Abstract
Clofarabine has shown impressive response rates in patients with acute leukemias. In vitro investigations with clofarabine in combination with cyclophosphamide in primary cells have demonstrated synergistic cytotoxicity and inhibition of DNA repair. Based on these clinical and laboratory observations, we designed a mechanism-based combination protocol with clofarabine and cyclophosphamide for patients with relapsed acute leukemias. Eighteen patients were treated with cyclophosphamide (200 mg/m2) alone on day 0 and with clofarabine plus cyclophosphamide on day 1. Clinical responses, toxicity, DNA damage measured as H2AX phosphorylation, and accumulation of clofarabine triphosphate (TP) were analyzed. At dose level 1 (20 mg/m2 clofarabine + cyclophosphamide, 6 patients) and dose level 0 (10 mg/m2 clofarabine + cyclophosphamide, 12 patients) overall response rates were 50% and 30%, respectively, with responses in 4 (67%) of 6 patients with refractory acute lymphoblastic leukemia. Dose-limiting toxicity occurred at dose level 1 with prolonged marrow aplasia. Four (22%) patients died from prolonged aplasia (1), fungal pneumonia (1), or multiorgan failure (2). In 12 of 13 patient samples, increased DNA damage (γH2AX) was observed with clofarabine and cyclophosphamide compared with cyclophosphamide alone. In conclusion, pharmacodynamic end points along with clinical results suggest usefulness of this combination strategy, whereas toxicity data suggest reduction in chemotherapeutic intensity. This clinical trial is registered with the National Cancer Institute's PDQ at www.clinicaltrials.gov as no. JHOC-J0561.
Introduction
Refractory and relapsed acute leukemias are characterized by an interplay of cellular mechanisms that shift the balance between cell survival and death pathways toward prolonged survival and, in turn, net drug resistance. The clinical outlook for such patients is very poor, with complete remissions (CRs) being achieved in 30% or fewer and a 1-year disease-free survival (DFS) of less than 10% with the use of current antileukemic agents. As a result, there is no standard of care for these diseases at the present time. In order to improve our results, one goal of any new therapeutic approach must be the ability to overcome drug resistance and promote drug-induced cell death. This may be achieved by mechanism-based combination chemotherapy
Clofarabine is a novel deoxyadenosine analog that is resistant to deamination by adenosine deaminase and phosphorolytic cleavage by bacterial purine nucleoside phosphorylase.1 Clofarabine has clinical activity as a single agent and in combination with cytosine arabinoside (ara-C) against refractory and relapsed pediatric acute lymphoblastic leukemia (ALL)2,3 and against relapsed and poor-risk, newly diagnosed acute myelogenous leukemia (AML) in adults, including those age 50 years and older.4–8 Clofarabine exerts its cytotoxicity through multiple mechanisms of action, with a major effect on ribonucleotide reductase (RR) inhibition9 and incorporation into DNA followed by inhibition of DNA polymerases.10,11 Additional effects on mitochondrial membrane polarization and disruption with resultant cytochrome c release and apoptosis induction have been reported.12
Ribonucleotide reductase converts ribonucleotides into deoxyribonucleotides and is therefore a pivotal enzyme in the processes of DNA synthesis and repair of DNA damage. Inhibition of RR depletes the intracellular pools of deoxyribonucleotides and their triphosphorylated forms (dNTPs). The depletion enhances the intracellular accumulation of nucleoside analogs and the incorporation of triphosphorylated nucleoside analogs into DNA, thereby heightening the cytotoxic effects of those agents.
Cyclophosphamide (CY; Cytoxan) is an alkylating agent that is effectively used for treatment of leukemias. A primary lesion of CY damage is DNA interstrand cross-links. These cross-links have been seen to be rapidly repaired in chronic lymphocytic leukemia (CLL) lymphocytes after in vitro exposure to activated CY.13 However, pretreatment with clofarabine impedes completion of the repair of DNA strand breaks, with resultant increase in apoptosis. This effect may be due to inhibition of both RR and DNA synthesis by clofarabine triphosphate (clofarabine-TP).13
On the basis of these findings and rationale, we designed a phase 1 clinical-laboratory correlative trial to test the hypothesis that pretreatment with clofarabine would impede the full repair of CY-induced DNA strand breaks and would therefore augment CY-driven cytotoxicity in vivo and possibly improve clinical responses in patients with relapsed or refractory acute leukemias. We delivered the drug combination in a timed sequential fashion.14,15 To confirm that CY-induced DNA damage was enhanced by clofarabine pretreatment, we measured leukemia cell DNA damage directly and serially during treatment. In order to test for potential augmentation of CY-induced DNA damage by clofarabine, we compared DNA damage with CY alone and with CY following clofarabine pretreatment.
Patients, materials, and methods
Patient eligibility and selection
Between December 2005 and August 2006, 18 adults (age ≥ 18 years) were entered on study at the Johns Hopkins Sidney Kimmel Cancer Center. Patients were required to have confirmed diagnoses of relapsed and/or refractory AML, including AML arising from myelodysplasia (MDS) or myeloproliferative disorder (MPD); ALL, including Philadelphia chromosome–positive (Ph+) ALL; or chronic myelogenous leukemia (CML) in accelerated phase (AP) or blast crisis (BC) of either myeloid or lymphoid origin that was resistant to imatinib.
Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower, serum creatinine level 176.8 μM (2.0 mg/dL) or lower, hepatic transaminase levels 5 × normal or lower, serum total bilirubin level 25.65 μM (1.5 mg/dL) or lower, negative pregnancy test within 48 hours prior to study, willingness to practice contraception, no more than 4 prior induction regimens (3 cytotoxic chemotherapy regimens), ineligibility for established curative regimens including stem-cell transplantation (SCT), and ability to provide informed consent. Patients who were unresponsive to or had relapsed after allogeneic or autologous SCT were eligible. The interval from prior treatment was at least 3 weeks for myelosuppressive cytotoxic agents (6 weeks for mitomycin or nitrosoureas), 2 weeks for radiotherapy, and 1 week for either nonmyelosuppressive treatments or treatment with hematopoietic growth factors. Hydroxyurea (HU) and/or imatinib were permitted for control of blast counts prior to clofarabine-CY therapy but had to be discontinued at least 72 hours before initiation of clofarabine-CY. All patients provided written informed consent in accordance with the Declaration of Helsinki and Johns Hopkins Medical Institutional Review Boards and guidelines.
Treatment schema
This phase 1 study was designed to determine the maximal tolerated dose (MTD) and assess the toxicity of clofarabine followed by fractionated CY in adults with relapsed or refractory acute leukemias. An additional objective was to test the hypothesis that clofarabine would inhibit the repair process initiated by CY-induced DNA damage. Single-agent CY was administered by 2-hour intravenous infusion on day 0 of cycle 1. On days 1 to 3 and 8 to 10, clofarabine was administered by intravenous infusion 2 hours before each dose of CY. For dose level 1, the day 0 and 1 CY doses were 200 mg/m2/dose and the day 2, 3, and 8 to 10 doses were 400 mg/m2/dose; the clofarabine dose was 20 mg/m2/day on days 1 to 3 and 8 to 10. Dose escalations for both clofarabine and CY were planned, with step-wise increases in clofarabine dose up to 50 mg/m2/dose and CY up to 800 mg/m2/day. However, because of dose-limiting toxicity (DLT) at dose level 1, clofarabine dosage was de-escalated to 10 mg/m2/day (dose level 0).
Supportive care
Clofarabine-related infusional toxicities.
Clofarabine administration has been associated with a systemic inflammatory response consisting of fever, respiratory distress, hypotension, capillary leak (pleural and pericardial effusions), and multiorgan failure.2,3,5,7 This constellation of symptoms has been most pronounced in the setting of drug-induced tumor lysis syndrome. To decrease the incidence and severity of this clofarabine-related cytokine-release syndrome, all patients were premedicated with acetaminophen 650 to 1000 mg orally 30 minutes prior to each dose of clofarabine to minimize the risk of fever and received prophylactic steroids (hydrocortisone 100 mg daily) on days 1, 2, and 3 prior to clofarabine dosing. Histamine (H1 and H2) blockade was also administered to patients with evidence of systemic inflammatory response syndrome or cytokine release manifested as tachypnea, tachycardia, fever, or hypotension temporally associated with clofarabine infusions.
Allopurinol.
All patients without known allergy to allopurinol received allopurinol 300 mg/day on days 0 through 5 to prevent occurrence and complications of tumor lysis–induced hyperuricemia.
Antibiotic prophylaxis.
Patients received prophylaxis against Pneumocystis carinii pneumonia, Gram-negative gastrointestinal infections, and reactivation of herpes simplex. Antifungal prophylaxis or therapy with triazoles or echinocandins was not given during administration of clofarabine in order to avoid CYP450-related drug-drug interactions.
Response and toxicity evaluation
To assess response to therapy, bone marrow aspiration and biopsy were performed prior to treatment, on day 14, and at the time of hematologic recovery or when leukemia regrowth was suspected clinically. Hematologic recovery was defined as absolute neutrophil count (ANC) 500/× 109/L or greater and a transfusion-independent platelet count 50 000/× 109/L or greater. CR required a normal bone marrow aspirate with absence of identifiable leukemia, absence of peripheral blood blasts, ANC 1000/× 109/L or greater, platelet count 100 000/× 109/L or greater, and clearance of extramedullary disease including splenomegaly. Clearance of cytogenetic abnormalities was not required for CR but was noted and described separately. Partial remission (PR) was defined as trilineage hematopoiesis in the marrow with normalization of peripheral blood counts but with 5% to 25% blasts in the marrow, at least 50% decrease in bone marrow blasts, or persistent splenomegaly. Response criteria for acute leukemias are consistent with those published by Cheson et al.16 Responses were determined following the first cycle of clofarabine-CY.
The National Cancer Institute (NCI) Common Toxicity Criteria (version 3.0) was the basis upon which all adverse events were described and graded, based on the treating physician's assessment. DLT was based on toxicities incurred during the first treatment cycle. If 2 of 3 or 2 of 6 patients developed DLT, the MTD was defined as 1 dose level below that which the DLTs were observed. If 2 of 3 or 2 of 6 patients at dose level 1 experienced DLT, the dose of clofarabine was reduced to 10 mg/m2/day with the CY dose remaining constant. Myelosuppression was not considered in evaluating toxicity except where bone marrow hypoplasia persisted for at least 42 days with marrow cellularity 5% or lower and no evidence of leukemia. Myelosuppression-related DLT occurred when ANC did not return to 1 × 109/L and platelet count did not return to 75 000/× 109/L or 20% of baseline values (whichever was less) by day 42 of therapy.
Laboratory correlates
All studies of peripheral blood and bone marrow blast populations were conducted following blast enrichment by Ficoll density separation. For the 13 patients whose circulating blasts were analyzed longitudinally for DNA damage and apoptosis, the median percentage of blasts prior to Ficoll enrichment was 75% (range, 31%-96%) on day 0 prior to treatment and 68% (range, 29%-100%) on day 1 after CY alone and before beginning the clofarabine infusion. The minimal percentage of blasts following Ficoll enrichment was 70% for all specimens.
Measurement of DNA damage
Histone H2AX is phosphorylated in response to the presence of DNA double-strand breaks.15 As such, the presence and magnitude of phosphorylated H2AX (γH2AX) is an indication of persistent, unrepaired DNA damage.17,18 We measured DNA damage by flow cytometry of leukemia blasts stained with antibody to γH2AX.18,19 Peripheral blood blasts were sampled before treatment, at the completion of day 0 CY infusion, 2 hours after completion of day 0 CY infusion, and 2 hours after completion of the combination of clofarabine followed by CY on day 1. When feasible, bone marrow blasts were assayed for the presence and magnitude of γH2AX prior to treatment, on day 0 at the completion of the CY infusion, and on day 3 after finishing clofarabine followed by CY. In brief, peripheral blood and bone marrow blasts were enriched by Ficoll density gradient separation, fixed in 70% ethanol, and stored at 4°C until staining in TST buffer (TBS, 4% FBS, 0.1% Triton X-100). Cells were incubated in either a 1:500 dilution of antiphosphohistone H2AX (Upstate Cell Signaling Solutions, Lake Placid, NY), a 1:500 dilution of mouse IgG1 isotype control (Southern Biotech, Birmingham, AL), or TST alone. After 2 hours incubation, cells were exposed for 1 hour to a 1:200 dilution of Alexa Fluor 488 goat antimouse IgG (H + L; Molecular Probes, Eugene, OR) with 5 μg/mL RNase, fixed in 2% formaldehyde in TBS, and stored at 4°C until flow-cytometric analysis. Cellular debris was gated out and nonspecific staining controls were used to mark the lower limit of the positive γH2AX-staining region (SR). Control SR was subtracted from SR of samples to determine the percentage of cells positive for γH2AX (expressed as % γH2AX). Each flow analysis was performed on a standard sampling of 1 × 104 cells. The standard deviation for this method (triplicate sampling) is 0.3-fold or less.
Measurement of apoptosis
Apoptosis was estimated for leukemic blasts obtained serially as described in “Patients, Materials and methods, Measurement of DNA damage,” on days 0 and 1 from peripheral blood and/or bone marrow by calculating sub-2N DNA from cell-cycle analysis using propidium iodide (PI)–stained cell nuclei. Following fixation in 70% ethanol, blasts were permeabilized in 0.1% Triton X-100, treated with 5 μg/mL RNase and 50 μg/mL PI, and stored at 4°C until flow-cytometric analysis. The sub-2N fractions, quantified in ungated histograms, were defined as the samples' apoptotic subpopulations.
Intracellular pharmacology
Peripheral blood cells were obtained on day 1 prior to clofarabine administration, at the end of the clofarabine infusion, at the end of the CY infusion, and 2 hours after completion of CY. Leukemic blasts were enriched by Ficoll density gradient separation and the cell number was determined by a Coulter counter (Coulter, Hialeah, FL). Cells were then processed for nucleotide extraction. In brief, nucleotides and clofarabine-TP were extracted from cells using standard procedures with HClO4 and separated on an anion-exchange Partisil-10 SAX column (Waters, Milford, MA) using high-performance liquid chromatography. The levels of triphosphates (clofarabine-TP and 4 NTPs) were calculated and expressed as the quantity of nucleotides contained in 1 × 107 cells. The lower limit of quantitation of the assay was approximately 1 pmol in an extract of 2 × 107 cells.20
Results
Patient demographics
Characteristics of the 18 adults (median age, 51 y; range, 27-67 y) who were enrolled and treated with clofarabine-CY are delineated in Table 1. All patients had refractory leukemia with multiple poor-risk features and had received multiple prior therapies. Median peripheral blood blast count for the entire group was 14 × 109/L (range, 0.8 × 109/L to 65 × 109/L), with the median for AML patients being 12 × 109/L and ALL patients being 29 × 109/L. Of 12 AML patients, 8 (67%) were primary refractory AML, 2 were refractory at second relapse, and 2 had first CR for less than 6 months. Similarly, 3 (50%) of 6 ALL patients had disease recurrence while on maintenance therapy and 2 relapsed after SCT (1 allogeneic, 1 autologous). The majority (14/18, 78%) had adverse cytogenetics,23–25 including 10 (83%) of 12 AML23,25 and 4 (67%) of 6 ALL patients (3 complex, 1 Ph+ ALL).24 Six patients were treated at dose level 1 (clofarabine 20 mg/m2/daily dose, CY 400 mg/m2/daily dose) and 12 were treated at dose level 0 (clofarabine 10 mg/m2/daily dose, CY 400 mg/m2/daily dose). Three of the 12 AML and 3 of the 6 ALL patients received dose level 1, and the remainder (9 AML and 3 ALL) received dose level 0. The decrease in clofarabine dose was prompted by the occurrence of prolonged marrow aplasia (≥ day 60) in 2 patients (1 AML, 1 ALL) and death in 2 patients from hepatic toxicity, prolonged marrow aplasia (1 ALL), and early onset multiorgan failure (1 AML; Table 2). These issues are discussed further in “Results, Toxicities.”
Characteristics of 18 adults undergoing therapy with clofarabine followed by cyclophosphamide
| Characteristics . | . |
|---|---|
| Male/female, no. | 8/10 |
| Median age, y (range) | 51 (27-67) |
| Disease type/status | |
| AML, no. patients | 12 |
| Relapse short CR1, less than 6 mo | 2 |
| Refractory (primary/multiple) | 10 (8/2) |
| ALL, no. patients | 6 |
| Relapsed T-ALL | 1 |
| Relapsed Ph+ ALL | 1 |
| Pre-B-ALL (relapsed on therapy/refractory) | 4 (1/3) |
| Biologic features (range) | |
| Pretreatment peripheral blasts, ×109/L | |
| AML | 10.3 (0.6-52) |
| ALL | 21 (14.1-51) |
| Secondary AML, no./total no. of patients | 7/12 |
| MDS/AML | 5 |
| Treatment-related AML | 2 |
| Adverse cytogenetics, no./total no. of patients | 14/18 |
| AML | 10/12* |
| ALL | 4/6† |
| Prior therapy, no. patients | |
| Stem-cell transplantation | 3 |
| Allogeneic | 2 (1 AML, 1 ALL) |
| Autologous | 1 (ALL) |
| Induction regimens, no./total no. | |
| 2 or more | 13/18 (9 AML, 4 ALL) |
| 3 or more | 7/18 (4 AML, 3 ALL) |
| Characteristics . | . |
|---|---|
| Male/female, no. | 8/10 |
| Median age, y (range) | 51 (27-67) |
| Disease type/status | |
| AML, no. patients | 12 |
| Relapse short CR1, less than 6 mo | 2 |
| Refractory (primary/multiple) | 10 (8/2) |
| ALL, no. patients | 6 |
| Relapsed T-ALL | 1 |
| Relapsed Ph+ ALL | 1 |
| Pre-B-ALL (relapsed on therapy/refractory) | 4 (1/3) |
| Biologic features (range) | |
| Pretreatment peripheral blasts, ×109/L | |
| AML | 10.3 (0.6-52) |
| ALL | 21 (14.1-51) |
| Secondary AML, no./total no. of patients | 7/12 |
| MDS/AML | 5 |
| Treatment-related AML | 2 |
| Adverse cytogenetics, no./total no. of patients | 14/18 |
| AML | 10/12* |
| ALL | 4/6† |
| Prior therapy, no. patients | |
| Stem-cell transplantation | 3 |
| Allogeneic | 2 (1 AML, 1 ALL) |
| Autologous | 1 (ALL) |
| Induction regimens, no./total no. | |
| 2 or more | 13/18 (9 AML, 4 ALL) |
| 3 or more | 7/18 (4 AML, 3 ALL) |
Data are numbers unless otherwise stated.
Seven complex (≥ 5 abnormalities), 2 monosomy 7 (+/- inv 3), 1 trisomy 21.
Three complex (≥ 5 abnormalities, including 6q- and trisomy 8), 1 Ph+.
Nonhematologic and hematologic toxicities of clofarabine followed by cyclophosphamide
| Toxicity . | Dose level 1 . | Dose level 0 . |
|---|---|---|
| No. patients | 6 | 12 |
| Clofarabine-related fever | 2 | 4 |
| Tumor lysis syndrome | 3; 1 grade 3 | 4; all grade ≤ 2 |
| Cytokine release/ARDS | 1 grade 3 | 1 grade 5 |
| Renal failure | 0 | 3; 2 grade 4 |
| Hepatic dysfunction | 4; all grade 3 | 2; 1 grade 3 |
| Multiorgan dysfunction | 1 grade 5 | 2; 1 grade 5 |
| Marrow aplasia longer than 42 d | 4*; 1 grade 5 | 3† |
| Documented infections | 8 events/5 patients | 7 events/6 patients |
| Bacteremia, Gram positive | 2; 1 grade 5 | 2 |
| Meningitis, S epidermis | 1 | |
| Ischemic colitis | 1 grade 5 | 0 |
| Fungal pneumonia | 4 | 3; 1 grade 5 |
| Death, no. patients (%) | 2 (33) | 2 (17) |
| Prolonged marrow aplasia | 1 | 0 |
| Fungal pneumonia | 0 | 1 |
| Multiorgan failure | 1 with ischemic colitis | 1 |
| Toxicity . | Dose level 1 . | Dose level 0 . |
|---|---|---|
| No. patients | 6 | 12 |
| Clofarabine-related fever | 2 | 4 |
| Tumor lysis syndrome | 3; 1 grade 3 | 4; all grade ≤ 2 |
| Cytokine release/ARDS | 1 grade 3 | 1 grade 5 |
| Renal failure | 0 | 3; 2 grade 4 |
| Hepatic dysfunction | 4; all grade 3 | 2; 1 grade 3 |
| Multiorgan dysfunction | 1 grade 5 | 2; 1 grade 5 |
| Marrow aplasia longer than 42 d | 4*; 1 grade 5 | 3† |
| Documented infections | 8 events/5 patients | 7 events/6 patients |
| Bacteremia, Gram positive | 2; 1 grade 5 | 2 |
| Meningitis, S epidermis | 1 | |
| Ischemic colitis | 1 grade 5 | 0 |
| Fungal pneumonia | 4 | 3; 1 grade 5 |
| Death, no. patients (%) | 2 (33) | 2 (17) |
| Prolonged marrow aplasia | 1 | 0 |
| Fungal pneumonia | 0 | 1 |
| Multiorgan failure | 1 with ischemic colitis | 1 |
Data are numbers of patients unless otherwise indicated.
One patient with MDS/AML recovered with leukemia day 75.
Two patients recovered with leukemia (days 55, 58).
Toxicities
The initial dose of CY 200 mg/m2 on day 0 induced a pronounced (≥ 50%) drop in peripheral blasts in only 1 of the 18 patients on study, whereas 5 patients (3 ALL, 2 AML) had at least 20% increases. Following day 1 clofarabine-CY administration, blast counts fell by at least 50% in 12 (67%) patients (5/6 [83%] dose level 1, and 7/12 [58%] dose level 0). By day 4, following completion of all 3 doses of clofarabine-CY, all patients had at least a 50% decrease in peripheral blasts and 15 (83%) had at least a 90% decrease. There was no statistical difference noted between the dose levels.
Table 2 details toxicity profiles for both dose levels. The initial dose of CY was not associated with fever, tumor lysis, or cytokine-release syndrome. In contrast, both dose levels of clofarabine followed by CY induced moderate tumor lysis in 7 patients (4 AML, 3 ALL), manifested predominantly by transient elevations in phosphate (≤ 6.5 mg/dL) and accompanied by fever. While 5 of these 7 patients had circulating blast counts of at least 10 000/mm3, there was no clear relationship between the occurrence of tumor lysis and the level of circulating blasts or the rate of fall in blasts in response to either CY or clofarabine-CY. One AML patient at dose level 1 experienced transient grade 3 respiratory distress. However, no patient required pressor support, renal dialysis, or mechanical ventilation during the initial tumor lysis period. Unlike other intensive antileukemia regimens with nucleoside analogs, anthracyclines, antimetabolites, and/or topoisomerase II–directed agents, oral and/or gastrointestinal mucositis were both uncommon and mild. Hepatic dysfunction as evidenced by grade 3 or lower transaminitis or hyperbilirubinemia or both occurred at both clofarabine doses but with somewhat greater frequency at the higher clofarabine dose (4/6 dose level 1 vs 2/12 dose level 0). Hepatic function returned to baseline values within 7 days in all but 1 patient at dose level 1 who died from multiorgan failure.
The median time to marrow recovery for patients treated on dose level 1 was 45 days (range, 24 to > 100 d). Hematologic DLT consisted of profound marrow aplasia lasting longer than 60 days in 2 of the 6 dose level 1 patients: 1 of who died with profound marrow aplasia and concomitant transaminitis on day 100, and the other who remained aplastic until day 75, at which time she recovered with initial trilineage hematopoiesis followed by recurrent acute leukemia. Hematologic DLT in 2 of the 6 patients led to a decrease in clofarabine to 10 mg/m2 per dose (dose level 0) without change in the CY dose. Since there was 1 DLT (death day 14 due to multiorgan failure) in the first 6 patients treated at dose level 0, this level was considered to be the MTD and the cohort was expanded to include a total of 12 patients. With the decrease in clofarabine dose, the median time to marrow recovery dropped to 35 days (range, 22-58 d), and no patient experienced marrow aplasia for longer than 60 days or died from failure to recover hematopoiesis.
Four (22%) of the 18 patients, 2 in each dose cohort, died during clofarabine-CY therapy. Causes of death in the dose level 1 patients were irreversible marrow failure without recurrent leukemia on day 100 and multiorgan failure on day 14. In the dose level 0 cohort, 1 died of fungal pneumonia and subarachnoid hemorrhage in the setting of prior central nervous system (CNS) leukemia on day 40 and 1 died of multiorgan failure on day 35. Three of the 4 patients who died on study had evaluable marrow on day 14 of therapy and all 3 had complete tumor clearance (CTC), as measured by bone marrow aspirate, biopsy, and flow-cytometric immunophenotype. None of the 4 patients had clinical evidence of normal or leukemic marrow recovery at the time of death.
Clinical outcome
Table 3 summarizes the clinical outcome after 1 cycle of clofarabine-CY. Responses occurred at both dose levels. The first response assessment occurred on day 14 of therapy, when marrow was assessed for presence or absence of residual leukemia by routine marrow aspiration, biopsy, and flow cytometry. CTC was documented in 4 (67%) of 6 patients at dose level 1 and in 8 (67%) of 12 at dose level 0. An additional patient (T-cell ALL) treated at dose level 0 had markedly reduced marrow blasts from 90% to 10%. CR was achieved in 2 (33%) of 6 dose level 1 patients, both with ALL (pre-B-ALL with complex cytogenetics including 6q−, relapsed after allogeneic SCT in CR2; Ph+ pre-B-ALL refractory to induction therapy plus imatinib). Of the 12 patients treated at dose level 0, 3 (25%) achieved CR including 1 primary refractory AML, 1 refractory AML with 3 prior induction regimens, and 1 pre-B-ALL relapsed on maintenance therapy with hyperleukocytosis and CNS involvement. An additional patient with T-ALL who relapsed on maintenance therapy achieved PR, for an overall response rate of 33% at dose level 0. Patients treated at both dose levels who achieved CR or PR were able to receive 1 to 3 additional cycles of clofarabine-CY (2 AML, 1 T-ALL), allogeneic SCT (2 AML), or donor lymphocyte infusion (DLI; 1 ALL), whereas patients who achieved PR (1 AML) or who did not have sustained response to a single cycle of clofarabine-CY (4 AML) were able to receive additional investigational salvage regimens.
Clinical outcome for 18 adults with refractory acute leukemia treated with clofarabine followed by cyclophosphamide
| Clofarabine dose . | n . | No. patients (%) . | |||||
|---|---|---|---|---|---|---|---|
| CTC day 14* . | CR . | PR . | NE . | NR . | Overall response . | ||
| Dose level 1, 20 mg/m2 | 6 | ||||||
| AML | 3 | 1 (33) | 0 | 1 (33) | 2 (67) | 0 | 1 (33) |
| ALL | 3 | 3 (100) | 2 (67) | 0 | 0 | 0 | 2 (67) |
| Total | NA | 4 (67) | 2 (33) | 1 (17) | 2 (33) | 0 | 3 (50) |
| Dose level 0, 10 mg/m2 | 12 | ||||||
| AML | 9 | 6 (67) | 2 (22) | 0 | 2 (22) | 5 (56) | 2 (22) |
| ALL | 3 | 1 (33) | 1 (33) | 1 (33) | 0 | 1 (33) | 2 (67) |
| Total | NA | 7 (58) | 3 (25) | 1 (8) | 2 (8) | 6 (50) | 4 (33) |
| Clofarabine dose . | n . | No. patients (%) . | |||||
|---|---|---|---|---|---|---|---|
| CTC day 14* . | CR . | PR . | NE . | NR . | Overall response . | ||
| Dose level 1, 20 mg/m2 | 6 | ||||||
| AML | 3 | 1 (33) | 0 | 1 (33) | 2 (67) | 0 | 1 (33) |
| ALL | 3 | 3 (100) | 2 (67) | 0 | 0 | 0 | 2 (67) |
| Total | NA | 4 (67) | 2 (33) | 1 (17) | 2 (33) | 0 | 3 (50) |
| Dose level 0, 10 mg/m2 | 12 | ||||||
| AML | 9 | 6 (67) | 2 (22) | 0 | 2 (22) | 5 (56) | 2 (22) |
| ALL | 3 | 1 (33) | 1 (33) | 1 (33) | 0 | 1 (33) | 2 (67) |
| Total | NA | 7 (58) | 3 (25) | 1 (8) | 2 (8) | 6 (50) | 4 (33) |
NE indicates not evaluable; NA, not applicable; and NR, no response.
DNA damage
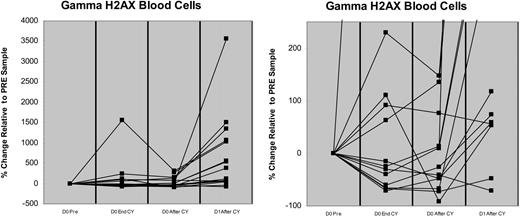
Previous in vitro studies have demonstrated the inability of CLL cells to fully repair CY-induced DNA damage when those cells are pretreated with clofarabine.13 To determine if clofarabine could have similar effects in vivo, we compared the presence and magnitude of damaged DNA by flow-cytometric measurement of γH2AX in peripheral blood blasts obtained longitudinally from 13 patients prior to day 0 at end of CY infusion (EOI), 2 hours after CY EOI, and on day 1 of clofarabine-CY 2 hours after CY EOI. As depicted in Figure 1 and Table 4, γH2AX increased immediately following day 0 (200 mg/m2) CY in 5 (38%) patients, with at least 3-fold increases seen in 2 (15%), but did not increase further over the next 2 hours. Administration of clofarabine prior to the day 1 dose of CY in the same patients, however, led to marked increases in γH2AX relative to day 0 pretreatment values in 12 (92%) of 13 patients, with 7 (54%) of 13 having striking (≥ 3-fold) increases that persisted for at least 2 hours after the end of the day 1 CY infusion (Figure 2). Moreover, cells obtained on day 1 from 11 (85%) of 13 patients evinced 2.4- to 38-fold increases in γH2AX relative to the comparable time point on day 0 (ie, 2 hours after CY EOI; Figure 2). For the 9 patients who achieved day 14 CTC, γH2AX in blasts obtained after the day 1 CY infusion increased a median 14.5-fold (range, 0.3-37 fold) relative to day 0 levels, with 6 (67%) having at least a 3-fold increase. In contrast, only 1 of the 3 patients who had residual leukemia on day 14 had at least a 3-fold increase (1.5, 1.7, 38) relative to day 0 γH2AX levels.
Changes in γH2AX induced by CY and clofarabine-CY. (A) Increase in H2AX phosphorylation in circulating leukemia cells from peripheral blood. The peripheral blood blasts were obtained from 13 patients and the presence and magnitude of DNA damage was measured by flow-cytometric analysis prior to initial CY (D0 pre), at the end of CY infusion (D0 End CY), 2 hours after EOI of day 0 CY (D0 After CY), and on day 1 following clofarabine-CY (2 hours after CY; D1 After CY). Cellular debris was gated out and nonspecific staining controls were used to mark the lower limit of the positive γH2AX-staining region (SR). Control SR was subtracted from SR of samples to determine the percentage of cells staining positive for γH2AX (expressed as % γH2AX). Pretreatment value was expressed as zero and all other values were percentage change. An expanded y-axis (−100% to 250%) is presented in panel B to highlight the differences in γH2AX at the measured time points.
Changes in γH2AX induced by CY and clofarabine-CY. (A) Increase in H2AX phosphorylation in circulating leukemia cells from peripheral blood. The peripheral blood blasts were obtained from 13 patients and the presence and magnitude of DNA damage was measured by flow-cytometric analysis prior to initial CY (D0 pre), at the end of CY infusion (D0 End CY), 2 hours after EOI of day 0 CY (D0 After CY), and on day 1 following clofarabine-CY (2 hours after CY; D1 After CY). Cellular debris was gated out and nonspecific staining controls were used to mark the lower limit of the positive γH2AX-staining region (SR). Control SR was subtracted from SR of samples to determine the percentage of cells staining positive for γH2AX (expressed as % γH2AX). Pretreatment value was expressed as zero and all other values were percentage change. An expanded y-axis (−100% to 250%) is presented in panel B to highlight the differences in γH2AX at the measured time points.
In vivo effects of clofarabine on cyclophosphamide-induced DNA damage and apoptosis in peripheral blood blasts
| . | Day 0, CY alone . | Day 1, clofarabine → CY, 2 h after end of infusion . | |
|---|---|---|---|
| End of infusion . | 2 h after end of infusion . | ||
| Fold increase in γH2AX relative to pretreatment | |||
| Median | 1.6 | 0.7 | 5.7 |
| Range | 0.4-6.2 | 0.6-6.8 | 0.3-38 |
| No. of 1.5-fold or greater increase (%) | 5/13 (38) | 3/13 (23) | 12/13 (92) |
| No. of 3-fold or greater increase (%) | 2/13 (15) | 1/13 (8) | 7/13 (54) |
| Fold increase in cells with sub-2N DNA content | |||
| Median | 1.3 | 1.2 | 1.6 |
| Range | 0.5-52 | 0.4-90 | 0.2-62 |
| No. of 1.5-fold or greater increase (%) | 3/13 (23) | 4/13 (32) | 8/13 (62) |
| . | Day 0, CY alone . | Day 1, clofarabine → CY, 2 h after end of infusion . | |
|---|---|---|---|
| End of infusion . | 2 h after end of infusion . | ||
| Fold increase in γH2AX relative to pretreatment | |||
| Median | 1.6 | 0.7 | 5.7 |
| Range | 0.4-6.2 | 0.6-6.8 | 0.3-38 |
| No. of 1.5-fold or greater increase (%) | 5/13 (38) | 3/13 (23) | 12/13 (92) |
| No. of 3-fold or greater increase (%) | 2/13 (15) | 1/13 (8) | 7/13 (54) |
| Fold increase in cells with sub-2N DNA content | |||
| Median | 1.3 | 1.2 | 1.6 |
| Range | 0.5-52 | 0.4-90 | 0.2-62 |
| No. of 1.5-fold or greater increase (%) | 3/13 (23) | 4/13 (32) | 8/13 (62) |
Where applicable, data are no./total no. of patients in that category.
Increase in γH2AX and sub-2N DNA with clofarabine-CY. Measurements of DNA damage (γH2AX; ○) and apoptosis (sub2N DNA; △) were made on peripheral blood blasts obtained from 13 patients before treatment, after CY on day 0, and after clofarabine-CY on day 1. Values obtained from day 1 after clofarabine-CY blasts were compared with values obtained from pretreatment blasts and day 0 after CY blasts. The relative changes are expressed as fold increase. ● indicates median fold change in γH2AX; and ▴, median fold change in sub-2N DNA.
Increase in γH2AX and sub-2N DNA with clofarabine-CY. Measurements of DNA damage (γH2AX; ○) and apoptosis (sub2N DNA; △) were made on peripheral blood blasts obtained from 13 patients before treatment, after CY on day 0, and after clofarabine-CY on day 1. Values obtained from day 1 after clofarabine-CY blasts were compared with values obtained from pretreatment blasts and day 0 after CY blasts. The relative changes are expressed as fold increase. ● indicates median fold change in γH2AX; and ▴, median fold change in sub-2N DNA.
Bone marrow blasts were obtained longitudinally from 4 patients on day 0 EOI CY and day 3 EOI clofarabine-CY. As depicted in Figure 3, there was no consistent increase in the amount of γH2AX detected in marrow blasts following the initial dose of CY on day 0. Following the day 3 doses of clofarabine-CY, however, the amount of detectable DNA damage was magnified in all 4 patients, with 2 patients having dramatic (> 40-fold) increases in γH2AX.
Increase in H2AX phosphorylation in leukemia cells obtained from bone marrow. The bone marrow blasts were obtained longitudinally from 4 patients and the presence and magnitude of damaged DNA was measured by flow-cytometric analysis on day 0 (Pre-Study), EOI CY (D0 End CY), and day 3 clofarabine followed by CY (D3 End). Cellular debris was gated out and nonspecific staining controls were used to mark the lower limit of the positive γH2AX-staining region (SR). Control SR was subtracted from sample SR to determine the percentage of cells staining positive for γH2AX (expressed as % γH2AX). Pretreatment value was expressed as 0 and all other values were percentage change.
Increase in H2AX phosphorylation in leukemia cells obtained from bone marrow. The bone marrow blasts were obtained longitudinally from 4 patients and the presence and magnitude of damaged DNA was measured by flow-cytometric analysis on day 0 (Pre-Study), EOI CY (D0 End CY), and day 3 clofarabine followed by CY (D3 End). Cellular debris was gated out and nonspecific staining controls were used to mark the lower limit of the positive γH2AX-staining region (SR). Control SR was subtracted from sample SR to determine the percentage of cells staining positive for γH2AX (expressed as % γH2AX). Pretreatment value was expressed as 0 and all other values were percentage change.
Apoptosis
The overall fraction of peripheral blood blasts undergoing apoptosis, as measured by the amount of sub-2N DNA in the blast population, increased 1.2- to 1.6-fold at all time points relative to day 0 pretreatment levels (Table 4). Nonetheless, 8 (62%) of 13 patients evinced at least a 1.5-fold increase in the sub-2N fraction following clofarabine-CY, whereas such increase was detected in 4 (32%) of 13 following CY alone. Median increase in day 1 post–clofarabine-CY sub-2N DNA was 2.2-fold (range, 0.2- to 62-fold) for the 9 patients achieving day 14 CTC and 1.4-fold for 3 with residual leukemia. Interestingly, all 3 patients who achieved CTC and CR had at least a 1.5-fold increase in sub-2N DNA on day 1 (1.6, 1.8, 2.0). In contrast, of the 4 patients who achieved CTC but were ultimately no response (NR), only 1 had a greater than 1.5-fold increase and 2 had major decreases (0.2-fold) in sub-2N DNA. The patient with the greatest increase in sub-2N DNA (62-fold increase over day 0) died of irreversible bone marrow failure.
Intracellular clofarabine metabolism
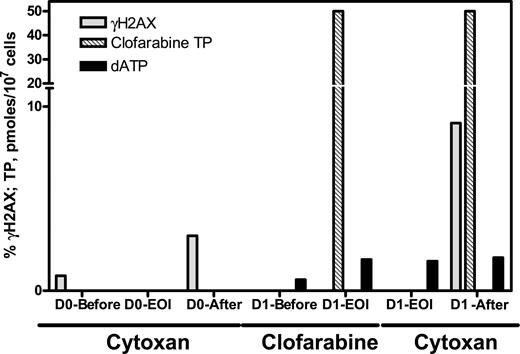
Clofarabine-TP accumulation was measured at the end of the clofarabine infusion in 4 patients, with intracellular clofarabine-TP levels being 6, 24, 28, and 50 pmol/107 cells. Ribonucleotides (ATP, CTP, GTP, TTP) were not affected by clofarabine treatment. For dNTPs, due to limited cell numbers, we measured dATP and dCTP only. The endogenous dCTP concentration was between 5 and 56 nmol/107 cells (n = 6). There was little effect on these values after clofarabine infusion (6-58 nmol/107 cells). Likewise, dATP levels varied widely among patients (range, 0.6-3.4 pmol/107 cells; n = 6) and did not change significantly after clofarabine or CY infusions. There was a high ratio of clofarabine-TP to dATP (1.7, 10, 34, and 83; n = 4). A representative compilation of serial γH2AX, clofarabine-TP, and dATP for 1 patient is depicted in Figure 4.
Pharmacokinetic and pharmacodynamic end points during therapy in leukemia blasts obtained from 1 representative patient. Leukemic blasts were obtained before treatment (D0-Pre), after end of infusion clofarabine (D1-EOI), and on day 1 following clofarabine followed by CY 2 hours after CY EOI (D1 After), and clofarabine-TP was extracted and analyzed (▩) using high-pressure liquid chromatography. Samples were obtained prior to pretreatment of clofarabine (D1-Before), after end of infusion clofarabine (D1-EOI), after end of infusion cyclophosphamide (D1-EOI), and on day 1 following clofarabine followed by CY 2 hours after CY EOI (D1 After) for dATP quantitation (■). Additional samples were obtained for γH2AX (□) and analyzed as described in Figure 1.
Pharmacokinetic and pharmacodynamic end points during therapy in leukemia blasts obtained from 1 representative patient. Leukemic blasts were obtained before treatment (D0-Pre), after end of infusion clofarabine (D1-EOI), and on day 1 following clofarabine followed by CY 2 hours after CY EOI (D1 After), and clofarabine-TP was extracted and analyzed (▩) using high-pressure liquid chromatography. Samples were obtained prior to pretreatment of clofarabine (D1-Before), after end of infusion clofarabine (D1-EOI), after end of infusion cyclophosphamide (D1-EOI), and on day 1 following clofarabine followed by CY 2 hours after CY EOI (D1 After) for dATP quantitation (■). Additional samples were obtained for γH2AX (□) and analyzed as described in Figure 1.
Discussion
This phase 1 trial was designed to test the hypothesis that the administration of clofarabine prior to CY would enhance the DNA damage induced by the alkylating agent and that such prolonged unrepaired damage, in turn, would lead to increased leukemic cell cytotoxicity,13 thereby overcoming 1 potential mechanism of drug resistance in refractory leukemias.26 Our clinical response and toxicity data and our laboratory studies of the in vivo effects of this drug sequence substantiate this hypothesis. In this highly refractory and heavily pretreated group of adults with acute leukemias, a single cycle of clofarabine followed by CY yielded clinical responses in 3 of (25%) 12 AML patients and 4 (67%) of 6 ALL patients. The net response rates in this small group of patients do not appear to relate to clofarabine dose. On the other hand, the achievement of day 14 marrow CTC and CR may relate to the amount of DNA damage achieved with clofarabine-CY, a hypothesis that needs further study in more patients in order to be validated. This notion is consistent with the observation that toxicity was to some extent dose related, particularly with regard to prolonged marrow aplasia. Nonetheless, there may be some apparent uncoupling of net DNA damage and apoptosis in some patients, particularly those who achieved CTC on day 14 but ultimately recovered with leukemia (NR). These findings are not surprising and suggest that additional pathways for DNA repair and net cellular survival may be operative in these poor-prognosis patients and may circumvent the enhancement of CY-induced DNA damage by clofarabine.
Clofarabine followed by CY was associated with increases in DNA damage and apoptosis in both AML and ALL blasts from both the peripheral blood and the marrow. The damage was measured by phosphorylation of the histone variant H2AX, an early event that occurs in response to DNA double-strand breaks. Data from longitudinal studies conducted in 13 patients suggest an increase in phosphorylation and accumulation of γH2AX after addition of clofarabine to CY, consistent with the notion that the addition of clofarabine augmented net CY-induced DNA damage. Since this work was performed in a limited number of patients to achieve its phase 1 objectives, a more extensive study will be needed to confirm these observations.
The inhibition of DNA synthesis may be due to inhibition of RR and incorporation of clofarabine-TP in the DNA repair patch.20 In this analysis of limited patient sample numbers, we observed no decrease in dATP during therapy, which may suggest a limited role of RR inhibition. However, in our study, we did not measure the effect of clofarabine on other dNTP pools. In this regard, in vivo studies accompanying a clinical trial of clofarabine given prior to the classic nucleoside analog ara-C detected depletion of intracellular cytosine pools by clofarabine and a resultant increase in ara-CTP incorporation into DNA.6,8 The in vivo data recapitulated the in vitro finding that clofarabine potentiated the intracellular metabolism of ara-C in leukemia cell line model systems.27 In our study, there was a narrow sample range and limited laboratory end points, making it difficult to define the total effect of clofarabine on CY-induced DNA damage and any resultant repair response. It will be of interest to examine these parameters in other diseases and in clofarabine combinations with other DNA-damaging agents.
Nucleoside analogs such as fludarabine,28,29 pentostatin,30 and cladribine31,32 have been combined effectively with CY in lymphoid malignancies such as CLL. Clinical data in patients who failed single-agent therapies with either nucleoside analogs or alkylating agents demonstrate that these patients can respond to the combination of such agents with an overall response rate of roughly 40%,33 suggesting that the combination may lower or eliminate resistance to each agent when used individually.
The combination of clofarabine with the classic alkylating agent CY, which damages DNA directly through adduct formation, is a new combination strategy based on preclinical studies in leukemia cells.20 At least in theory, this mechanism-based combination requires the presence of clofarabine-TP during the DNA repair phase of CY-induced DNA damage. Thus, an effective combination would be expected to require simultaneous or sequential administration, namely clofarabine followed by CY, in order to afford the presence of clofarabine-TP while the DNA repair machinery is activated.34
There are several mechanisms that could interplay to create the net enhancement of unrepaired DNA damage. One such mechanism may relate to direct DNA damage from the incorporation of clofarabine-TP into DNA. Further, the high ratio of clofarabine-TP to dATP may serve to enhance the direct CY-related DNA damage. As is true for the clinical responses seen thus far in this patient cohort, the effect of clofarabine on CY-induced DNA damage does not appear to be closely related to the clofarabine dose tested, although only 2 doses were studied. It may be that the combined effects of clofarabine and CY override any dose-dependent effect of clofarabine alone or that the ability of clofarabine to augment CY-induced DNA damage is independent of dose once sufficient intracellular clofarabine-TP levels are achieved.
In sum, clofarabine followed by CY induces significant leukemia cell kill in patients with refractory AML and ALL on both the molecular and clinical levels. The activity of this combination warrants further exploration in patients with relapsed and refractory acute leukemias. The toxicities of this regimen are noteworthy and suggest that modifications in total doses of one or both drugs may afford equal efficacy while improving tolerability.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank Anchalee Jiemjit, James P. Barber, and Kimberly Mirjifay for their expert technical assistance.
This work was supported in part by a grant from Genzyme Corporation (J.E.K.) and by CA57629 from the National Cancer Institute, National Institutes of Health (V.G.).
National Institutes of Health
Authorship
Contribution: J.E.K. designed and performed research, analyzed data, and wrote the paper; R.M.R. performed laboratory assays for DNA damage and apoptosis, prepared specimens for pharmacokinetic studies, and helped to analyze data and write the paper; K.B. conducted pharmacokinetic analysis including clofarabine triphosphate accumulation and intracellular dATP levels; J.B. coordinated clinical drug administration and sample acquisition for laboratory correlates; J.G. performed research, collected data, and assisted with data analysis; S.D.G., B.D.S., M.A.M., H.C., and M.J.L. participated in protocol design, performed research, and helped to analyze data; and V.G. participated in protocol design, directed pharmacokinetic laboratory end points, and helped to write the paper. All authors reviewed and approved the manuscript prior to submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judith E. Karp, Sidney Kimmel Cancer Center at Johns Hopkins, 1650 Orleans St, CRB Room 289, Baltimore, MD 21231-1000; e-mail: jkarp2@jhmi.edu.