Abstract
By comparing mature CD8-cell turnover in different organs, we previously demonstrated that CD8 cells proliferate predominantly in the bone marrow (BM). To investigate the mechanisms underlying such increased turnover, we compared BM, lymph nodes, and spleen CD8 cells from untreated C57BL/6 mice regarding in vivo proliferation within the organ; in vitro response to interleukin-7 (IL-7), IL-15, IL-21; ex vivo expression of membrane CD127 (IL-7Rα), intracellular Bcl-2, phospho–STAT-5 (signal transducer and activator of transcription 5), phospho-p38 mitogen activated protein kinase (MAPK); and in vivo proliferation on adoptive transfer. In the BM, the proliferation rate was increased for either total CD8 cells or individual CD44 and CD122 subsets. In contrast, purified CD8+ cells from the BM did not show an enhanced in vitro proliferative response to IL-7, IL-15, and IL-21 compared with corresponding spleen cells. After transfer and polyinosinic-polycytidylic acid (polyI:C) treatment, both spleen-derived and BM-derived CD8 cells from congenic donors proliferated approximately twice more in the recipient BM than in spleen and lymph nodes. Our results suggest that BM CD8 cells are not committed to self-renewal, but rather are stimulated in the organ. Molecular events constantly induced in the CD8 cells within the BM of untreated mice include increase of both phosphorylated STAT-5 and phosphorylated p38 intracellular levels, and the reduction of CD127 membrane expression.
Introduction
The size of the T-cell pool remains fairly stable, despite the input of new T cells from the thymus, the expansion of antigen-specific T cells during primary and anamnestic responses, the slow proliferation mostly concerning memory T cells, and the losses caused by cell death.1 This dynamic equilibrium implies that proliferation is compensated by cell death, and that positive and negative signals influencing proliferation and cell survival are balanced under normal conditions. Such regulation is manifested by the finding that T cells undergo acute “homeostatic” proliferation on adoptive transfer into lymphopenic syngeneic hosts.2–4
The lymphopenia-induced proliferation of CD8 T cells has different requirements for naive and memory cells.5 It has been reported that naive CD8 cells require contact with self major histocompatibility complex (MHC)/peptide complexes to proliferate in T-cell–depleted hosts,3,4 whereas memory CD8 cells do not,6 except for a recently characterized cell subset expressing low levels of CD122 (ie, the interleukin [IL]-2/IL-15Rβ chain).7 IL-7 and IL-15 jointly regulate both CD8-cell proliferation in lymphopenic hosts and CD8-cell survival in healthy individuals.8–10
Memory, but not naive, CD8 cells proliferate in response to agents that stimulate the innate immune system and induce inflammation, for example, lipopolysaccharides (LPS) from Gram-negative bacteria and polyinosinic-polycytidylic acid (poly I:C), a synthetic molecule mimicking viral double-stranded RNA.11,12 Such “bystander” CD8-cell proliferation is T-cell receptor (TCR)–independent and requires the poly I:C–induced release of interferon-I (IFN-I), which in turn either stimulates IL-15 production13 or possibly has a direct effect on T cells.14 A second pathway involving IFN-γ, IL-12, IL-18, and stimulating the proliferation of memory CD8 cells has also been described.15
In addition to proliferation in response to T-cell depletion and to IFNs, memory CD8 cells undergo intermittent cell division under normal T-cell–sufficient conditions.16,17 Such physiologic “background” or “basal” cell division greatly contributes to maintain stable over time the frequency of antigen-specific memory cells within the CD8 cells, thus sustaining long-term memory. It has been proposed that different subsets of memory T cells have diverse expansion potentials and that the so-called central memory T cells have a high self-renewal capacity.18,19 Recent work has shed light on the signals regulating background proliferation of memory CD8 cells. An important role is played by IL-15, which stimulates proliferation of memory phenotype (CD44high) but not naive phenotype (CD44int/low) CD8 cells when injected into mice or when added to purified CD8 cells in vitro.13,20 Experiments with genetically engineered mice and blocking antibodies have demonstrated that IL-15 and IL-7 are critical for memory CD8-cell maintenance.8–10,21 IL-7 appears to play a greater role in cell survival, whereas IL-15 is primarily responsible for driving memory cells into cell cycle.22 Recently, one group has shown that IL-21 can synergize with either IL-7 or IL-15 in inducing CD8 cell proliferation,23 whereas other groups have reported opposite findings.24,25
It is worth noting that each of these cytokines, such as IL-7, IL-15, and IL-21, activates STAT-5 through tyrosine phosphorylation22,26,27 and that STAT-5 deficient mice have impaired memory CD8-cell proliferation.28 Recent evidence suggests that IL-15 and possibly IL-7 can affect memory CD8-cell maintenance by modulating CD8-cell membrane expression of molecules involved in the control of CD8-cell activation and survival. It has been demonstrated that IL-15 induces 4-1BB membrane expression by CD8 cells, by a mechanism requiring mitogen activated protein kinase (MAPK) p38 activation.29 The 4-1BB molecule belongs to the tumor necrosis factor (TNF) receptor family and, on interaction with its counterreceptor 4-1BBL, promotes CD8-cell activation and survival.30,31 Several other members of the TNF/TNF receptor family have been implicated in survival and proliferation of memory CD8 cells, such as OX40/OX40L, CD27/CD70, and CD30/CD30L.30,31 Intracellular signaling through TNF receptor family members involves p38 activation by phosphorylation.31 Thus, p38 is an important molecule in the control of CD8-cell activation and survival involved in the down-stream signaling of either IL-15/IL-7 receptors or TNF receptor family members.29,31–34
We have previously documented that the in vivo proliferation of total CD8 cells and memory CD8 cells is higher in the bone marrow (BM) than in either spleen or lymph nodes (LN) of untreated C57BL/6 (B6) mice.35 One possibility is that CD8 cells proliferate in the BM because they gain access to proliferation-inducing cytokines, such as IL-7 and IL-15, present in this organ. Increasing knowledge supports the notions that mature lymphocyte proliferation occurs in specialized anatomical “niches,”2 and that the BM contains such “niches” for mature CD8 cells.36 An alternative possibility is that the CD8 cells localized in the BM have an intrinsic higher proliferative potential, possibly attributable to a diverse differentiation stage. To discriminate between these possibilities, in this report we took 4 approaches. First, we examined the in vivo proliferation of CD44 and CD122 CD8-cell subsets. Second, we measured the in vitro CD8-cell response to the cytokines IL-7, IL-15, IL-21. Third, we studied CD8 cells ex vivo regarding membrane expression of the IL-7 receptor-α chain, phosphorylation state of the signal-transducing molecules STAT-5 (signal transducer and activator of transcription 5) and p38, and intracellular levels of the antiapoptotic molecule Bcl-2.37–39 Fourth, we performed adoptive transfer experiments to compare the in vivo proliferative response to polyI:C treatment of spleen- and BM-derived CD8 cells.
Materials and methods
Mice
C57BL/6 (B6, expressing the CD45.2 allele) mice were either purchased from Harlan Nossan (Corezzana, Italy) and Charles River (Calco, Italy), or obtained from the Regina Elena Cancer Institute facility (Rome, Italy). CD45.1 B6 mice were purchased from Charles River. OT-I CD45.1 mice were a gift from Matteo Bellone (San Raffalele Hospital, Milan, Italy). Mice were housed at the Institute of Genetics and Biophysics A. Buzzati-Traverso and Univerity of Rome La Sapienza animal facilities, according to the institutional guidelines, and used at 2 to 4 months of age. Sentinel mice were screened for seropositivity to Sendai virus, rodent Coronavirus, and Mycoplasma pulmonis by Murine Immunocomb test (Charles River) and found negative.
In vivo BrdU incorporation
B6 mice were treated with 0.8 mg/mL bromodeoxyuridine (BrdU; Sigma, Milan, Italy) in their drinking water for 3 days. BrdU staining was performed as previously described.35
Immunization
B6 mice were adoptively transferred with 4 × 106 LN cells from CD45–1+ OT-I mice (comprising approximately 2.5 × 106 CD8 cells expressing the OT-I TCR).35 After 24 hours, adoptively transferred mice (here called OT-I-B6 mice) were injected intraperitoneally with 2.5 mg ovalbumin (OVA) and 150 μg polyinosinic-polycytidylic acid (polyI:C); 2 weeks later, mice were boosted intraperitoneally with 250 μg OVA and 150 μg polyI:C.35 After approximately 2 weeks, we analyzed blood samples by OVA-tetramer staining35 and found that the frequency of anti-OVA 257-264 peptide CD8 cells was 24.2% (± 10.0%) in immunized mice versus 2.2% (± 1.0%) in untreated controls. Immunized OT-I-B6 mice were used 7 to 12 months after priming. At that time, the frequency of anti-OVA 257-264 peptide CD8 cells was 13.2% (±3.8%) in the spleen and 20.6% (± 2.2%) in the BM. Values are reported as means (± SD).
CD8+-cell purification
For in vitro tests with cytokines and Western blotting, CD8+ cells (> 97% pure) were obtained from spleen and BM single-cell suspensions by positive magnetic selection with anti-CD8β fluorescein isothiocyanate (FITC) monoclonal antibody (mAb) (Pharmingen-Becton Dickinson, San Jose, CA) and anti-FITC magnetic microbeads (Miltenyi Biotec, Auburn, CA). CD8 cell forward scatter, CD44, and CD122 expression were not statistically different before and after purification.
Membrane staining and flow cytometric analysis
Cells were stained in 100 μL of phosphate-buffered saline containing 0.5% bovine serum albumin, 0.02% sodium azide, and 2 mM EDTA (ethylenediaminetetraacetic acid), pH 8. Nonspecific staining was blocked with anti-FcγR (clone 2.4G2) mAb. The following mAbs were used (all from Pharmingen-Becton Dickinson, conjugated with FITC, phycoerythrin (PE), Cychrome, peridinin chlorophyll protein (PerCP)-Cy5.5, Alexa 647, and biotin): anti-CD8α (53-67), anti-CD8β (53-5.8), anti-CD122 (TM-β1), anti-CD44 (IM7), anti-TCR (H57-597), anti-CD45.1 (A20), and anti-CD45.2 (104). Anti-CD127 (A7R34) mAbs were purchased from eBioscience (San Diego, CA). After incubation with the appropriate mAbs for 10-15 minutes on ice, cells were washed and analyzed by flow cytometry using Cell Quest software (v 3.3, BD BioScience, San Jose, CA).
Cell cultures
Purified CD8+ cells (5 × 104 cells/well) were cultured at 37°C, 5% CO2 in 96-well, flat-bottomed plates (BD Labware, Bedford, MA) in 200 μL of medium. Our medium was RPMI 1640 medium (Cambrex, Baltimore, MD) containing L-glutamine supplemented with 10% fetal calf serum (Hyclone, Outh Logan, UT), antibiotic antimycotic (Sigma), and β-mercaptoethanol (Sigma). Recombinant mouse IL-15, IL-7 and IL-21 (all from R & D Systems Minneapolis, MN) were added at the beginning of the culture, at indicated concentrations. Every 3 to 4 days, half of the medium was replaced with the same volume of fresh medium with cytokines.
Analysis of in vitro proliferation
Proliferation was assessed after 5 days of culture by measuring the uptake of tritiated thymidine (1 μCi/mL; Amersham, Uppsala, Sweden) during a 12-hour pulse. Cells were harvested (FilterMate Harvester; Packard, Downers Grove, IL) and incorporated radioactivity was measured by a β-counter apparatus (TopCount; Packard). All cultures were performed at least in duplicates and the average count per minute (cpm) values were calculated (except for average values < 100 cpm, SD was routinely approximately10% of the average). In some experiments, proliferation was assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) labeling. Purified CD8+ cells from either spleen or BM were labeled with CFSE as previously described40 and cultured in medium with different stimuli for 7 days in 96-well, flat-bottomed plates. For each culture condition, cells were placed in 5 to 10 wells (5 × 104 cells/well) and pooled at the end of incubation. Cells were analyzed by flow cytometry and 10 000 to 30 000 events per sample were acquired.
Ex vivo staining of phospho-STAT-5 and phospho-p38 MAPK
Staining of phosphorylated STAT-5 and p38 was performed as described.41 Briefly, untreated B6 mice were killed and, within 10 minutes, single-cell suspensions from spleen and BM were fixed in 1.6% formaldehyde/phosphate-buffered saline. After 15 minutes at room temperature, cell suspensions were filtered with 70-μm pore size filters. Cells were permeabilized in 80% methanol for 1 to 2 hours on ice and left overnight at −80°C. After rehydratation in 0.5% bovine serum albumin and phosphate-buffered saline for 1 hour on ice, cells were incubated with anti-FcγR (2.4G2) mAb and subsequently with anti-TCR PE and anti-CD8α Alexa 488 mAbs for 30 minutes on ice. After washings, cells were incubated for 30 minutes on ice with either anti-phospho-STAT-5 (Y694) Alexa 647 mAb (clone 47) or control Alexa 647 mAb. Cells were washed and analyzed by flow cytometry. Similar experiments were performed using anti-phospho-p38 (T180/Y182) Alexa 647 mAb (clone 36). Antibodies were purchased from Pharmingen-Becton Dickinson. As positive control, spleen cells from untreated B6 mice were stimulated in vitro in 24-well plates at 2 × 106 cells/well in 1 mL medium at 37°C (5% CO2). To induce phospho-STAT-5, IL-15 was added at the final concentration of 25 ng/mL. Regarding phospho-p38, LPS was added at the final concentration of 1 μg/mL. In both cases, cells were incubated for 15 minutes, harvested, and stained in parallel with fresh spleen and BM samples. TCR+CD8+ cells were analyzed for phospho-STAT-5 and CD11b+ cells for phospho-p38.41 For additional controls, see Document S1 (on the Blood website via the Supplemental Materials link at the top of the online article).
Adoptive transfer experiments
CD8-enriched cell populations were obtained from single-cell suspension of spleen and BM by magnetic selection using Dynal mouse CD8 negative isolation kit (Invitrogen, San Giuliano Milanese, Italy). CD8- cell percentage was 80% to 90% for spleen and 40% to 50% for BM-cell preparations. Spleen and BM-cell preparations from 2 different CD45-congenic B6 mice were mixed in a 1:1 CD8 ratio, labeled with CFSE, and injected intravenously in CD45.1 B6 mice (approximately 6 × 106 cells/mouse, containing 2 × 106 spleen CD8 cells and 2 × 106 BM CD8 cells). The next day, mice were either injected with 150 μg polyI:C intraperitoneally or left untreated.42 After 3 days, proliferation of CFSE-labeled CD8 cells was assessed on spleen, peripheral lymph node (PLN), and BM samples by flow cytometry (200 000-400 000 events were acquired in the TCR+ gate).
Statistical analysis
Statistical analysis was performed by Student t test. Differences were considered significant when P was .05 or less and highly significant when P was .01 or less.
Results
In vivo proliferation of BM, LN, and spleen CD8 cells
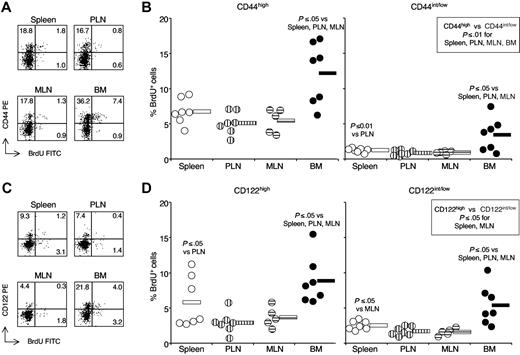
To gain insight into the phenotype of CD8 cells proliferating in the BM versus those proliferating in either spleen or LN, we examined BrdU incorporation within either CD44high or CD44int/low CD8 cells in each organ. For both CD8-cell subsets, BrdU incorporation in the BM was 2 to 3 times higher than in the spleen and 2 to 4 times higher than in the LN (Figure 1A,B). Results were similar with either PLNs or mesenteric lymph nodes (MLNs). As expected, proliferation of CD44int/low CD8 cells was always lower than that of CD44high cells. Even in the BM, the average percentage of BrdU+ cells within CD44int/low CD8 cells was only 3.4%, approximately 4-times lower than the corresponding percentage within BM CD44high CD8 cells, and half of that within spleen CD44high CD8 cells.
BrdU incorporation by CD8 cells in spleen, PLN, MLN and BM (BM). Single-cell suspensions were prepared from spleen, PLN (ie, inguinal and brachial), MLN, and BM of BrdU-treated B6 mice. After staining with anti-TCR cychrome, anti-CD8 biotin-streptavidin-APC, anti-CD44 PE (A,B) or anti CD122-PE (C,D) mAbs plus either control FITC or anti-BrdU FITC mAb, cells from individual mice were analyzed by flow cytometry. (A and C) BrdU staining. Representative staining profiles of spleen, PLN, MLN, and BM samples after gating on TCR+CD8+ cells. The scales on x-axis and y-axis are logarithmic, in arbitrary units. The numbers represent the percentages of cells within CD8 cells in the indicated quadrant. (B and D) BrdU+ cell percentages within CD8-cell subsets. The percentages of BrdU+ cells within CD44high and CD44int/low (B), CD122high and CD122int/low (D) TCR+CD8+ cells from each organ were determined, after subtraction of background staining with control mAb (≤ 1%). The percentages of BrdU+ cells from individual mice are represented, as well as the average value of each group (horizontal bar). The panels summarize the results obtained in 3 independent experiments. The P values are indicated when P ≤ .05 and P ≤ .01.
BrdU incorporation by CD8 cells in spleen, PLN, MLN and BM (BM). Single-cell suspensions were prepared from spleen, PLN (ie, inguinal and brachial), MLN, and BM of BrdU-treated B6 mice. After staining with anti-TCR cychrome, anti-CD8 biotin-streptavidin-APC, anti-CD44 PE (A,B) or anti CD122-PE (C,D) mAbs plus either control FITC or anti-BrdU FITC mAb, cells from individual mice were analyzed by flow cytometry. (A and C) BrdU staining. Representative staining profiles of spleen, PLN, MLN, and BM samples after gating on TCR+CD8+ cells. The scales on x-axis and y-axis are logarithmic, in arbitrary units. The numbers represent the percentages of cells within CD8 cells in the indicated quadrant. (B and D) BrdU+ cell percentages within CD8-cell subsets. The percentages of BrdU+ cells within CD44high and CD44int/low (B), CD122high and CD122int/low (D) TCR+CD8+ cells from each organ were determined, after subtraction of background staining with control mAb (≤ 1%). The percentages of BrdU+ cells from individual mice are represented, as well as the average value of each group (horizontal bar). The panels summarize the results obtained in 3 independent experiments. The P values are indicated when P ≤ .05 and P ≤ .01.
We investigated the in vivo proliferation of CD122 CD8-cell subsets in our system and found that both CD122high and CD122int/low CD8 cells from the BM had a significantly higher turnover than the corresponding cells from spleen, PLN, and MLN (Figure 1C,D). In agreement with reported findings on pooled LN cells,13 in the spleen and MLN the percentage of BrdU+ cells was higher within CD122high than within CD122int/low CD8 cells.
BM CD8 cells always had the highest proliferation rate and LN CD8 cells the lowest one, without major differences between PLN and MLN. Although the percentages of either CD44high or CD122high cells were higher in BM CD8 cells than in spleen and LN CD8 cells (for mice in Figure 1, CD44high percentages: BM 52.9% ± 7.7%, spleen 23.8% ± 2.8%, PLN 21.6%± 3.7%, MLN 18.8%± 2.4%; CD122high percentages: BM 30.0% ± 7.7%, spleen 12.8% ± 1.9%, PLN 9.7% ± 2.8%, MLN 6.7% ± 3.2%), our results clearly show that the increased rate of CD8-cell turnover in the BM cannot simply be explained by the high proportion of CD44high and CD122high CD8 cells in this organ.
In vitro proliferation of BM and spleen CD8 cells in response to IL-15, IL-7, and IL-21
To test whether BM CD8 cells had an increased ability to respond to IL-15, IL-7, and IL-21 in comparison with spleen CD8 cells, we examined the in vitro proliferation by thymidine incorporation of highly purified (> 97% pure) CD8 cells obtained from each of the 2 organs.
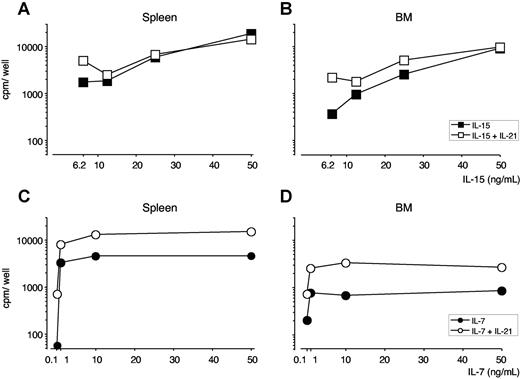
BM and spleen CD8 cells displayed similar proliferative curves in response to IL-15, with a mitogenic effect starting at 25 ng/mL of IL-15 and reaching a plateau at 50-100 ng/mL of IL-15 (Figures 2 and S1A). On stimulation with IL-7, spleen CD8 cells displayed a higher proliferative response than BM CD8 cells, at each of the mitogenic IL-7 doses tested, ranging from 1 to 100 ng/mL (Figures 2 and S1B). Results obtained by thymidine incorporation were reproducible and similar to those obtained by CFSE labeling (data not shown).
Proliferative response of purified CD8 cells from spleen and BM to IL-21, in combination with either IL-15 or IL-7. Purified CD8+ cells (5 × 104 cells/well) from spleen and BM of untreated B6 mice were cultured for 5 days in the presence of graded concentrations of either IL-15 or IL-7, alone or in combination with IL-21. H3-thymidine incorporation was determined after a 12-hour pulse. (A,B) IL-21 and IL-15. Spleen (A) and BM (B) CD8-cell response to graded dose of IL-15, alone or in combination with 50 ng/mL of IL-21. (C,D) IL-21 and IL-7. Spleen (C) and BM (D) CD8-cell response to graded dose of IL-7, alone or in combination with 50 ng/mL of IL-21. For both organs, background proliferation with medium resulted in less than 60 cpm and incubation with 10, 50, and 100 ng/mL of IL-21 in approximately 100 cpm. One representative experiment is shown of 3. For each experiment, cells were pooled from 4 to 5 mice.
Proliferative response of purified CD8 cells from spleen and BM to IL-21, in combination with either IL-15 or IL-7. Purified CD8+ cells (5 × 104 cells/well) from spleen and BM of untreated B6 mice were cultured for 5 days in the presence of graded concentrations of either IL-15 or IL-7, alone or in combination with IL-21. H3-thymidine incorporation was determined after a 12-hour pulse. (A,B) IL-21 and IL-15. Spleen (A) and BM (B) CD8-cell response to graded dose of IL-15, alone or in combination with 50 ng/mL of IL-21. (C,D) IL-21 and IL-7. Spleen (C) and BM (D) CD8-cell response to graded dose of IL-7, alone or in combination with 50 ng/mL of IL-21. For both organs, background proliferation with medium resulted in less than 60 cpm and incubation with 10, 50, and 100 ng/mL of IL-21 in approximately 100 cpm. One representative experiment is shown of 3. For each experiment, cells were pooled from 4 to 5 mice.
We also examined the effects on BM and spleen CD8 cells of IL-21. We found that IL-21 was unable to stimulate either spleen or BM CD8-cell proliferation, but it increased the proliferative effects of low doses of IL-15 and synergized with any tested dose of IL-7. BM and spleen CD8 cells gave similar results (Figure 2).
Our results show that BM CD8 cells do not have a strongly increased proliferative response to IL-7, IL-15, and IL-21 compared with spleen CD8 cells. The response to IL-7 is consistently more pronounced in the case of spleen CD8 cells. This might be attributable to the higher proportion of CD44int/low CD8 cells in the spleen versus the BM, considering that IL-7 is especially effective on naive phenotype CD8 cells.22 Another possibility is that differences in IL-7Rα expression by spleen and BM CD8 cells contribute to the diverse IL-7 response.35
To gain insight into the survival capacity of BM and spleen CD8 cells, we measured by flow cytometry the intracellular levels of Bcl-2 expressed by freshly isolated CD8 cells from the 2 organs. Within each CD44 subset, no statistically significant differences were found between spleen and BM CD8 cells (Figure S2A,B). Both in the BM and in the spleen, CD44high CD8 cells expressed higher levels of Bcl-2 than CD44int/low CD8 cells, as expected.37,43 We then compared the survival of purified spleen and BM CD8 cells in response to low doses of either IL-15 or IL-7, including submitogenic concentrations. No relevant differences were found between BM and spleen (Figure S2C,D). Taken together, these findings suggest that BM CD8 cells do not have a greater resistance to apoptosis than spleen CD8 cells.
In vitro proliferation of BM and spleen antigen-specific memory CD8 cells in response to IL-15, IL-7, and IL-21
In the light of our previous findings on the preferential in vivo proliferation of antigen-specific cells in the BM of immune mice,35 we undertook another set of experiments in which we analyzed OVA 257-264–specific memory CD8 cells, using the so-called OT-I-B6 mice.35 Briefly, B6 mice were transferred with a small number of LN cells from anti-OVA 257-264 peptide TCR transgenic OT-I CD45.1 mice, immunized with OVA, and used as a source of CD8 cells for in vitro studies 7-12 months after immunization.
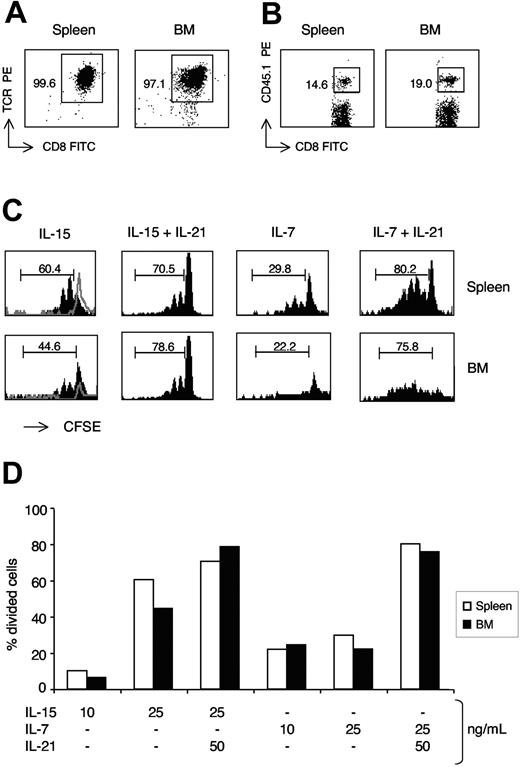
We purified total CD8 cells from spleen and BM, and, after CFSE labeling, stimulated them for 7 days with IL-15 and IL-7 and tested each alone or in combination with IL-21. At the end of incubation, we performed flow cytometric analysis on gated CD45.1+ cells (OVA 257-264–specific memory OT-I CD8 cells). The percentage of divided cells among CD45.1+ CD8 cells was not higher in the BM than in the spleen cell cultures (Figure 3). CD45.1+ CD8 cells from both organs had a strong proliferative response to IL-15, and proliferated to a less extent on incubation with IL-7. IL-21 synergized with both cytokines (Figure 3). The percentage of CD45.1+ cells within CD8 cells increased on average 1.3-times in stimulated BM-cell cultures and 1.7-times in corresponding spleen cell cultures (data not shown). Our results show that BM and spleen antigen-specific memory CD8 cells have a similar in vitro proliferative response to IL-15, IL-7, and IL-21.
Proliferative response of spleen and BM antigen-specific memory CD8 cells to IL-15, IL-7, and IL-21. OT-I-B6 mice were immunized against OVA as described in “Materials and methods, Immunization.” After 7 to 12 months, CD8+ cells were purified from spleen and BM, labeled with CFSE, and cultured (5 × 104 cells/well) for 7 days in the presence of graded concentrations of IL-15 and IL-7, each tested alone or in combination with IL-21. (A,B) CD8-cell purification and CD45.1 staining. Typical cytometric profiles of total CD8+ cells purified from spleen and BM (A) and of CD45.1 staining within CD8+ cells (B). The scales on x-axis and y-axis are logarithmic, in arbitrary units. Numbers indicate the percentages in the gated region, showing the purity of CD8 cell preparation (A) and the frequency of CD45.1+ OT-I cells within CD8 cells (B). All CD45.1+CD8+ cells were CD45.2− and OVA-tetramer+. (C,D) Analysis of CD45.1+-cell proliferation by CFSE labeling. After 7 days of culture with IL-15, IL-7, and IL-21, cells were analyzed by flow cytometry and the percentage of divided cells, those with decreased CFSE fluorescence intensity, was determined within CD45.1+ cells. For each organ, the marker was set based on CFSE intensity of unstimulated cells. Example of CFSE staining profiles of spleen and BM CD45.1+ CD8 cells in response to IL-15 and IL-7 (each at 25 ng/mL), with or without IL-21 (at 50 ng/mL) (C) and comparison of spleen and BM CD45.1+ CD8-cell proliferative response to graded concentrations of IL-15 and IL-7, each tested alone or in combination with 50 ng/mL of IL-21 (D). In (C), the scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value 20, except for IL-15+IL-21 panels, which have a maximum value of 80). The unstimulated cell profiles are shown with gray lines. The numbers represent the percentages of cells in the marked region. One representative experiment is shown of 3. For each experiment, cells were pooled from 4 to 5 mice. Differences between BM and spleen were similar in the 3 experiments and not statistically significant (average difference never > 20% for any cytokine concentration).
Proliferative response of spleen and BM antigen-specific memory CD8 cells to IL-15, IL-7, and IL-21. OT-I-B6 mice were immunized against OVA as described in “Materials and methods, Immunization.” After 7 to 12 months, CD8+ cells were purified from spleen and BM, labeled with CFSE, and cultured (5 × 104 cells/well) for 7 days in the presence of graded concentrations of IL-15 and IL-7, each tested alone or in combination with IL-21. (A,B) CD8-cell purification and CD45.1 staining. Typical cytometric profiles of total CD8+ cells purified from spleen and BM (A) and of CD45.1 staining within CD8+ cells (B). The scales on x-axis and y-axis are logarithmic, in arbitrary units. Numbers indicate the percentages in the gated region, showing the purity of CD8 cell preparation (A) and the frequency of CD45.1+ OT-I cells within CD8 cells (B). All CD45.1+CD8+ cells were CD45.2− and OVA-tetramer+. (C,D) Analysis of CD45.1+-cell proliferation by CFSE labeling. After 7 days of culture with IL-15, IL-7, and IL-21, cells were analyzed by flow cytometry and the percentage of divided cells, those with decreased CFSE fluorescence intensity, was determined within CD45.1+ cells. For each organ, the marker was set based on CFSE intensity of unstimulated cells. Example of CFSE staining profiles of spleen and BM CD45.1+ CD8 cells in response to IL-15 and IL-7 (each at 25 ng/mL), with or without IL-21 (at 50 ng/mL) (C) and comparison of spleen and BM CD45.1+ CD8-cell proliferative response to graded concentrations of IL-15 and IL-7, each tested alone or in combination with 50 ng/mL of IL-21 (D). In (C), the scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value 20, except for IL-15+IL-21 panels, which have a maximum value of 80). The unstimulated cell profiles are shown with gray lines. The numbers represent the percentages of cells in the marked region. One representative experiment is shown of 3. For each experiment, cells were pooled from 4 to 5 mice. Differences between BM and spleen were similar in the 3 experiments and not statistically significant (average difference never > 20% for any cytokine concentration).
