Abstract
Ligation of CD40 on dendritic cells (DCs) induces early production of inflammatory mediators via canonical NF-κB signaling, as well as late expression of the anti-inflammatory enzyme indoleamine 2,3-dioxygenase (IDO) via unknown signal transduction. By selective blocking of either the canonical NF-κB pathway using the NEMO-binding domain peptide or the noncanonical NF-κB pathway by small interfering RNA, we demonstrate that IDO expression requires noncanonical NF-κB signaling. Also, noncanonical NF-κB signaling down-regulates proinflammatory cytokine production in DCs. In addition, selective activation of the noncanonical NF-κB pathway results in noninflammatory DCs that suppress T-cell activation and promote the development of T cells with regulatory properties. These findings reveal an important role of the noncanonical NF-κB pathway in the regulation of immunity.
Introduction
Dendritic cells (DCs) are key regulators of adaptive immunity by selectively promoting or suppressing T-cell responses.1 One of the suppressive mechanisms involves the expression of the enzyme indoleamine 2,3-dioxygenase (IDO) by DCs.2 IDO degrades the essential amino acid tryptophan into kynurenine, which leads to tryptophan depletion resulting in suppression of T-cell proliferation3-5 or induction of apoptosis in activated T cells both in vitro and in vivo,6 and, consequently, the induction of tolerance.7,8 IDO can be induced in DCs by a variety of stimuli, including ligation of CD40 or CD80/CD86 by, respectively, CD40L3,9,10 or CTLA-411,12 on activated T cells, as well as soluble factors such as IFN-γ and IL-1 (reviewed in Mellor and Munn2 ). Some other factors, such as LPS, require additional signals such as IFN-γ to effectively induce IDO in DCs.3,13 Remarkably, the conditions resulting in the expression of anti-inflammatory IDO also result in the expression of proinflammatory cytokines.
NF-κB transcription factors are essential for the expression of proinflammatory cytokines in DCs14 and have been implicated in IDO induction.15 NF-κB can be activated via 2 distinct signal transduction pathways. The canonical (also known as classical) NF-κB pathway requires activation of the IKK complex, consisting of the catalytic subunits IKKα and IKKβ, and the regulatory subunit NEMO/IKKγ, and controls NF-κB activation in response to proinflammatory stimuli such as LPS, TNFα, and CD40L.16-19 Activation of this pathway results predominantly in the activation, nuclear translocation, and DNA binding of the classical NF-κB dimer p50-RelA. In this pathway, IKKβ is essential for NF-κB activation, whereas IKKα is dispensable for the activation and induction of NF-κB DNA-binding activity in most cell types.19-21
In contrast, the noncanonical (also known as alternative) pathway is strictly dependent on IKKα homodimers and requires neither IKKβ nor NEMO/IKKγ.22,23 The target for IKKα homodimers is NF-κB2/p100, which upon activation of IKKα by NF-κB-inducing kinase (NIK) is incompletely degraded into p52, resulting in the release and nuclear translocation of mainly p52-RelB dimers. This pathway can be triggered by the activation of members of the TNF-receptor superfamily such as the lymphotoxin β receptor, B-cell activating factor belonging to the TNF family (BAFF) receptor, and CD40 (which also induce canonical NF-κB signaling), but not via pattern recognition receptors such as Toll-like receptor 4 (TLR4), the receptor for LPS.24
It has been suggested that the canonical and noncanonical NF-κB pathways play distinct roles in immunity (reviewed in Bonizzi and Karin25 ). Recent literature proposes a role for the noncanonical pathway in the regulation of immune responses, as IKKα is implicated in the negative regulation of inflammation26,27 and NIK has a role in the development of regulatory T cells (Tregs).28 In addition, it has been demonstrated that IKKα has an important function in thymic organogenesis for the establishment of central tolerance in cooperation with NIK.29 However, the precise mechanisms involved have not been fully elucidated yet.
Tregs are known to induce IDO in DCs,12 and IDO is involved in tolerance induction (reviewed in Mellor and Munn2 ). Interestingly, Fallarino et al recently demonstrated in a mouse model that IDO-expressing DCs induce a regulatory phenotype in naive T cells through tryptophan starvation and tryptophan catabolites.30
Therefore, we investigated whether the induction of IDO and the induction of proinflammatory cytokines require different NF-κB activation pathways by selectively blocking the canonical pathway using the NEMO-binding domain (NBD) peptide, a recently developed inhibitor of the classical IKK complex that prevents the association of IKKβ with IKKγ,31 and the noncanonical pathway using small interference RNA (siRNA) for NIK and/or IKKα. We demonstrate that the noncanonical NF-κB pathway is essential for the induction of regulatory functions in human DCs, including IDO expression and the control of proinflammatory cytokine production.
Materials and methods
Abs, cytokines, and reagents
Human IL-4 (20 × 108 U/mg) was obtained from Pharma Biotechnology (Hanover, Germany). Human rGM-CSF (specific activity, 1.11 × 107 U/mg) was a gift of Schering-Plough (Uden, the Netherlands). Human IL-3 (10 ng/mL) was obtained from Strathmann Biotech (Hanover, Germany). Mouse mAbs to human CD28 (CLB-CD28/1) and human CD3 (CLB-T3/4E-1XE) were obtained from Central Laboratory of The Netherlands Red Cross Blood Transfusion Center (Amsterdam, the Netherlands). Mouse mAbs to phosphorylated IκBα (phospho-IκBα) and total IκBα, and polyclonal rabbit anti–β-actin were obtained from Cell Signaling Technology (Beverly, MA). Polyclonal rabbit antihuman RelB, anti-p65, and anti-IKKα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse mAb to human IDO was a kind gift of Dr O. Takikawa (Hokkaido University, Sapporo, Japan). Approval was obtained from the AMC/University of Amsterdam Institutional Review Board for these studies.
In vitro generation and maturation of DCs from monocytes or direct isolation of BDCA1+ and BDCA4+ cells from peripheral blood
Monocyte-derived DCs were obtained as described previously.32 On day 6, maturation of immature DCs was induced by the addition of LPS (100 ng/mL; Sigma Adrich, Zwijndrecht, the Netherlands) or CD40 ligand (CD40L)–expressing mouse plasmacytoma cells (irradiated J558 cells, 1:1 ratio with DCs; a kind gift from Dr P. Lane, University of Birmingham, Birmingham, United Kingdom) in the presence or absence of 1-methyl-dl-tryptophan (MT; 50 μg/mL). After 48 hours, full maturation into CD1a+CD83+ mature effector DCs was confirmed by flow cytometric analysis. BDCA1+ and BDCA4+ cells were isolated from peripheral blood using specific isolation kits from Miltenyi Biotec (Bergisch Gladbach, Germany). BDCA1+ and BDCA4+ cells were cultured in the presence of GM-CSF and IL-3, respectively.
NBD peptides and NBD-mediated NF-κB inhibition
NEMO-binding domain (NBD) peptides were synthesized as described previously31 and subsequently dissolved in DMSO to stocks of 50 mM. The sequences of the wild-type and mutant (MUT) NBD peptides have been described previously.31 To study the effect of IKKβ inhibition, immature DCs were incubated for 2 hours with the NBD peptide or controls (MUT/medium) prior to induction of maturation or stimulation by LPS/CD40L. NBD/MUT peptides were used at a concentration of 50 μM, unless indicated otherwise. All subsequent tests were performed after harvesting and extensive washing of the cells to remove all factors as described previously.33
Western blotting
After the indicated times of incubation and stimulation, cells were washed twice with ice-cold PBS to remove all serum proteins and then lysed in 1 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Western blotting was performed as described previously.33 Densitometry was performed using Quantity One software (Bio-Rad, Hercules, CA).
IDO activity
IDO activity was determined according to Feng and Taylor.34 In brief, cells were harvested, lyophilized, resuspended in PBS, and cleared from insoluble material by centrifugation. The supernatant was incubated with l-tryptophan in a reaction buffer for 30 minutes at 37°C, after which the reaction was terminated with TCA. The resulting N-formylkynurenine, was hydrolyzed to kynurenine at 50°C for 30 minutes, followed by the addition of an equal volume of Ehrlich reagent (Sigma Aldrich). The product was read at 490 nm in a microplate reader (Titertek multiskan MCC/340; Titertek, Huntsville, AL). OD values above background (PBS only) were used to calculate fold induction. The basal level of expression in untreated cells was set at 1.0. IDO activities were corrected for protein content (Bio-Rad protein assay; Bio-Rad). In addition, high-performance liquid chromatography (HPLC) analysis was performed to determine tryptophan and kynurenine levels in the supernatants of cultured cells, as described previously.35 Results were expressed as kynurenine-tryptophan ratio.
Immunofluorescence staining of RelB and confocal microscopy analysis
NBD- or MUT-pretreated (50 μM) DCs were stimulated for 4 hours with LPS or CD40L, washed 3 times, and then centrifuged onto glass slides (Superfrost) at a density of 500 cells per slide. Subsequently, cells were air-dried and fixed in cold acetone for 10 minutes. The slides were then washed extensively with PBS, and stained for RelB expression using an anti-RelB primary Ab (Santa Cruz Biotechnology) as described previously.33 RelB expression was visualized using a Leica TCS SP (Leica Microsystems, Heidelberg, Germany) confocal system, equipped with an Ar/Kr/HeNe laser combination. Images were taken using a 40×/1.25 NA objective.
siRNA experiments
To date, no specific pharmacological inhibitors for IKKα exist to selectively block the noncanonical pathway of NF-κB activation.36 Therefore, we used siRNA to target this pathway. Immature DCs were seeded into 24-well plates (1.5 × 105 cells; 400 μL) in serum-free IMDM. Lipofectamine 2000 (Invitrogen, Breda, the Netherlands) was prediluted 4:100 in serum-free IMDM (40 μL), and stock concentrations of siRNA for NIK (siNIK), IKKα (siIKKα), or control scrambled, nonblocking RNA (siC) (50 nmol/mL; Ambion, Cambridge, United Kingdom) were prediluted 3:100 in serum-free IMDM (40 μL) for 5 minutes. Subsequently, Lipofectamine 2000 and siRNA predilutions were mixed and incubated for 30 minutes at room temperature (RT). Next, this mix was slowly added to the DCs (80 μL/ well) and incubated for 4 hours at 37°C. After that, 120 μL IMDM containing 50% FCS was added, and DCs were either left unstimulated (200 μL IMDM 10% FCS) or stimulated with CD40L (irradiated J558 cells, 1:1 ratio with DCs; 200 μL IMDM 10% FCS) for the indicated times. To evaluate transfection efficiency, FAM-labeled control RNA was used instead of siRNA. After 24 hours, transfected DCs were analyzed by flow cytometry for FAM content. The siRNA sequences used to target human mRNA sequences were as follows: siNIK, 5′-GCUCCGUCUACAAGCUUGAtt-3′ (sense) and siIKKα, 5′-GGCCUGUGAUGUUCCUGAAtt-3′ (sense).
Cytokine production by DCs
DCs (2 × 104 cells/well) were stimulated with CD40 ligand (CD40L)–expressing mouse plasmacytoma cells (J558 cells, 2 × 104 cells/well; a kind gift from Dr P. Lane, University of Birmingham, Birmingham, United Kingdom), in 96-well flat-bottom culture plates (Corning Life Sciences, Schiphol-Rijk, the Netherlands) in IMDM containing 10% FCS in a final volume of 200 μL. Supernatants were harvested after 24 hours and stored at −20°C until the levels of IL-12p70 or IL-6 were measured by specific solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) as described previously.37
Mixed lymphocyte reactions
Highly purified CD4+CD45RA+CD45RO− naive Th cells (> 98% as assessed by flow cytometry) were isolated from peripheral blood mononuclear cells (PBMCs) using a CD4+ T-cell isolation kit from Miltenyi Biotec, followed by depletion of CD4+CD45RO+ T cells using PE-labeled anti-CD45RO (Dako, Glostrup, Denmark) and anti–PE-beads (Miltenyi Biotec). Naive Th cells (2.5 × 104 cells per 200 μL) were cocultured in 96-well flat-bottomed culture plates with different concentrations of mature DCs. After 5 days, cell proliferation was assessed by the incorporation of [3H]thymidine (Radiochemical Center, Amersham, Little Chalfont, United Kingdom) after a pulse with 13 kBq per well during the last 16 hours, as measured by liquid scintillation counting. In other experiments, 5 × 104 anti-CD3 (1 μg/mL) and anti-CD28 (0.5 μg/mL) preactivated total CD4+ T cells were cocultured with 1 × 104 effector DCs in the presence or absence of 1-methyl-dl-tryptophan (MT; 50 μg/mL) for 24 hours to evaluate IDO-mediated T-cell apoptosis. Apoptosis was assessed by annexin V and propidium iodine (PI) staining using the annexin V–FITC apoptosis detection kit (BD Biosciences, San Jose, CA) as described by the manufacturer. Staining of the cells was evaluated by FACScan (Becton Dickinson, Lincoln Park, NJ). To investigate the effects of IDO on T-cell proliferation, 5 × 104 anti-CD3/anti-CD28 preactivated CD4+ T cells were cocultured with 1 × 104 DCs in the presence or absence of 1-methyl-dl-tryptophan (MT; 50 μg/mL) for 3 days and proliferation was assessed by the incorporation of [3H]thymidine as described above.
Suppressor assay
On day 12, resting T cells were harvested and washed 3 times with serum-free medium. Cells (1 × 106) were stained with 1.0 μM CellTrace Far Red DDAO-SE (Molecular Probes, Eugene, OR), a fluorescent dye (FL4), for 15 minutes at room temperature according to the manufacturer's instructions. After thorough washing, 2.5 × 104 DDAO-SE–labeled CD4+ T cells (DC-primed T cells) were stimulated by anti-CD3 (1 μg/mL) and anti-CD28 (0.5 μg/mL) in round-bottom 96-well plates. After overnight preactivation, 2.5 × 104 peripheral CD4+ T cells were added, representing the responder T cells. Prior to this, the responder T cells were labeled with CFSE (0.5 μM; Molecular Probes), a green cell cycle tracking dye (FL1), for 15 minutes at room temperature. After 5 days, the content of DDAO-SE and CFSE in the DC-primed and responder T cells, respectively, was analyzed by flow cytometry, and the proliferation index or precursor frequency was determined with Modfit (BD Pharmingen, San Diego, CA) as described earlier.38
Statistical analysis
Data were analyzed for statistical significance (GraphPad, InStat, version 2.02; GraphPad Software, San Diego, CA) using ANOVA or Student t test. A P value less than .05 was taken as the level of significance.
Results
CD40 ligation on DCs results in high levels of IDO
It has previously been shown that CD40 ligation, which activates both the canonical and the noncanonical NF-κB pathway, strongly increases IDO expression in human DCs and macrophages,3,9,10,39 although there is some controversy on this issue.2,40 To confirm the ability of human DCs to express IDO upon CD40 ligation, human monocyte-derived DCs were stimulated either with CD40L or with LPS, which mainly activates the canonical NF-κB pathway.24 After 2 days, DCs were lysed and IDO protein levels in the cytoplasmic extracts determined by Western blotting. In these experiments, CD40L stimulation resulted in marked IDO expression in DCs, in contrast to stimulation with LPS, which did not induce IDO (Figure 1A). LPS priming of DCs, followed by CD40L stimulation, resulted in IDO expression comparable with CD40 ligation alone, showing that LPS does not irreversibly impair IDO induction by CD40L (data not shown). Furthermore, equally high IDO levels were detected in DCs stimulated with soluble CD40L trimers, CD40L-expressing activated CD4+ T cells, or CD40L-transfected J558 cells (Figure 1B), whereas mock-transfected J558 cells did not induce IDO. J558-CD40L cells were used for CD40 ligation in the remainder of the experiments. The CD40L-induced IDO protein was enzymatically active as shown by its ability to degrade the essential amino acid tryptophan into kynurenine (Figure 1C) and HPLC analysis of kynurenine and tryptophan levels in culture supernatants (Figure 1D). IDO activity could be blocked by the addition of the competitive inhibitor 1-methyl-tryptophan (MT) (P < .05). Next, we performed coculture experiments to show that CD40L-stimulated IDO-expressing DCs induced more apoptosis in activated T cells compared with LPS-stimulated DCs (P < .05), which could be abolished by addition of MT to the culture (Figure 1E). Furthermore, blocking of IDO activity by MT in cocultures of CD40L-stimulated DCs and preactivated CD4+ T cells resulted in increased T-cell proliferation (Figure 1F). In addition, CD40L stimulation also increased IDO expression in human BDCA1+ myeloid DCs and BDCA4+ plasmacytoid DCs, freshly isolated from peripheral blood (Figure 1G), underlining that CD40L induces IDO expression in naturally occurring DCs as well. Altogether, these findings stress that CD40L induces high levels of IDO in human DCs, which is enzymatically active and down-regulates effector T-cell responses.
CD40L stimulation of DCs results in functional IDO expression. (A) CD40L stimulation of DCs induces IDO protein expression. Monocyte-derived DCs were matured with LPS or CD40L. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (B) IDO is efficiently induced by various sources of CD40L. DCs were either left unstimulated or stimulated with J558-CD40L–transfected cells, sCD40L (500 ng/mL; Immunex, Seattle, WA), or activated CD4+ T cells. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (C) CD40L-induced IDO protein is enzymatically functional. Monocyte-derived DCs were either unstimulated or matured with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, cells were harvested, extensively washed in cold PBS, and freeze-dried. Subsequently, IDO enzymatic activity in the samples was evaluated by testing the capacity to degrade tryptophan into kynurenine. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05). (D) HPLC analysis of kynurenine and tryptophan levels in culture supernatants. Monocyte-derived DCs were either unstimulated or matured with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, supernatants were harvested and IDO enzymatic activity in the samples was evaluated by measuring tryptophan and kynurenine by HPLC. Results are expressed as kynurenine-tryptophan ratio and represent mean plus or minus SEM from 3 independent experiments (*P < .05). (E) CD40L-induced IDO induces apoptosis of preactivated T cells. Monocyte-derived DCs were stimulated with CD40L or LPS in the presence or absence of MT. After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 24 hours. Subsequently, T-cell apoptosis was assessed by annexin V/propidium iodine (PI) staining and evaluated by flow cytometry. The frequency of apoptosis in T cells stimulated with anti-CD3/CD28 Ab alone is 3% to 5%. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05). (F) CD40L-induced IDO reduces proliferation of preactivated T cells. Monocyte-derived DCs were stimulated with CD40L or LPS in the presence or absence of MT. After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 3 days. Subsequently, T-cell proliferation was evaluated by [3H]TdR incorporation. The cpm for preactivated CD4+ T cells in the absence of DCs was 2515 plus or minus 389 without MT, and 1694 plus or minus 183 in the presence of MT. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .05). (G) CD40L stimulation of BDCA1+ and BDCA4+ cells also results in IDO protein expression. BDCA1+ and BDCA4+ DCs were freshly isolated from peripheral blood and stimulated with CD40L-expressing J558 cells. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content. Representative blots from 4 independent experiments are shown.
CD40L stimulation of DCs results in functional IDO expression. (A) CD40L stimulation of DCs induces IDO protein expression. Monocyte-derived DCs were matured with LPS or CD40L. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (B) IDO is efficiently induced by various sources of CD40L. DCs were either left unstimulated or stimulated with J558-CD40L–transfected cells, sCD40L (500 ng/mL; Immunex, Seattle, WA), or activated CD4+ T cells. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (C) CD40L-induced IDO protein is enzymatically functional. Monocyte-derived DCs were either unstimulated or matured with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, cells were harvested, extensively washed in cold PBS, and freeze-dried. Subsequently, IDO enzymatic activity in the samples was evaluated by testing the capacity to degrade tryptophan into kynurenine. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05). (D) HPLC analysis of kynurenine and tryptophan levels in culture supernatants. Monocyte-derived DCs were either unstimulated or matured with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, supernatants were harvested and IDO enzymatic activity in the samples was evaluated by measuring tryptophan and kynurenine by HPLC. Results are expressed as kynurenine-tryptophan ratio and represent mean plus or minus SEM from 3 independent experiments (*P < .05). (E) CD40L-induced IDO induces apoptosis of preactivated T cells. Monocyte-derived DCs were stimulated with CD40L or LPS in the presence or absence of MT. After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 24 hours. Subsequently, T-cell apoptosis was assessed by annexin V/propidium iodine (PI) staining and evaluated by flow cytometry. The frequency of apoptosis in T cells stimulated with anti-CD3/CD28 Ab alone is 3% to 5%. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05). (F) CD40L-induced IDO reduces proliferation of preactivated T cells. Monocyte-derived DCs were stimulated with CD40L or LPS in the presence or absence of MT. After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 3 days. Subsequently, T-cell proliferation was evaluated by [3H]TdR incorporation. The cpm for preactivated CD4+ T cells in the absence of DCs was 2515 plus or minus 389 without MT, and 1694 plus or minus 183 in the presence of MT. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .05). (G) CD40L stimulation of BDCA1+ and BDCA4+ cells also results in IDO protein expression. BDCA1+ and BDCA4+ DCs were freshly isolated from peripheral blood and stimulated with CD40L-expressing J558 cells. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content. Representative blots from 4 independent experiments are shown.
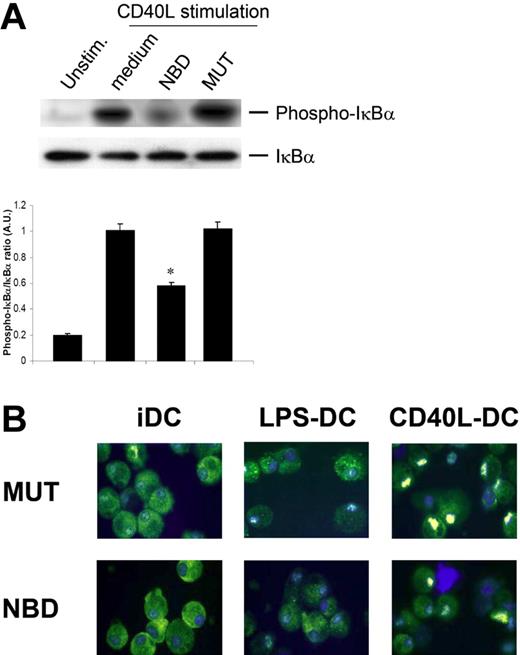
NBD peptide selectively blocks canonical CD40L-induced NF-κB activation
CD40L activates both the canonical and noncanonical pathways of NF-κB activation, while LPS activates only the canonical pathway in DCs. RelA is the transactivating subunit of the classical NF-κB heterodimer p50-RelA that is principally activated via the canonical pathway and is involved in many inflammatory processes, whereas RelB is the most prominent NF-κB subunit activated by the noncanonical pathway.25 The NBD peptide is a highly selective inhibitor of the canonical NF-κB pathway.31 We have previously demonstrated that the NBD peptide blocks LPS-induced activation of the canonical NF-κB pathway in human DCs.33 To investigate to what extent the NBD peptide blocks the canonical arm of CD40L-induced NF-κB activation, the phosphorylation status of IκBα in cytoplasmic extracts from DCs was determined by Western blotting. CD40 ligation induced IκBα phosphorylation in DCs, which was reduced after NBD pretreatment of DCs resulting in a decreased phosphorylated (phospho)IκBα/IκBα ratio (P < .05). As expected, the mutant control peptide (MUT) did not reduce IκBα phosphorylation (Figure 2A). This finding demonstrates that the NBD peptide selectively inhibits the canonical arm of NF-κB signaling in DCs, by inhibition of IκBα phosphorylation.
NBD peptide selectively blocks canonical NF-κB activation. (A) NBD peptide blocks CD40L-induced IκBα phosphorylation. Monocyte-derived DCs were preincubated with either NBD peptide or MUT peptide for 2 hours. Subsequently, cells were stimulated with CD40L for 30 minutes, extensively washed, and lysed in sample buffer. Cell lysates were analyzed by Western blotting and densitometry was performed. One representative experiment of 3 is shown; densitometry includes data from all experiments (*P < .05). (B) NBD peptide completely blocks nuclear translocation of RelB following LPS stimulation, whereas CD40L-induced RelB translocation is only marginally affected. Series of confocal images of monocyte-derived DCs stimulated for 4 hours with LPS or CD40L in the presence or absence of NBD/MUT peptides or controls. Cells were centrifuged onto glass slides, fixed in cold acetone, and stained for RelB expression. Nuclei were stained with Hoechst, and cells were analyzed by scanning the entire cell using a confocal laser microscope. In the displayed overlay pictures, RelB nuclear translocation can be evaluated. Representative pictures from one experiment are shown. Results are representative of 3 independent experiments. See “Materials and methods, Immunofluorescence staining” for image acquisition information.
NBD peptide selectively blocks canonical NF-κB activation. (A) NBD peptide blocks CD40L-induced IκBα phosphorylation. Monocyte-derived DCs were preincubated with either NBD peptide or MUT peptide for 2 hours. Subsequently, cells were stimulated with CD40L for 30 minutes, extensively washed, and lysed in sample buffer. Cell lysates were analyzed by Western blotting and densitometry was performed. One representative experiment of 3 is shown; densitometry includes data from all experiments (*P < .05). (B) NBD peptide completely blocks nuclear translocation of RelB following LPS stimulation, whereas CD40L-induced RelB translocation is only marginally affected. Series of confocal images of monocyte-derived DCs stimulated for 4 hours with LPS or CD40L in the presence or absence of NBD/MUT peptides or controls. Cells were centrifuged onto glass slides, fixed in cold acetone, and stained for RelB expression. Nuclei were stained with Hoechst, and cells were analyzed by scanning the entire cell using a confocal laser microscope. In the displayed overlay pictures, RelB nuclear translocation can be evaluated. Representative pictures from one experiment are shown. Results are representative of 3 independent experiments. See “Materials and methods, Immunofluorescence staining” for image acquisition information.
RelB is predominantly activated via the noncanonical pathway, but can also be activated to some extent via the canonical pathway.24 Therefore, we further characterized the effects of the NBD peptide on cellular RelB localization in CD40L- and LPS-stimulated DCs. Using confocal microscopy, we showed that nuclear translocation of RelB is far more pronounced following CD40 ligation than after LPS stimulation (Figure 2B). The low rate of LPS-induced RelB translocation was completely abrogated by NBD pretreatment, whereas CD40L-induced translocation was only marginally decreased. These results further substantiate that the NBD peptide effectively blocks the canonical pathway, while leaving noncanonical NF-κB signaling intact.
Essential requirement of the noncanonical NF-κB pathway for regulatory mechanisms in DCs, including induction of IDO
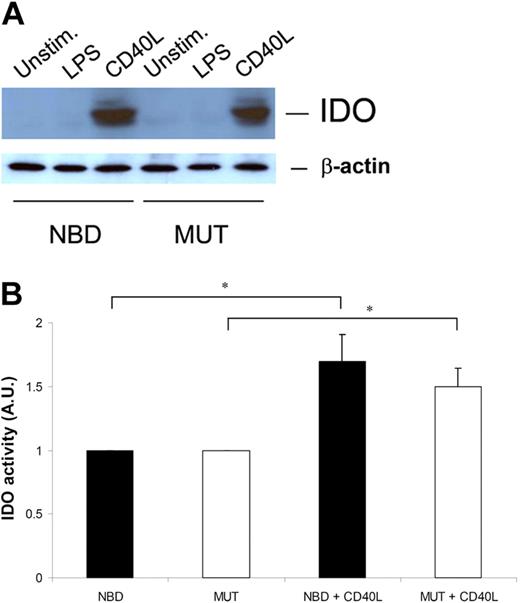
To first investigate the contribution of the canonical NF-κB pathway to CD40L-induced IDO expression, we tested the effects of NBD treatment on IDO protein levels in DCs. We have previously demonstrated that the NBD peptide blocks LPS-induced functional maturation of DCs and the production of proinflammatory cytokines.33 Canonical NF-κB blockade in CD40L-stimulated DCs showed comparable reduction in the expression of HLA-DR and costimulatory molecules and the production of IL-12, which are all key to T-cell immunity (Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, canonical NF-κB blockade in CD40L- or LPS-stimulated DCs (CD40L-NBD DCs and LPS-NBD DCs, respectively) did not affect IDO protein levels, either positively or negatively (Figure 3A). In addition, the activity of CD40L-induced IDO was not changed (Figure 3B). Taken together, these findings rule out an important role for the canonical NF-κB pathway in the induction of IDO following CD40L stimulation in DCs.
Effective IDO induction in DCs does not require canonical NF-κB activation. (A) Monocyte-derived DCs were either left unstimulated or matured with LPS or CD40L in the presence or absence of NBD/MUT peptides. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (B) Monocyte-derived DCs were either left unstimulated or matured with CD40L in the presence or absence of NBD/MUT peptides. After 48 hours, cells were harvested, extensively washed in cold PBS, and freeze-dried. Subsequently, IDO enzymatic activity in the samples was evaluated by testing the capacity to degrade tryptophan into kynurenine. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05).
Effective IDO induction in DCs does not require canonical NF-κB activation. (A) Monocyte-derived DCs were either left unstimulated or matured with LPS or CD40L in the presence or absence of NBD/MUT peptides. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (B) Monocyte-derived DCs were either left unstimulated or matured with CD40L in the presence or absence of NBD/MUT peptides. After 48 hours, cells were harvested, extensively washed in cold PBS, and freeze-dried. Subsequently, IDO enzymatic activity in the samples was evaluated by testing the capacity to degrade tryptophan into kynurenine. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05).
To investigate the contribution of the noncanonical NF-κB pathway to CD40L-induced IDO expression, we tested the requirement of different signaling intermediates of this pathway. Therefore, we used siRNA technology to specifically knock down the noncanonical pathway-associated kinases NIK and IKKα, and studied the consequences for IDO expression at the protein level. This technique always yielded transfection efficiencies of more than 95% (Figure S2A). Knockdown of both NIK and IKKα in CD40L-stimulated DCs resulted in significantly reduced IDO expression compared with the control nonfunctional siRNA-treated cells (0.44 ± 0.03 vs 1 [P < .01] and 0.19 ± 0.01 vs 1 [P < .001], respectively) (Figure 4A lanes 2-3), demonstrating that IDO expression in DCs requires activation of the noncanonical NF-κB pathway. No effects were observed of siRNA-mediated knockdown of the noncanonical NF-κB pathway in J558-mock stimulated DCs (Figure S2B).
Essential requirement of the noncanonical NF-κB pathway for CD40L-induced IDO in DCs. (A) Immature DCs were treated with control nonblocking siRNA (siC) or siRNA for the noncanonical NF-κB pathway–associated kinases NIK (siNIK) and IKKα (siIKKα). Subsequently, cells were matured for 2 days with CD40L, extensively washed, and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IKKα and IDO content, and β-actin as loading control. Densitometry was performed on IDO blots; numbers indicate percent reduction of IDO expression by siIKKα. One representative experiment of 3 is shown; densitometry includes data from all experiments (*P < .01; **P < .001). (B) Increased IL-12p70 production by DCs after siRNA-mediated knockdown of the noncanonical pathway. Immature DCs were preincubated with NBD/MUT peptides and matured for 2 days with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, the cells were thoroughly washed and stimulated with CD40L-expressing mouse plasmacytoma cells in the presence or absence of 1-methyl-tryptophan (MT). Supernatants were harvested after 24 hours and secreted IL-12p70 was measured by ELISA. Results are expressed as mean plus or minus SD from one representative experiment of 3 performed in triplicate (*P < .001). (C) Increased IL-6 production by DCs after siRNA-mediated knockdown of the noncanonical pathway. Immature DCs were preincubated with NBD/MUT peptides and matured for 2 days with CD40L in the presence or absence of 1-methyl-tryptophane (MT). After 48 hours, the cells were thoroughly washed and stimulated with CD40L-expressing mouse plasmacytoma cells in the presence or absence of 1-methyl-tryptophan (MT). Supernatants were harvested after 24 hours and secreted IL-6 was measured by ELISA. Results are expressed as mean plus or minus SD from 1 representative experiment of 3 performed in triplicate (*P < .001). (D) IDO-mediated inhibition of preactivated T-cell proliferation is noncanonical NF-κB pathway dependent. Monocyte-derived DCs were stimulated with CD40L in the presence or absence of methyl-tryptophan (MT). After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 3 days. Subsequently, T-cell proliferation was evaluated by [3H]thymidine incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .05).
Essential requirement of the noncanonical NF-κB pathway for CD40L-induced IDO in DCs. (A) Immature DCs were treated with control nonblocking siRNA (siC) or siRNA for the noncanonical NF-κB pathway–associated kinases NIK (siNIK) and IKKα (siIKKα). Subsequently, cells were matured for 2 days with CD40L, extensively washed, and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IKKα and IDO content, and β-actin as loading control. Densitometry was performed on IDO blots; numbers indicate percent reduction of IDO expression by siIKKα. One representative experiment of 3 is shown; densitometry includes data from all experiments (*P < .01; **P < .001). (B) Increased IL-12p70 production by DCs after siRNA-mediated knockdown of the noncanonical pathway. Immature DCs were preincubated with NBD/MUT peptides and matured for 2 days with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, the cells were thoroughly washed and stimulated with CD40L-expressing mouse plasmacytoma cells in the presence or absence of 1-methyl-tryptophan (MT). Supernatants were harvested after 24 hours and secreted IL-12p70 was measured by ELISA. Results are expressed as mean plus or minus SD from one representative experiment of 3 performed in triplicate (*P < .001). (C) Increased IL-6 production by DCs after siRNA-mediated knockdown of the noncanonical pathway. Immature DCs were preincubated with NBD/MUT peptides and matured for 2 days with CD40L in the presence or absence of 1-methyl-tryptophane (MT). After 48 hours, the cells were thoroughly washed and stimulated with CD40L-expressing mouse plasmacytoma cells in the presence or absence of 1-methyl-tryptophan (MT). Supernatants were harvested after 24 hours and secreted IL-6 was measured by ELISA. Results are expressed as mean plus or minus SD from 1 representative experiment of 3 performed in triplicate (*P < .001). (D) IDO-mediated inhibition of preactivated T-cell proliferation is noncanonical NF-κB pathway dependent. Monocyte-derived DCs were stimulated with CD40L in the presence or absence of methyl-tryptophan (MT). After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 3 days. Subsequently, T-cell proliferation was evaluated by [3H]thymidine incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .05).
Simultaneously, knockdown of IKKα or NIK resulted in strongly increased proinflammatory IL-12p70 (*P < .001) and IL-6 (*P < .001) production in DCs, which could be completely blocked by inhibition of the canonical pathway by the NBD peptide. Addition of MT to the culture had no effect on cytokine production. (Figure 4B,C). IL-10 production in CD40L-stimulated DCs was just above background level and was not affected by inhibition of canonical and/or noncanonical NF-κB signaling (data not shown). These findings reveal a more general role for noncanonical NF-κB signaling in the negative regulation of inflammation, via down-regulation of proinflammatory cytokine production in DCs.
Subsequently, we tested the functional consequences of reduced IDO expression in CD40L-stimulated DCs on the proliferative response of recently activated effector T cells. siRNA-mediated knockdown of the noncanonical pathway resulted in significantly increased effector T-cell proliferation (P < .05) (Figure 4D). Blockade of IDO activity through addition of MT to the culture also resulted in a significant increase in effector T-cell proliferation (P < .05). Addition of MT did not further increase the effector T-cell proliferation that was observed after knockdown of IKKα or NIK in DCs. These results demonstrate that, while the canonical NF-κB pathway in DCs mediates T-cell immunity, the noncanonical NF-κB pathway plays a crucial role in the negative regulation of recently activated effector T-cell responses via the induction of IDO in DCs and down-regulation of proinflammatory cytokine production in DCs.
Role of the canonical and noncanonical NF-κB pathways in the initiation and regulation of immunity
Having demonstrated the regulatory effects of IDO and the noncanonical NF-κB pathway in DCs on proinflammatory cytokine production and recently activated effector T-cell proliferation, we next set out to elucidate the consequences of CD40L-induced canonical and noncanonical NF-κB activation in DCs for the ability of these DCs to initiate adaptive immunity. Therefore, we investigated to what extent NBD or siIKKα treatment of CD40L-stimulated DCs affects the capacity of DCs to activate naive T cells. First, we examined the potential of these DCs to induce proliferation in naive T cells in a mixed lymphocyte reaction (MLR). Blockade of the canonical pathway by NBD peptide pretreatment significantly reduced naive T-cell proliferation in this model (Figure 5A). This finding may be explained by the inhibitory effects of the NBD peptide on the expression of HLA-DR and costimulatory molecules, as well as reduced levels of the T-cell stimulatory cytokine IL-12p70 in NBD-treated DCs (Figure S1A,B). However, selective knockdown of IKKα in DCs did not affect naive T-cell proliferation positively or negatively, either in NBD-treated DCs or in control DCs (Figure 5B). In addition, NBD-treated DCs did not increase apoptosis of naive CD4+ T cells and no effect of IKKα knockdown in DCs on naive T-cell apoptosis was observed (Figure 5C). Also, no effect of MT addition to the culture was observed (data not shown). In summary, canonical NF-κB inhibition in CD40L-stimulated DCs results in the generation of immunomodulatory DCs that lead to hypoproliferative naive CD4 T cells. These experiments demonstrate an important role for the canonical NF-κB pathway in DCs in the initiation of T-cell responses, whereas the noncanonical NF-κB pathway, which regulates effector responses, neither initiates nor regulates this process.
Differential contribution of canonical and noncanonical NF-κB signaling in DCs to naive T-cell proliferation and apoptosis induction. (A) Blockade of the canonical NF-κB pathway inhibits the capacity of DCs to induce proliferation in naive T cells. Immature DCs were incubated with NBD/MUT peptides and matured for 2 days with LPS or CD40L. Subsequently, the cells were thoroughly washed and loaded with Staphylococcus aureus enterotoxin B (SEB; 10 pg/mL), and different concentrations of DCs were used to stimulate naive CD4+ Th cells. The proliferative response was determined at day 5 of coculture by [3H]TdR incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments. (B) Blockade of noncanonical NF-κB signaling does not change the capacity of DCs to induce T-cell proliferation. Immature DCs were treated with siRNA, incubated with NBD/MUT peptides, and matured for 2 days with CD40L. Subsequently, the cells were thoroughly washed, loaded with SEB, and used to stimulate naive CD4+ Th cells. The proliferative response was determined at day 5 of coculture by [3H]TdR incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .01). (C) Blockade of noncanonical NF-κB signaling does not alter apoptosis induction in naive CD4+ T cells by CD40L-NBD DCs. Monocyte-derived DCs were treated with siRNA, incubated with NBD/MUT peptides and stimulated with CD40L. After 48 hours, DCs were washed extensively, loaded with SEB, and cocultured with naive CD4+ T cells for 24 hours. Subsequently, T-cell apoptosis was assessed by annexin V/propidium iodine (PI) staining and evaluated by flow cytometry. Results represent mean plus or minus SEM from 3 independent experiments.
Differential contribution of canonical and noncanonical NF-κB signaling in DCs to naive T-cell proliferation and apoptosis induction. (A) Blockade of the canonical NF-κB pathway inhibits the capacity of DCs to induce proliferation in naive T cells. Immature DCs were incubated with NBD/MUT peptides and matured for 2 days with LPS or CD40L. Subsequently, the cells were thoroughly washed and loaded with Staphylococcus aureus enterotoxin B (SEB; 10 pg/mL), and different concentrations of DCs were used to stimulate naive CD4+ Th cells. The proliferative response was determined at day 5 of coculture by [3H]TdR incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments. (B) Blockade of noncanonical NF-κB signaling does not change the capacity of DCs to induce T-cell proliferation. Immature DCs were treated with siRNA, incubated with NBD/MUT peptides, and matured for 2 days with CD40L. Subsequently, the cells were thoroughly washed, loaded with SEB, and used to stimulate naive CD4+ Th cells. The proliferative response was determined at day 5 of coculture by [3H]TdR incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .01). (C) Blockade of noncanonical NF-κB signaling does not alter apoptosis induction in naive CD4+ T cells by CD40L-NBD DCs. Monocyte-derived DCs were treated with siRNA, incubated with NBD/MUT peptides and stimulated with CD40L. After 48 hours, DCs were washed extensively, loaded with SEB, and cocultured with naive CD4+ T cells for 24 hours. Subsequently, T-cell apoptosis was assessed by annexin V/propidium iodine (PI) staining and evaluated by flow cytometry. Results represent mean plus or minus SEM from 3 independent experiments.
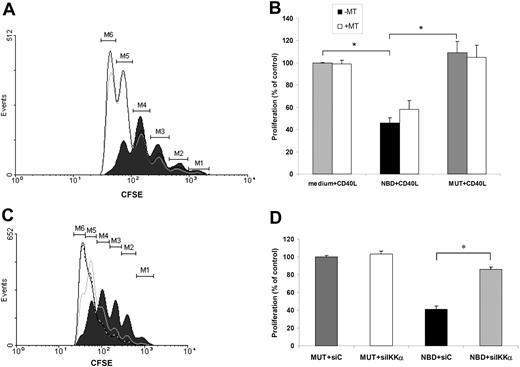
The noncanonical NF-κB pathway in DCs promotes the development of T cells with regulatory properties
Regulatory T cells (Tregs) are closely linked to the induction of IDO in DCs, for instance through ligation of CTLA-4,11,12 and, vice versa, IDO-expressing DCs have been demonstrated to give rise to Tregs in the murine system.30 Tregs have many different faces, but a general feature is their low, intrinsic proliferative capacity (reviewed in Fehervari and Sakaguchi41 and von Boehmer42 ). The low proliferative capacity of the T cells induced by CD40L-NBD DCs (CD40L-NBD T cells; Figure 5A), in combination with the massive IDO expression in the instructing DCs, could thereforepoint to the development of T cells with a suppressive effect on bystander T cells. Therefore, CD40L-NBD T cells were tested for their possible regulatory activity in a suppressor assay using 2 cell cycle tracking dyes as described previously.38 CD40L-NBD T cells clearly exhibited suppressive function, indicated by a slower rate of target cell division and progression through fewer cell cycles following anti-CD3/anti-CD28 stimulation in the coculture (Figure 6A filled histogram). CD4+ T cells derived from CD40L-MUT DCs (CD40L-MUT T cells) did not suppress target cell proliferation as the vast majority of the cells progressed through all cell cycles (Figure 6A open histogram). Setting the mean fluorescence intensity of the target cells cultured with T cells instructed by CD40L-stimulated DCs without inhibitors at 100%, we calculated the percentage of target cell proliferation in the presence of either CD40L-NBD T cells or CD40L-MUT T cells. Addition of CD40L-NBD T cells to the target cells resulted in a dramatic reduction in target cell proliferation compared with incubation with CD40L-MUT T cells (46.1% ± 2.5% vs 109.1% ± 10.2%, respectively; P < .001). The suppressive effect was not solely induced by IDO expression in CD40L-NBD DCs, as addition of MT to the DC culture did not unequivocally block suppression (Figure 6B). These data demonstrate that CD40 ligation of DCs in combination with canonical NF-κB inhibition results in the generation of T cells with suppressive function from naive CD4+ precursors, which is not absolutely dependent on IDO. No suppressor activity was observed in CD4+ T cells derived from naive precursors that interacted with LPS-NBD DCs or LPS-MUT DCs (data not shown), suggesting that noncanonical NF-κB signaling in CD40L-NBD DCs is vital to the development of these suppressive T cells. Indeed, IKKα knockdown in CD40L-NBD DCs almost completely abolished the suppressive capacity of CD40L-NBD T cells, indicated by normal proliferation of target cells in the suppressor assay (Figure 6C gray line) and an increase in target cell proliferation from 41.2% ± 3.7% to 86.0% ± 2.5% after siIKKα treatment of DCs (*P < .001) (Figure 6D). siRNA treatment of DCs by itself did not result in altered T-cell proliferation of naive CD4+ T cells that could account for the observed differences (Figure S3). Collectively, these data demonstrate an essential requirement of the noncanonical NF-κB pathway in DCs for the generation of T cells with suppressive function from naive T cells.
CD40L-NBD T cells suppress proliferation of CFSE-labeled CD4+ target cells and are generated via a noncanonical NF-κB pathway–mediated mechanism in DCs. (A) CD40L-NBD T cells suppress proliferation of CFSE-labeled CD4+ target T cells. Naive CD4+ T cells instructed by NBD-treated CD40L-stimulated DCs (CD40L-NBD T cells) were tested for their suppressive activity on proliferation of anti-CD3/CD28–stimulated CFSE-labeled CD4+ target T cells. CFSE profiles of target cells cocultured with CD40L-NBD T cells (filled histogram), target cells cocultured with CD40L-MUT T cells (black line), or target cells alone (gray line) are shown. Histograms are representative profiles from 1 of 5 independent experiments that yielded similar results. (B) Quantification of target cell proliferation in coculture with CD40L-NBD T cells or CD40L-MUT T cells, instructed by DCs in the presence or absence of 1-methyl-tryptophan (MT). Data are expressed as percent proliferation compared with control (medium + CD40L) and represent mean plus or minus SEM from 5 independent experiments (*P < .001). (C) Suppressive capacity of CD40L-NBD T cells is induced by DCs via a noncanonical NF-κB pathway–mediated mechanism. CD40L-NBD DCs were treated with siRNA for IKKα (siIKKα) or control siRNA (siC), and subsequently the suppressive activity of T cells instructed by these DCs (siIKKα or siC CD40L-NBD T cells, respectively) was tested in the same assay as described in panel A. CFSE profiles of target cells cocultured with siC CD40L-NBD T cells (filled histogram) and target cells cocultured with siIKKα CD40L-NBD T cells (gray line) were compared with CD40L-MUT T cells (black line). Shown are representative profiles from 1 of 3 independent experiments that yielded similar results. (D) Quantification of target cell proliferation in coculture with T cells derived from siRNA-treated CD40L-stimulated NBD/MUT DCs. Data are expressed as percent proliferation compared with control (medium + CD40L) and represent mean plus or minus SEM from 3 independent experiments (*P < .001).
CD40L-NBD T cells suppress proliferation of CFSE-labeled CD4+ target cells and are generated via a noncanonical NF-κB pathway–mediated mechanism in DCs. (A) CD40L-NBD T cells suppress proliferation of CFSE-labeled CD4+ target T cells. Naive CD4+ T cells instructed by NBD-treated CD40L-stimulated DCs (CD40L-NBD T cells) were tested for their suppressive activity on proliferation of anti-CD3/CD28–stimulated CFSE-labeled CD4+ target T cells. CFSE profiles of target cells cocultured with CD40L-NBD T cells (filled histogram), target cells cocultured with CD40L-MUT T cells (black line), or target cells alone (gray line) are shown. Histograms are representative profiles from 1 of 5 independent experiments that yielded similar results. (B) Quantification of target cell proliferation in coculture with CD40L-NBD T cells or CD40L-MUT T cells, instructed by DCs in the presence or absence of 1-methyl-tryptophan (MT). Data are expressed as percent proliferation compared with control (medium + CD40L) and represent mean plus or minus SEM from 5 independent experiments (*P < .001). (C) Suppressive capacity of CD40L-NBD T cells is induced by DCs via a noncanonical NF-κB pathway–mediated mechanism. CD40L-NBD DCs were treated with siRNA for IKKα (siIKKα) or control siRNA (siC), and subsequently the suppressive activity of T cells instructed by these DCs (siIKKα or siC CD40L-NBD T cells, respectively) was tested in the same assay as described in panel A. CFSE profiles of target cells cocultured with siC CD40L-NBD T cells (filled histogram) and target cells cocultured with siIKKα CD40L-NBD T cells (gray line) were compared with CD40L-MUT T cells (black line). Shown are representative profiles from 1 of 3 independent experiments that yielded similar results. (D) Quantification of target cell proliferation in coculture with T cells derived from siRNA-treated CD40L-stimulated NBD/MUT DCs. Data are expressed as percent proliferation compared with control (medium + CD40L) and represent mean plus or minus SEM from 3 independent experiments (*P < .001).
Discussion
In the current study, we demonstrate that the noncanonical NF-κB pathway is essential for IDO expression, and controls the production of proinflammatory cytokines induced by the canonical NF-κB pathway in CD40L-activated DCs. We propose that the noncanonical NF-κB pathway, induced by CD40L expressed on T cells, controls T-cell activation in vivo. The regulation of adaptive immunity by the noncanonical NF-κB pathway is substantiated by the finding that CD40L-induced IDO is enzymatically active and mediates apoptosis of recently activated effector T cells. In addition, noncanonical NF-κB signaling, in combination with inhibition of the canonical pathway in DCs, promotes the development of T cells with suppressive function from naive CD4+ T-cell precursors.
Activation of canonical NF-κB signaling is well controlled by the induction of IκBα transcription following NF-κB DNA binding, which attenuates the activation of this important proinflammatory pathway. To date, immune regulation by products of the noncanonical NF-κB pathway is largely unknown, except for recent studies in macrophages that suggest a role for IKKα in the negative regulation of inflammation via either control of IKKβ activity27 or accelerating the turnover of proinflammatory RelA and c-Rel–containing dimers and their removal from proinflammatory gene promoters.26 We found that selective knockdown of the noncanonical pathway using siRNA for IKKα or NIK in DCs also resulted in increased proinflammatory cytokine production, suggesting a similar negative regulation also takes place in DCs. Our present data also indicate that IKKα-mediated IDO expression could provide an additional mechanism of immune regulation, as IDO not only prevents further activation of DCs via tryptophan catabolism,11 but also regulates adaptive immunity induced by these DCs by controlling the size and the activity of the induced effector T-cell population and the generation of Tregs.30 Based on these findings, we propose that noncanonical NF-κB signaling in DCs is important in the negative regulation of inflammation and immunity, to some extent by IDO-expressing cells.
Functional IDO expression following CD40 ligation has been described previously,3,9,10 although a recent report demonstrates that CTLA-4-Ig is unable to induce tryptophan catabolism in previously CD40L-primed DCs.43 This difference could be explained by different experimental conditions, such as longer maturation time in our experiments (48 hours vs 24 hours) and different sensitivity of the assays used. In addition, the indicated report did not evaluate IDO expression by CD40 ligation alone, without CTLA-4-Ig stimulation.43 Conversely, we did not study the effects of B7 engagement on CD40L-induced IDO expression, but investigated the contribution of canonical and noncanonical NF-κB signaling to this process in DCs. Interestingly, we found that CD40L stimulation in combination with specific inhibition of canonical, IKK complex–mediated NF-κB activation resulted in the generation of a regulatory DC phenotype. These cells were characterized by low expression of MHC class II, costimulatory molecules (eg, CD86), and cytokines (eg, IL-12p70) but unaltered expression of IDO. These DCs had a poor capacity to induce proliferation in naive T cells, which was not caused by IDO expression (data not shown) and may very well be the result of poor MHC class II and CD86 expression due to IKKβ inhibition,44 but promoted the development of T cells with suppressive function, which was entirely dependent on noncanonical NF-κB signaling. Of interest, addition of MT to neutralize IDO activity in DCs did not block the induction of suppressive T cells. This may in part be explained by the fact that MT has been demonstrated to also have other effects on DCs that do not correlate with inhibition of IDO activity, such as activation of MAPK.45 Based on these findings, we propose that noncanonical NF-κB signaling in DCs negative regulates immunity through the induction of Tregs.
Various Treg categories have been described, such as naturally occurring CD4+CD25+ T cells or extrathymically induced Tr1 and Th3 cells (reviewed in Fehervari and Sakaguchi41 and von Boehmer42 ). The CD40L-NBD T cells do not seem to have clear characteristics of known Treg categories. They hardly produce IL-10, and addition of neutralizing antibodies against IL-10 and/or TGF-β did not inhibit their suppressive function. Furthermore, CD40L-NBD T cells nonsignificantly differentially express the Treg marker FoxP3 and the surface markers GITR, Lag3, and CTLA-4 (Table S1), which may indicate an overall suppressive character of these cells. Interestingly, CD40L-NBD T cells produce significantly less IFN-γ and more IL-4 (Figure S4), a cytokine that has been associated with maintenance and even enhancement of regulatory T-cell function.46-49 Furthermore, induced Tregs have often reported to be IL-4 positive.50-54 This finding is in line with earlier observations that IDO is able to induce apoptosis selectively in Th1 cells, but not in Th2 cells.6 In short, the NBD-CD40L DC-derived T cells are induced via a noncanonical NF-κB–dependent mechanism, but the exact phenotype of these cells and their mechanism of suppression require further investigations. Nevertheless, these induced T cells with suppressive function promise to have great potential for cellular immunotherapy using DCs.
In the current study, we demonstrate that the regulatory properties of the noncanonical NF-κB pathway in DCs are optimal when canonical NF-κB activity is suppressed. We propose that the balance between the expression of inflammatory and T-cell stimulatory molecules on the one hand, and tolerogenic molecules such as IDO on the other hand, is of key importance for the capacity of DCs to induce or suppress immunity. All known activators of the noncanonical NF-κB pathway simultaneously activate the canonical pathway (reviewed in Hayden and Ghosh24 ), but whether these factors also induce IDO remains to be investigated. However, the IKKα-mediated accelerated turnover of proinflammatory canonical NF-κB dimers will certainly add to the control of immune responses.26 We propose that selective inhibition of canonical NF-κB signaling will tip the balance and results in a relative increase in mechanisms associated with noncanonical NF-κB signaling, such as IDO expression after CD40 ligation. Another possible explanation for the enhanced effects of IDO in NBD-treated DCs may be the fact that IL-6 has been demonstrated to negatively regulate IDO expression through the induction of SOCS3, thereby preventing tryptophan catabolism.55 Canonical NF-κB blockade results in reduced IL-6 production in DCs, which will therefore result in increased IDO activity. Therefore, antigen presented by IKKβ-inhibited DCs under inflammatory conditions in the abundant presence of CD40L-expressing T cells may lead to tolerance induction and the development of Tregs. This can be exploited in a number of therapeutic settings, for example in treatment of autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis, as well as in allergy or transplant rejection. Specific inhibition of the canonical pathway-associated kinase IKKβ, while leaving the noncanonical pathway intact, would potentiate regulatory mechanisms. Indeed, IKKβ inhibition in DCs has been demonstrated to result in immunoregulation both in vitro48,56 and in vivo.57 Here, we suggest that the induction of such Tregs occurs via noncanonical NF-κB signaling in DCs. Conversely, when swift immune activation is required, for example in severe infections, IKKα inhibitors may be applied to boost the innate immune response via both IDO inhibition and the recently discovered prevention of RelA and c-Rel turnover26 or delayed restoration of IκBα,27 resulting in protracted NF-κB activation.
In conclusion, we demonstrate an essential requirement of the noncanonical NF-κB pathway for regulatory functions in DCs, including effective IDO induction and negative regulation of proinflammatory cytokine production. Selective canonical NF-κB inhibition results in DCs that induce T cells with suppressive function via a noncanonical pathway-dependent mechanism. This discovery presents a novel mechanism of action via which IKKβ inhibitors may exert their beneficial effects and has important implications for the use of anti-inflammatory drugs that selectively inhibit the canonical NF-κB pathway and therefore would be beneficial for immunotherapy of transplantation, and autoimmune and allergic diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant NR 01-1-302 from the Dutch Arthritis Association (S.W.T.).
We thank Dr R. Lutter for helpful discussions and supply of reagents, and Drs R.A.W. van Lier and E. A. Wierenga for critically reading the paper.
Authorship
Contribution: S.W.T. designed and performed research, collected, analyzed and interpreted data, performed statistical analysis, and wrote the paper; N.H., J.H.N.S., and K.F.S. performed research, and collected, analyzed, and interpreted data; M.J.M. and S.G. contributed vital new reagents or analytical tools, and analyzed and interpreted data; M.J.V., M.L.K., and P.P.T. analyzed and interpreted data, and drafted the paper; and E.C.J. designed research, analyzed and interpreted data, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul P. Tak, Clinical Immunology & Rheumatology, F4-218, AMC/University of Amsterdam, PO Box 22660, 1100 DD Amsterdam, the Netherlands; e-mail: p.p.tak@amc.uva.nl.

![Figure 1. CD40L stimulation of DCs results in functional IDO expression. (A) CD40L stimulation of DCs induces IDO protein expression. Monocyte-derived DCs were matured with LPS or CD40L. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (B) IDO is efficiently induced by various sources of CD40L. DCs were either left unstimulated or stimulated with J558-CD40L–transfected cells, sCD40L (500 ng/mL; Immunex, Seattle, WA), or activated CD4+ T cells. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content, and β-actin as loading control. One representative experiment of 3 is shown. (C) CD40L-induced IDO protein is enzymatically functional. Monocyte-derived DCs were either unstimulated or matured with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, cells were harvested, extensively washed in cold PBS, and freeze-dried. Subsequently, IDO enzymatic activity in the samples was evaluated by testing the capacity to degrade tryptophan into kynurenine. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05). (D) HPLC analysis of kynurenine and tryptophan levels in culture supernatants. Monocyte-derived DCs were either unstimulated or matured with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, supernatants were harvested and IDO enzymatic activity in the samples was evaluated by measuring tryptophan and kynurenine by HPLC. Results are expressed as kynurenine-tryptophan ratio and represent mean plus or minus SEM from 3 independent experiments (*P < .05). (E) CD40L-induced IDO induces apoptosis of preactivated T cells. Monocyte-derived DCs were stimulated with CD40L or LPS in the presence or absence of MT. After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 24 hours. Subsequently, T-cell apoptosis was assessed by annexin V/propidium iodine (PI) staining and evaluated by flow cytometry. The frequency of apoptosis in T cells stimulated with anti-CD3/CD28 Ab alone is 3% to 5%. Results represent mean plus or minus SEM from 3 independent experiments (*P < .05). (F) CD40L-induced IDO reduces proliferation of preactivated T cells. Monocyte-derived DCs were stimulated with CD40L or LPS in the presence or absence of MT. After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 3 days. Subsequently, T-cell proliferation was evaluated by [3H]TdR incorporation. The cpm for preactivated CD4+ T cells in the absence of DCs was 2515 plus or minus 389 without MT, and 1694 plus or minus 183 in the presence of MT. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .05). (G) CD40L stimulation of BDCA1+ and BDCA4+ cells also results in IDO protein expression. BDCA1+ and BDCA4+ DCs were freshly isolated from peripheral blood and stimulated with CD40L-expressing J558 cells. After 48 hours, the cells were extensively washed and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IDO content. Representative blots from 4 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2006-11-056010/6/m_zh80160705880001.jpeg?Expires=1769138213&Signature=VZBl1aeypAYoLZ92eKUiJZF0Jq6mqeaeR71NKyYosIYlaFkrL0fILx5lq8V72qjAXdqKQkfFq1cTbjzGHLN-Rc63p1YCETfq~rRFGEIYvXBF3hNyjdfryyBUa0btDThZ1PS8ljIbGfLayAdBshT32ocl-2MUG3UFT1h99k0P4kzfL-jWUGhBXkFtleyqmZof2NSboFG4t-m~SU12rFnvc77jh9bNLhGqIPOpvFP00tPjucFhaCKabLEecV6Nb7Aqtq6XmiCqz4YRl4c0d4MfNWUz5~DP0vRrImlSnpy4xqJF~wjrLeBKxZNz3NaHvbF6k4uf44knMVVXsaj20HFW5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Essential requirement of the noncanonical NF-κB pathway for CD40L-induced IDO in DCs. (A) Immature DCs were treated with control nonblocking siRNA (siC) or siRNA for the noncanonical NF-κB pathway–associated kinases NIK (siNIK) and IKKα (siIKKα). Subsequently, cells were matured for 2 days with CD40L, extensively washed, and lysed in sample buffer. Cell lysates were analyzed by Western blotting for IKKα and IDO content, and β-actin as loading control. Densitometry was performed on IDO blots; numbers indicate percent reduction of IDO expression by siIKKα. One representative experiment of 3 is shown; densitometry includes data from all experiments (*P < .01; **P < .001). (B) Increased IL-12p70 production by DCs after siRNA-mediated knockdown of the noncanonical pathway. Immature DCs were preincubated with NBD/MUT peptides and matured for 2 days with CD40L in the presence or absence of 1-methyl-tryptophan (MT). After 48 hours, the cells were thoroughly washed and stimulated with CD40L-expressing mouse plasmacytoma cells in the presence or absence of 1-methyl-tryptophan (MT). Supernatants were harvested after 24 hours and secreted IL-12p70 was measured by ELISA. Results are expressed as mean plus or minus SD from one representative experiment of 3 performed in triplicate (*P < .001). (C) Increased IL-6 production by DCs after siRNA-mediated knockdown of the noncanonical pathway. Immature DCs were preincubated with NBD/MUT peptides and matured for 2 days with CD40L in the presence or absence of 1-methyl-tryptophane (MT). After 48 hours, the cells were thoroughly washed and stimulated with CD40L-expressing mouse plasmacytoma cells in the presence or absence of 1-methyl-tryptophan (MT). Supernatants were harvested after 24 hours and secreted IL-6 was measured by ELISA. Results are expressed as mean plus or minus SD from 1 representative experiment of 3 performed in triplicate (*P < .001). (D) IDO-mediated inhibition of preactivated T-cell proliferation is noncanonical NF-κB pathway dependent. Monocyte-derived DCs were stimulated with CD40L in the presence or absence of methyl-tryptophan (MT). After 48 hours, cells were cocultured with anti-CD3/anti-CD28 preactivated CD4+ T cells in the presence or absence of MT for 3 days. Subsequently, T-cell proliferation was evaluated by [3H]thymidine incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2006-11-056010/6/m_zh80160705880004.jpeg?Expires=1769138213&Signature=u4mGXmUDDnXfMZm25nofwF-DDwjAwFIQQmXjIfK6VzNkMAjjlaKTZTvXj3MrsmmVNcv25PEIxLYocxOTjzUOkRWLighZVunuAq1g6bMOFr5zaggQLiSWWAmKSByQ8mHJVWWXYBBr9aNoCn-SPhWfi18bGWKM-YUEsTCh1QrhskAr7Cu5An26JZohikjyBhVTjeEd7WlArZXs90of9wEjHHIBEk~-994pkvLaG6BXB-5pjScg1pz2mNh9OVbUO3TDcxkraJe9ixKd4UwvDukYf-X29DuDMPNkKJ~NLvoM71nNAvCNenXZosybdwoDwjhKCd3UMbWPmzEFWiAW6glPJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Differential contribution of canonical and noncanonical NF-κB signaling in DCs to naive T-cell proliferation and apoptosis induction. (A) Blockade of the canonical NF-κB pathway inhibits the capacity of DCs to induce proliferation in naive T cells. Immature DCs were incubated with NBD/MUT peptides and matured for 2 days with LPS or CD40L. Subsequently, the cells were thoroughly washed and loaded with Staphylococcus aureus enterotoxin B (SEB; 10 pg/mL), and different concentrations of DCs were used to stimulate naive CD4+ Th cells. The proliferative response was determined at day 5 of coculture by [3H]TdR incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments. (B) Blockade of noncanonical NF-κB signaling does not change the capacity of DCs to induce T-cell proliferation. Immature DCs were treated with siRNA, incubated with NBD/MUT peptides, and matured for 2 days with CD40L. Subsequently, the cells were thoroughly washed, loaded with SEB, and used to stimulate naive CD4+ Th cells. The proliferative response was determined at day 5 of coculture by [3H]TdR incorporation. Data are presented as mean cpm plus or minus SD of triplicate cultures. Results are representative of 3 independent experiments (*P < .01). (C) Blockade of noncanonical NF-κB signaling does not alter apoptosis induction in naive CD4+ T cells by CD40L-NBD DCs. Monocyte-derived DCs were treated with siRNA, incubated with NBD/MUT peptides and stimulated with CD40L. After 48 hours, DCs were washed extensively, loaded with SEB, and cocultured with naive CD4+ T cells for 24 hours. Subsequently, T-cell apoptosis was assessed by annexin V/propidium iodine (PI) staining and evaluated by flow cytometry. Results represent mean plus or minus SEM from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2006-11-056010/6/m_zh80160705880005.jpeg?Expires=1769138213&Signature=FOoPYbqfItM3yELYnCbNh5A2BMVWMjF8~ku1brGllcGgTYXRKhjWMltosis9PqOcieXItnEGYaiqJXWgv3OIlrxA-tfInU6BxyAemJs3D839aecg7CGnZxmVOrRoO7zcdh4ZIm~Gyh-ZQ7DCcz-AncvsxtmBOrE2uF84P8a5zWTZACkwThcIMINj7t~TXfYp0VsCxxztVQsp018UX46vuJeyks6ZYo4iTE82qR1xxbBbBIzW1e7bqc6ai1m6btpLEqn0CUo9KKQxLX6bc2utboTeN-oN2QGuGij1bXEM5X1zgvzMkiXd3BLgVbVU-oO4TUFYfqHTrGNvAPDPFssu4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal