Abstract
Systemic delivery of lentiviral vector (LV) in immunocompetent mice leads to efficient in vivo cell transduction and expression of the encoded protein under the control of the ubiquitous promoter of human cytomegalovirus (CMV). However, antitransgene immune response results in clearance of transduced cells 4 weeks after injection. T regulatory cells (Tregs), which have been demonstrated to control immune responses in vivo, were tested for their ability to suppress antitransgene response leading to stable long-term expression. Adoptive transfer of natural CD4+CD25+ Tregs (nTregs) isolated from wild type (wt) mice or from transgene tolerant transgenic (tg) mice did not suppress the antitransgene immune response after LV delivery. These data demonstrate that neither increasing the endogenous pool of natural Tregs nor transferring nTregs selected in a transgene-expressing thymus can modulate the immune response and mediate sustained transgene expression. Conversely, adoptive transfer of antigen-presenting cells (APCs) isolated from transgene-tolerant tg mice efficiently reduced the immune response leading to stable LV-encoded protein expression in vivo. Reduction of CD8+ effector T cells was observed in LV-treated mice coinjected with transgene-expressing APCs compared with control mice. These data indicate that antitransgene immune response can be modulated by transgene-expressing APCs possibly through deletion of effector T cells.
Introduction
Stable gene replacement by direct in vivo administration of gene transfer vectors has great potential as an effective, long-lasting, and relatively simple therapy to administer for several inherited disorders.1 The efficacy of most gene therapy protocols depends on persistent high-level expression of transgene-encoded proteins. However, in many instances gene therapy–derived products are recognized as foreign antigens by the host immune system, which mounts an immune response leading to clearance of gene-modified cells. Indeed, many gene therapy studies in mouse models and in humans2 have shown a common pattern in which the immune system initially reacts against proteins derived from the delivery vector and subsequently against the transgene product itself (reviewed in Zhou et al3 ). Therefore, the development of novel strategies to halt the host immune response to gene therapy–derived products is fundamental for the success of gene therapy trials.
It has been demonstrated that the type of vector, transgene, and target cells; the time of onset and average level of transgene expression; and the route of vector delivery4 can significantly influence the type and strength of host immune response after gene therapy.3 The use of immunosuppressive drugs is a common approach to suppress immune responses toward transgene products and vector.5 Drugs such as cyclosporin, tacrolimus, and cyclophosphamide can indeed inhibit synthesis and release of cytokines, and prevent activation/expansion of T cells, thereby blocking an immune response. Unfortunately, these therapies are not antigen specific and patients remain in a general state of immunosuppression with high risk of infections. Novel approaches aimed at preventing or blocking transgene-specific immune responses are more desirable and can be accomplished by inducing antigen-specific immunotolerance.
Tolerance is controlled by several mechanisms including clonal deletion of antigen-specific T cells, functional inactivation, clonal anergy, and active suppression mediated by T regulatory cells (Tregs). The CD4+ Tregs that constitutively express the interleukin (IL)–2Rα chain (CD4+CD25+) are one of the best characterized Treg subsets. They are termed natural Tregs (nTregs) because they develop in the thymus and, once generated, migrate to peripheral tissues where they may prevent the activation of reactive T cells.6 Deficiency in or dysfunction of these cells can cause autoimmune diseases and a reduction in their function can also boost antitumor immunity.7 Adoptive transfer of nTregs can revert established diseases such as autoimmune diabetes, inflammatory bowel disease, experimental autoimmune encephalomyelitis, and allergy, and induce transplantation tolerance in preclinical animal models.8 Therefore, nTregs are particularly attractive for designing ways to induce immunologic tolerance to new antigens introduced by gene transfer. Alternatively, clonal deletion of effector T cells can occur when a high amount of antigen is presented in lymphoid organs by antigen-presenting cells (APCs) for a prolonged period of time.9
Human immunodeficiency virus–based lentiviral vectors (LVs) are a promising class of vectors capable of integrating into both dividing and nondividing cells.10 We and others have shown that intravenous administration of late-generation LVs into immunodeficient (severe combined immunodeficiency [SCID]) mice results in stable transgene expression mainly in liver cells and splenocytes without signs of toxicity.11–13 However, when we delivered LV driven by an ubiquitous promoter (cytomegalovirus [CMV]) into immunocompetent C57BL/6 mice, clearance of transduced cells was observed.14
In this study, we further characterized the in vivo immune response after systemic injection of LV expressing the green fluorescent protein (GFP) in C57BL/6 mice and subsequently explored the potential of nTregs to induce GFP-specific tolerance. Results show that neither wild-type (wt) syngeneic nTregs nor GFP+ nTregs isolated from GFP-transgenic (tg) mice can actively modulate the in vivo immune response to LV-delivered GFP. Conversely, adoptive transfer of GFP+-APCs from GFP-tg mice significantly reduces the in vivo immune response to LV-delivered GFP, allowing a sustained in vivo expression of GFP+ transduced cells.
Materials and methods
Vector
The self-inactivating pRRLsin.cPPT.CMV.eGFP.Wpre construct was used to generate vesicular stomatitis virus (VSV)–pseudotyped lentiviral vector (LV-CMV-GFP) as previously described.11 Titers, determined on HeLa, were 2 × 109 to 2 × 1010 transducing units HeLa (TUHeLa)/mL with 55 to 300 μg HIV-1 p24/mL. Transduced cells were analyzed by flow cytometry.
Mice
C57BL/6 mice were purchased from Charles River Laboratories (Calco, Milan, Italy). C57BL/6 GFP transgenic mice, which ubiquitously express GFP under the direction of the ubiquitin C promoter (UBI-GFP/BL6),15 and C57BL/6-SCID mice were purchased from Jackson Laboratories (Bar Harbor, ME). LV-CMV-GFP (7-12 μg HIVp24/mouse, 5-8 × 108 TUHeLa/mouse, 0.2 mL final volume) or phosphate-buffered saline (PBS) was injected into the tail vein of 6- to 8-week-old mice.
All mice were kept under specific pathogen-free conditions and all Animal Care procedures were performed according to protocols approved by the Hopital San Raffaele (HSR) Institutional Animal Care and Use Committee, IACUC no. 213.
Cells sorting and adoptive transfer
CD4+CD25+ nTregs were isolated from splenocytes of C57BL/6 mice with the regulatory T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) (average purity, ≥ 80%). Alternatively, CD4+ T cells were first purified by positive selection with anti-CD4 monoclonal antibody (mAb)-coated microbeads (Miltenyi Biotec) and thereafter were stained with anti-CD4 and anti-CD25 mAbs (BD Biosciences, Mountain View, CA). CD4+CD25+ T cells were then sorted by fluorescence-activated cell sorting (FACS) on a FAC-Star (BD Biosciences). Splenic APCs were isolated from GFP-tg mice by negative selection with anti-CD90 microbeads (Miltenyi Biotec). Two million purified nTregs or 15 × 106 GFP-tg APCs were injected intravenously the day before the administration of LV-CMV-GFP.
Immune reconstitution of C57BL/6-SCID mice
Splenic T cells were isolated from wt C57BL/6 mice by positive selection with anti-CD90 microbeads (Miltenyi Biotec). Splenic APCs were isolated from wt or GFP-tg mice by negative selection with anti-CD90 microbeads (Miltenyi Biotec). C57BL/6-SCID mice were reconstituted by intravenous injection with 16.5 × 106wt T cells (CD90+) and 33.5 × 106wt APCs or GFP-tg APCs (CD90−). Cells were transferred intravenously the day before administration of LV-CMV-GFP.
Tissue analysis
LV-CMV-GFP– or PBS-injected mice were killed at the indicated time points after LV injection and liver was fixed in 4% paraformaldehyde, embedded in optimal cutting temperature (OCT), and frozen in isopenthane precooled in liquid nitrogen. Cryostat sections (5-μm thick) were postfixed with 4% paraformaldehyde, blocked with 5% goat serum (Vector Laboratories, Burlingame, CA), 1% bovine serum albumin (BSA), 0.1% Triton X-100 in PBS, and incubated with rabbit anti-GFP (Molecular Probes, Eugene, OR) and rat antimouse CD8 (BD Pharmingen, San Diego, CA). Sections were washed and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) and tetramethylrhodamine-5-isothiocyanate (TRITC)-conjugated goat anti-rat IgG (Molecular Probes). Nuclei were stained with TOPRO-3 (Molecular Probes). Images were visualized with a Zeiss Axioskop2 microscope using 3-laser confocal microscopy with a Zeiss Plan-Neofluar 20×/0.5 numeric aperture objective lens and Zeiss W-PI 10×/0.23 objective lens as eyepiece (Zeiss, Arese, Italy). Images were acquired using a Radiance-2100 camera and LaserSharp-2000 acquisition software (Bio-Rad, Segrate, Italy). The percentage of GFP-expressing hepatocytes was evaluated dividing the total number of GFP+ hepatocytes by the number of total nuclei in each acquired image. A total of 10 images from different sections were analyzed.
Flow cytometry
Spleens and livers were mashed to obtain a single cell suspension, and the obtained cells were stained with the following Abs: allophycocyanin (APC)–conjugated anti-CD11c (H3L), anti-TCRβ (H57-597); R-phycoerythrin–cyanine 5 (PE-Cy5)–conjugated anti-B220 (RA3-6B2), anti-CD4 (RM4-5), anti-CD8α (53-6.7); R-phycoerythrin (PE)–conjugated anti-CD62L (MEL14), anti-CD25 (PC61), anti-CD3 (17A2), anti-CD19 (1D3), anti-CD11b (M1/70), anti-CD45RB (16A), anti-CD14 (RmC5-3); and peridinin chlorophyll (PerCP)–conjugated NK1.1 (PK136) (all from BD Biosciences). Labeled cells were analyzed with a FACScan flow cytometer equipped with CellQuest software (BD Biosciences).
IFN-γ ELISPOT assay
GFP-specific IFN-γ–secreting cells were enumerated by enzyme-linked immunospot (ELISPOT) assay as previously described.16 Briefly, 5 × 104 magnetically sorted splenic CD8+ T cells (Miltenyi Biotec) were plated in ELISPOT plates (Millipore, Bedford, MA) coated with anti–IFN-γ capture mAb (5 μg/mL, R46A2; BD Biosciences) in the presence of IL-2 (50 U/mL; BD Biosciences) and 5 × 104 irradiated (30 Gray [3000 rad]) wt EL-4 or GFP+ EL-4 cells (see the following paragraph). After 42 hours of incubation at 37°C, 5% CO2, plates were washed and IFN-γ–producing cells were detected by anti–IFN-γ detection mAb (XMG 1.2; BD). Spots were counted by a KS ELISPOT system (Zeiss Vision, Göttingen, Germany). The number of spots in control wells was subtracted from the spots in test samples.
GFP+ EL-4 cells were obtained by incubating wt EL-4 murine tumor cell lines with LV-CMV-GFP (136 ng HIVp24/mL). Five days after transduction, EL-4 cells were collected and FACS sorted to obtain 99% pure GFP+ EL-4 cells.
Anti-GFP Abs enzyme-linked immunosorbent assay
Sera of the treated mice were tested for the presence of anti-GFP antibodies via enzyme-linked immunosorbent assay (ELISA). Microtiter plates were coated with rGFP (Clontech, Palo Alto, CA) (0.3 μg/well in 0.1 M carbonate buffer, pH 9.6). Ten-fold dilutions of mouse sera (1:10 000) were added, and anti-GFP antibodies were detected with peroxidase-conjugated rabbit anti-mouse Ig (Dako Cytomation, Glostrup, Denmark). Plates were reacted with H2O2 and OPD (Sigma Aldrich, Steinheim, Germany) and analyzed at 492 nm.
In vitro suppression experiments
CD4+CD25− T cells isolated with magnetic beads from spleens of naive C57BL/6 mice were cocultured with increasing doses of magnetically sorted splenic CD4+CD25+ T cells in 96-well plates coated with 10 μg/mL anti-CD3 mAb (BD Biosciences). 3[H]-thymidine was added after 4 days of culture and pulsed cells were harvested after an additional 16 hours.
Statistical analysis
All statistical analyses were performed using the Student t test.
Results
Systemic delivery of LV-CMV-GFP in immunocompetent mice leads to anti-GFP immune response and consequent GFP clearance
Systemic administration of LVs expressing the GFP marker from the immediate early enhancer/promoter of human cytomegalovirus (LV-CMV-GFP) into the tail vein of immunodeficient CB-17-SCID mice led to stable transgene expression. Conversely, in immunocompetent C57BL/6 mice, GFP expression peaked 2 weeks after injection in the spleen, bone marrow, and liver but started to decrease soon after11 (data not shown and Figure 1A). These data confirm and extend our previous observations that GFP is highly immunogenic when delivered into immunocompetent mice via LVs controlled by an ubiquitous promoter.14 Using this mouse model, which is well established in our laboratory, we further characterized the in vivo anti-GFP immune response and subsequently explored new approaches to achieve stable long-term GFP expression through induction of tolerance.
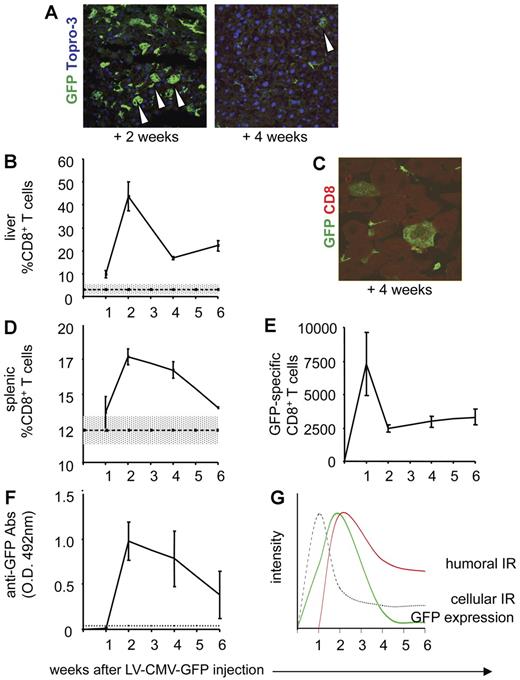
Characterization of the anti-GFP immune response in C57BL/6 mice injected with LV-CMV-GFP. C57BL/6 mice were injected with LV-CMV-GFP and were killed 1, 2, 4, and 6 weeks after injection. Liver sections from C57BL/6 mice were analyzed by confocal microscopy, after immunostaining for GFP (green) and Topro-3 (blue), 2 weeks (A, left panel) and 4 weeks (A, right panel) after LV administration. Original magnification, × 400. (B) CD8+ T cells infiltrating the liver of LV-CMV-GFP–injected mice were detected by FACS after mechanical destruction of the tissue. Data are expressed as average of percentage of CD8+ T cells gated on lymphocytes (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). Dotted line represents the average of CD8+ T cells detected in livers of vehicle-injected mice (± SD, gray area) (n = 8). (C) CD8+ T cells infiltrating the liver of C57BL/6 mice killed at 4 weeks after LV-CMV-GFP injection were detected by confocal immunofluorescence analysis of liver sections immunostained with anti-GFP (green) and anti-CD8 (red) mAbs. The image is representative of 15 images analyzed from tissues of 2 mice. Original magnification, × 1000. (D) CD8+ T cells in the spleen of LV-CMV-GFP–injected mice were detected by FACS. Data are expressed as average of percentage of CD8+ T cells gated on lymphocytes (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). Dotted line represents average of CD8+ T cells detected in spleens of vehicle-injected mice (n = 8) (± SD, gray area). (E) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen of LV-CMV-GFP–injected mice were counted by ELISPOT. Data are expressed as average of CD8+ GFP-specific T cells of 1 × 106 total CD8+ T cells (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). (F) GFP-specific antibodies present in the sera of LV-CMV-GFP–injected mice were measured by ELISA. Absorbance of 1:10 000 dilution are shown as average (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). Dotted line represents the cut off, which was calculated as average OD of vehicle-injected mice (± 3 SD). Based on the experimental data, (G) depicts the kinetics of GFP expression in immunocompetent mice after LV-CMV-GFP injection (green solid line). As soon as 1 week after LV injection, the anti-GFP cellular immune response takes place (gray dotted line) followed by the humoral immune response (red solid line), which mediates clearance of GFP+ cells 6 weeks after vector administration.
Characterization of the anti-GFP immune response in C57BL/6 mice injected with LV-CMV-GFP. C57BL/6 mice were injected with LV-CMV-GFP and were killed 1, 2, 4, and 6 weeks after injection. Liver sections from C57BL/6 mice were analyzed by confocal microscopy, after immunostaining for GFP (green) and Topro-3 (blue), 2 weeks (A, left panel) and 4 weeks (A, right panel) after LV administration. Original magnification, × 400. (B) CD8+ T cells infiltrating the liver of LV-CMV-GFP–injected mice were detected by FACS after mechanical destruction of the tissue. Data are expressed as average of percentage of CD8+ T cells gated on lymphocytes (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). Dotted line represents the average of CD8+ T cells detected in livers of vehicle-injected mice (± SD, gray area) (n = 8). (C) CD8+ T cells infiltrating the liver of C57BL/6 mice killed at 4 weeks after LV-CMV-GFP injection were detected by confocal immunofluorescence analysis of liver sections immunostained with anti-GFP (green) and anti-CD8 (red) mAbs. The image is representative of 15 images analyzed from tissues of 2 mice. Original magnification, × 1000. (D) CD8+ T cells in the spleen of LV-CMV-GFP–injected mice were detected by FACS. Data are expressed as average of percentage of CD8+ T cells gated on lymphocytes (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). Dotted line represents average of CD8+ T cells detected in spleens of vehicle-injected mice (n = 8) (± SD, gray area). (E) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen of LV-CMV-GFP–injected mice were counted by ELISPOT. Data are expressed as average of CD8+ GFP-specific T cells of 1 × 106 total CD8+ T cells (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). (F) GFP-specific antibodies present in the sera of LV-CMV-GFP–injected mice were measured by ELISA. Absorbance of 1:10 000 dilution are shown as average (± SD). One representative experiment of 6 is presented (3 animals per group per experiment). Dotted line represents the cut off, which was calculated as average OD of vehicle-injected mice (± 3 SD). Based on the experimental data, (G) depicts the kinetics of GFP expression in immunocompetent mice after LV-CMV-GFP injection (green solid line). As soon as 1 week after LV injection, the anti-GFP cellular immune response takes place (gray dotted line) followed by the humoral immune response (red solid line), which mediates clearance of GFP+ cells 6 weeks after vector administration.
In C57BL/6 mice systemically injected with LV-CMV-GFP (vector) or PBS (vehicle) and killed 1, 2, 4, and 6 weeks later, analysis of liver cell suspensions revealed the presence of infiltrating CD8+ T cells in vector-injected but not in vehicle-injected mice, with the peak of cell infiltration 2 weeks after injection (Figure 1B). Conversely, the percentage of infiltrating CD4+ T cells was not different at any time point in mice receiving the vector compared with vehicle-injected mice (data not shown). Confocal microscopy analysis showed frequent colocalization of LV-CMV-GFP–transduced hepatocytes and CD8+ T cells infiltrating the liver in vector-injected mice, demonstrating a direct interaction between GFP+ cells and CD8+ T cells (Figure 1C). A similar increase in CD8+ T cells was detected in the spleens of vector-injected mice (Figure 1D), while the number of CD4+ T cells was unchanged compared with vehicle-injected mice (data not shown). The CD8+ T cells purified from spleens of vector-injected mice showed a GFP-specific production of IFN-γ, which was evident 1 week after injection and that, although decreasing thereafter, persisted at significant levels until 6 weeks after injection (Figure 1E). Anti-GFP antibodies were also present in vector-injected mice starting 2 weeks after injection (Figure 1F). Whereas the induction of IgG antibodies was expected to be mediated by CD4+ T cells,17 we could not detect a specific increase in CD4+ T cells and anti-GFP–specific CD4+ T cells in either spleens or lymph nodes of vector-injected mice by testing GFP-driven proliferation with a conventional [3H]-thymidine incorporation assay (data not shown).
Overall, these data demonstrate that systemic delivery of LV-CMV-GFP into immunocompetent mice leads to efficient in vivo transduction and GFP expression as early as 1 week after injection with a peak of expression 2 weeks later. However, the host defense mechanisms trigger a cellular immune response, with induction of GFP-specific CD8+ T cells quickly after injection, and a humoral immune response with anti-GFP antibodies, leading to complete clearance of GFP+ cells (model depicted in Figure 1G).
Adoptive transfer of wt nTregs or nTregs isolated from GFP-tolerant mice does not modulate the anti-GFP immune response
Several experimental approaches were tested to modulate the in vivo anti-GFP immune response with the aim of inducing persistent GFP expression after LV-CMV-GFP injection. The outcome of all the different approaches was assessed 4 weeks after vector injection, the time point at which GFP clearance is observed. The following immunologic parameters were measured: number of CD8+ T cells infiltrating the liver, anti-GFP–specific CD8+ T cells present in the spleen, and anti-GFP–specific Abs in serum of vector- versus vehicle-injected mice. The average percentage of CD8+ T cells infiltrating the liver 4 weeks after LV administration was 9.9% (± 1.3 SE), the average number of GFP-specific CD8+ T cells was 1717 (± 301.5 SE) of 106 CD8+ T cells, and the average serum anti-GFP–specific Abs was 1.33 OD (± 0.1 SE). These values were calculated by pooling the data generated in 17 mice within the LV-CMV-GFP–injected group. The high variability observed among experiments can be ascribed to the use of differentvector preparations in the different experiments that may lead to a variable anti-GFP immune response. Liver GFP-expressing cells were also monitored by confocal microscopy to prove that reduced anti-GFP immune response results in sustained in vivo GFP expression.
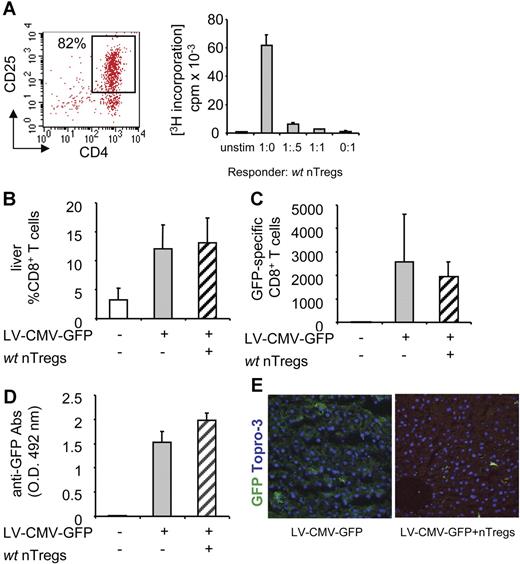
First, we tested whether adoptive transfer of nTregs is effective in prolonging transgene expression. nTregs magnetically sorted from spleens of wt C57BL/6 mice (wt nTregs) were assessed for their suppressive ability in vitro (Figure 2A) and in a model of immunization in vivo (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Once the nTreg suppressive ability was assessed, nTregs were injected intravenously into syngeneic mice the day before LV-CMV-GFP administration. The number of CD8+ T cells infiltrating the liver (Figure 2B), the frequency of splenic GFP-specific CD8+ T cells (Figure 2C), and the titers of anti-GFP–specific Abs (Figure 2D) were similar in control vector-injected mice and in mice coinjected with the vector and wt nTregs. Accordingly, similar clearance in GFP-expressing hepatocytes was detected in the livers of control and coinjected mice (Figure 2E), demonstrating that adoptive transfer of wt nTregs is not effective in modulating the anti-GFP immune response.
The anti-GFP immune response in mice coinjected with LV-CMV-GFP and syngeneic wt nTregs. nTregs isolated from spleens of syngeneic wt C57BL/6 mice were sorted by magnetic beads, and (A, left panel) FACS profile of purified CD4+CD25+ T cells is shown (nTregs purity 82%). (A, right panel) nTregs' suppressive capacity was tested in vitro in coculture assay with CD4+CD25− responder T cells stimulated with anti-CD3 mAb. (B) C57BL/6 mice were injected with 2 × 106 purified nTregs the day before LV-CMV-GFP injection. Four weeks after injection, the anti-GFP immune response was analyzed in mice injected with vehicle (□), LV-CMV-GFP (▒), or LV-CMV-GFP + wt nTregs (▨). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 3 is presented (3 animals per group per experiment). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
The anti-GFP immune response in mice coinjected with LV-CMV-GFP and syngeneic wt nTregs. nTregs isolated from spleens of syngeneic wt C57BL/6 mice were sorted by magnetic beads, and (A, left panel) FACS profile of purified CD4+CD25+ T cells is shown (nTregs purity 82%). (A, right panel) nTregs' suppressive capacity was tested in vitro in coculture assay with CD4+CD25− responder T cells stimulated with anti-CD3 mAb. (B) C57BL/6 mice were injected with 2 × 106 purified nTregs the day before LV-CMV-GFP injection. Four weeks after injection, the anti-GFP immune response was analyzed in mice injected with vehicle (□), LV-CMV-GFP (▒), or LV-CMV-GFP + wt nTregs (▨). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 3 is presented (3 animals per group per experiment). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
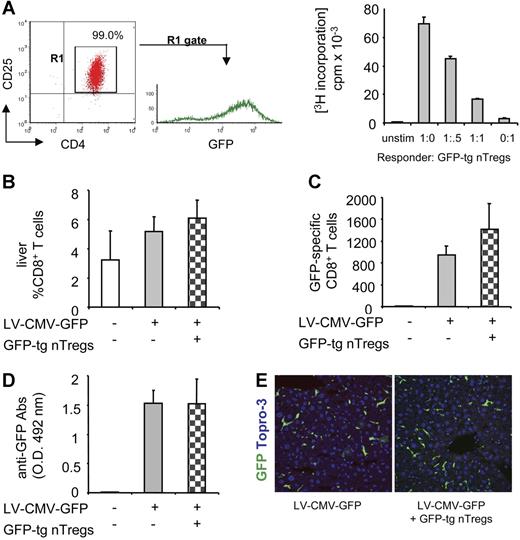
One possible explanation for the negative results shown in Figure 2 could be that nTregs isolated from wt mice are selected in a thymus in which GFP is not presented as self antigen. It has indeed been demonstrated that nTregs preferentially suppress anti-self immune responses once they migrate from the thymus into the periphery.18 To address this point, we next tested whether nTregs require selection in a GFP-expressing thymus in order to suppress the anti-GFP immune response once transferred in LV-CMV-GFP recipient mice. nTregs were purified from spleens of GFP-tolerant transgenic mice (GFP-tg nTregs) and their suppressive ability was tested in vitro before their adoptive transfer into wt C57BL/6 mice (Figure 3A). Mice were injected with LV-CMV-GFP the day after GFP-tg nTreg transfer. Four weeks later, the number of CD8+ T cells infiltrating the liver (Figure 3B), the frequency of splenic GFP-specific CD8+ T cells (Figure 3C), and the titer of anti-GFP Abs (Figure 3D) were similar in control vector-injected mice and in mice coinjected with the vector and GFP-tg nTregs. Confocal microscopy of liver sections confirmed clearance of GFP-expressing hepatocytes in both control and coinjected mice (Figure 3E). These data prove that GFP-tg nTregs selected in a GFP-expressing thymus do not modulate the anti-GFP immune response when transferred in recipients that recognize GFP as foreign antigen. These negative results might be ascribed to an insufficient number of nTregs adoptively transferred (ie, 2 × 106). However, we previously demonstrated that transfer of as low as 0.16 × 106 purified nTregs prevents autoimmune diabetes development in vivo.19 It is therefore unlikely that the number of nTregs transferred in vector-injected mice was too low to provide an effective GFP-specific immune modulation.
The anti-GFP immune response in mice coinjected with LV-CMV-GFP and GFP-tg nTregs. (A, left panel) nTregs isolated from spleens of GFP-tg mice were sorted by flow cytometry, and FACS profile of sorted CD4+CD25+GFP+ T cells is shown (GFP-tg nTregs purity 99%). (A, right panel) GFP-tg nTregs' suppressive capacity was tested in vitro in coculture assay with CD4+CD25− responder T cells isolated from C57BL/6 mice and stimulated with anti-CD3 mAb. (B) C57BL/6 mice were injected with 2 × 106 purified GFP-tg nTregs the day before LV-CMV-GFP injection. Four weeks after injection, the anti-GFP immune response was analyzed in mice injected with vehicle (□), LV-CMV-GFP (▒), or LV-CMV-GFP + GFP-tg nTregs ( ). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals per group per experiment). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals per group per experiment). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
The anti-GFP immune response in mice coinjected with LV-CMV-GFP and GFP-tg nTregs. (A, left panel) nTregs isolated from spleens of GFP-tg mice were sorted by flow cytometry, and FACS profile of sorted CD4+CD25+GFP+ T cells is shown (GFP-tg nTregs purity 99%). (A, right panel) GFP-tg nTregs' suppressive capacity was tested in vitro in coculture assay with CD4+CD25− responder T cells isolated from C57BL/6 mice and stimulated with anti-CD3 mAb. (B) C57BL/6 mice were injected with 2 × 106 purified GFP-tg nTregs the day before LV-CMV-GFP injection. Four weeks after injection, the anti-GFP immune response was analyzed in mice injected with vehicle (□), LV-CMV-GFP (▒), or LV-CMV-GFP + GFP-tg nTregs ( ). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals per group per experiment). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals per group per experiment). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
Adoptive transfer of GFP-expressing antigen-presenting cells modulates the anti-GFP immune response and leads to in vivo persistence of GFP+ cells
Several studies have demonstrated that APCs, such as B cells or dendritic cells (DCs), can present antigens in a tolerogenic way and consequently can modulate antigen-specific immune responses in vivo by activating/expanding Tregs20,21 or by inducing deletion of effector T cells.9 We therefore tested whether adoptive transfer of GFP+ APCs isolated from GFP-tg tolerant mice can abrogate the anti-GFP immune response when transferred in mice injected with LV-CMV-GFP. GFP+ APCs were obtained from spleens of GFP-tg mice after depletion of CD4+ and CD8+ T cells (Figure 4A), and the remaining cell subsets were defined and quantified by expression of specific surface markers. As shown in Table 1, the majority of APCs consisted of B cells.
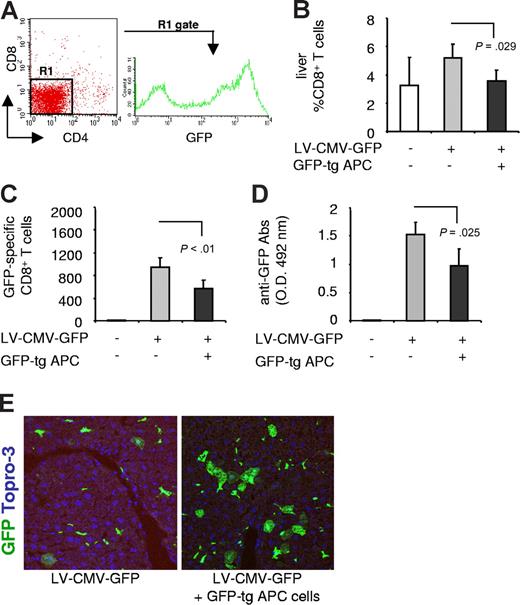
The anti-GFP immune response in mice coinjected with LV-CMV-GFP and GFP-tg APCs. (A) Splenic APCs were isolated from GFP-tg mice, and the FACS profile is shown. (B) C57BL/6 mice were injected with 15 × 106 GFP-tg APCs the day before LV-CMV-GFP injection. Four weeks after injection, the anti-GFP immune response was analyzed in mice injected with vehicle (□), LV-CMV-GFP (▒), or LV-CMV-GFP + GFP-tg APCs (■). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals in the control group, which is the same included in Figure 3; and 5 animals in the other groups). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
The anti-GFP immune response in mice coinjected with LV-CMV-GFP and GFP-tg APCs. (A) Splenic APCs were isolated from GFP-tg mice, and the FACS profile is shown. (B) C57BL/6 mice were injected with 15 × 106 GFP-tg APCs the day before LV-CMV-GFP injection. Four weeks after injection, the anti-GFP immune response was analyzed in mice injected with vehicle (□), LV-CMV-GFP (▒), or LV-CMV-GFP + GFP-tg APCs (■). CD8+ T cells infiltrating the liver were detected by FACS after mechanical destruction of the tissue. (C) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. (D) GFP-specific antibodies present in the sera diluted 10 000-fold were measured by ELISA. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals in the control group, which is the same included in Figure 3; and 5 animals in the other groups). (E) Liver sections were analyzed by confocal microscopy after immunostaining for GFP (green) and Topro-3 (blue).
The cell subsets comprising GFP-tg APCs
| Cell subset . | Surface markers . | % in APCs, plus and minus 1 SD* . | % GFP+ . | No. of injected cells × 103† . |
|---|---|---|---|---|
| B cells | B220+ CD19+ | 84.4 ± 2.7 | 81.4 | 12659 |
| Lymphoid DCs | CD11c+ CD11b− CD8α+ | 1.5 ± 0.3 | 97.7 | 229 |
| CD45RBhi DCs | CD11clow CD45RBhi | 0.8 ± 0.1 | 85.2 | 121 |
| Myeloid DCs | CD11c+ CD11b+ CD8α− | 0.5 ± 0.01 | 91.7 | 73 |
| Plasmacytoid DCs | CD11c+ B220+ GR-1+ | 0.3 ± 0.02 | 92.8 | 48 |
| NK cells | NK1.1+ | 2.3 ± 0.4 | 90.9 | 352 |
| Monocytes/MΦ | CD14+ | 2.0 ± 0.2 | 81.4 | 303 |
| CD4+ T cells | CD3+ CD4+ | 1.5 ± 0.4 | 83.6 | 228 |
| Granulocytes | B220− GR1+ | 1.4 ± 0.7 | 88.4 | 211 |
| CD8+ T cells | CD3+ CD8+ | 0.3 ± 0.003 | 80.4 | 51 |
| NK T cells | NK1.1+ TCRβ+ | 0.1 ± 0.002 | 81.6 | 20 |
| Cell subset . | Surface markers . | % in APCs, plus and minus 1 SD* . | % GFP+ . | No. of injected cells × 103† . |
|---|---|---|---|---|
| B cells | B220+ CD19+ | 84.4 ± 2.7 | 81.4 | 12659 |
| Lymphoid DCs | CD11c+ CD11b− CD8α+ | 1.5 ± 0.3 | 97.7 | 229 |
| CD45RBhi DCs | CD11clow CD45RBhi | 0.8 ± 0.1 | 85.2 | 121 |
| Myeloid DCs | CD11c+ CD11b+ CD8α− | 0.5 ± 0.01 | 91.7 | 73 |
| Plasmacytoid DCs | CD11c+ B220+ GR-1+ | 0.3 ± 0.02 | 92.8 | 48 |
| NK cells | NK1.1+ | 2.3 ± 0.4 | 90.9 | 352 |
| Monocytes/MΦ | CD14+ | 2.0 ± 0.2 | 81.4 | 303 |
| CD4+ T cells | CD3+ CD4+ | 1.5 ± 0.4 | 83.6 | 228 |
| Granulocytes | B220− GR1+ | 1.4 ± 0.7 | 88.4 | 211 |
| CD8+ T cells | CD3+ CD8+ | 0.3 ± 0.003 | 80.4 | 51 |
| NK T cells | NK1.1+ TCRβ+ | 0.1 ± 0.002 | 81.6 | 20 |
Averages of 3 animals are shown in columns 3, 4, and 5. Plus and minus 1 SD is included in column 3.
Calculated on R1 gate that excludes red blood cells (R1 = 97.75% of the total events acquired).
Calculated on a total of 15 × 106 APCs injected per mouse.
C57BL/6 mice were coinjected with GFP-tg APCs and LV-CMV-GFP or with vector alone. Interestingly, the number of CD8+ T cells infiltrating the liver (Figure 4B), the frequency of splenic GFP-specific CD8+ T cells (Figure 4C), and the titer of anti-GFP–specific Abs (Figure 4D) were all significantly reduced in mice coinjected with vector and GFP-tg APCs. Consistent with the reduced anti-GFP immune response, confocal microscopy analysis showed persistent GFP-expressing cells in the liver of animals coinjected with the vector and GFP-tg APCs (5.6% ± 1.6%) and not in mice injected with the vector alone (0.8% ± 0.7%) (Figure 4E). These data prove that adoptive transfer of transgene-expressing APCs reduces the antitransgene immune response following LV-CMV-GFP administration.
Adoptive transfer of GFP-expressing antigen-presenting cells modulates the anti-GFP immune response through deletion of CD8+ effector T cells
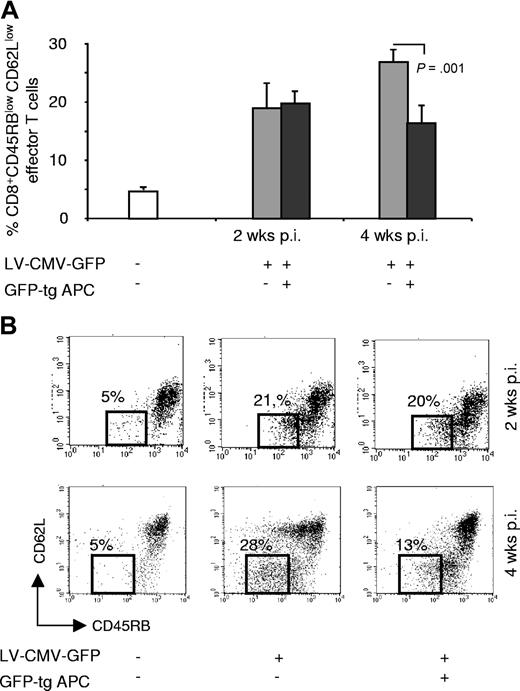
The reduced immune response to GFP after adoptive transfer of GFP-tg APCs could be due to (1) lack of induction of GFP-specific CD8+ T cells; (2) deletion of GFP-specific CD8+ T cells; and/or (3) immunomodulation by in vivo activated Tregs. CD8+ effector T cells, defined by the expression of CD45RBlow and CD62Llow,22 were present in similar amounts in mice coinjected with the vector and GFP-tg APCs and in control mice at 2 weeks after injection. Conversely, CD8+ effector T cells were significantly reduced at 4 weeks after injection only in mice receiving GFP-tg-APCs (Figure 5A,B). nTregs, detected by coexpression of CD4, CD25, and Foxp3, were not expanded in mice tested either at 2 or 4 weeks after vector injection (Figure S2). To define whether nTregs, although not expanded in vivo, were responsible for the reduced anti-GFP immune response after adoptive transfer of GFP-tg-APCs, purified CD4+CD25+CD62Lhigh T cells from the tolerant mice were transferred in newly vector-injected mice. Adoptive transfer of nTregs from tolerant mice did not modulate the anti-GFP immune response (data not shown). Overall these data show that modulation of the anti-GFP immune response by GFP-tg-APCs is not due to lack of induction of CD8+ T cells or to activation of nTregs. Therefore, we conclude that persistence of transgene expressed by APCs for at least 7 days after transfer (as shown in Figure S3) leads to clonal deletion of Ag-specific CD8+ T cells.
CD8+ effector T cells in mice coinjected with LV-CMV-GFP and GFP-tg APCs. (A) C57BL/6 mice were injected with 15 × 106 GFP-tg APCs the day before LV-CMV-GFP injection. Two and 4 weeks after vector administration splenocytes were isolated from mice injected with vehicle (□), LV-CMV-GFP (▒), or coinjected with LV-CMV-GFP and GFP-tg APCs (■), and percentages of CD8+ effector T cells, defined by expression of CD45RBlow CD62Llow, were assessed. (B) A representative dot plot analysis for each experimental group at the indicated time points is shown. Numbers indicate the percentages of CD8+ CD45RBlow CD62Llow in each group. Data are expressed as average of percentages (± SD). Two experiments were performed to allow analysis at different time points and are both shown (the 2 animals in the control group were pooled from the 2 different experiments; 5 animals in the other groups per each time point are shown).
CD8+ effector T cells in mice coinjected with LV-CMV-GFP and GFP-tg APCs. (A) C57BL/6 mice were injected with 15 × 106 GFP-tg APCs the day before LV-CMV-GFP injection. Two and 4 weeks after vector administration splenocytes were isolated from mice injected with vehicle (□), LV-CMV-GFP (▒), or coinjected with LV-CMV-GFP and GFP-tg APCs (■), and percentages of CD8+ effector T cells, defined by expression of CD45RBlow CD62Llow, were assessed. (B) A representative dot plot analysis for each experimental group at the indicated time points is shown. Numbers indicate the percentages of CD8+ CD45RBlow CD62Llow in each group. Data are expressed as average of percentages (± SD). Two experiments were performed to allow analysis at different time points and are both shown (the 2 animals in the control group were pooled from the 2 different experiments; 5 animals in the other groups per each time point are shown).
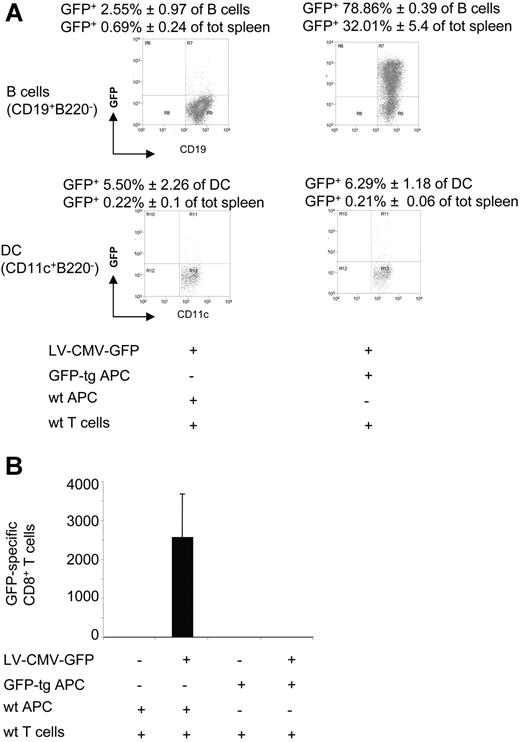
To define whether high numbers of GFP-expressing cells, persisting for a long period in vivo, could mediate deletion of T cells, as demonstrated in several animal models (reviewed in Zinkernagel9 ), immunodeficient C57BL/6-SCID mice were immune-reconstituted with wt T cells and GFP-tg APCs before LV-CMV-GFP injection. In this mouse model, high levels of GFP-expressing APCs, which were mainly B cells, coexist with functional wt T cells (Figure 6A). Control C57BL/6-SCID mice reconstituted with wt T cells plus wt APCs and injected with LV-CMV-GFP displayed an effective anti-GFP immune response, as shown by the high proportion of GFP-specific IFN-γ–producing CD8+ T cells (Figure 6B). On the contrary, in mice reconstituted with wt T cells plus GFP-tg APCs, CD8+ T cells specific for GFP were undetectable (Figure 6B).
The cellular immune response to GFP in SCID mice injected with LV-CMV-GFP previously immune-reconstituted. C57BL/6-SCID mice were immune-reconstituted with 16.5 × 106wt T cells (wt CD90+) and 33.5 × 106wt APCs or GFP-tg APCs (wt or GFP-tg CD90−) and injected with LV-CMV-GFP the day after. Two weeks after injection, mice were killed and splenocytes analyzed. (A) The presence of GFP+ APCs was investigated by FACS within the B-cell (B220+CD19+) (top dot plots) and the DC (CD11c+B220−) (bottom dot plots) subsets. One representative dot plot analysis is presented. (B) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals per group per experiment).
The cellular immune response to GFP in SCID mice injected with LV-CMV-GFP previously immune-reconstituted. C57BL/6-SCID mice were immune-reconstituted with 16.5 × 106wt T cells (wt CD90+) and 33.5 × 106wt APCs or GFP-tg APCs (wt or GFP-tg CD90−) and injected with LV-CMV-GFP the day after. Two weeks after injection, mice were killed and splenocytes analyzed. (A) The presence of GFP+ APCs was investigated by FACS within the B-cell (B220+CD19+) (top dot plots) and the DC (CD11c+B220−) (bottom dot plots) subsets. One representative dot plot analysis is presented. (B) GFP-specific IFN-γ–producing CD8+ T cells present in the spleen were counted by ELISPOT. Data are expressed as average (± SD). One representative experiment of 2 is presented (3 animals per group per experiment).
Overall these data demonstrate that persistence and high frequency of GFP presentation by GFP-tg APCs results in deletion of anti-GFP CD8+ effector T cells leading to stable transgene expression after LV-CMV-GFP delivery.
Discussion
Gene transfer via systemic administration of LVs in immunocompetent C57BL/6 mice leads to transgene expression that is transient because of the quick development of the antitransgene immune response, which mediates clearance of transduced cells. CD8+ effector T cells producing IFN-γ in response to GFP and anti-GFP antibodies can be detected in vector-injected mice 1 and 2 weeks after LV injection, respectively. Adoptive transfer of nTregs isolated either from wt syngeneic mice or from GFP-tg tolerant mice is not effective in reducing the anti-GFP immune response induced by LV-CMV-GFP injection. Conversely, GFP+ APCs isolated from GFP-tg tolerant mice efficiently modulate the anti-GFP immune response leading to stable transgene expression in vivo. Long-term GFP expression correlates with a significant reduction in CD8+ T effector cells but not with the expansion of CD4+CD25+Foxp3+ nTregs. Indeed, experiments in which immunodeficient mice were immune reconstituted show that GFP-tg APCs mediate deletion of GFP-specific CD8+ effector T cells.
The immunogenicity of GFP delivered into an immunocompetent host has been previously reported.12 CD8+ T-cell responses have been described in rhesus monkeys receiving a low nonmyeloablative dose of radiation and hematopoietic cells transduced with murine leukemia virus expressing GFP.23 Furthermore, syngeneic tumor cells transduced with retroviral vectors expressing GFP were rejected, following transplantation in immunocompetent Balb/c recipients, through development of CD8+ T cells recognizing the foreign GFP peptides.24 However, when tumor cells retrovirally transduced with GFP were injected in C57BL/6 mice, the anti-GFP immune response was not observed.24,25 These results differ from our data, which show that GFP systemically delivered via LVs in C57BL/6 mice is immunogeneic. It is possible that GFP-transduced tumor cells are less immunogeneic than systemic in vivo LV-GFP delivery.26 In our experimental model, efficient LV transduction of APCs may be responsible for the induction of the anti-GFP immune response. LV-mediated APC transduction has indeed been exploited as an efficient tool for vaccine development and immunotherapy approaches.27 Accordingly, we and others have previously demonstrated that LV-transduced APCs are activated and therefore prone to stimulate an active immune response.13 However, the LV efficacy at inducing immune responses is a considerable obstacle when attempting stable in vivo LV-mediated gene expression.
We previously demonstrated that the anti-GFP immune response after systemic LV delivery can be avoided by hepatocyte-specific LV expression,14 probably due to low or absent transgene expression within APCs in vivo and/or an active tolerogenic effect of hepatic gene expression.4,28 Indeed, when GFP expression was limited to nonhematopoietic cells via systemic delivery of LVs encoding target sequences of endogenous micro RNAs, stable long-term transgene expression was observed.29 In the present study, we attempted to directly modulate the host immune response rather than modifying the delivery vector. The nTregs, which play a key role in maintaining peripheral tolerance through suppression of both CD4+ and CD8+ effector T cells,6 represent the ideal candidate for in vivo immunomodulation. However, in our model, neither increasing the pool of endogenous nTregs by adoptive transfer of wt nTregs nor transferring nTregs selected in a GFP+ environment was effective in modulating the anti-GFP immune response.
Gross et al demonstrated that adoptive transfer of transgene-specific nTregs isolated from mice harboring a transgene-specific T-cell receptor (TCR) abolished the antitransgene CD8+ T cells and Ab production, while allowing sustained transgene expression. In contrast, adoptive transfer of wt nTregs did not lead to stable transgene expression.30 In our experimental system, tg nTregs, which have been isolated from mice that express GFP ubiquitously but do not harbor GFP-specific TCR, are not able to modulate the anti-GFP immune response. Collectively, Gross et al's data together with our new results indicate that nTregs, in order to be effective in down-modulating an antigen-specific immune response in vivo, require antigen TCR specificity and TCR engagement rather than being selected in an antigen-expressing environment.
APCs are crucial components of the immune system, acting as sentinels in various tissues and monitoring the presence of foreign antigens. When non-self/dangerous antigens are sensed, the antigens collected by APCs are processed into peptides and presented on MHC molecules at the APC surface. Thereafter, APCs move through lymphatic vessels to lymph nodes where they interact with T cells inducing either an immunogeneic or a tolerogenic response. The intensity of the stimulus from MHC-antigen complexes and/or the presence of second signals from costimulatory molecules are considered to be determining factors for the type of immune response. APCs that express low amounts of surface MHC molecules and lack costimulatory signals would tolerize T cells, whereas APCs that express higher amounts of surface MHC and express high levels of costimulatory molecules, such as CD80 and CD86, would activate T cells into an effector immune response. In our experimental model, both resting and in vitro–activated GFP-tg APCs (data not shown) modulate the in vivo anti-GFP immune response, indicating that the GFP-tg APC modulatory activity may be independent from their activation state.
Alternatively, it has been proposed that specialized APC subsets are “dedicated” to tolerance induction by activating/inducing Ag-specific Tregs (reviewed in Bacchetta et al31 ). It is possible that, in our model, GFP-tg APCs contain tolerogenic GFP-expressing cells, which are not otherwise transduced in vivo by LV-CMV-GFP, and that might activate endogenous nTregs. Consequently, only GFP-tg APCs can trigger a regulatory immune response. However, this hypothesis could be excluded based on the observation that nTregs were not expanded in mice coinjected with GFP-tg APCs either 2 weeks or 4 weeks after vector delivery. Furthermore adoptive transfer of nTregs isolated from long-term expressing GFP mice did not modulate the anti-GFP immune response, further confirming lack of nTreg-mediated immune modulation.
An alternative model, which might explain our findings, proposes that antigen localization, dose, and persistence are critical factors that determine reactivity patterns as follows: (1) antigens that do not reach secondary lymphoid organs in minimum doses or for a sufficiently long time are immunologically ignored; (2) antigens that either commonly exist in the lymphoid system or reach it and persist in high amounts for long periods delete T cells; and (3) antigens that are transported to secondary lymphoid organs in sufficient (but not excessive) amounts and for a sufficient time period (but not excessive) induce an effective immune response (reviewed in Zinkernagel9 ). In our system, LV-CMV-GFP delivery gradually generates a small pool of APCs that expresses a low amount of GFP in secondary lymphoid organs that probably triggers an active immune response. Conversely, GFP-tg APCs transport an excessive amount of GFP to secondary lymphoid organs where GFP persists for at least 7 days in immunocompetent mice and 14 days in immunodeficient mice reconstituted with wt T cells plus GFP-tg APCs. Significant reduction of CD8+ effector T cells, occurring after T-cell activation, was observed in mice injected with GFP-tg APCs. Further deletion of CD8+ effector T cells was observed in immunodeficient mice immune-reconstituted with wt T cells plus GFP-tg APCs and injected with LV-CMV-GFP. Overall, these data suggest that the amount and persistence of GFP is crucial in determining the outcome of the anti-GFP immune response. The observation that transfer of a low number of GFP-tg APCs (1.5 × 106 cells, which represents 10% of the standard dose) did not modulate the anti-GFP immune response (data not shown) further supports our hypothesis.
The cells that modulate the in vivo anti-GFP immune response (GFP-tg APCs) comprise a mix population of T-cell–depleted splenocytes. In order to understand whether B cells, which represent approximately 80% of the transferred APCs, are the key players in this in vivo function we performed experiments in which purified GFP-tg B cells were transferred in LV-CMV-GFP–injected mice. We observed that B cells modulate the cellular antitransgene immune response and lead to deletion of CD8+ T effector cells as efficiently as total GFP-tg APCs (data not shown). However, purified GFP-tg B cells do not reduce the antitransgene Ab production (data not shown), suggesting that cell subsets other than B cells play an important role in modulating the anti-GFP humoral response in our animal model.
Taken together, our results demonstrate that an antitransgene immune response, triggered after systemic LV-CMV delivery, cannot be modulated in vivo by nTregs isolated from wt syngeneic animals or from transgene-expressing tolerant mice. Only adoptive transfer of transgene-expressing APCs, independently from their activation state, modulates the antitransgene-specific immune response. Persistence and frequency of transgene-expressing APCs are likely to be key factors that lead to clonal deletion of antitransgene-specific CD8+ effector T cells and consequent in vivo stable and long-term transgene expression. These results could lead to novel approaches in which transgene-expressing APCs can be used to induce transgene tolerance in gene therapy trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research funding from the Italian Telethon Foundation to M.G.R.
We thank Angela Stabilini (HSR-TIGET, Milano) for helpful technical assistance.
Authorship
Contribution: A.A. designed and performed research experiments, collected and analyzed data, and contributed to writing the paper; M.B. designed and performed research experiments, collected and analyzed data, and wrote the paper; A.F. designed and performed research experiments, and collected and analyzed data; A.L. and L.S.S. performed research experiments; L.N. supervised research; MG.R. supervised research and research funds, and contributed to writing the paper. A.A., M.B., and A.F. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria-Grazia Roncarolo, HSR-TIGET Via Olgettina 58, 20132 Milan, Italy; e-mail: m.roncarolo@hsr.it.