Abstract
Angiotensin II (Ang-II) exerts inflammatory activity and is involved in different cardiovascular disorders. This study has evaluated the involvement of tumor necrosis factor alpha (TNFα) in the leukocyte accumulation elicited by Ang-II. Ang-II (1 nM intraperitoneally in rats) induced TNFα release at 1 hour followed by neutrophil and mononuclear cell recruitment. The administration of an antirat TNFα antiserum had no effect on Ang-IIinduced neutrophil accumulation but inhibited the infiltration of mononuclear cells and reduced CC chemokine content in the peritoneal exudate. Pretreatment with either an anti-TNFα or an anti-IL-4 antiserum decreased Ang-II-induced arteriolar mononuclear leukocyte adhesion by 68% and 60%, respectively, in the rat mesenteric microcirculation. While no expression of TNFα was found in the postcapillary venules of Ang-II-injected animals, this cytokine was clearly up-regulated in the arterioles. Stimulation of human umbilical arterial endothelial cells (HUAECs) or isolated human mononuclear cells with 1 μM Ang-II caused increased TNFα mRNA expression and protein. Neutralization of TNFα activity reduced Ang-II-induced MCP-1, MCP-3, and RANTES release from HUAECs and MIP-1α from blood cells. In conclusion, the selective mononuclear leukocyte adhesion to Ang-II-stimulated arterioles is largely mediated by TNFα in cooperation with constitutive IL-4. Therefore, neutralization of TNFα activity may help to prevent mononuclear cell infiltration and the progression of the atherogenic process.
Introduction
Atherosclerosis is the major cause of myocardial infarction, stroke, and peripheral vascular disease, which accounts for nearly half of all mortality in developed countries.1 Adhesive interactions between leukocytes and arterial endothelium precede leukocyte infiltration to the subendothelial space in pathological events such as atherosclerosis.1,2 The migration of leukocytes from the blood to sites of extravascular injury is mediated through a sequential cascade of leukocyte-endothelial cell adhesive interactions that involve an array of cell adhesion molecules (CAMs) present on leukocytes and endothelial cells.3 Interestingly, whereas leukocyte-endothelial cell interactions in postcapillary venules are induced by a wide range of stimuli, leukocyte interactions with the arterial endothelium are only induced by certain risk factors for atherosclerosis such as cytokines like interleukin-1β (IL-1β) or tumor necrosis factor alpha (TNFα), oxidized low density lipoproteins (LDL), cigarette smoke, or angiotensin-II (Ang-II).4–8
Activation of the renin-angiotensin system has been demonstrated in myocardial ischemia, acute myocardial infarction, coronary occlusion, and reperfusion models as well as in chronic left ventricular dysfunction after myocardial infarction.9–11 Ang-II, the main effector peptide of the renin-angiotensin system, is implicated in atherogenesis beyond its hemodynamic effects.12 We demonstrated that 4 hours of exposure to Ang-II in vivo caused arteriolar leukocyte adhesion in the rat mesenteric microcirculation through interaction with its AT1 receptor subtype,8 and this effect was not observed under acute (1-hour) stimulation with this peptide hormone.13 Furthermore, mononuclear cells were found to be the primary cells attached to the arteriolar endothelium whereas the leukocytes interacting with the venular endothelium at the same time were predominantly neutrophils. Despite these findings, the same CAMs were expressed in both the arteriolar and venular endothelia in response to Ang-II,8 suggesting that other mechanisms were responsible for the differential cellular distribution within the microcirculation.
Therefore, this differential effect displayed by Ang-II may be due to different activation mechanisms. Thus, in postcapillary venules, the early response of neutrophil accumulation is primarily mediated by the direct effect of Ang-II on endothelial cells via the expression of P selectin and the rapid release of CXC chemokines.13,14 In arterioles, leukocyte adhesion probably requires the additional release of other inflammatory mediators. With regard to this, there is indirect evidence that TNFα might be involved in the proinflammatory activity elicited by Ang-II, because administration of an Ang-II AT1 receptor antagonist, valsartan, inhibited the expression of this cytokine in a murine model of arterial injury.15
On the other hand, another interesting cytokine is IL-4. IL-4 increases P-selectin expression through a transcriptionally dependent mechanism with delayed kinetics that are compared with the action of other cytokines such as TNFα.16 In addition, IL-4 can cause the adhesion of eosinophils and mononuclear cells to the endothelium but not neutrophils.17,18 It can also provoke the release of different CC chemokines such as MCP-1 and MCP-4, resulting in the selective accumulation of mononuclear cells.19,20 Furthermore, the combination of TNFα with IL-4 results in a selective and synergistic increase in VCAM-1 expression that accounts for the increased adhesion of lymphocytes.21 Ang-II causes selective mononuclear leukocyte adhesion to the arteriolar endothelium with delayed kinetics compared with that observed in postcapillary venules.8
Therefore, in the present study we have investigated in vivo whether TNFα, IL-4, or both are involved in induced leukocyte infiltration and the consequences of TNFα or IL-4 neutralization on the arteriolar mononuclear leukocyte adhesion induced by Ang-II. Furthermore, in vitro, we have also tested whether this peptide hormone can promote the synthesis and release of TNFα from human umbilical arterial endothelial cells (HUAECs) as well as from human peripheral blood mononuclear cells (HPBMCs). Finally, the possible generation of IL-4 from HPBMCs stimulated with Ang-II was also investigated.
Materials and methods
Leukocyte migration into the peritoneal cavity
All of these studies were approved by the Institutional Ethics Committee, Faculty of Medicine, University of Valencia. Blood was obtained from healthy human donors after written informed consent was given by all participants in accordance with the Declaration of Helsinki. Male Sprague-Dawley rats weighing 200 to 250 g were sedated with ether and injected intraperitoneally with 5 mL PBS or 1 nM Ang-II. After 1, 4, 8, and 24 hours of the injection, animals were killed by an anesthetic overdose and the peritoneal cavity was first lavaged with 5 mL PBS and then with 30 mL heparinized PBS (10 U/mL). The 5 and 30 mL lavages were centrifuged separately to obtain the cell pellets that were then combined for total leukocyte counts in a hemocytometer and differential cell analysis of 500 cells per slide on cytospins stained with May-Grünwald and Giemsa stains. The results are expressed as the number of neutrophils and mononuclear leukocytes recovered from each cavity. The supernatant from the first (5 mL) lavage, after addition of carrier protein (0.5% bovine serum albumin [BSA]) and storage at −20°C, was used for determination of TNFα and IL-4 concentrations. Rat TNFα and IL-4 levels were determined by conventional sandwich enzyme-linked immunosorbent assays (ELISAs). Results are expressed as pM cytokine in the supernatant from the 5 mL lavage.
In another set of experiments, rats were administered 15 minutes prior to Ang-II injection with 1 mL per rat of an antirat TNFα antiserum intravenously, and its effect on Ang-II-induced neutrophil and mononuclear cell recruitment as well as CINC/KC, MIP-2, MCP-1, RANTES, and MIP-1α release was evaluated 1, 4, and 8 hours after the intraperitoneal injection of the peptide. Rat chemokine levels were determined by conventional sandwich ELISAs. Results are expressed as pM chemokine in the supernatant from the 5 mL lavage. The anti-TNFα polyclonal antiserum was raised in sheep as previously described,22 and it has been found to reduce neutrophil accumulation by 90% in a rat model of ischemia/reperfusion-induced injury.23
In an additional group of experiments, after 1 hour of PBS or Ang-II intraperitoneal administration in animals untreated or treated with the anti-rat TNFα antiserum (1 mL per rat intravenously), a 100 mg sample of the mesenteric tissue was collected. Chemokine mRNA expression was determined by real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) as previously described.14 TaqMan predevelopment assay reagents were used to determine chemokine mRNA and TaqMan RT reagents to generate cDNA. Glyceraldehyide 3-phosphate dehydrogenase (GAPDH) was used as endogenous control gene.
Intravital microscopy
The details of the experimental preparation have been described previously.8 Briefly, male Sprague-Dawley rats weighing 200 to 250 g were anesthetized with sodium pentobarbital (Sigma Química, Madrid, Spain; 65 mg/kg intraperitoneally), and the trachea, right jugular vein, and carotid artery were cannulated. After performing a midline abdominal incision, a segment of the midjejunum was exteriorized and placed over an optically clear viewing pedestal maintained at 37°C, which facilitated tissue transillumination. The exposed mesentery was continuously superfused with warmed bicarbonate buffer saline ([BBS] pH 7.4) equilibrated with 5% CO2 in nitrogen. An orthostatic microscope (Nikon Optiphot-2, SMZ1; Nikon, Badhoevedor, the Netherlands) equipped with a 20× objective lens (Nikon SLDW) and 10× eyepiece permitted tissue visualization. A video camera (Sony SSC-C350P; Sony, Koeln, Germany) mounted on the microscope projected images onto a color monitor (Sony Trinitron PVM-14N2E), and these images were captured on a videotape (Sony SVT-S3000P) for playback analysis (final magnification of the video screen, × 1300). Arterioles (20 to 30 μm diameter) and single unbranched mesenteric venules (25 to 40 μm diameter) were selected and the diameters measured online using a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX). Centerline red blood cell velocity (Vrbc) was also measured online with an optical Doppler velocimeter (Microcirculation Research Institute). Venular blood flow and wall shear rate (γ) were calculated as previously described.24 The number of rolling, adherent, and emigrated leukocytes was determined offline during playback analysis of videotaped images.
Experimental protocol
Animals were sedated and intraperitoneally injected with 5 mL PBS or Ang-II (1 nM), as described above. After 4 hours the mesentery was exposed in preparation for measurements of venular leukocyte rolling flux, velocity, adhesion, emigration, arteriolar leukocyte adhesion, mean arterial blood pressure (MABP) and venular and arteriolar Vrbc, shear rate, and diameter over a 5-minute period.
To investigate the involvement of TNFα or IL-4, rats were pretreated 15 minutes prior to Ang-II injection with an antirat TNFα antiserum, or an antirat IL-4 antiserum (1 mL intravenously), or with a combination of both antisera. To determine the effect of these sera on levels of circulating leukocytes, blood samples were taken from the rats when all the measurements were performed. The anti-IL-4 antiserum was raised in sheep following a similar procedure to that used for the anti-TNFα antiserum.22
Immunohistochemistry
After the completion of the intravital microscopy measurements, the mesentery was isolated, fixed in 4% paraformaldehyde, dehydrated using graded acetone washes at 4°C, and embedded in paraffin wax for immunolocalization of TNFα and IL-4 using a modified avidin and biotin immunoperoxidase technique as previously described.25 Tissue sections (4 μm thick) were incubated for 24 hours with 100 μg/mL of an antibody against rat TNFα or rat IL-4. Positive staining was defined as an arteriole or venule displaying brown reaction product.
Cell culture
HUAECs were isolated by collagenase treatment26 and maintained in human endothelial cells' specific medium EBM-2 supplemented with EGM-2 and 10% FCS. Cells up to passage 2 were grown to confluence on 24-well culture plates. Prior to every experiment, cells were incubated for 16 hours in medium containing 1% FCS and then returned to the 10% FCS medium at the start of all experimental incubations.
Cells were stimulated with 1 μM Ang-II for 1 and 4 hours. Selective antagonists of AT1 (losartan, 100 μM), or AT2 (PD123319, 100 μM) receptors, or a combination of both were added to some wells 1 hour prior to Ang-II (1 μM) stimulation. At the end of the experiment, cell free supernatants were stored at −20°C for TNFα ELISA.
In another set of experiments, we investigated the effect of TNFα neutralization on Ang-II-induced chemokine release in HUAECs. Cells were stimulated with medium or 1 μM Ang-II for 4 and 24 hours. Anti-TNFα monoclonal antibody (mAb) (2 μg/mL) was added to some wells 1 hour prior to Ang-II (1 μM) stimulation. At the end of the experiment, cell free supernatants were stored at −20°C for ELISA. Human chemokines (IL-8, MCP-1, MCP-3, and RANTES) were measured by conventional sandwich ELISA, and the results are expressed as pM chemokine.
Effect of Ang-II on TNFα and IL-4 generation in HPBMCs
HPBMCs were obtained from buffy coats of healthy donors by Ficoll Hypaque density gradient centrifugation. Mononuclear leukocytes, 5 × 106 cells per tube, were stimulated with 1 μM Ang-II for 1, 4, and 24 hours. Selective antagonist of AT1 receptor (losartan, 100 μM) was added to some wells 1 hour prior to Ang-II (1 μM) stimulation. At the end of the experiment, cell free supernatants were stored at −20°C for TNFα and IL-4 ELISA.
Quantitative RT-PCR
TNFα mRNA was determined by real-time quantitative RT-PCR. HUAECs and HPBMCs were incubated with medium or 1 μM Ang-II for 1, 4, and 24 hours in the presence or absence of losartan (100 μM), and total RNA was extracted using TRIzol. Quantitative data of relative gene expression were determined by the comparative Ct method (ΔΔCt) as described by the manufacturer (PE-ABI PRISM 7700 Sequence Detection System, Foster City, CA) and previously reported.27 GAPDH was used as endogenous control gene. TaqMan predevelopment assay reagents were used to determine TNFα or IL-4 mRNA and TaqMan RT reagents to generate cDNA.
Effect of anti-TNFα on Ang-II-induced MIP-1α and IL-8 release in human whole blood
Human whole blood (10 U/mL heparin from healthy volunteers) was incubated with saline or 1 μM Ang-II for 4 hours. Anti-TNFα mAb (2 μg/mL) was added to some preparations 1 hour prior to Ang-II (1 μM) stimulation. Before centrifugation to obtain plasma, further heparin was added (to 100 U/mL) to promote the release of any chemokines bound to erythrocytes. Plasma samples were stored at −80°C for MIP-1α and IL-8 ELISA.
Effect of TNFα and IL-4 on HPBMC adhesion to HUAECs under flow conditions
Previous studies have shown that the costimulation with TNFα and IL-4 causes a synergistic increase in VCAM-1 expression in HUVECs.21 Following a similar protocol, HUAECs were stimulated with medium or TNFα (0.2 ng/mL, equivalent to 10 pM), or with IL-4 (20 ng/mL, equivalent to 100 U/mL), or with a combination of both cytokines for 24 hours. After this time period, the incubation buffer was removed, the Glycotech flow chamber was assembled and placed onto an inverted microscope stage, and freshly isolated HPBMCs (1 × 106/mL) were perfused across endothelial monolayers. Accumulation was determined after 5 minutes at 1 dyn/cm2 in all experiments. After 5 minutes of perfusion, the inlet line was transferred to HBSS to prevent the binding of new HPBMCs, and shear was maintained at 1 dyn/cm2. Arrested cells on the surface of the endothelium were visualized and recorded (×20 objective, ×10 eyepiece) using phase contrast microscopy.
Statistical analysis
All values are reported as mean ± SEM. Data within groups were compared using an analysis of variance (1-way ANOVA) with a Newman-Keuls post hoc correction for multiple comparisons. Statistical significance was set at P less than .05.
Materials
Sodium pentobarbital, Ang-II, neutralizing antihuman TNFα mAb (clone 28401.111, mouse IgG1), and PD123319 were purchased from Sigma Química. EBM-2 medium supplemented with EGM-2 was from Innogenetics (Barcelona, Spain). Ficoll Hypaque density gradient was from Pharmacia Biotech (Uppsala, Sweden). Human TNFα, IL-4, IL-8, MCP-1, MCP-3, RANTES, MIP-1α, rat CINC/KC, MIP-2, MCP-1, RANTES, MIP-1α, and antibodies to rat TNFα, IL-4, CINC/KC, MIP-2, MCP-1, RANTES, and MIP-1α were from PeproTech (London, United Kingdom). The antibody pair for human TNFα, IL-4, IL-8, MCP-1, MCP-3, and RANTES ELISA was from R&D Systems (Madrid, Spain); neutravidin-horseradish peroxidase was from Perbio Science (Cheshire, United Kingdom); K-Blue substrate was from Neogen (Lexington, KY). TRIzol was from Life Technologies (Paisley, United Kingdom); TaqMan predevelopment and RT reagents were from PE Biosystems (Morrisville, NC). Losartan was kindly donated by Merck Sharp & Dohme (Madrid, Spain).
Results
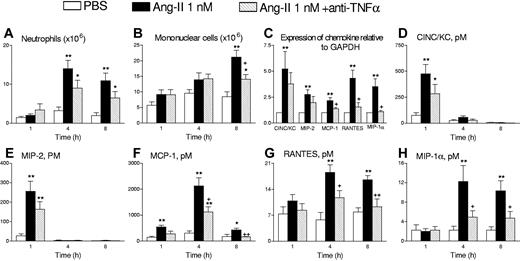
Intraperitoneal administration of 5 mL of 1 nM Ang-II in rats induced a significant increase in TNFα levels 1 hour after its intraperitoneal injection (Figure 1A), before significant leukocyte accumulation was detected (Figure 1B,C). We found no increases in IL-4 content. This effect was followed by neutrophil and mononuclear leukocyte recruitment, which was maximal at 4 and 8 hours, respectively (Figure 1B,C). We have previously demonstrated14 that Ang-II causes neutrophil accumulation into the peritoneal cavity of the rat, which was dependent on the rapid release of 2 CXC chemokines CINC/KC and MIP-2. In the present study, when animals were pretreated with a neutralizing antiserum against rat TNFα, neither the neutrophil infiltration into the peritoneal cavity (Figure 2A) nor CINC/KC and MIP-2 mRNA and protein expression was significantly affected (Figure 2C-E). In another study, we also showed that Ang-II was capable of inducing mononuclear cell infiltration into the peritoneal cavity of the rat, and this effect was due to the generation and release of different CC chemokines such as MCP-1, RANTES, and MIP-1α.28 Interestingly, pretreatment with an antiserum against rat TNFα resulted in a significant reduction of mononuclear leukocyte numbers 8 hours after Ang-II intraperitoneal injection by 55% (Figure 2B). In addition, as illustrated in Figure 2, the TNFα antiserum significantly decreased the generation and release of these 3 CC chemokines at 4 and 8 hours after Ang-II intraperitoneal administration (Figure 2C,F-H). The peritoneal content of MCP-1, RANTES, and MIP-1α after 4 hours of Ang-II exposure was reduced by 45%, 54%, and 73%, respectively, in animals treated with the antiserum.
Effect of Ang-II on TNFα release and leukocyte accumulation into the rat peripheral cavity. Time course of Ang-II-induced TNFα release (A), neutrophil polymorphonuclear leukocytes [PMNs] (B), and mononuclear leukocyte accumulation (C). Rats were intraperitoneally injected with 5 mL PBS or 1 nM Ang-II. Results are mean (± SEM) for 5 animals per group: *P < .05 or **P < .01 relative to values in the PBS-injected group.
Effect of Ang-II on TNFα release and leukocyte accumulation into the rat peripheral cavity. Time course of Ang-II-induced TNFα release (A), neutrophil polymorphonuclear leukocytes [PMNs] (B), and mononuclear leukocyte accumulation (C). Rats were intraperitoneally injected with 5 mL PBS or 1 nM Ang-II. Results are mean (± SEM) for 5 animals per group: *P < .05 or **P < .01 relative to values in the PBS-injected group.
Effects of an antirat TNFα antiserum on Ang-II-induced leukocyte accumulation and chemokine mRNA expression and generation in the rat peritoneal cavity. Neutrophil accumulation (A), mononuclear leukocyte accumulation (B), chemokine mRNA expression (C), and the generation of CINC/KC (D), MIP-2 (E), MCP-1 (F), RANTES (G), and MIP-1α (H) in the rat peritoneal cavity. Cell counts and chemokine levels in the peritoneal exudate as well as chemokine mRNA expression in the mesenteric tissue were determined in the following experimental groups: untreated rats intraperitoneally injected with 5 mL PBS, untreated rats exposed to 1 nM Ang-II intraperitoneally, and Ang-II-exposed rats pretreated with the antirat TNFα antiserum (1 mL per rat intravenously) 15 minutes prior to Ang-II intraperitoneally. Results are the mean (± SEM) for 5 to 8 animals per group. *P < .05 or **P < .01 relative to values in the PBS-injected group; + P < .05 or ++ P < .01 relative to the Ang-II untreated group.
Effects of an antirat TNFα antiserum on Ang-II-induced leukocyte accumulation and chemokine mRNA expression and generation in the rat peritoneal cavity. Neutrophil accumulation (A), mononuclear leukocyte accumulation (B), chemokine mRNA expression (C), and the generation of CINC/KC (D), MIP-2 (E), MCP-1 (F), RANTES (G), and MIP-1α (H) in the rat peritoneal cavity. Cell counts and chemokine levels in the peritoneal exudate as well as chemokine mRNA expression in the mesenteric tissue were determined in the following experimental groups: untreated rats intraperitoneally injected with 5 mL PBS, untreated rats exposed to 1 nM Ang-II intraperitoneally, and Ang-II-exposed rats pretreated with the antirat TNFα antiserum (1 mL per rat intravenously) 15 minutes prior to Ang-II intraperitoneally. Results are the mean (± SEM) for 5 to 8 animals per group. *P < .05 or **P < .01 relative to values in the PBS-injected group; + P < .05 or ++ P < .01 relative to the Ang-II untreated group.
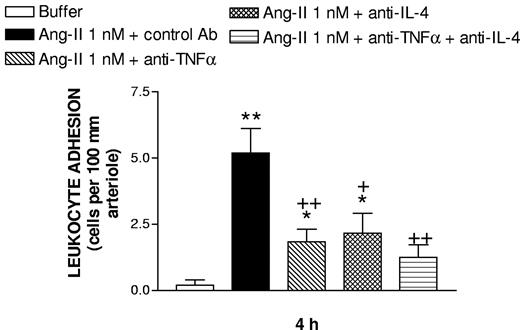
Intravital microscopy was used to examine the effect of an anti-TNFα and an anti-IL-4 antisera on Ang-II-induced leukocyte trafficking in the mesentery. Exposure to 1 nM Ang-II for 4 hours caused a significant enhancement of arteriolar leukocyte adhesion (Figure 3) without inducing changes in the number of circulating leukocytes, MABP, arteriolar diameter, or shear rate (Table 1). The enhancement of arteriolar leukocyte adhesion was inhibited by antirat TNFα, antirat IL-4, and a combination of both antisera by 68%, 60%, and 79%, respectively, but the number of circulating leukocytes, MABP, arteriolar diameter, and shear rate were unaffected by this treatment (Table 1).
Effect of antirat TNFα and antirat IL-4 antisera on subacute (4-hour) Ang-II-induced leukocyte adhesion to rat mesenteric arterioles. Rats were treated intraperitoneally with PBS (buffer; n = 5) or 1 nM Ang-II (n = 5). Some animals were pretreated with the antirat TNFα antiserum (1 mL per rat intravenously; n = 6), or the antirat IL-4 antiserum (1 mL per rat intravenously; n = 6), or a combination of both antisera (n = 4) 15 minutes before the administration of Ang-II. Results are mean (± SEM). *P < .05 or **P < .01 relative to the PBS group; + P < .05 or ++ P < .01 relative to the Ang-II group.
Effect of antirat TNFα and antirat IL-4 antisera on subacute (4-hour) Ang-II-induced leukocyte adhesion to rat mesenteric arterioles. Rats were treated intraperitoneally with PBS (buffer; n = 5) or 1 nM Ang-II (n = 5). Some animals were pretreated with the antirat TNFα antiserum (1 mL per rat intravenously; n = 6), or the antirat IL-4 antiserum (1 mL per rat intravenously; n = 6), or a combination of both antisera (n = 4) 15 minutes before the administration of Ang-II. Results are mean (± SEM). *P < .05 or **P < .01 relative to the PBS group; + P < .05 or ++ P < .01 relative to the Ang-II group.
Hemodynamic parameters and systemic leukocyte counts
| . | Treatment . | ||||
|---|---|---|---|---|---|
| PBS . | Ang-II . | Ang-II and anti-TNFα . | Ang-II and anti-IL-4 . | Ang-II and anti-TNFαand anti-IL-4 . | |
| MABP, mm Hg | 111.8 ± 2.7 | 110.2 ± 4.6 | 107.6 ± 6.2 | 112.6 ± 2.0 | 113.9 ± 2.9 |
| Arteriolar diameter | 22.4 ± 1.7 | 21.0 ± 1.6 | 21.0 ± 1.3 | 22.2 ± 1.9 | 23.4 ± 2.7 |
| Arteriolar shear rate, s−1 | 1367.4 ± 122.8 | 1234.6 ± 98.6 | 1147.8 ± 97.1 | 1158.0 ± 95.7 | 1161.7 ± 80.5 |
| Venular diameter | 28.6 ± 1.2 | 29.6 ± 1.3 | 30.7 ± 2.1 | 29.3 ± 1.6 | 31.0 ± 4.0 |
| Venular shear rate, s−1 | 527.4 ± 83.9 | 468.6 ± 71.0 | 541.6 ± 57.8 | 503.3 ± 46.8 | 562.6 ± 64.9 |
| Systemic leukocyte counts, cells/μL | 11 860.2 ± 809.2 | 10 092.3 ± 685.6 | 10 427.3 ± 494.1 | 9995.2 ± 836.7 | 11 104.0 ± 525.8 |
| . | Treatment . | ||||
|---|---|---|---|---|---|
| PBS . | Ang-II . | Ang-II and anti-TNFα . | Ang-II and anti-IL-4 . | Ang-II and anti-TNFαand anti-IL-4 . | |
| MABP, mm Hg | 111.8 ± 2.7 | 110.2 ± 4.6 | 107.6 ± 6.2 | 112.6 ± 2.0 | 113.9 ± 2.9 |
| Arteriolar diameter | 22.4 ± 1.7 | 21.0 ± 1.6 | 21.0 ± 1.3 | 22.2 ± 1.9 | 23.4 ± 2.7 |
| Arteriolar shear rate, s−1 | 1367.4 ± 122.8 | 1234.6 ± 98.6 | 1147.8 ± 97.1 | 1158.0 ± 95.7 | 1161.7 ± 80.5 |
| Venular diameter | 28.6 ± 1.2 | 29.6 ± 1.3 | 30.7 ± 2.1 | 29.3 ± 1.6 | 31.0 ± 4.0 |
| Venular shear rate, s−1 | 527.4 ± 83.9 | 468.6 ± 71.0 | 541.6 ± 57.8 | 503.3 ± 46.8 | 562.6 ± 64.9 |
| Systemic leukocyte counts, cells/μL | 11 860.2 ± 809.2 | 10 092.3 ± 685.6 | 10 427.3 ± 494.1 | 9995.2 ± 836.7 | 11 104.0 ± 525.8 |
Parameters (mean ± SEM in animals used for intravital microscopy studies) were measured 4 hours after the intraperitoneal injection of PBS (n = 5) or Ang-II (1 nM) in animals untreated (n = 5) or pretreated with anti-TNFα, anti-IL-4 (n = 6), or a combination of both antisera (n = 4).
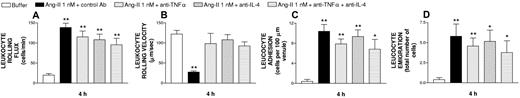
In the postcapillary venules of the same animals, 4 hours of exposure to Ang-II induced a significant increase in venular leukocyte rolling flux, adhesion, and emigration as well as a concomitant decrease in venular leukocyte rolling velocity (Figure 4) without modifying venular diameter or venular shear rate (Table 1). Surprisingly, pretreatment with the anti-rat TNFα or with the antirat IL-4 antiserum had no significant effect on Ang-II-induced leukocyte-endothelial cell interactions (Figure 4) despite the fact that there is a clear effect on leukocyte rolling velocity, which returns to basal. The administration of the antisera did not affect the venular diameter or shear rate (Table 1).
Effect of antirat TNFα and antirat IL-4 antisera on subacute (4-hour) Ang-II-induced leukocyte responses within rat mesenteric postcapillary venules. These responses were measured in the same rats as those described in the legend to Figure 3: venular responses of leukocyte rolling flux (A), leukocyte rolling velocity (B), leukocyte adhesion (C), and leukocyte emigration (D) are mean (± SEM). *P < .05 or **P < .01 relative to the PBS group.
Effect of antirat TNFα and antirat IL-4 antisera on subacute (4-hour) Ang-II-induced leukocyte responses within rat mesenteric postcapillary venules. These responses were measured in the same rats as those described in the legend to Figure 3: venular responses of leukocyte rolling flux (A), leukocyte rolling velocity (B), leukocyte adhesion (C), and leukocyte emigration (D) are mean (± SEM). *P < .05 or **P < .01 relative to the PBS group.
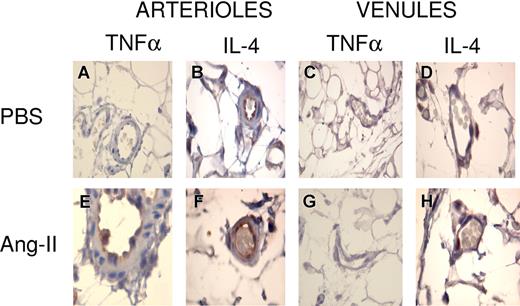
Interestingly, immunohistochemistry studies revealed no expression of TNFα in the arterioles and postcapillary venules of PBS-injected animals (Figure 5A,C). In contrast, constitutive IL-4 expression was found in the arterioles of the same animals (Figure 5B) and in the postcapillary venules although in moderated amounts (Figure 5D). Surprisingly, while marked expression of TNFα was observed in the arterioles of Ang-II-treated animals (Figure 5E), no expression was detected in the postcapillary venules (Figure 5G). In addition, in the arteriolar endothelium, after 4 hours of Ang-II exposure IL-4 was also expressed, but its expression was similar to that encountered in PBS-injected animals (Figure 5F), and it was weakly expressed in the postcapillary venules exposed to Ang-II (Figure 5H).
Representative photomicrographs showing immunolocalization of TNFα and IL-4 in rat mesenteric arterioles and postcapillary venules. Mesentery was fixed for staining with anti-TNFα (A,C,E,G) or IL-4 (B,D,F,H) antibodies 4 hours after the intraperitoneal injection of PBS (A-D) or Ang-II (1 nM; E-H). Brown reaction product indicates positive immunoperoxidase localization on the vascular endothelium. All panels are lightly counterstained with hematoxylin. Results are representative of 4 to 5 experiments with each treatment. A 100 × 1.25 oil objective lens was used. Leica IM 1000 software capture imaging (Leica Microsystems, Wetzlar, Germany) was used to obtain the images.
Representative photomicrographs showing immunolocalization of TNFα and IL-4 in rat mesenteric arterioles and postcapillary venules. Mesentery was fixed for staining with anti-TNFα (A,C,E,G) or IL-4 (B,D,F,H) antibodies 4 hours after the intraperitoneal injection of PBS (A-D) or Ang-II (1 nM; E-H). Brown reaction product indicates positive immunoperoxidase localization on the vascular endothelium. All panels are lightly counterstained with hematoxylin. Results are representative of 4 to 5 experiments with each treatment. A 100 × 1.25 oil objective lens was used. Leica IM 1000 software capture imaging (Leica Microsystems, Wetzlar, Germany) was used to obtain the images.
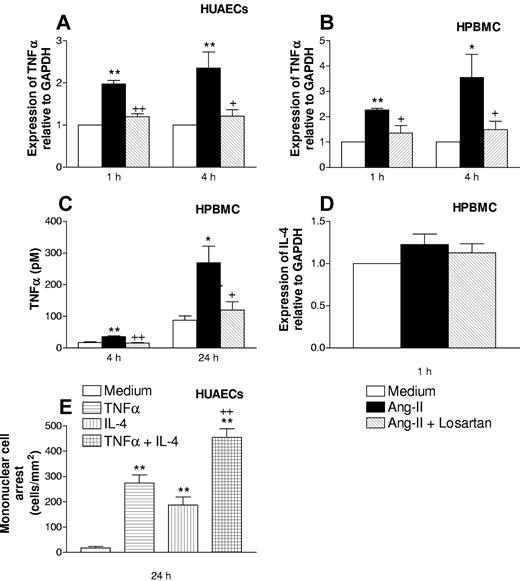
To extend these findings to humans, we first investigated whether Ang-II can induce TNFα release from arterial endothelial cells. For this purpose, we used cultures of HUAECs. TNFα mRNA was increased within 1 and 4 hours after stimulation with 1 μM Ang-II (Figure 6A). These effects appear to be mediated through interaction of Ang-II with its AT1 receptor, because losartan inhibited Ang-II-induced TNFα mRNA expression (Figure 6A). In contrast, TNFα protein in HUAEC supernatants was not detected after incubation with 1 μM Ang-II for 1, 4, and 24 hours (data not shown), indicating that the amount of TNFα released by Ang-II is below the limit of detection of the ELISA assay (3.1 pM).
Effect of Ang-II on TNFα and IL-4 in human cells. Effect of TNFα and/or IL-4 on HPBMC recruitment by HUAECs. Time course of Ang-II-induced TNFα mRNA increase in HUAECs (A), HPBMCs (B), TNFα release in HPBMCs, (C) IL-4 mRNA expression in HPBMs (D), and recruitment of HPBMCs by TNFα and/or IL-4-stimulated HUAECs (E). HUAECs and HPBMCs (5 × 106/mL) were stimulated with 1 μM Ang-II in the presence or absence of losartan (100 μM) for 1, 4, and 24 hours. Total mRNA was extracted. Relative quantification of the mRNA levels of TNFα, IL-4, and GAPDH was determined by using real-time quantitative RT-PCR by the comparative Ct method (ΔΔCt method). Columns show the fold increase in expression of TNFα and IL-4 mRNA relative to control GAPDH values, calculated as mean ± SEM of the 2-ΔΔ Ct values of 4 to 5 experiments. Protein content in the supernatant was determined by conventional sandwich ELISA and expressed as pM concentration of the cytokine of mean ± SEM from 4 to 5 experiments. *P < .05 or **P < .01 relative to values in the control group. + P < .05 relative to the Ang-II untreated group. HUAECs were incubated with medium, TNFα (0.2 ng/mL), IL-4 (20 ng/mL), or with a combination of both cytokines for 24 hours. Isolated HPBMCs (1 × 106/mL) were perfused over the HUAECs for 5 minutes at 1 dyn/cm2 and leukocyte accumulation quantified. Results are the mean (± SEM) for 4 experiments. **P < .01 relative to values in the control group; ++ P < .01 relative to the values in the TNFα or IL-4 groups.
Effect of Ang-II on TNFα and IL-4 in human cells. Effect of TNFα and/or IL-4 on HPBMC recruitment by HUAECs. Time course of Ang-II-induced TNFα mRNA increase in HUAECs (A), HPBMCs (B), TNFα release in HPBMCs, (C) IL-4 mRNA expression in HPBMs (D), and recruitment of HPBMCs by TNFα and/or IL-4-stimulated HUAECs (E). HUAECs and HPBMCs (5 × 106/mL) were stimulated with 1 μM Ang-II in the presence or absence of losartan (100 μM) for 1, 4, and 24 hours. Total mRNA was extracted. Relative quantification of the mRNA levels of TNFα, IL-4, and GAPDH was determined by using real-time quantitative RT-PCR by the comparative Ct method (ΔΔCt method). Columns show the fold increase in expression of TNFα and IL-4 mRNA relative to control GAPDH values, calculated as mean ± SEM of the 2-ΔΔ Ct values of 4 to 5 experiments. Protein content in the supernatant was determined by conventional sandwich ELISA and expressed as pM concentration of the cytokine of mean ± SEM from 4 to 5 experiments. *P < .05 or **P < .01 relative to values in the control group. + P < .05 relative to the Ang-II untreated group. HUAECs were incubated with medium, TNFα (0.2 ng/mL), IL-4 (20 ng/mL), or with a combination of both cytokines for 24 hours. Isolated HPBMCs (1 × 106/mL) were perfused over the HUAECs for 5 minutes at 1 dyn/cm2 and leukocyte accumulation quantified. Results are the mean (± SEM) for 4 experiments. **P < .01 relative to values in the control group; ++ P < .01 relative to the values in the TNFα or IL-4 groups.
When human peripheral blood mononuclear leukocytes (HPBMCs, 5 × 106/mL) were stimulated with 1 μM Ang-II for 1, 4, and 24 hours, TNFα mRNA was increased at 1 and 4 hours (Figure 6B). This effect was mediated through interaction of Ang-II with its AT1 receptor, because losartan blocked it (Figure 6B). In addition, significant release of TNFα was detected after 4- and 24-hour stimulation with the peptide hormone (Figure 6C), and losartan also reduced these increases (Figure 6C). By contrast, IL-4 mRNA expression was not found in mononuclear leukocytes stimulated with Ang-II for 1 hour (Figure 6D) or 4 hours (data not shown).
In another set of experiments HUAECs were stimulated with a low dose of TNFα, or with IL-4, or with a combination of both cytokines for 24 hours. As illustrated in Figure 6E, when HUAECs were stimulated with either cytokine alone, significant mononuclear leukocyte arrest was observed compared with the values obtained in unstimulated HUAECs. However, the stimulation of endothelial cells with both cytokines caused a clear additive effect on mononuclear leukocyte arrest (Figure 6E).
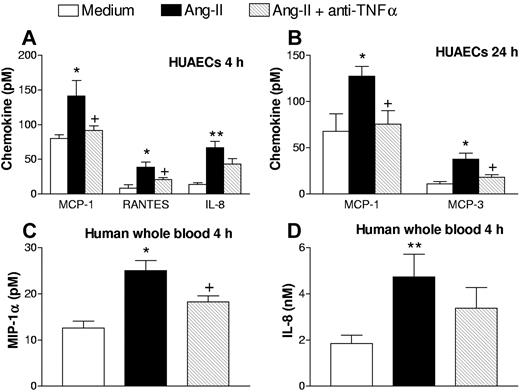
We have previously shown that Ang-II causes the release of IL-8, MCP-1, MCP-3, and RANTES from HUVECS and HUAECs.14,28 In the present study significant increases in MCP-1 were detected at 4 and 24 hours after stimulation of HUAECs with 1 μM Ang-II (Figure 7). Pretreatment of the cells with a neutralizing mAb against human TNFα significantly decreased the release of this chemokine after 4 and 24 hours of stimulation with Ang-II (Figure 7). IL-8 and RANTES were increased at 4 hours in HUAECs (Figure 7A), and MCP-3 was detected at 24 hours (Figure 7B). Similarly, reductions in RANTES and MCP-3 levels in the presence of the anti-TNFα mAb were also observed whereas reduction of IL-8 content was not significant (Figure 7).
Effect of a neutralizing antihuman TNFα mAb on Ang-II-induced MCP-1, RANTES, IL-8, and MCP-3 release from HUAECs, MIP-1α, and IL-8 release in human whole blood. HUAECs and human whole blood were stimulated with Ang-II (1 μM) or with 1 μM Ang-II plus anti-TNFα mAb (2 μg/mL). The release of MCP-1, RANTES, and IL-8 at 4 hours (A) and MCP-1 and MCP-3 at 24 hours (B; pM in the cell supernatant) as well as the release of MIP-1α (C) and IL-8 (D; pM in the plasma) in response to Ang-II was measured by ELISA and is expressed as mean (± SEM) of 4 to 6 experiments: *P < .05 or **P < .01 relative to values in the medium control group; + P < .05 relative to the 1 μM Ang-II group.
Effect of a neutralizing antihuman TNFα mAb on Ang-II-induced MCP-1, RANTES, IL-8, and MCP-3 release from HUAECs, MIP-1α, and IL-8 release in human whole blood. HUAECs and human whole blood were stimulated with Ang-II (1 μM) or with 1 μM Ang-II plus anti-TNFα mAb (2 μg/mL). The release of MCP-1, RANTES, and IL-8 at 4 hours (A) and MCP-1 and MCP-3 at 24 hours (B; pM in the cell supernatant) as well as the release of MIP-1α (C) and IL-8 (D; pM in the plasma) in response to Ang-II was measured by ELISA and is expressed as mean (± SEM) of 4 to 6 experiments: *P < .05 or **P < .01 relative to values in the medium control group; + P < .05 relative to the 1 μM Ang-II group.
Finally, when human whole blood was stimulated with 1 μM Ang-II for 4 hours, significant MIP-1α (Figure 7C) and IL-8 (Figure 7D) release was encountered. Preincubation with the neutralizing mAb directed against human TNFα significantly decreased Ang-II-induced MIP-1α release (Figure 7C) but had no significant effect on IL-8 generation (Figure 7D).
Discussion
Endothelial dysfunction leads to a proinflammatory and prothrombotic phenotype of the endothelium,2 which provokes the attachment and the subsequent migration of leukocytes, events that are linked with the onset of the atherosclerotic lesion formation. In this regard, Ang-II has been implicated in atherogenesis and endothelial dysfunction.12 Ang-II promotes the accumulation of both neutrophils and mononuclear cells into the peritoneal cavity, and these responses are preceded by the generation of CXC and CC chemokines.14,28 Despite these findings, examination of the rat mesenteric microvasculature using intravital microscopy demonstrates important differences in the accumulation of these cell types in response to Ang-II. Indeed, Ang-II promotes the adhesion of mononuclear leukocytes, but not of neutrophils, to arterioles.8 This is in contrast to responses in the postcapillary venules where neutrophils are the major leukocyte subtype recruited by this peptide hormone.8,14 In the present study we have investigated whether possible different activation mechanism might explain this differential accumulation of leukocytes in the arteriolar and venular endothelia induced by Ang-II.
We have shown that Ang-II causes a rapid release of TNFα that was detected within the first hour after its intraperitoneal injection; this was followed by the recruitment of neutrophils at 4 hours and the subsequent infiltration of mononuclear cells (8 hours). Interestingly, when a neutralizing antiserum against rat TNFα was administered, Ang-II-induced neutrophil accumulation was not affected, but significant decreases in the mononuclear cell recruitment were observed. Furthermore, the inhibition of TNFα activity resulted in the significant decrease of the CC chemokine content in the peritoneal exudates of animals injected with Ang-II without significantly affecting the levels of the CXC chemokines investigated. TNFα provokes the release of different CXC and CC chemokines, including KC, MIP-2, MCP-1, RANTES, or MIP-1a,29,30 and we have also found that Ang-II can cause the generation and release of these inflammatory mediators.14,28 On the other hand, in other in vivo models of inflammation, it has been shown that the release of KC and MIP-2 can be independent of TNFα production.31
Despite these findings, the lack of effect of TNFα antiserum on the neutrophil recruitment elicited by Ang-II led us to hypothesize that perhaps another inflammatory mediator released by this peptide hormone might contribute to the observed responses. As previously described, IL-4 increases P-selectin expression through a transcriptionally dependent mechanism with kinetics that are delayed compared with the action of other cytokines such as TNFα.16 The combination of TNFα with IL-4 can cause a selective and synergistic increase in VCAM-1 expression that results in the increased adhesion of mononuclear leukocytes.21 On the other hand, Ang-II causes selective mononuclear leukocyte adhesion to the arteriolar endothelium, and P-selectin and VCAM-1 are the main CAMs up-regulated by this peptide hormone.8 Therefore, we next examined the possible involvement of IL-4 in the mononuclear leukocyte recruitment elicited by Ang-II. For this purpose, we first determined the IL-4 content in the peritoneal exudates of the animals intraperitoneally injected with the peptide hormone and found no IL-4 protein in them.
Because the preceding events of rolling and adhesion to the endothelium could be investigated in detail by intravital microscopy of the mesenteric microcirculation, we next investigated the effect of the anti-TNFα and an anti-IL-4 antisera on leukocyte-endothelial cell interactions induced by Ang-II. We established that both antisera inhibited the adhesion of leukocytes to the arteriolar endothelium and in a similar order of magnitude. To our knowledge this is the first report that involves TNFα and IL-4 in the arteriolar leukocyte adhesion caused by this peptide hormone. In contrast, neither the anti-TNFα antiserum nor the anti-IL-4 significantly affected the Ang-II-induced leukocyte-endothelium interactions in postcapillary venules where neutrophils are the prominent cells interacting with this vascular bed. However, leukocyte rolling velocity was the only parameter slightly increased after the pretreatment with the antisera. This effect might be explained by the ability of these antisera to block VCAM-1 expression, which is involved in the slow rolling of leukocytes.32,33
These intriguing observations led us to investigate the expression of TNFα and IL-4 in the mesenteric arterioles and postcapillary venules of the animals treated with Ang-II. Interestingly we first found that Ang-II only caused significant expression of TNFα in the arterioles but not in the postcapillary venules of these rats. Although this finding gives the primary difference between the arteriolar and venular endothelia in response to Ang-II, we thought that it would be of relevance to study the expression of IL-4 in these microvessels. Surprisingly, we found marked constitutive IL-4 expression in the arterioles of PBS-injected animals and a weaker expression of this cytokine in the postcapillary venules. In addition, Ang-II exposure for 4 hours did not result in increased expression of IL-4 in both endothelia. Although IL-4 cellular expression is assumed to be restricted to leukocyte cells, a recent study34 has found that rat as well as human endothelia can express this cytokine constitutively. Therefore, it is conceivable that TNFα and IL-4 act in a paracrine manner provoking the selective adhesion of mononuclear cells to the arterial endothelium likely through increased expression of CAMs such as P selectin and VCAM-1 and through the release of mononuclear cell chemoattractants.
On the other hand, Ang-II receptors are present on both endothelial and mononuclear cells,35 and it can promote their activation.36,37 Therefore, we next examined the possible involvement of TNFα and IL-4 on Ang-II-induced responses in vitro using human cells. The induction of TNFα was investigated at the mRNA and protein level in endothelial cells and in isolated mononuclear leukocytes, whereas IL-4 was only studied in the latter in vitro system. Ang-II, acting at its AT1 receptor, increased TNFα mRNA in HUAECs. However, we did not detect any protein in the supernatants, indicating that the amount of TNFα released by Ang-II is below the limit of detection of the ELISA assay (3.1 pM). In isolated mononuclear leukocytes, Ang-II induced TNFα mRNA synthesis within 1 hour and secretion of the cytokine at 4 hours, and these responses were found to be mediated through AT1 receptor interaction. In contrast, neither IL-4 mRNA nor IL-4 protein was encountered in these supernatants. When HUAECs were stimulated with TNFα and/or IL-4, either cytokine alone caused a significant mononuclear cell recruitment. However, an additive and significant increase in leukocyte arrest was observed when HUAECs were stimulated with both cytokines. As opposed to Iademarco et al21 who used HUVECs, but in agreement with Stocker et al38 who employed porcine aortic endothelial cells, we did not observe a synergistic effect between both cytokines but did note an additive effect. In addition, at this shear rate no rolling interactions were detected. These findings add further support to our in vivo observations on mesenteric arterioles when they were stimulated with Ang-II for 4 hours.
Finally, Ang-II promotes the release of MCP-1 in vivo and in vitro28,39 as well as IL-8, RANTES, and MCP-3 from HUAECs.28 In agreement with the in vivo experiments, we found that the neutralization of TNFα activity on HUAECs inhibited the Ang-II-induced synthesis and release of these CC chemokines whereas IL-8 generation was not significantly affected. In addition, when human whole blood was stimulated with Ang-II, both IL-8 and MIP-1α were secreted after 4 hours of stimulation with the peptide hormone. We also found that neutralization of TNFα activity inhibited Ang-II-induced MIP-1α synthesis. Although IL-8 release was reduced, the differences encountered did not reach statistical significance. These results suggest that blood elements as well as the vasculature are potential sources of CC chemokines upon Ang-II stimulation, and these events are partly mediated by TNFα.
In conclusion, we have demonstrated for the first time that TNFα and IL-4 cooperate to promote Ang-II-induced selective mononuclear cell adhesion to the mesenteric arterioles. Neutralization of TNFα activity inhibits the recruitment of mononuclear leukocytes and the synthesis of CC chemokines that can amplify the inflammatory cascade elicited by this peptide hormone. We suggest that TNFα receptor antagonists may represent a reasonable therapeutic target for the inhibition of mononuclear cell recruitment to the arterial wall, which precedes atherosclerotic lesion formation, without compromising other neutrophilic responses necessary for host defense.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants SAF-2005-01649, SAF-2005-0669, and SAF-2006-01002 from CICYT, Spanish Ministry of Education and Science; Research Group 03/166 of Conselleria of Education and Culture (Generalitat Valenciana); a grant from Conselleria of Industry, University and Science (Generalitat Valenciana) (T.M.); a grant from Spanish Ministry of Education and Science (Y.N.A.N., B.B., C.C.); a grant from AlBan office and CDCH-UCV (M.L.); and a grant from the University of Valencia (P.J.J.).
Authorship
Contribution: T.M., Y.N.A.N., M.L., R.E., C.C., B.B., J.M.C.-N., P.J.J., J.C., E.J.M., and M.-J.S. performed research and analyzed data; S.P. contributed vital reagents; and M.-J.S. designed research and drafted the manuscript in collaboration with J.C. and E.J.M.
T.M. and Y.N.A.N. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria-Jesus Sanz, Departamento de Farmacología, Facultad de Medicina, Universidad de Valencia, Av Blasco Ibañez, 17 E-46010 Valencia, Spain; e-mail: maria.j.sanz@uv.es.

![Figure 1. Effect of Ang-II on TNFα release and leukocyte accumulation into the rat peripheral cavity. Time course of Ang-II-induced TNFα release (A), neutrophil polymorphonuclear leukocytes [PMNs] (B), and mononuclear leukocyte accumulation (C). Rats were intraperitoneally injected with 5 mL PBS or 1 nM Ang-II. Results are mean (± SEM) for 5 animals per group: *P < .05 or **P < .01 relative to values in the PBS-injected group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2007-01-070607/7/m_zh80190707880001.jpeg?Expires=1769812958&Signature=nWaChLQbXNL4UKcejZ9BhQNjZ8Mcro0NlvCEFZIcRnuAvyfL8VJANWxesFEm9ImcecAXkbVCwUm3FRm63~OoPB9KCuoqikCah6zKIVCnYJAtNefcqoluXtltMH6ANEG0XoDXjXJiPu1rsBCF~4Ok833vn2Abv2~C0lWPTXqRy5CYI0HPrHQdZLSSejU-kV6R6y-OweGdvqwoOJi0Q5zjCY-7rTzmAO8szBKfvEMIsub8RAmHcUQXXPqh31GJH6SIsNCDDauS2ajMdO2j25JshmNAa2pbtHFOynxO~7~v-OX9TuWqXI8ZQ~8Q9CK8DR6S0~Nt8bAIGsjKbnMD6li62g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)