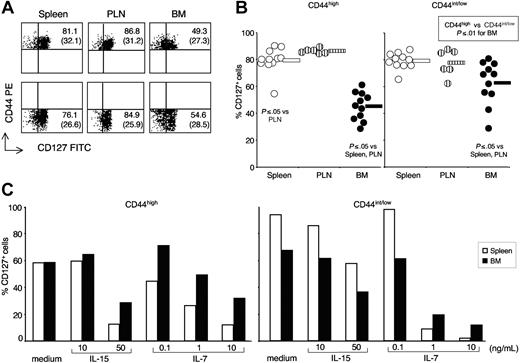
Ex vivo expression and in vitro modulation of membrane IL-7Rα (CD127) by BM, LN, and spleen CD8 cells
We previously documented that antigen-specific memory CD8 cells had a lower membrane expression of IL-7Rα (CD127) in the BM than they had in spleen and LN.35 Here, we analyzed CD127 expression by freshly isolated CD8 cells from BM, PLN, and spleen, gating separately on CD44high and CD44int/low CD8 cells, because CD127 has been proposed as a memory differentiation marker.38 We found that the percentage of CD127+ cells was significantly lower in BM than in either spleen or PLN CD8 cells, both in the case of CD44high and in that of CD44int/low subset (Figure 4A,B). Such differences in CD127 expression might contribute to the diverse proliferative response to IL-7 of BM and spleen CD8 cells (Figures 2 and S1B). Moreover, in the BM but not in the spleen and PLN, CD127+ cells were significantly less abundant among CD44high than among CD44int/low CD8 cells (Figure 4B).
CD127 expression by spleen, PLN, and BM CD8 cells. (A,B) CD127 phenotype. Single-cell suspensions were prepared from spleen, PLN, and BM of untreated B6 mice. In some experiments, cells from individual mice were stained with anti-CD8 cychrome, anti-CD44 PE plus either control FITC or anti-CD127 FITC mAb, and analyzed by flow cytometry. In other experiments, purified spleen and BM cells were pooled from 8 to 10 mice and CD8+ cells were purified before CD127 staining. No differences were observed in the 2 sets of experiments. Representative staining profiles of spleen, PLN, and BM samples, after gating on CD8+ cells (A) and percentages of CD127+ cells within CD44high and CD44int/low CD8 cells from spleen, PLN, and BM (B). In (A), the scales on x-axis and y-axis are logarithmic, in arbitrary units. The numbers represent the percentages of CD127+ cells in the right quadrant within CD44high (top panels) and CD44int/low (bottom panels) CD8 cells. Mean fluorescence intensity of cells in the right quadrant is indicated in parentheses. In panel B, individual values are represented, as well as the average of each group (horizontal bar). (C) Modulation of CD127 after culture with IL-15 and IL-7. Purified CD8+ cells (5 × 104 cells/well) from BM and spleen of untreated B6 mice were cultured for 3 days in the presence of medium, IL-15, or IL-7, as indicated. At the end of incubation, the percentages of CD127+ cells within PI− CD44high and CD44int/low cells were determined. One representative experiment is shown of 3. For each experiment, BM and spleen cells were pooled from 8 to 10 mice. Differences between BM and spleen were similar in the 3 experiments and not statistically significant (average difference never > 20% for any cytokine concentration).
CD127 expression by spleen, PLN, and BM CD8 cells. (A,B) CD127 phenotype. Single-cell suspensions were prepared from spleen, PLN, and BM of untreated B6 mice. In some experiments, cells from individual mice were stained with anti-CD8 cychrome, anti-CD44 PE plus either control FITC or anti-CD127 FITC mAb, and analyzed by flow cytometry. In other experiments, purified spleen and BM cells were pooled from 8 to 10 mice and CD8+ cells were purified before CD127 staining. No differences were observed in the 2 sets of experiments. Representative staining profiles of spleen, PLN, and BM samples, after gating on CD8+ cells (A) and percentages of CD127+ cells within CD44high and CD44int/low CD8 cells from spleen, PLN, and BM (B). In (A), the scales on x-axis and y-axis are logarithmic, in arbitrary units. The numbers represent the percentages of CD127+ cells in the right quadrant within CD44high (top panels) and CD44int/low (bottom panels) CD8 cells. Mean fluorescence intensity of cells in the right quadrant is indicated in parentheses. In panel B, individual values are represented, as well as the average of each group (horizontal bar). (C) Modulation of CD127 after culture with IL-15 and IL-7. Purified CD8+ cells (5 × 104 cells/well) from BM and spleen of untreated B6 mice were cultured for 3 days in the presence of medium, IL-15, or IL-7, as indicated. At the end of incubation, the percentages of CD127+ cells within PI− CD44high and CD44int/low cells were determined. One representative experiment is shown of 3. For each experiment, BM and spleen cells were pooled from 8 to 10 mice. Differences between BM and spleen were similar in the 3 experiments and not statistically significant (average difference never > 20% for any cytokine concentration).
It has been shown that splenic T cells cultured in vitro with T-cell pro-survival cytokines have a decreased membrane expression of CD127, caused by a transcriptional inhibition.44 Here, we examined CD127 expression by CD44high and CD44int/low CD8 cells from BM and spleen after in vitro incubation with either IL-15 or IL-7. In agreement with the reported findings on splenic T cells,44 we observed that the percentage of CD127+ cells was reduced in both spleen and BM CD8 cells after treatment with IL-15 and IL-7 (Figure 4C). In contrast with the findings on CD127 expression, the majority of CD44high cells from either spleen or BM became CD122high after incubation with either IL-15 or IL-7 (Figure S3). Regarding CD44int/low cells from the BM and the spleen, few of them were CD122high, either freshly isolated from the organs or cultured with IL-15 and IL-7 (Figure S3).
Our findings suggest that prosurvival cytokines inhibit CD127 expression by CD8 cells in the BM environment, possibly reflecting a high local concentration of IL-15 and/or IL-7.
Ex vivo expression of phospho-STAT-5 and phospho-p38 MAPK by BM, LN, and spleen CD8 cells
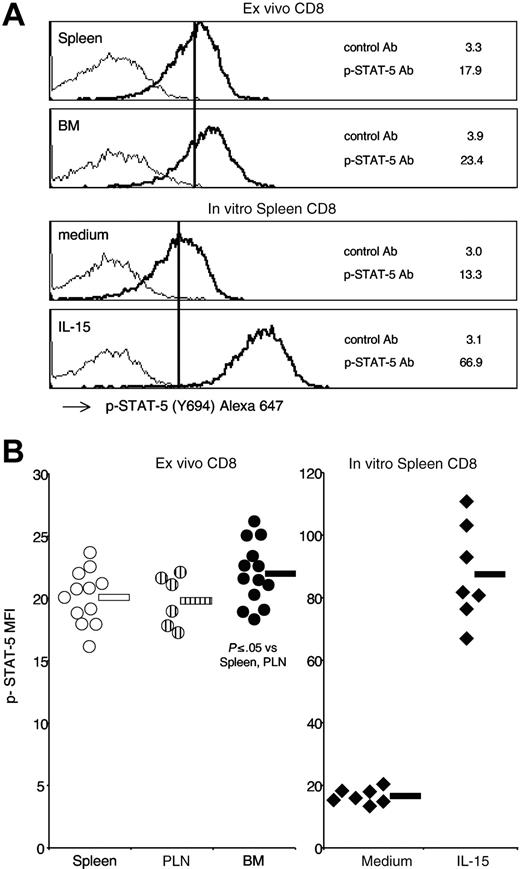
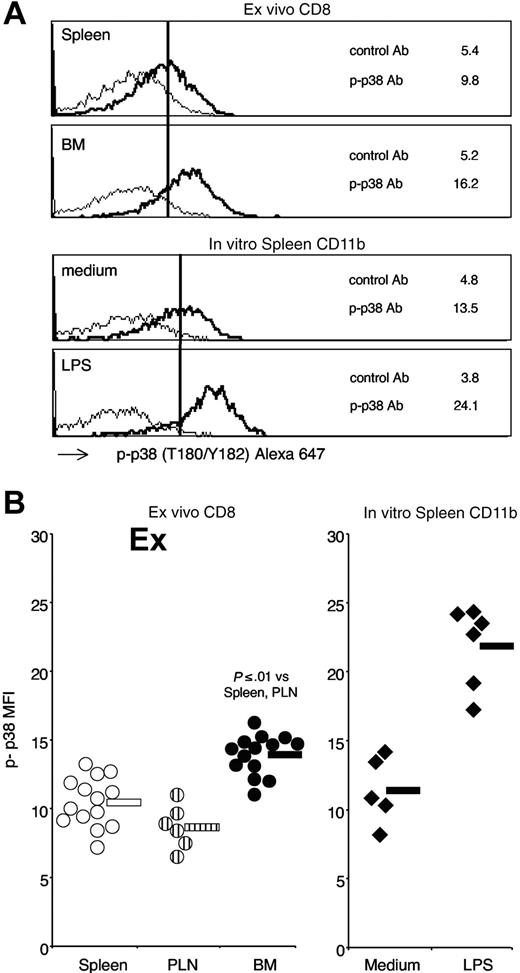
To gain insight into the intracellular pathways possibly activated in BM CD8 cells by IL-15 and other stimuli in the organ, we examined the phosphorylation state of the signal-transducing molecules STAT-5 and p38 expressed by freshly isolated spleen, PLN, and BM CD8 cells. To this aim, we took advantage of a powerful technique that allowed direct analysis by flow cytometry of the intracellular levels of phospho-proteins expressed by individual cells in a heterogeneous sample.45 By using such a technique, we successfully reproduced published results on phospho-STAT-5 induction in splenic CD4 cells after intravenous injection of IL-2,41 demonstrating that this method allowed us to detect ex vivo a signal generated in vivo in the spleen by the injected IL-2 (Document S1). Applying phospho-protein flow cytometric analysis on freshly isolated thymocytes from B6 mice, another report documented that it is possible to detect ex vivo a signal generated in vivo in the absence of any treatment.46 Here, we analyzed spleen, PLN, and BM CD8 cells from untreated B6 mice by gating on TCR+CD8+ cells and measured their intracellular levels of phospho-STAT-5 and phospho-p38. We found that BM CD8 cells expressed significantly higher levels of phospho-STAT-5 than spleen CD8 cells (Figure 5). BM CD8 cells had also significantly increased levels of phospho-p38 compared with spleen CD8 cells (Figure 6). Intracellular levels of either phospho-STAT-5 or phospho-p38 of PLN CD8 cells were similar to those of spleen CD8 cells (Figures 5,6). Because cell manipulation altered CD44 staining (data not shown), we could not analyze CD44 subsets separately. In line with a recent report showing that activated STAT-5 is degraded in the nucleus,47 we observed that the intracellular levels of total STAT-5 measured by flow cytometry were lower in BM CD8 cells than in either spleen or PLN CD8 cells (total STAT-5 mean fluorescence intensity: spleen 33.1 ± 6.3; PLN 31.6 ± 5.5; BM 23.8 ± 2.0; n = 9; BM versus spleen P ≤ .01). As a negative control, we measured total Bcl-2 levels in parallel and did not find a statistically significant difference between CD8 cells from BM, spleen, and PLN (data not shown). A higher level of phospho-STAT-5 was found in BM CD8 cells compared with spleen CD8 cells also by Western blot analysis (Figure S4). Our results suggest that the BM environment is highly stimulatory for CD8 cells, and both STAT-5 and p38 are locally activated to a further extent than they are in the spleen environment.
Phospho-STAT-5 expression by spleen, PLN, and BM CD8 cells. Untreated B6 mice were killed and, within 10 minutes, single-cell suspensions from spleen, PLN, and BM were fixed in 1.6% formaldehyde phosphate-buffered saline. After permeabilization with 80% methanol and rehydration with 0.5% bovine serum albumin phosphate-buffered saline, cells were stained with anti-TCR PE, anti-CD8 Alexa 488, plus either control mAb or anti-STAT-5(Y694) mAb, both conjugated with Alexa 647. After gating on TCR+CD8+ cells, the mean fluorescence intensity of phospho-STAT-5 mAb and control mAb were determined on spleen, PLN, and BM samples. (A) Phospho-STAT-5 staining profiles. Representative staining profiles of spleen and BM samples. As a control, spleen cells were incubated for 15 minutes at 37°C with either medium or IL-15 at 25 ng/mL and TCR+CD8+ cells were analyzed. The scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value, 55). The numbers represent the mean fluorescence intensity of phospho-STAT-5 mAb (–) and control mAb (–). (B) Phospho-STAT-5 mean fluorescence intensity. Phospho-STAT-5 mean fluorescence intensity values of spleen, PLN, and BM CD8 cells from individual mice and averages are shown in the left panel. In vitro stimulated spleen CD8 cells from individual experiments and averages are shown in the right panel. Average values are shown by the horizontal bar. The panels summarize the results obtained in 7 independent experiments.
Phospho-STAT-5 expression by spleen, PLN, and BM CD8 cells. Untreated B6 mice were killed and, within 10 minutes, single-cell suspensions from spleen, PLN, and BM were fixed in 1.6% formaldehyde phosphate-buffered saline. After permeabilization with 80% methanol and rehydration with 0.5% bovine serum albumin phosphate-buffered saline, cells were stained with anti-TCR PE, anti-CD8 Alexa 488, plus either control mAb or anti-STAT-5(Y694) mAb, both conjugated with Alexa 647. After gating on TCR+CD8+ cells, the mean fluorescence intensity of phospho-STAT-5 mAb and control mAb were determined on spleen, PLN, and BM samples. (A) Phospho-STAT-5 staining profiles. Representative staining profiles of spleen and BM samples. As a control, spleen cells were incubated for 15 minutes at 37°C with either medium or IL-15 at 25 ng/mL and TCR+CD8+ cells were analyzed. The scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value, 55). The numbers represent the mean fluorescence intensity of phospho-STAT-5 mAb (–) and control mAb (–). (B) Phospho-STAT-5 mean fluorescence intensity. Phospho-STAT-5 mean fluorescence intensity values of spleen, PLN, and BM CD8 cells from individual mice and averages are shown in the left panel. In vitro stimulated spleen CD8 cells from individual experiments and averages are shown in the right panel. Average values are shown by the horizontal bar. The panels summarize the results obtained in 7 independent experiments.
Phospho-p38 MAPK expression by spleen, PLN, and BM CD8 cells. Membrane and intracellular staining of spleen, PLN, and BM CD8 cells were performed as in Figure 5, except that anti-p38 (T180/Y182) mAb was used. (A) Phospho-p38 staining profiles. Representative staining profiles of spleen and BM samples. As a control, spleen cells were incubated for 15 minutes at 37°C with either medium or LPS at 1 μg/mL and CD11b+ cells were analyzed. The scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value 60 for CD8 and 30 for CD11b panels). The numbers represent the mean fluorescence intensity of phospho-p38 mAb (–) and control mAb (–). (B) Phospho-p38 mean fluorescence intensity. Phospho-p38 mean fluorescence intensity values of spleen, PLN, and BM CD8 cells from individual mice and averages are shown in the left panel. In vitro stimulated spleen CD11b cells from individual experiments and averages are shown in the right panel. Average values are shown by the horizontal bar. The panels summarize the results obtained in 6 independent experiments.
Phospho-p38 MAPK expression by spleen, PLN, and BM CD8 cells. Membrane and intracellular staining of spleen, PLN, and BM CD8 cells were performed as in Figure 5, except that anti-p38 (T180/Y182) mAb was used. (A) Phospho-p38 staining profiles. Representative staining profiles of spleen and BM samples. As a control, spleen cells were incubated for 15 minutes at 37°C with either medium or LPS at 1 μg/mL and CD11b+ cells were analyzed. The scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value 60 for CD8 and 30 for CD11b panels). The numbers represent the mean fluorescence intensity of phospho-p38 mAb (–) and control mAb (–). (B) Phospho-p38 mean fluorescence intensity. Phospho-p38 mean fluorescence intensity values of spleen, PLN, and BM CD8 cells from individual mice and averages are shown in the left panel. In vitro stimulated spleen CD11b cells from individual experiments and averages are shown in the right panel. Average values are shown by the horizontal bar. The panels summarize the results obtained in 6 independent experiments.
In vivo proliferation of transferred BM and spleen CD8 cells in response to polyI:C treatment
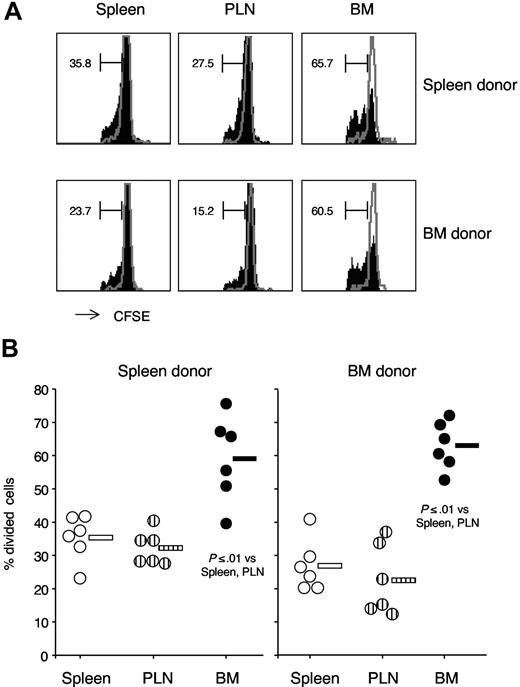
We examined the possibility that BM CD8 cells had a cell-intrinsic proliferative advantage that could be detected only in vivo. We thus compared the in vivo proliferative response to polyI:C treatment of adoptively transferred CD8 cells obtained from either BM or spleen. CD8-enriched cell preparations from either spleen or BM of 2 different CD45-congenic donors were mixed, labeled with CFSE, and injected in recipient CD45.1 B6 mice. Such settings were designed to control for the potential effects of cotransferred cells, other than CD8 cells.48 Mice were treated with polyI:C and 3 days later CFSE-labeled CD8 cells from each of the 2 donors were analyzed by flow cytometry.42 We observed that transferred CD8 cells from either BM or spleen had a similar proliferative response to polyI:C treatment, independently of the donor organ (Figure 7). Both BM- and spleen-derived CD8 cells proliferated approximately 2-times more in the BM than in either spleen or PLN of polyI:C-treated mice (Figure 7). These results suggest that, within the same organ environment, CD8 cells originally obtained from the BM have a similar capacity to proliferate in vivo compared with those from the spleen.
PolyI:C treatment–induced proliferation of adoptively transferred spleen and BM CD8 cells in spleen, PLN, and BM of recipient mice. CD8-enriched cell preparations were obtained by negative magnetic selection from either spleen of CD45.1 B6 mice or BM of B6 mice. In some experiments, the reverse combination of spleen and BM donor mice was used and results were similar. In each experiment, CD8 cell enrichment was checked by flow cytometry and cell preparations from spleen (80%–90% CD8 cells) and BM (40%–50% CD8 cells) were mixed in a 1:1 CD8-cell ratio. After CFSE labeling, donor cells were injected intravenously in CD45.1 B6 mice. The next day, mice were either injected with 150 μg polyI:C or left untreated and CFSE-labeled CD8 cell proliferation was examined after 3 days. Spleen, PLN, and BM cells were stained with TCR Alexa 647, CD8 PerCP-Cy5.5, and CD45.1 PE. CFSE-labeled TCR+CD8+ cells were examined and the percentage of divided cells was determined either within CD45.1+ or CD45.1 − cells. For each experiment, the marker was set based on CFSE intensity of cells from an untreated mouse. (A) CFSE labeling profile. Representative CFSE profiles are shown for either CD45.1+ (spleen donor) or CD45.1 − (BM donor) cells, obtained from spleen, PLN and BM of a polyI:C-treated mouse. Control-cell profiles from an untreated mouse are shown with gray lines. The numbers represent the percentages of divided cells in the marked region. The scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value 50, except for recipient BM panels, which have maximum value 20). (B) Percentages of divided cells within CFSE-labeled cells. Average values are shown by the horizontal bar. The panels summarize the results obtained in 3 independent experiments.
PolyI:C treatment–induced proliferation of adoptively transferred spleen and BM CD8 cells in spleen, PLN, and BM of recipient mice. CD8-enriched cell preparations were obtained by negative magnetic selection from either spleen of CD45.1 B6 mice or BM of B6 mice. In some experiments, the reverse combination of spleen and BM donor mice was used and results were similar. In each experiment, CD8 cell enrichment was checked by flow cytometry and cell preparations from spleen (80%–90% CD8 cells) and BM (40%–50% CD8 cells) were mixed in a 1:1 CD8-cell ratio. After CFSE labeling, donor cells were injected intravenously in CD45.1 B6 mice. The next day, mice were either injected with 150 μg polyI:C or left untreated and CFSE-labeled CD8 cell proliferation was examined after 3 days. Spleen, PLN, and BM cells were stained with TCR Alexa 647, CD8 PerCP-Cy5.5, and CD45.1 PE. CFSE-labeled TCR+CD8+ cells were examined and the percentage of divided cells was determined either within CD45.1+ or CD45.1 − cells. For each experiment, the marker was set based on CFSE intensity of cells from an untreated mouse. (A) CFSE labeling profile. Representative CFSE profiles are shown for either CD45.1+ (spleen donor) or CD45.1 − (BM donor) cells, obtained from spleen, PLN and BM of a polyI:C-treated mouse. Control-cell profiles from an untreated mouse are shown with gray lines. The numbers represent the percentages of divided cells in the marked region. The scale on x-axis is logarithmic, in arbitrary units, and that on y-axis is linear (maximum value 50, except for recipient BM panels, which have maximum value 20). (B) Percentages of divided cells within CFSE-labeled cells. Average values are shown by the horizontal bar. The panels summarize the results obtained in 3 independent experiments.
Discussion
In this report, we show that both naive and memory CD8 cells have a 2- to 4-fold higher turnover in the BM than in either spleen or LN. When the numbers of cells are taken into account, it becomes clear that the BM gives an essential contribution to CD8-cell proliferation.35,36 We previously reported that the BM contains approximately 40% of the total BrdU+ CD44high CD8 cells present in spleen, LN, and BM taken all together.35 A similar calculation for CD44int/low CD8 cells shows that approximately 20% of the total BrdU+ cells are contained in the BM (data not shown).
By measuring ex vivo the proportion of cycling cells within BM, spleen, or LN CD8 cells from untreated mice, we previously demonstrated that the difference between these organs is mostly attributable to the local rate of proliferation.35 This is in agreement with other studies showing ex vivo cell-cycle analysis on LCMV-specific CD8 cells.42 Our present findings suggest that BM CD8 cells are not committed to an enhanced proliferation or a better survival, but rather are stimulated to proliferate in the organ. After isolation from the organ environment, BM CD8 cells transiently retain some traits of such stimulation, such as a higher level of phospho-STAT-5 and phospho-p38 and a lower CD127 expression compared with corresponding spleen and LN cells. Still, when stimulated for a few days in vitro, purified CD8 cells obtained from the BM did not show an enhanced proliferative response to IL-7, IL-15, and IL-21. Notably, we confirmed these findings with antigen-primed TCR-transgenic CD8 cells (all stably CD44high)35 from BM and spleen, making it unlikely that differences in TCR clonality, antigen priming, and CD44 phenotype contributed to our results. After isolation from the organ and adoptive transfer, BM- and spleen-derived CD8 cells display a similar in vivo proliferative response to polyI:C treatment. Both cell populations divide approximately 2 times more in the BM than in spleen and LN of the recipient. These results further support the concept that BM CD8 cells do not have by themselves a higher proliferative capacity than spleen CD8 cells, but rather behave accordingly to the organ environment in which they are located.
We previously showed that antigen-specific memory CD8 cells from the BM produced IFN-γ after a 40-hour in vitro stimulation, whereas the corresponding spleen cells required a 6-day stimulation. We also documented that freshly isolated BM CD8 cells contain a greater percentage of cells with high forward scatter than the corresponding spleen CD8 cells, possibly reflecting enrichment in blast CD8 cells in the BM. This occurs either within CD44high and CD44int/low CD8 cells, reinforcing the concept that CD8 cells from the BM are in a different activation state than spleen CD8 cells.49
It is possible that the difference in the local rate of proliferation between BM and spleen CD8 cells is amplified by either homing or survival. As regards homing, we and others40,50 have previously shown that adoptively transferred memory T cells preferentially seed the BM, but it is not known whether endogenous memory CD8 cells that migrate to the BM are enriched in cells that have recently proliferated in other organs. It is also possible that, after cell division in the BM, daughter CD8 cells preferentially exit to seed other organs, and/or to leave space in the BM for other incoming CD8 cells. Regarding survival, we showed that BM and spleen CD8 cells have a very similar capacity to survive in vitro. Further studies are required to investigate whether the BM environment supports CD8-cell survival better than the spleen.
We observed that the expression of Bcl-2, a molecule controlling CD8-cell survival,43 was similar between BM and spleen CD44high CD8 cells, as well as between BM and spleen CD44int/low CD8 cells. In contrast, in both organs, there was a significant difference between CD44high and CD44int/low CD8 cells, supporting the concept that Bcl-2 levels increase with the transition from virgin to memory CD8 cells20,37 and do not change in different organs. The possibility is still open that other members of the Bcl-2 family of proteins and other molecules involved in the control of CD8-cell survival are differently modulated in the BM and in the spleen.
Considering our previous results on CD127 expression by BM, spleen, and LN antigen-specific CD8 cells,35 we analyzed the expression of CD127 by CD44 CD8-cell subsets from the 3 organs. CD127 is a putative memory differentiation marker, which is first downregulated and subsequently upregulated by CD8 cells during immune responses.38,39 Cell-transfer studies have suggested that the early CD127+ cells are the precursors of memory cells.38 We previously demonstrated that the kinetics of expression of CD127 by antigen-primed CD8 cells is similar in BM, spleen, and LN, but the percentage of CD127+ cells is constantly lower in the BM.35 We show here that both CD44high and CD44int/low CD8 cells have a reduced percentage of CD127+ cells in the BM compared with corresponding cells in either spleen or LN. Thus, we confirm our initial hypothesis that CD127 expression is not only regulated during CD8-cell differentiation but also modulated by the organ environment.35 Notably, we observed here that, within the BM, the proportion of CD127+ cells was lower among CD44high than CD44int/low CD8 cells, possibly reflecting a preferential stimulation of memory CD8 cells in the local environment. IL-15, IL-7, and other stimuli likely play a role in downregulating membrane CD127 in BM CD8 cells.
To gain a better insight in the signaling pathways activated in CD8 cells by cytokines and other molecules present in the BM, we analyzed ex vivo the intracellular levels of phosphorylated STAT-5 and p38 MAPK.
STAT-5 is a downstream effector of membrane signaling initiated by IL-2 family cytokines, including IL-7, IL-15, and IL-21. STAT-5 is activated by JAK1 and JAK3, which are associated with IL-2Rβ and γ chain and directly phosphorylate STAT-5 Y694 residue. Phospho-STAT-5 proteins dimerize and translocate to the nucleus, where they regulate the transcription of several genes involved in cytokine-induced activation and proliferation.22,26 We found that the levels of phospho-STAT-5 were significantly higher and those of total STAT-5 significantly lower in BM compared with spleen CD8 cells. The decrease of total STAT-5 in BM CD8 cells might be attributable to activation-induced degradation.47 When the levels of total STAT-5 are taken into account, the difference in phospho-STAT-5 expression between BM and spleen CD8 cells is even more pronounced. These results suggest that IL-2 family cytokines (most likely IL-15 and IL-7) stimulate CD8 cells in the BM.
We also found a significant increase in the intracellular levels of phospho-p38 in BM CD8 cells compared with spleen CD8 cells. The p38 MAPK molecules are activated by phosphorylation in response to stress, LPS, and inflammatory cytokines such as TNFα and other members of the TNF family.30,31,34,51 Several members of the TNF family are involved in the control of CD8- cell survival and proliferation.30,31 In particular, an important role in antigen-independent CD8-cell survival has been ascribed to 4-1BB/4-1BBL interaction, and it has been shown that spleen and BM CD8 cells express 4-1BB in response to IL-15 by a mechanism dependent on p38 activation.29 Considering also the essential role played by p38 in integrating TCR and CD28 signaling, as well as in driving Th1 differentiation and IFN-γ production,52 it is clear that several pathways converge on p38, making it a crucial molecule of T-cell activation.34 Interestingly, p38 has been involved also in the apoptotic pathway in CD8 cells,53 and activation of p38 is induced by TGF-β, an inhibitory molecule for T-cell proliferation and survival.34,54 Thus, several candidates might be involved in the p38 activation observed in BM CD8 cells, including TNF family members, IL-15, IL-7, TGF-β, VCAM-1, type I IFN, and others.51,55
Taken together, our findings on phospho-STAT-5 and phospho-p38 suggest that BM CD8 cells integrate several signals received in the organ. Further studies are required to define the contribution of different extracellular factors, as well as to identify other intracellular molecules involved in the signaling pathways.
In conclusion, we reported here that BM CD8 cells are stimulated in the BM, so that at any given time a fraction of them is driven into proliferation. This is not a peculiarity of memory CD8 cells, because it occurs also in the case of CD8 cells with low expression of either CD44 or CD122. The slow turnover of naive phenotype CD8 cells in the BM might contribute to the self-renewal of these cells occurring at some degree under normal conditions. Still, in the absence of a thymus, the lifespan of naive T cells is limited.56 Our findings suggest that BM CD8 cells are not committed to self-renewal, but rather are stimulated in the organ. It is tempting to speculate that, among the heterogeneous mixture of CD8 cells recirculating in the periphery, those having the ability to migrate to the BM will gain an advantage over other cells, because of their access to proliferative signals in the BM “niches.” Such CD8 cells will expand, at the expenses of other rival CD8 cells. By this mechanism, the BM will imprint a mark on the composition of the recirculating CD8-cell pool.
By increasing the knowledge on the peculiarities of BM CD8 cells, our results are relevant for the current therapies of hematologic malignancies based on T-cell–replete BMT.57 Moreover, our present findings together with our previous results on long-term antigen-specific memory35,40,49 support the notion that the BM can be an excellent source of effector T cells for adoptive immunotherapy for hematologic malignancies and solid cancers.36,58
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Bellone for OT-I mice; R. Manz, G. Matarese, A. Kettner for reading the manuscript; the Immunology group at the IGB-IBP (particularly G. Del Pozzo and P. G. De Berardinis) for discussion; and the Santoni laboratory (particularly G. Bernardini, G. Sciumè, A. Gismondi, H. Stabile) for discussion and help with Western blotting and adoptive transfer experiments. A special thank you goes to P. Matzinger for her generous intellectual support.
This work was supported by grants from EU (Project QLK2-CT-2002-0062, EPI-PEP-VAC), Italian Government (FIRB 2001 Project RBNE01RB9B) and the Italian Association for Cancer Research (AIRC).
Authorship
G.C., A.S., J.G., and F.D. designed research. G.C. and F.D. wrote the paper. G.C., E.P., and L.P. performed research, collected data, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesca Di Rosa, CNR Staff Scientist, c/o University of Rome “La Sapienza,” Department of Experimental Medicine, viale Regina Elena 324, 00161 Rome; e-mail: dirosa@igb.cnr.it or francesca.dirosa@uniroma1.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal