In this issue of Blood, Hsieh and colleagues report on the evaluation of a novel, orally bioavailable prolyl-hydroxylase inhibitor (PHI) as an agent to stimulate erythropoiesis. Using a nonhuman primate model, the authors observed significant stimulation of endogenous erythropoietin production and erythropoiesis with chronic oral administration of FG-2216, which acts by inducing the hypoxia-inducible factor (HIF) transcriptional pathway.
Hypoxia has long been recognized as a factor that stimulates erythropoiesis.1 In the late 1800s, Bert and Jourdanet reported the initial observation that altitude caused “thick blood” in both animals and humans. Over half a century later, Faura and colleagues demonstrated erythropoietic activity in the urine of lowlanders moved to high altitude, consistent with other work suggesting a humoral basis for stimulation of red cell production. Indeed, Erslev had earlier reported in 1953 that transfusion of plasma from anemic rabbits into normal rabbits resulted in stimulation of erythropoiesis. Later work documented production of erythropoietin (Epo) in isolated, perfused kidneys. In the last 25 years, human Epo was purified, cloned, and produced as a recombinant protein (rhEpo), which has been utilized clinically with much success to treat the anemia of renal failure, cancer, AIDS, and other anemic disorders. Despite its benefit, treatment with recombinant Epo remains expensive, must be administered parenterally, and is potentially immunogenic.2
Recent efforts by scientists at FibroGen to identify orally administered new agents that are equally as effective and safe as rhEpo have capitalized on the discoveries regarding the molecular basis of Epo regulation by the blood oxygen load. It is now understood that low levels of Epo are constitutively produced in renal peritubular interstitial cells and that reduced oxygen delivery to these cells (and likely others in the liver) leads to up-regulation of Epo transcription through the HIF transcriptional pathway.3 In the absence of hypoxia or anemia, HIF-2α protein is normally efficiently degraded by the proteasome after hydroxylation and subsequent interaction with the von Hippel–Lindau protein, allowing polyubiquintination. The hydroxylation reaction is carried out in an oxygen- and iron-dependent manner by specific HIF–prolyl hydroxylases (HIF-PHD) with 2-oxoglutarate as a cofactor. Decreased oxygen in the blood, whether due to reduced hemoglobin concentration or diminished pulmonary loading, is “sensed” by the PHD in the way of decreased O2 available for hydroxylation, and HIF-2α levels rise.2,3 HIF-2α then becomes available to heterodimerize with constitutively expressed HIF-β, leading to transcriptional activation of the Epo gene through heterodimer binding to a 3′ enhancer region.
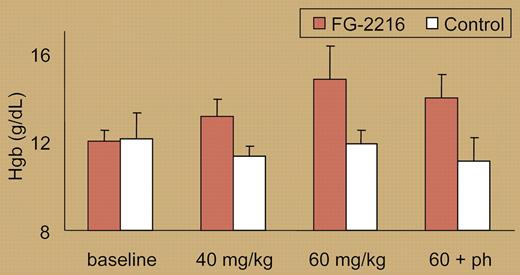
Here, the Tisdale group utilized a panel of HIF PHD inhibitors to stabilize HIF-2α in vivo, demonstrating significant induction of Epo production in mice after oral administration. One compound, FG-2216, also showed potent activity in inducing Epo when administered twice weekly over 6 to 8 weeks to rhesus macaques, resulting in a several-gram-per-deciliter rise in hemoglobin concentration (see figure). Additionally, administration of FG-2216 protected macaques against the development of anemia, compared with control animals, when subjected to weekly large-volume phlebotomy. Small increases in fetal hemoglobin were also noted, and this is likely to be a topic of future studies. Although no deleterious effects were observed over the 2-month dosing period, long-term studies will be needed to address possible adverse effects given the involvement of the HIF pathway in vasculogenesis and carcinogenesis.2 Overall, these data, along with recent meeting reports from other FibroGen investigators reporting activity of FG-2216 in human volunteers and patients, bring great promise that an orally administered agent will be available in the clinic in the near future to treat a variety of anemias.
Hemoglobin levels in treated and control rhesus macaques. See the complete figure in the article beginning on page2140.
Hemoglobin levels in treated and control rhesus macaques. See the complete figure in the article beginning on page2140.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal