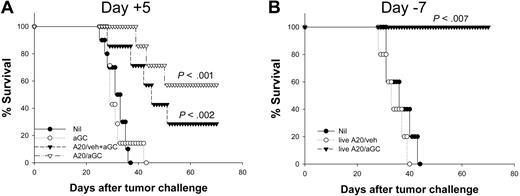
A previous report indicates that systemic αGC injection together with tumor showed significant protection against subsequent tumor challenge.14 We sought to compare the antitumor activity elicited by vaccination with αGC alone or together with apoptotic tumor cells (A20 + αGC) with that by A20/αGC. When we vaccinated mice with 1 μg of αGC (intraperitoneally) plus apoptotic A20, we observed that A20/αGC vaccination was superior to A20 + αGC in suppressing tumor growth in mice carrying A20 tumor for 5 days (Figure S1B, available on the Blood website; see the Supplemental Materials link at the top of the online article) (P < .001, A20/αGC versus A20/veh + αGC). To further compare the efficacy of A20/αGC with other regimen, mice were treated 5 days after receiving A20 tumor with 2 μg αGC (intravenously) alone or together with irradiated A20, or A20/αGC, respectively. As shown in Figure 4A, injection of αGC alone was not effective in suppressing tumor growth in tumor-bearing mice. By contrast, mice vaccinated with A20 + αGC showed significantly prolonged survival (P < .001). Again, A20/αGC vaccination induced a profound antitumor activity in 5-day tumor-bearing mice (P < .001), which was comparable to that elicited by A20 + αGC in this experimental setting (P=.318, A20/αGC versus A20/veh + αGC). Together, these observations suggest that antitumor activity generated by A20/αGC vaccination may be at least comparable to that elicited by free αGC plus apoptotic A20 vaccination.
Comparison of antitumor efficacy by free or tumor-loaded αGC. (A) BALB/c mice received 1 × 106 live A20 cells intravenously (day 0) before they were vaccinated with A20/αGC, intravenous injection of αGC (2 μg/mouse) plus A20/veh (A20/veh + αGC), or αGC alone (2 μg/mouse) at day 5 (n=7 per group). (B) Mice were vaccinated with the indicated regimen with nonirradiated A20 cells (live A20/veh, live A20/αGC) on day −7 (n=5 per group). On day 0, all mice received 106 live A20 cells intravenously. Nonvaccinated mice were used as a control (Nil). The survival was monitored daily. P values were calculated in comparison with the nonvaccinated group.
Comparison of antitumor efficacy by free or tumor-loaded αGC. (A) BALB/c mice received 1 × 106 live A20 cells intravenously (day 0) before they were vaccinated with A20/αGC, intravenous injection of αGC (2 μg/mouse) plus A20/veh (A20/veh + αGC), or αGC alone (2 μg/mouse) at day 5 (n=7 per group). (B) Mice were vaccinated with the indicated regimen with nonirradiated A20 cells (live A20/veh, live A20/αGC) on day −7 (n=5 per group). On day 0, all mice received 106 live A20 cells intravenously. Nonvaccinated mice were used as a control (Nil). The survival was monitored daily. P values were calculated in comparison with the nonvaccinated group.
We also asked whether irradiation of A20 cells is essential for triggering the observed antitumor effects. Coculture of A20 with αGC or coculture of A20/αGC with BALB/c splenocytes did not induce any additional apoptosis in A20 cells (Figure S2). However, as shown in Figure 4B, vaccination with live A20/αGC also resulted in complete protection, demonstrating that irradiation of lymphoma before vaccination is not necessary for generating antitumor immunity in our model.
Vaccination with αGC-loaded A20 does not alter immune cell populations in the spleen and blood
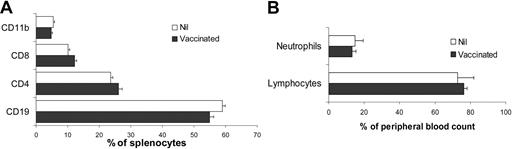
Vaccination with TAAs from A20 was previously reported to manifest autoimmune disorders, characterized by decreased numbers of T cells and increased numbers of macrophage in lymphoid organs and neutrophilia in blood as well as hepatosplenomegaly.26 To assess whether vaccination with A20/αGC induced autoimmune reaction, we analyzed mice 8 weeks after vaccination. Phenotypic analysis of lymphoid cells in the spleen revealed that the composition of CD4+ and CD8+ T cells, CD19+ B cells, and CD11b+ macrophages was comparable between A20/αGC-vaccinated and nontreated mice (Figure 5A). No hepatosplenomegaly was observed, and the total cellularity of spleen and lymph nodes was normal in vaccinated mice (not shown). Moreover, the numbers of circulating neutrophils and lymphocytes were both within normal ranges in the vaccinated mice (Figure 5B). Therefore, although our autologous vaccine strategy generated strong antitumor activity against B lymphoma, this approach did not induce a reduction in the number of B cells, or an increase of macrophages or neutrophils.
Analysis of immune cell composition in the spleens and blood of A20/αGC-vaccinated mice. BALB/c mice were vaccinated with A20/αGC or left untreated (Nil) (n=3 per group). At 8 weeks after vaccination, splenocytes (A) and blood leukocytes (B) were isolated from vaccinated or untreated mice (Nil). Cells were analyzed by flow cytometer after staining with anti-CD19, anti-CD4, anti-CD8, and anti-CD11b Abs (A). Neutrophils were defined by forward scatter (FSC) and side scatter (SSC) profiles in combination with anti-Gr1 and anti-CD11b Ab staining by flow cytometer (B). Values are means (± SEM).
Analysis of immune cell composition in the spleens and blood of A20/αGC-vaccinated mice. BALB/c mice were vaccinated with A20/αGC or left untreated (Nil) (n=3 per group). At 8 weeks after vaccination, splenocytes (A) and blood leukocytes (B) were isolated from vaccinated or untreated mice (Nil). Cells were analyzed by flow cytometer after staining with anti-CD19, anti-CD4, anti-CD8, and anti-CD11b Abs (A). Neutrophils were defined by forward scatter (FSC) and side scatter (SSC) profiles in combination with anti-Gr1 and anti-CD11b Ab staining by flow cytometer (B). Values are means (± SEM).
Antitumor immunity generated by αGC-loaded A20 is mediated by conventional CD4+ T cells
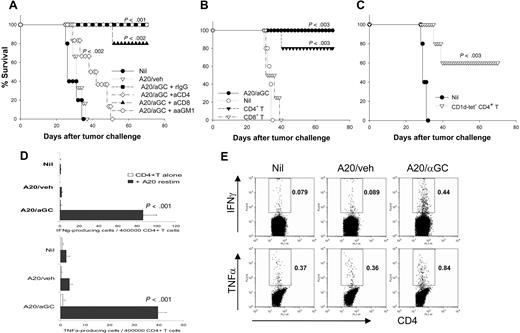
A20/αGC vaccination appears to effectively establish antitumor immune memory (Figures 2,3). We sought to identify the mechanisms and which immune cell type(s) is responsible for tumor eradication. First, we depleted CD4, CD8, or NK cells by specific antibodies in vaccinated mice. Vaccinated mice depleted of CD8+ cells or NK cells were still resistant to live A20 tumor challenge (Figure 6A). Interestingly, depletion of CD4+ cells was associated with abrogation of most of the protective effect, although their survival was modestly prolonged compared with that of the control groups (Figure 6A). To identify more precisely the effector cell types mediating antitumor immunity, we next used an adoptive transfer model. CD4+ cells or CD8+ cells were isolated from lymphoid tissues of A20/αGC-vaccinated mice and transferred intravenously into syngeneic naive mice before challenge with live A20. Mice receiving CD8+ cells showed almost identical survival kinetics with nonvaccinated mice (Figure 6B), suggesting these cells are not sufficient in tumor rejection. However, 80% mice receiving CD4+ cells showed complete protection against subsequent A20 challenge (Figure 6B). Since NKT cells are mainly CD4+, the antitumor activity by CD4+ cells could be mediated by either CD4+ NKT cells or conventional CD4+ T cells. To clarify this, we sorted CD1d tetramer−CD4+ T cells from A20/αGC-vaccinated mice and transferred them into syngeneic naive mice before challenging the mice with live A20. Mice receiving the CD1d tetramer−CD4+ T cells showed significantly prolonged survival, and 60% of mice were completely protected against live A20 challenge (Figure 6C). Taken together, these results indicate that effector CD4+ T cells exclusively are both necessary and sufficient for the antitumor activity generated by A20/αGC.
Exclusive role of CD4+ T cells in mediating antitumor activity. (A) A group of mice were vaccinated with A20/veh, A20/αGC, or left untreated (Nil) on day −7. A20/αGC-vaccinated mice were injected intraperitoneally with depleting Abs of CD4, CD8, or NK cells, respectively, on days −3 and −1 (n=5-7 mice per group) before all mice were inoculated intravenously with live A20 on day 0. Survival was monitored daily. (B) BALB/c mice were vaccinated with A20/αGC. At 7 days later, CD4+ or CD8+ cells from lymphoid cells of A20/αGC-vaccinated mice were sorted and transferred intravenously into syngeneic naive mice (2-3 × 107/transfer) on day 0 (n=4-5 mice per group). A20/αGC-vaccinated mice (day −7) were used as a positive control. All mice were inoculated intravenously with live A20 on day 0, and the survival was monitored daily. P values are calculated in comparison with the nonvaccinated (Nil) group. (C) BALB/c mice were vaccinated with A20/αGC. At 7 days later, CD1d tetramer−CD4+ cells from lymphoid cells of A20/αGC-vaccinated mice were sorted and transferred intravenously into syngeneic naive mice (2 × 107/transfer) on day 0. Nontreated naive mice were used as a control (Nil). All mice were inoculated intravenously with live A20 cells on day 0, and the survival was monitored. P values are calculated in comparison with nontreated group. (D,E) CD1d tetramer−CD4+ T cells were sorted from vaccinated mice by using FACSAria (n=3 per group). Sorted CD4+ T cells (4 × 105/well) were cultured in the presence or absence of irradiated A20 cells (1 × 105/well) for 24 hours, and ELISPOT (D) or intracellular cytokine staining (E) was performed for analysis of IFN-γ and TNF-α. Values in panel D are means (±SEM). Values in panel E are percentages of the gated cells.
Exclusive role of CD4+ T cells in mediating antitumor activity. (A) A group of mice were vaccinated with A20/veh, A20/αGC, or left untreated (Nil) on day −7. A20/αGC-vaccinated mice were injected intraperitoneally with depleting Abs of CD4, CD8, or NK cells, respectively, on days −3 and −1 (n=5-7 mice per group) before all mice were inoculated intravenously with live A20 on day 0. Survival was monitored daily. (B) BALB/c mice were vaccinated with A20/αGC. At 7 days later, CD4+ or CD8+ cells from lymphoid cells of A20/αGC-vaccinated mice were sorted and transferred intravenously into syngeneic naive mice (2-3 × 107/transfer) on day 0 (n=4-5 mice per group). A20/αGC-vaccinated mice (day −7) were used as a positive control. All mice were inoculated intravenously with live A20 on day 0, and the survival was monitored daily. P values are calculated in comparison with the nonvaccinated (Nil) group. (C) BALB/c mice were vaccinated with A20/αGC. At 7 days later, CD1d tetramer−CD4+ cells from lymphoid cells of A20/αGC-vaccinated mice were sorted and transferred intravenously into syngeneic naive mice (2 × 107/transfer) on day 0. Nontreated naive mice were used as a control (Nil). All mice were inoculated intravenously with live A20 cells on day 0, and the survival was monitored. P values are calculated in comparison with nontreated group. (D,E) CD1d tetramer−CD4+ T cells were sorted from vaccinated mice by using FACSAria (n=3 per group). Sorted CD4+ T cells (4 × 105/well) were cultured in the presence or absence of irradiated A20 cells (1 × 105/well) for 24 hours, and ELISPOT (D) or intracellular cytokine staining (E) was performed for analysis of IFN-γ and TNF-α. Values in panel D are means (±SEM). Values in panel E are percentages of the gated cells.
These observations prompted us to further analyze CD4+ T cells after A20/αGC vaccination. We isolated CD1d tetramer−CD4+ T cells (to remove NKT-cell contamination) from the spleens of vaccinated mice and cocultured them with irradiated A20. Both enzyme-linked immunospot (ELISPOT) assay and intracellular cytokine analysis showed significant numbers of CD4+ T cells from A20/αGC-vaccinated mice produced IFN-γ and TNF-α (Figure 6D,E). Production of these cytokines was A20-specific, since few to no cytokine-producing cells were detected in the absence of A20 in the culture. Thus, vaccination with A20/αGC generated tumor-specific CD4+ T-cell immunity.
Discussion
Although autologous tumors are ideal sources of tumor antigens for vaccination, their poor immunogenicity has been a major obstacle. Our results demonstrated that B lymphoma, when coated with αGC, activated NKT cells and elicited potent protective and therapeutic antitumor immunity in a CD4+ T-cell-dependent manner.
In our model, conventional CD4+ T cells play a major role in the generation of antitumor immunity after vaccination. First, depletion of CD4+ cells abolished the antitumor effect of A20/αGC vaccination. Second, transfer of CD1d tetramer−CD4+ T cells from vaccinated mice was sufficient for suppressing tumor growth. Moreover, survivors from the initial tumor challenge were all protected against secondary tumor challenge, indicative of an established adaptive immunity rather than innate immunity. This line of data is supported by recent studies by others showing that CD4+ T cells could mediate antitumor activity in a CD8+ T-cell-independent manner, even against MHC II− tumors.27–29 Also, Liu et al have described that injection of apoptotic tumors in combination with free-form αGC induced antitumor T-cell immunity, which is dependent on both CD4+ and CD8+ T cells.14 On the other hand, Hong et al demonstrated the crucial role of CD4+ T cells in mediating antitumor immunity induced by vaccination with apoptotic tumors plus αGC in the absence of CD8+ T cells.30 It remains elusive whether the tumor regression by CD4+ T cells in the present study were due to direct interaction between the tumor and CD4+ T cells or through activation of other innate immune cells, such as macrophage.
Although vaccination with A20/αGC promptly activates NKT cells in vivo in our model, how antilymphoma CD4+ T-cell effector function is generated is unclear at this stage. We proposed that A20 cells presenting αGC-activated NKT cells provide a “danger signal” to TAA-specific T cells during their interaction with A20 or A20-capturing host antigen-presenting cells. With help of NKT cells, the TAA-specific CD4+ T cells differentiate into effector T cells that can mediate tumor killing. Interestingly, we could generate antitumor activity by vaccination of live A20/αGC. A recent study showed that αGC-loaded tumor cells can be killed by innate lymphocytes,31 such as NKT cells. Apoptotic A20 cells might be captured by host DCs and further enhance A20-specific adaptive immunity, since activation of NKT cells results in the full maturation of DCs in vivo.14 Shimizu et al recently reported that live tumor cells loaded with αGC induce tumor resistance that depends on innate immunity, including NK and NKT cells. By using CD4−/− and CD8−/− mice, they showed that the tumor resistance is independent of CD4+ and CD8+ T cells. In our model, however, we observed that the A20/αGC-vaccinated mice established memory-type antitumor immunity to A20, and antitumor activity was dependent on CD4+ T cells. The reason for this discrepancy between their study and ours is not clear at this moment. Since live tumor cells were used in most of their study, whereas irradiated tumor cells were used in our study, it seems possible that the status of tumor cells at the time of vaccination affects the antitumor effector mechanism. It is also possible that the type of tumor cells affects the type of effector mechanism, since we used B lymphoma that expresses MHC II. Future studies will need to clarify the exact mechanism of antitumor immunity elicited by our vaccine. Nonetheless, the presence of αGC on the surface of CD1d+ tumors triggers innate immunity by NKT cells, which can result in adaptive T-cell immunity specific for the tumor.
Compared with other current approaches using autologous tumors, the vaccination strategy we have described in the current study is simpler and more time- and labor-effective. Importantly, recent clinical trials have shown that use of NKT ligand in humans is nontoxic and safe.32,33 In the present study, we found no evident autoimmune phenotypes in vaccinated mice, demonstrating specificity of tumor immunity. In our experimental settings, we observed that vaccination with the tumor-associated form of αGC triggered at least equivalently effective tumor regression compared with apoptotic tumor plus high doses of intravenous αGC in tumor-bearing mice. Moreover, A20/αGC vaccination was more potent in tumor regression to vaccination with low doses of αGC (intraperitoneally) plus apoptotic tumor (intravenously). This is perhaps due to increased local concentration of αGC, which is likely safer than systemic immune activation by αGC.
Although NKT cells represent a minor population in humans, injection of the cell-associated form of αGC efficiently expands this population in patients with cancer.16 Therefore, we think that our vaccine strategy can be developed into a novel patient- and tumor-specific immunotherapy against lymphomas. Importantly, our vaccine approach induced long-term memory antitumor activity. T-cell priming is generally normal after depletion of normal B cells by antibody-mediated immunotherapy of lymphoma in humans;34 thus, our vaccine strategy could be applied to patients with lymphoma in combination with conventional immunotherapy. Since CD1d is widely expressed on most hematopoietic cells and therefore also on tumors of blood origin, including B and T lymphoma, myeloma, and leukemia,17,18 further studies may extend our vaccine strategy to other hematologic malignancies and to human patients.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the FACS Core Facility at M. D. Anderson for assistance with cell sorting and the NIH tetramer core facility for providing PBS57-loaded CD1d tetramers.
This work was supported in part by grants from National Institute of Health (to C.D.), a Multidisciplinary Research Project of M. D. Anderson Cancer Center (to L.K.), and a Specialized Center of Research (SCOR) grant from the Leukemia and Lymphoma Society (to C.D. and L.K.). C.D. is a Cancer Research Institute Investigator and a Trust Fellow of the M. D. Anderson Cancer Center.
National Institutes of Health
Authorship
Contribution: Y.C., L.W.K., and C.D. designed research; Y.C. performed research; C.Y.K. and S.K. contributed a new reagent; Y.C., H.Q., and C.D. analyzed and interpreted data; and Y.C., L.W.K., and C.D. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chen Dong, Department of Immunology and Center for Cancer Immunology Research, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: cdong@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal