Abstract
The prognostic impact of tyrosine kinase domain (TKD) mutations of the fms-like tyrosine kinase-3 (FLT3) gene in acute myeloid leukemia (AML) is currently uncertain. To resolve this issue we screened 1107 young adult nonacute promyelocytic leukemia AML patients with known FLT3 internal tandem duplication (ITD) status for FLT3/TKDs; they were detected in 127 (11%) cases. Mutations were associated with a high white cell count (P =.006) and patients with inv(16) (P = .005) but were infrequent in patients with adverse cytogenetics and secondary AML. Overall survival (OS) at 5 years was 53% and 37% for FLT3/TKD mutant and wild-type patients respectively (odds ratio, 0.72; 95% confidence interval, 0.58 to 0.89; P = .002). For both the cumulative incidence of relapse and OS the difference in outcome between FLT3/ITDs and FLT3/TKDs was highly significant (P < .001). In multivariate analysis, impact of FLT3/TKDs on OS when including all mutant-positive patients was not significant, but patients with high-level mutations (more than 25% mutant) had a significantly improved outcome (P = .004). The novel finding that biologically distinct activating mutations of the same gene can be associated with markedly different clinical outcomes has implications for risk stratification and therapy and is significant to the understanding of chemoresistance in AML.
Introduction
The identification of aberrant protein tyrosine kinase (TK) signaling pathways in many human cancers has led to important diagnostic and therapeutic advances in recent years.1 A classic example is Fms-like tyrosine kinase 3 (FLT3), a class III TK receptor expressed on the surface of normal hematopoietic progenitors and blast cells from most cases of acute myeloid leukemia (AML),2 which is constitutively activated in many cases of AML due to the presence of internal tandem duplications (ITDs) within the juxtamembrane (JM) domain.3–5 FLT3/ITDs occur in approximately 25% of cases of AML in younger adults, and in nonacute promyelocytic leukemia (non-APL) AML they predict for increased relapse rate from complete remission (CR) and reduced survival.3–7
Subsequent to the identification of FLT3/ITDs in AML, it was noted that primary AML blasts from a number of patients had constitutively phosphorylated FLT3 in the absence of an FLT3/ITD,8 raising the possibility of other mechanisms of aberrant FLT3 activation. Shortly afterward, 2 groups independently reported activating mutations affecting codons 835 and 836 in the FLT3 second TK domain (TKD).9,10 Presence of these mutations has been confirmed by others, and the overall incidence in AML was reported to be approximately 7%.11–15 A number of different mutations have been reported. Most are point mutations such as substitution of asparate residue 835 with a tyrosine (D835Y, the most frequent mutation), histidine, valine, or glutamate; other alterations include small deletions (Δ835, Δ836) and insertions. All mutations initially described disrupted an EcoRV restriction enzyme cutting site that facilitated a simple screening technique using digestion of polymerase chain reaction (PCR) products. Subsequently, however, a number of other point mutations have been reported at codons 839, 841, and 84216–18 as well as a 2 amino acid insertion between codons 840 and 841,19 all of which occur outside of the EcoRV restriction digest site.
The clinical significance of FLT3/TKD mutations in AML is unclear, and different groups have reported either no significant impact of an FLT3/TKD mutation on overall survival (OS)9,12,13,15 or an adverse prognostic impact.11 The differences in outcome in these studies may partly be due to the low incidence of the mutations, such that relatively small numbers of mutant-positive patients have been investigated, as well as heterogeneity in both treatment protocols and characteristics of the patients included. A recent meta-analysis of the above studies has suggested that the outcome of FLT3/TKD mutations may be similar to that of FLT3/ITDs.20 This has important implications for the application of risk-adapted treatment in these patients, particularly in view of the debate regarding optimal therapy for FLT3/ITD+ patients6,21 and the current interest in molecularly targeted therapy with FLT3 inhibitors.22
To address this issue, we have screened a large cohort of young adult non-APL AML patients with known FLT3/ITD status, treated in the United Kingdom Medical Research Council (MRC) AML10 and AML12 trials, for FLT3/TKD mutations using denaturing high-performance liquid chromatography (dHPLC). We have examined the impact of mutations on clinical outcome and compared this with the impact of FLT3/ITDs.
Patients, materials, and methods
Patients
Genomic DNA (gDNA) was available from blast cells of 1107 adults with AML entered into either the United Kingdom MRC AML10 (n = 380) or AML12 (n = 727) trials. Most patients had de novo AML (n = 1024, 93%). Median age at trial entry was 42 years of age; all patients were 15 years of age or older, and only 27 were over 60 years of age. Demographic details of the patient cohort are given in Table 1. Ethical approval for the trials and tissue collection for research was obtained from the Multi-Center Research Ethics Committee of Wales and local research ethics committees as appropriate, and informed consent was obtained in accordance with the Declaration of Helsinki.
Clinical and demographic characteristics of 1107 non-APL AML patients in the total cohort
| . | Total no. . | FLT3/TKD− (% of total TKD−) . | FLT3/TKD+ (% of total TKD+) . | Percent FLT3/TKD+ . | P . | Percent FLT3/ITD+ . |
|---|---|---|---|---|---|---|
| Total | 1107 | 980 | 127 | 11 | .3 | 26 |
| AML10 | 380 | 331 (34) | 49 (39) | 13 | N/A | 23 |
| AML12 | 727 | 649 (66) | 78 (61) | 11 | N/A | 27 |
| Type of AML | .02 | |||||
| De novo | 1024 | 900 (92) | 124 (98) | 12 | N/A | 26 |
| Secondary | 83 | 80 (8) | 3 (2) | 4 | N/A | 23 |
| FAB type | .02 | |||||
| M0 | 38 | 35 (4) | 3 (3) | 8 | N/A | 8 |
| M1 | 215 | 196 (21) | 19 (16) | 9 | N/A | 29 |
| M2 | 323 | 295 (32) | 28 (23) | 9 | N/A | 24 |
| M4 | 277 | 231 (25) | 46 (38) | 17 | N/A | 30 |
| M5 | 130 | 112 (12) | 18 (15) | 14 | N/A | 32 |
| M6 | 30 | 24 (3) | 6 (5) | 20 | N/A | 7 |
| M7 | 15 | 15 (2) | 0 | 0 | N/A | 7 |
| RAEB-t | 19 | 19 (2) | 0 | 0 | N/A | 5 |
| Bilineage | 1 | 1 (<.5) | 0 | 0 | N/A | 100 |
| Unknown | 59 | 52 | 7 | 12 | N/A | 19 |
| Sex | .07 | |||||
| Female | 570 | 495 (51) | 75 (59) | 13 | N/A | 27 |
| Male | 537 | 485 (49) | 52 (41) | 10 | N/A | 25 |
| Performance status | .3 | |||||
| 0 | 445 | 398 (41) | 47 (37) | 11 | N/A | 27 |
| 1 | 285 | 253 (26) | 32 (25) | 11 | N/A | 23 |
| 2 | 259 | 227 (23) | 32 (25) | 12 | N/A | 25 |
| 3 | 102 | 89 (9) | 13 (10) | 13 | N/A | 27 |
| 4 | 16 | 13 (1) | 3 (2) | 19 | N/A | 25 |
| Age, y | .6 | |||||
| 15-29 | 216 | 192 (20) | 24 (19) | 11 | N/A | 21 |
| 30-39 | 242 | 220 (22) | 22 (17) | 9 | N/A | 24 |
| 40-49 | 317 | 272 (28) | 45 (35) | 14 | N/A | 28 |
| 50-59 | 305 | 273 (28) | 32 (25) | 10 | N/A | 27 |
| 60 or older | 27 | 23 (2) | 4 (3) | 15 | N/A | 41 |
| Median | 42 | 42 | 43 | N/A | N/A | N/A |
| WBC, × 109/L | .006 | |||||
| Less than 10 | 317 | 292 (30) | 25 (20) | 8 | N/A | 10 |
| 10-19.9 | 173 | 156 (16) | 17 (13) | 10 | N/A | 23 |
| 20-49.9 | 252 | 220 (23) | 32 (25) | 13 | N/A | 27 |
| 50-99.9 | 173 | 144 (15) | 29 (23) | 17 | N/A | 35 |
| 100 or above | 171 | 148 (15) | 23 (18) | 13 | N/A | 47 |
| Unknown | 21 | 20 | 1 | 5 | N/A | 19 |
| Median | 24.5 | 23 | 38.3 | N/A | N/A | N/A |
| . | Total no. . | FLT3/TKD− (% of total TKD−) . | FLT3/TKD+ (% of total TKD+) . | Percent FLT3/TKD+ . | P . | Percent FLT3/ITD+ . |
|---|---|---|---|---|---|---|
| Total | 1107 | 980 | 127 | 11 | .3 | 26 |
| AML10 | 380 | 331 (34) | 49 (39) | 13 | N/A | 23 |
| AML12 | 727 | 649 (66) | 78 (61) | 11 | N/A | 27 |
| Type of AML | .02 | |||||
| De novo | 1024 | 900 (92) | 124 (98) | 12 | N/A | 26 |
| Secondary | 83 | 80 (8) | 3 (2) | 4 | N/A | 23 |
| FAB type | .02 | |||||
| M0 | 38 | 35 (4) | 3 (3) | 8 | N/A | 8 |
| M1 | 215 | 196 (21) | 19 (16) | 9 | N/A | 29 |
| M2 | 323 | 295 (32) | 28 (23) | 9 | N/A | 24 |
| M4 | 277 | 231 (25) | 46 (38) | 17 | N/A | 30 |
| M5 | 130 | 112 (12) | 18 (15) | 14 | N/A | 32 |
| M6 | 30 | 24 (3) | 6 (5) | 20 | N/A | 7 |
| M7 | 15 | 15 (2) | 0 | 0 | N/A | 7 |
| RAEB-t | 19 | 19 (2) | 0 | 0 | N/A | 5 |
| Bilineage | 1 | 1 (<.5) | 0 | 0 | N/A | 100 |
| Unknown | 59 | 52 | 7 | 12 | N/A | 19 |
| Sex | .07 | |||||
| Female | 570 | 495 (51) | 75 (59) | 13 | N/A | 27 |
| Male | 537 | 485 (49) | 52 (41) | 10 | N/A | 25 |
| Performance status | .3 | |||||
| 0 | 445 | 398 (41) | 47 (37) | 11 | N/A | 27 |
| 1 | 285 | 253 (26) | 32 (25) | 11 | N/A | 23 |
| 2 | 259 | 227 (23) | 32 (25) | 12 | N/A | 25 |
| 3 | 102 | 89 (9) | 13 (10) | 13 | N/A | 27 |
| 4 | 16 | 13 (1) | 3 (2) | 19 | N/A | 25 |
| Age, y | .6 | |||||
| 15-29 | 216 | 192 (20) | 24 (19) | 11 | N/A | 21 |
| 30-39 | 242 | 220 (22) | 22 (17) | 9 | N/A | 24 |
| 40-49 | 317 | 272 (28) | 45 (35) | 14 | N/A | 28 |
| 50-59 | 305 | 273 (28) | 32 (25) | 10 | N/A | 27 |
| 60 or older | 27 | 23 (2) | 4 (3) | 15 | N/A | 41 |
| Median | 42 | 42 | 43 | N/A | N/A | N/A |
| WBC, × 109/L | .006 | |||||
| Less than 10 | 317 | 292 (30) | 25 (20) | 8 | N/A | 10 |
| 10-19.9 | 173 | 156 (16) | 17 (13) | 10 | N/A | 23 |
| 20-49.9 | 252 | 220 (23) | 32 (25) | 13 | N/A | 27 |
| 50-99.9 | 173 | 144 (15) | 29 (23) | 17 | N/A | 35 |
| 100 or above | 171 | 148 (15) | 23 (18) | 13 | N/A | 47 |
| Unknown | 21 | 20 | 1 | 5 | N/A | 19 |
| Median | 24.5 | 23 | 38.3 | N/A | N/A | N/A |
P values relate to the incidence of FLT3/TKD mutations using the Mantel-Haenszel test for trend in age and WBC count and, otherwise, χ2 or the Fisher exact test for heterogeneity.
N/A indicates not applicable.
Analysis of FLT3 mutations
Screening for FLT3/ITD and FLT3/TKD mutations.
Screening for FLT3/ITDs was performed as previously described.23 For dHPLC analysis to screen for FLT3/TKD mutations, PCR was used to amplify a 278 base pair (bp) fragment covering FLT3 exon 20 (previously designated exon 17) and the flanking intronic regions from approximately 100 ng gDNA with Optimase polymerase (Transgenomic, Elancourt, France), manufacturer's recommended conditions, primers 20F and 20R (Table 2), 32 cycles of amplification, and an annealing temperature of 63°C. Products were denatured and run on dHPLC (Transgenomic WAVE) at optimal melting temperatures of 59.0°C and 62.0°C calculated using WAVEMAKER software (Transgenomic). Screening using PCR and EcoRV restriction enzyme digestion was performed as previously described.7
PCR primers and restriction enzyme digests used for the detection of FLT3/TKD mutations
| . | Primers . | PCR temperature, °C . | Restriction endonuclease . | WT PCR product size, bp . | Digest, mutant, bp . |
|---|---|---|---|---|---|
| dHPLC | |||||
| gDNA | 20F: 5′-CATCACCGGTACCTCCTACTG-3′ | 63 | N/A | 278 | N/A |
| 20R: 5′-TAACGACACAACACAAAATAGCCGT-3′ | |||||
| Mutation | |||||
| D835Y | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGATCGA-3′ | 62 | ClaI | 149 | 122 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| D835H | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGGTCGA-3′ | 64 | HincII | 149 | 121 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| D835E | F: 5′-CATCACCGGTACCTCCTACTG-3′ | 63 | DpnII | 278 | 188 |
| R: 5′-TAACGACACAACACAAAATAGCCGT-3′ | |||||
| D835V | F: 5′-CATCACCGGTACCTCCTACTG-3′ | 64 | HincII | 217 | 187 |
| R(mm): 5′-CCTGACAACATAGTTGGAATCACTCATGTT-3′ | |||||
| Δ836 | F(mm): 5′-AAGATATGTGACTTTGGATTGGCTCGACAT-3′ | 64 | NdeI | 146 | 114 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| Δ835 | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGTTCGA-3′ | 64 | BstBI | 149 | 119 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| D835N | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGTTCGA-3′ | 64 | BstBI | 149 | 122 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ |
| . | Primers . | PCR temperature, °C . | Restriction endonuclease . | WT PCR product size, bp . | Digest, mutant, bp . |
|---|---|---|---|---|---|
| dHPLC | |||||
| gDNA | 20F: 5′-CATCACCGGTACCTCCTACTG-3′ | 63 | N/A | 278 | N/A |
| 20R: 5′-TAACGACACAACACAAAATAGCCGT-3′ | |||||
| Mutation | |||||
| D835Y | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGATCGA-3′ | 62 | ClaI | 149 | 122 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| D835H | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGGTCGA-3′ | 64 | HincII | 149 | 121 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| D835E | F: 5′-CATCACCGGTACCTCCTACTG-3′ | 63 | DpnII | 278 | 188 |
| R: 5′-TAACGACACAACACAAAATAGCCGT-3′ | |||||
| D835V | F: 5′-CATCACCGGTACCTCCTACTG-3′ | 64 | HincII | 217 | 187 |
| R(mm): 5′-CCTGACAACATAGTTGGAATCACTCATGTT-3′ | |||||
| Δ836 | F(mm): 5′-AAGATATGTGACTTTGGATTGGCTCGACAT-3′ | 64 | NdeI | 146 | 114 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| Δ835 | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGTTCGA-3′ | 64 | BstBI | 149 | 119 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ | |||||
| D835N | F(mm): 5′-GTGAAGATATGTGACTTTGGATTGGTTCGA-3′ | 64 | BstBI | 149 | 122 |
| R: 5′-CAGTGAGTGCAGTTGTTTACCATGATAACG-3′ |
F indicates forward primer; R, reverse primer; mm, mismatch primer (mismatch underlined).
Identification of FLT3/TKD mutations.
PCR products were obtained using 35 cycles of amplification with BIOTAQ DNA polymerase (Bioline, London, United Kingdom), manufacturer's recommended conditions, and annealing temperatures as specified (Table 2). They were sequenced using the DTCS Quick Start kit (Beckman Coulter UK, Buckinghamshire, United Kingdom) and analyzed on a CEQ8000 DNA Genetic Analysis System (Beckman Coulter). Some mutations were confirmed using mutation-specific restriction enzyme digestion of PCR products by designing mismatch primers that discriminated between wild-type (WT) and mutant alleles (Table 2). For samples in which the mutant level was too low to be identified by sequencing or restriction digest, PCR products were cloned (TOPO TA Cloning; Invitrogen, Paisley, United Kingdom) and sequenced.
Quantification of FLT3/TKD mutations.
For FLT3/TKD mutations, the relative level of mutant was quantified using PCR as described but with a fluorescently labeled primer and fragment analysis on the CEQ8000 DNA Genetic Analysis System. For deletions and insertions, undigested PCR products were analyzed; for other mutations products were first digested with either a mutation-specific restriction enzyme or EcoRV.
Therapy
End points
CR was defined as a normocellular bone marrow (BM) aspirate with normal trilineage maturation and less than 5% blasts without a requirement for regeneration of peripheral counts. In practice, 97% of patients who achieved the protocol-prescribed definition of CR had peripheral regeneration of neutrophils to 1.0 × 109/L and platelets to 100 × 109/L. Remission failures were classified by the clinicians as either partial remission (5% to 15% blasts or less than 5% blasts but a hypocellular BM), resistant disease (RD) defined as greater than 15% blasts in the BM, or induction death (ID) (ie, related to treatment or hypoplasia). When a clinician's evaluation was not available, deaths within 30 days of trial entry were classified as IDs and all other failures to achieve remission as RD. OS was defined as the time from trial entry to death. For patients entering CR, relapse-free survival (RFS) was the time from first CR to an event (death in first CR or relapse). Cumulative incidence of relapse (CIR) was the incidence of relapse for which death during CR was considered a competing risk.
Statistical methods
The Mantel-Haenszel test for trend (for ordinal data) and Fisher exact test (in 2 × 2 tables) or χ2 tests (for larger tables) were used to test for differences in clinical and demographic data by FLT3/TKD positivity. Kaplan-Meier life tables were constructed for survival data and were compared by means of the log-rank test, with surviving patients in AML10 censored on April 1, 2004, and surviving patients in AML12 censored on April 1, 2005. Follow-up was up-to-date for most patients, and the small number of patients lost to follow-up are censored at the date they were last known to be alive. Analysis of time to event data was carried out using standard log-rank methods. Odds ratio (OR) plots, with tests for heterogeneity, were used to investigate whether the prognostic relevance of FLT3/TKD differed between FLT3/ITD subgroups. Multivariate analysis was used to find the factors most closely associated with CR rate, and multivariate Cox models were used to analyze OS, RFS, and CIR. Models were fitted using forward selection, with variables added to the model if they reached significance at the P = .01 level. Univariate analysis was not done before the multivariate analysis. All variables were candidates for the model at all times. Because of multiple testing, the level of significance was set at P = .01. All P values are 2-tailed.
Results
Incidence, sequence, and quantification of FLT3/TKD mutations
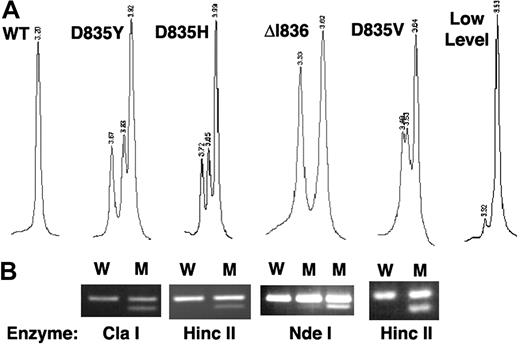
In the total cohort of 1107 patients, 127 (11%) were FLT3/TKD mutant-positive (FLT3/TKD+) by WAVE screening, of which 122 had a pattern consistent with a single mutant and 5 had evidence of 2 mutants. Mutations were initially determined by direct sequencing; however, it soon became apparent that the WAVE chromatogram pattern was specific to the underlying mutation and, therefore, most were identified by WAVE pattern and confirmed by mutation-specific restriction enzyme digest (Table 2; Figure 1). All mutants were identified and confirmed except those in 7 patients in whom the heteroduplex peak was very small, suggesting that the mutant present was only a small percentage of the total FLT3 alleles (Figure 1A). Overall, 13 different mutations were detected (Table 3). D835Y was the most common, accounting for 50% of mutant-positive patients; D835H, D835V, D835E, and Δ836 were all found at a similar frequency of 6% to 12% of positive patients. Two novel mutations were detected, each in a single case: (1) a 3 bp insertion that replaced aspartate codon 835 with glycine and proline and (2) a 16 bp tandem duplication plus 10 bp deletion within the kinase domain that replaced codons 835 and 836 (DI) with VIPT. Five of the mutations detected (4%) occurred outside the EcoRV restriction digest site.
Detection of FLT3/TKD mutations. (A) dHPLC chromatograms of a 278 bp PCR product generated from genomic DNA showing the WT homoduplex and heteroduplex patterns from higher-level D835Y, D835H, ΔI836, and D835V mutations and a lower-level D835Y mutant. (B) Corresponding mismatch PCR and specific restriction enzyme analysis confirming the mutation. W indicates wild-type; M, mutant.
Detection of FLT3/TKD mutations. (A) dHPLC chromatograms of a 278 bp PCR product generated from genomic DNA showing the WT homoduplex and heteroduplex patterns from higher-level D835Y, D835H, ΔI836, and D835V mutations and a lower-level D835Y mutant. (B) Corresponding mismatch PCR and specific restriction enzyme analysis confirming the mutation. W indicates wild-type; M, mutant.
FLT3/TKD mutations detected in total cohort of 1107 patients
| . | Higher-level mutant, no. . | Lower-level mutant, no. . | Total (% FLT3/TKD+ patients) . |
|---|---|---|---|
| D835Y | 33 | 30 | 63 (50) |
| D835E | 4 | 7 | 11 (9) |
| D835H | 9 | 5 | 14 (11) |
| D835N | 1 | 0 | 1 (1) |
| D835V | 5 | 3 | 8 (6) |
| Δ835 | 1 | 0 | 1 (1) |
| Δ836 | 4 | 11 | 15 (12) |
| ΔDI + InsVIPT | 0 | 1 | 1 (1) |
| D835GP | 1 | 0 | 1 (1) |
| D839G | 1 | 0 | 1 (1) |
| N841I | 1 | 1 | 2 (2) |
| N841K | 1 | 0 | 1 (1) |
| N841Y | 1 | 0 | 1 (1) |
| Unknown | 0 | 7 | 7 (6) |
| . | Higher-level mutant, no. . | Lower-level mutant, no. . | Total (% FLT3/TKD+ patients) . |
|---|---|---|---|
| D835Y | 33 | 30 | 63 (50) |
| D835E | 4 | 7 | 11 (9) |
| D835H | 9 | 5 | 14 (11) |
| D835N | 1 | 0 | 1 (1) |
| D835V | 5 | 3 | 8 (6) |
| Δ835 | 1 | 0 | 1 (1) |
| Δ836 | 4 | 11 | 15 (12) |
| ΔDI + InsVIPT | 0 | 1 | 1 (1) |
| D835GP | 1 | 0 | 1 (1) |
| D839G | 1 | 0 | 1 (1) |
| N841I | 1 | 1 | 2 (2) |
| N841K | 1 | 0 | 1 (1) |
| N841Y | 1 | 0 | 1 (1) |
| Unknown | 0 | 7 | 7 (6) |
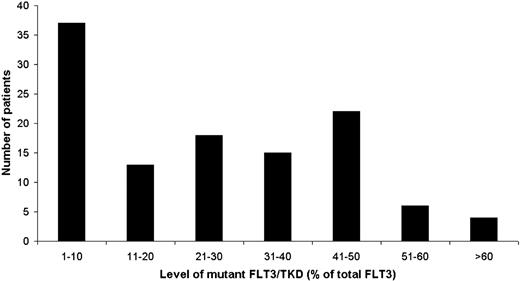
The relative mutant level as a percentage of total FLT3 alleles was quantified in 115 samples using labeled PCR products and size separation after mutation-specific or EcoRV restriction enzyme digestion if required. Quantification was not performed in 12 cases—either because the mutation had not been identified (7 cases) or due to lack of material—and these were scored by visual estimation of the chromatogram. The distribution of the relative mutant level is shown in Figure 2. Mutants were arbitrarily classified as “higher” level, with greater than the median level of 25%, or “lower” level when less than or equal to this. Of the total cohort of 127 mutant-positive cases, a higher level of mutant was present in 62 (49%) (ie, 6% of all patients in the cohort), and the median mutant level in this group was 43% (range, 26% to 93%). Of note, only 2 patients showed evidence of biallelic mutation or loss of WT alleles, with a 88% and 93% mutant level, respectively. A lower level of mutant was present in 65 cases (51%) (ie, 6% of all patients), with a median level of 6% (range, 1% to 25%). In the 5 cases with 2 mutants, 4 of them had 1 major mutant of higher level plus a minor mutant of very low level (approximately 2% of total), and in the remaining case both mutants were lower level (less than 25% of total).
EcoRV digestion and dHPLC analysis were both performed in 247 patients, including all 127 with an abnormal WAVE pattern: 120 were WT by both techniques, but 13 (10%) that were mutant by dHPLC were WT by EcoRV digestion. Four of these 13 cases had a mutant of higher level, but the mutations were outside of the EcoRV cutting site. Nine cases had a lower-level mutant by dHPLC. Of these, 5 were identified, all of which disrupted the EcoRV cutting site; 3 were quantified and found to be 3%, 3%, and 1% of total FLT3 alleles, respectively. Consequently, approximately one third of the mutants that were missed by EcoRV digestion were because the mutation did not change this cutting site, and the rest were below the detection level of the EcoRV technique.
Demographics of patients with an FLT3/TKD mutation
Details of the presenting features of the 1107 patients in the cohort are given in Table 1. The presence of a mutation was not related to sex or age. Mutations were found in all French-American-British (FAB) subtypes except M7. There was some evidence that FLT3/TKD mutations were less frequent in patients with secondary AML compared with de novo disease (P = .02). The presence of an FLT3/TKD mutation correlated with a high presenting white blood cell count (WBC): median WBC, 38.3 × 109/L in mutant-positive patients and 23.0 × 109/L in WT patients (P = .006). This correlation was restricted to higher-level TKD mutants: median WBC, 49.0 × 109/L in higher-level mutants and 24.4 × 109/L in lower-level mutants (P = .002). Information on karyotype was available in 882 patients (Table 4). There was a high incidence of FLT3/TKD mutations in patients with inv(16) (24%, P = .005) and a low incidence in cases with adverse cytogenetics (3%, P = .008).
Incidence of FLT3/TKD+ patients in specific cytogenetic risk groups and subgroups
| Cytogenetics . | Total no. . | FLT3/TKD−, no. . | FLT3/TKD+, no. . | FLT3/TKD+, % . | P . | FLT3/ITD+, % . |
|---|---|---|---|---|---|---|
| Favorable | 129 | 111 | 18 | 14 | N/A | 12 |
| t(8;21) | 74 | 69 | 5 | 7 | .3 | 15 |
| inv(16) | 55 | 42 | 13 | 24 | .005 | 7 |
| Intermediate | 648 | 575 | 73 | 11 | N/A | 31 |
| Normal | 437 | 386 | 51 | 12 | .4 | 35 |
| del(7q) | 23 | 21 | 2 | 9 | 1.0 | 4 |
| 11q23 | 30 | 28 | 2 | 7 | .8 | 3 |
| +8 | 69 | 61 | 8 | 12 | .8 | 23 |
| +22 | 16 | 13 | 3 | 19 | .2 | 6 |
| Adverse | 105 | 102 | 3 | 3 | N/A | 7 |
| Complex | 61 | 60 | 1 | 2 | .02 | 3 |
| del (5q) | 22 | 20 | 2 | 9 | 1.0 | 0 |
| −5 | 21 | 21 | 0 | 0 | .15 | 0 |
| −7 | 42 | 40 | 2 | 5 | .3 | 2 |
| abn(3q) | 31 | 30 | 1 | 3 | .2 | 16 |
| Unknown | 225 | 192 | 33 | 15 | N/A | 28 |
| Cytogenetics . | Total no. . | FLT3/TKD−, no. . | FLT3/TKD+, no. . | FLT3/TKD+, % . | P . | FLT3/ITD+, % . |
|---|---|---|---|---|---|---|
| Favorable | 129 | 111 | 18 | 14 | N/A | 12 |
| t(8;21) | 74 | 69 | 5 | 7 | .3 | 15 |
| inv(16) | 55 | 42 | 13 | 24 | .005 | 7 |
| Intermediate | 648 | 575 | 73 | 11 | N/A | 31 |
| Normal | 437 | 386 | 51 | 12 | .4 | 35 |
| del(7q) | 23 | 21 | 2 | 9 | 1.0 | 4 |
| 11q23 | 30 | 28 | 2 | 7 | .8 | 3 |
| +8 | 69 | 61 | 8 | 12 | .8 | 23 |
| +22 | 16 | 13 | 3 | 19 | .2 | 6 |
| Adverse | 105 | 102 | 3 | 3 | N/A | 7 |
| Complex | 61 | 60 | 1 | 2 | .02 | 3 |
| del (5q) | 22 | 20 | 2 | 9 | 1.0 | 0 |
| −5 | 21 | 21 | 0 | 0 | .15 | 0 |
| −7 | 42 | 40 | 2 | 5 | .3 | 2 |
| abn(3q) | 31 | 30 | 1 | 3 | .2 | 16 |
| Unknown | 225 | 192 | 33 | 15 | N/A | 28 |
P values are for Fisher exact test for the incidence of TKD mutations in each individual cytogenetic abnormality versus all other known karyotypes.
There were a number of features associated with FLT3/TKD+ cases that differed markedly from those of FLT3/ITD+ cases. FLT3/TKD mutations were uncommon in patients with secondary AML, whereas in our cohort the incidence of FLT3/ITDs did not differ significantly between de novo and secondary AML,23 although others have reported that FLT3/ITDs are less frequent in secondary AML.11,12,26 Unlike FLT3/ITDs, which are particularly frequent in patients with normal cytogenetics, the only group in which FLT3/TKD mutations were more common were cases with inv(16).
Relationship between the presence of an FLT3/TKD mutation and clinical outcome
Of the 1107 non-APL AML patients, 723 (65%) had WT FLT3, 257 (23%) were FLT3/ITD+ only, 100 (9%) FLT3/TKD+ only, and 27 (2%) had both mutations. Outcome data were available on the whole cohort with a median follow-up of 7.9 years (range, 6 to 193 months). Of the 127 FLT3/TKD+ patients, 14 received an autograft and 17 an allograft.
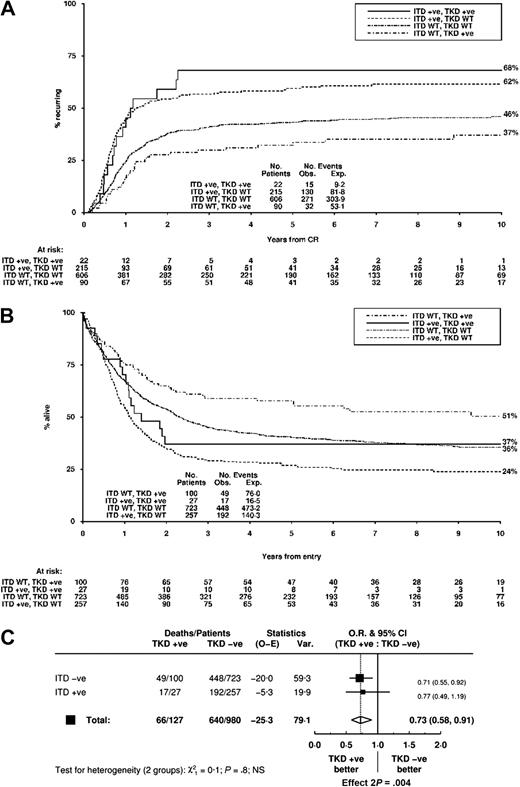
The CR rate for all 1107 patients was 85%, and the presence of an FLT3/TKD mutation did not influence the rate of CR, ID, or RD. In univariate analysis, patients with an FLT3/TKD mutation had a reduced CIR, improved RFS, and improved OS at 5 years (Table 5). Outcome in a significant proportion of the FLT3/TKD mutant-negative cases would be influenced by the presence of an FLT3/ITD; therefore, the impact in the cohort of both types of mutation was examined. The CIR at 10 years was 68% for FLT3/ITD+TKD+ patients, 62% for FLT3/ITD+TKD− patients, 46% for FLT3/ITD−TKD− patients, and 37% for FLT3/TKD+ITD− patients (Figure 3A). OS at 10 years was 37%, 24%, 36%, and 51%, respectively (Figure 3B). A direct comparison of FLT3/TKD+ and FLT3/ITD+ cases demonstrated a highly significant difference in CIR (OR, 0.45; 95% confidence interval [CI], 0.33 to 0.61; P < .001) and OS (OR, 0.53; 95% CI, 0.41 to 0.69; P < .001). There was no evidence that the impact of an FLT3/TKD mutation on OS differed according to FLT3/ITD status (test for interaction, P = .8), although there were only 27 patients with both types of mutation (Figure 3C).
Clinical outcome in FLT3/TKD− and FLT3/TKD+ non-APL AML patients
| . | TKD−, % . | TKD+, % . | OR (CI); P . | Adjusted result: OR (CI); P . | TKD low, % . | TKD high, % . | OR (CI); P . | Adjusted result: OR (CI); P . |
|---|---|---|---|---|---|---|---|---|
| Initial response to therapy | ||||||||
| CR | 84 | 88 | 0.73 (0.44-1.21); .2 | 0.80 (0.38-1.69); .6 | 83 | 94 | 0.72 (0.49-1.06); .07 | 0.74 (0.44-1.29); .3 |
| ID | 6 | 4 | 0.68 (0.31-1.50); .3 | 0.64 (0.19-2.15); .5 | 6 | 2 | 0.65 (0.33-1.28); .17 | 0.57 (0.22-1.47); .2 |
| RD | 10 | 8 | 0.79 (0.42-1.48); .5 | 0.94 (0.38-2.28); .9 | 11 | 5 | 0.78 (0.49-1.24); .3 | 0.87 (0.48-1.57); .6 |
| Outcome at 5 y | ||||||||
| CIR | 48 | 39 | 0.75 (0.57-0.98); .03 | 0.75 (0.53-1.07); .11 | 50 | 30 | 0.78 (0.64-0.94); .01 | 0.80 (0.64-1.00); .05 |
| RFS | 35 | 48 | 0.74 (0.59-0.93); .008 | 0.71 (0.52-0.97); .03 | 35 | 60 | 0.77 (0.65-0.91); .003 | 0.78 (0.64-0.95); .01 |
| OS | 37 | 53 | 0.72 (0.58-0.89); .002 | 0.71 (0.52-0.96); .03 | 37 | 71 | 0.74 (0.63-0.87); .004 | 0.74 (0.61-0.91); .004 |
| . | TKD−, % . | TKD+, % . | OR (CI); P . | Adjusted result: OR (CI); P . | TKD low, % . | TKD high, % . | OR (CI); P . | Adjusted result: OR (CI); P . |
|---|---|---|---|---|---|---|---|---|
| Initial response to therapy | ||||||||
| CR | 84 | 88 | 0.73 (0.44-1.21); .2 | 0.80 (0.38-1.69); .6 | 83 | 94 | 0.72 (0.49-1.06); .07 | 0.74 (0.44-1.29); .3 |
| ID | 6 | 4 | 0.68 (0.31-1.50); .3 | 0.64 (0.19-2.15); .5 | 6 | 2 | 0.65 (0.33-1.28); .17 | 0.57 (0.22-1.47); .2 |
| RD | 10 | 8 | 0.79 (0.42-1.48); .5 | 0.94 (0.38-2.28); .9 | 11 | 5 | 0.78 (0.49-1.24); .3 | 0.87 (0.48-1.57); .6 |
| Outcome at 5 y | ||||||||
| CIR | 48 | 39 | 0.75 (0.57-0.98); .03 | 0.75 (0.53-1.07); .11 | 50 | 30 | 0.78 (0.64-0.94); .01 | 0.80 (0.64-1.00); .05 |
| RFS | 35 | 48 | 0.74 (0.59-0.93); .008 | 0.71 (0.52-0.97); .03 | 35 | 60 | 0.77 (0.65-0.91); .003 | 0.78 (0.64-0.95); .01 |
| OS | 37 | 53 | 0.72 (0.58-0.89); .002 | 0.71 (0.52-0.96); .03 | 37 | 71 | 0.74 (0.63-0.87); .004 | 0.74 (0.61-0.91); .004 |
P values relating to TKD− versus TKD+ analysis are for the Mantel-Haenszel test for the initial response and the log-rank test for long-term outcomes. P values relating to TKD− versus TKD low versus TKD high analysis were obtained using logistic regression analysis. Adjusted results show results of multivariate analyses adjusted for significant variables in forward selection regression model.
Clinical outcome for non-APL AML patients stratified according to FLT3/ITD and FLT3/TKD status. (A) CIR and (B) OS. (C) Analysis of OS showing effect of FLT3/TKD status stratified by FLT3/ITD.
Clinical outcome for non-APL AML patients stratified according to FLT3/ITD and FLT3/TKD status. (A) CIR and (B) OS. (C) Analysis of OS showing effect of FLT3/TKD status stratified by FLT3/ITD.
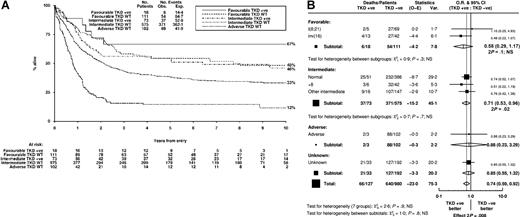
Kaplan-Meier curves for OS in the 3 cytogenetic risk groups stratified by FLT3/TKD status are shown in Figure 4A. When the cohort was stratified according to cytogenetic risk group, there was no heterogeneity or trend between the groups in the effect of an FLT3/TKD mutation on OS (P = .8, Figure 4B). Similarly, stratification according to specific cytogenetic abnormalities with sufficient FLT3/TKD+ patients (normal karyotype, inv(16), +8) did not reveal any heterogeneity in the impact of an FLT3/TKD mutation on OS (P = .9, Figure 4B).
Impact of FLT3/TKD mutation status on OS stratified according to cytogenetic abnormalities. (A) Kaplan-Meier curves stratified according to FLT3/TKD status and cytogenetic risk group. Because only 3 patients with adverse cytogenetics carried a FLT3/TKD mutation, this curve is not shown. (B) Analysis of OS showing the effect of FLT3/TKD status stratified according to cytogenetic abnormality and category.
Impact of FLT3/TKD mutation status on OS stratified according to cytogenetic abnormalities. (A) Kaplan-Meier curves stratified according to FLT3/TKD status and cytogenetic risk group. Because only 3 patients with adverse cytogenetics carried a FLT3/TKD mutation, this curve is not shown. (B) Analysis of OS showing the effect of FLT3/TKD status stratified according to cytogenetic abnormality and category.
A Cox multivariate analysis was performed, the variables considered being trial, cytogenetic risk group, age, sex, presentation WBC count, de novo or secondary AML, World Health Organization (WHO) performance status, and presence of an FLT3/ITD or TKD mutation. The favorable prognostic impact of an FLT3/TKD mutation on OS (OR, 0.71; CI, 0.52 to 0.96) had a P value of .03, which was not deemed to be significant using the criteria adopted in this analysis. Other variables were more powerful including presence of an FLT3/ITD (OR, 1.43; 95% CI, 1.19 to 1.72; P < .001), cytogenetics (OR, 1.80; 95% CI, 1.51 to 2.16; P < .001), age (OR, 1.02; 95% CI, 1.01 to 1.02; P < .001), sex (OR, 0.79; 95% CI, 0.67 to 0.94; P = .007), and WHO performance status (OR, 1.13; 95% CI, 1.04 to 1.22; P = .003). The point estimate for the reduction in CIR associated with an FLT3/TKD mutation was of similar magnitude to the reduction in OS (OR, 0.75; 95% CI, 0.53 to 1.07; P = .13). The only variables predicting for increased CIR were the presence of an FLT3/ITD (OR, 1.82; 95% CI, 1.46 to 2.27; P < .001) and cytogenetic risk group (OR, 1.70; 95% CI, 1.35 to 2.13; P < .001).
Relationship between the level of an FLT3/TKD mutation and clinical outcome
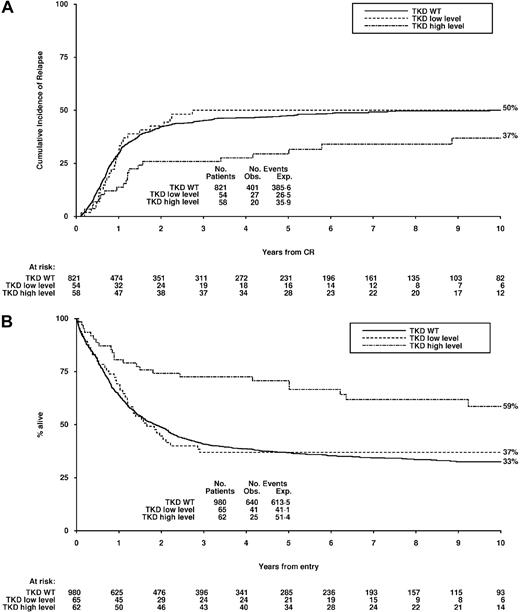
Proportional hazards regression analysis did not demonstrate any natural cutoff of mutant FLT3/TKD level that acted as an independent predictor of outcome, and therefore patients were stratified into 2 groups according to the median mutant level (greater than 25% and equal to or less than 25%). There was no evidence of any impact of mutant level on the rate of CR, ID, or RD (Table 5). Patients with a higher-level FLT3/TKD mutant, however, had an improved CIR and OS compared with patients with a lower level of mutation whose outcome was similar to that of FLT3/TKD WT patients (Table 5). The CIR at 10 years was 50% for FLT3/TKD WT patients, 50% for patients with a lower-level FLT3/TKD mutant, and 37% for patients with a higher-level mutant (Figure 5A). The OS at 10 years was 33%, 37%, and 59%, respectively (Figure 5B). This improved OS remained significant in a Cox multivariate analysis (OR, 0.74; 95% CI, 0.61 to 0.91; P = .004), although the difference was not significant for the CIR (OR, 0.80; 95% CI, 0.64 to 1.00; P = .05).
Clinical outcome for non-APL AML patients stratified according to FLT3/TKD mutant level. (A) CIR and (B) OS. High-level mutations, more than 25% of FLT3 alleles; low-level mutations, 25% of FLT3 alleles or less.
Clinical outcome for non-APL AML patients stratified according to FLT3/TKD mutant level. (A) CIR and (B) OS. High-level mutations, more than 25% of FLT3 alleles; low-level mutations, 25% of FLT3 alleles or less.
Discussion
In this study we have screened a large cohort of younger adult non-APL AML patients for the presence of FLT3/TKD mutations using dHPLC analysis and found the incidence to be 11%. There are a number of advantages of this technique. Firstly, it is exquisitely sensitive and does not depend on the efficient digestion of a restriction enzyme to be confident of the presence of a mutation. Small heteroduplex peaks, which were subsequently quantified as mutants present as low as 2% of total FLT3 alleles, were detected with ease (Figure 1A). This level of sensitivity may explain the increased incidence of mutant-positive patients in our cohort compared with the 7% reported in most other studies,9–11,13–15 although incidences up to 13% have been reported.12,27 In fact, 6% of cases in our cohort were found to have a higher level of mutant, defined as greater than the median level of 25% of total FLT3 alleles. Secondly, the technique picks up mutations that are outside of the EcoRV digestion site, which has been used in most published studies to date to screen for mutations, and in our cohort these cases accounted for 4% of the mutant-positive patients. Thirdly, the chromatogram profile produced by different mutations is specific and highly reproducible, in most cases enabling direct mutation identification from the WAVE pattern and confirmation using a mutation-specific restriction enzyme digest without the need for sequencing.
The 2 different types of FLT3 mutation, ITDs and TKD mutations, were associated with distinct biologic entities that have been noted in some but not all other cohorts.9–11,28,29 For example, in our cohort there were differences in the frequency with which FLT3/TKDs were detected in de novo or secondary disease, which were not observed for FLT3/ITDs. With regard to cytogenetic subgroups, an increased frequency of FLT3/TKD mutations was noted in cases with inv(16), unlike FLT3/ITDs, which were uncommon in this subgroup. It has been suggested that cases of FLT3/TKD+ with inv(16) are restricted to those with variant CBFbeta-MYH11 fusion transcripts,28 but breakpoint analysis was not available in our series. The differences between FLT3/TKDs and FLT3/ITDs may be a consequence of distinct mechanisms underlying the formation of each type of mutation, perhaps reflecting variable susceptibilities of the target cell of transformation to different types of DNA damage. It is also likely that the phenotype of the cell will be influenced by intracellular events downstream of the activated receptor; for example, distinct patterns of gene expression have been reported in primary leukemic samples containing FLT3/ITDs and TKD mutations.30 In vitro analysis in a cell model system transfected with the different types of mutant also found that differential signaling was induced by the 2 mutations.31 Furthermore, in a mouse model transplanting retrovirally transduced BM progenitors, the phenotype differed significantly according to the type of mutation transplanted.32 Mice undergoing transplantation with an FLT3/ITD developed a lethal oligoclonal myeloproliferative disorder and those with an FLT3/TKD mutation an oligoclonal lymphoproliferative disorder.
The most striking difference between FLT3/ITDs and FLT3/TKDs in our cohort of patients was the different impact on clinical outcome, which was highly significant: the 10-year OS for FLT3/TKD+ patients was 51% compared with 24% for FLT3/ITD+ patients (Figure 3B). This difference in OS is clearly related to the difference in CIR (CIR at 10 years = 37% in FLT3/TKD+ patients compared with 62% in FLT3/ITD+ patients; Figure 3A). There was no association of an FLT3/TKD mutation nor an FLT3/ITD23 with attainment of CR. This suggests that any leukemic progenitors that remain after induction chemotherapy may be more chemosensitive if they are FLT3/TKD+ and therefore more likely to be eradicated, with the converse being likely in FLT3/ITD+ cases. In univariate analysis, outcome in FLT3/TKD mutant-positive patients was significantly more favorable than in FLT3/TKD mutant-negative patients, both for RFS and OS. However, in multivariate analysis, with the level of significance set at P = .01, this difference in OS was no longer significant (P = .03). Nevertheless, an adverse impact of an FLT3/TKD mutation compared with FLT3/WT disease is clearly excluded. These results are in contrast to those of previous studies. A recent meta-analysis of the major studies concluded that FLT3/TKD mutations are associated with inferior disease-free survival, although there was no significant evidence of TKD mutation adversely affecting OS.20 The differences from our study may be related to heterogeneity of patient inclusion criteria, length of follow-up, or the play of chance owing to the relatively small number of mutant-positive patients in previous studies. Alternatively, although in our view unlikely, it may reflect differences in treatment schedules.
This study contains a sufficient number of FLT3/TKD+ patients to allow meaningful analysis of clinical outcome in relation to the level of an FLT3/TKD mutation. Both a higher WBC, indicative of a strong proliferative signal, and better survival were associated with FLT3/TKD+ patients with a higher level of mutant. For example, patients with a higher-level mutant had an OS at 10 years of 59% compared with 37% in patients with a lower-level mutant and 33% in patients without an FLT3/TKD mutation (Figure 5B). This difference remained significant in multivariate analysis (P = .004). It is possible that the presence of an FLT3/TKD mutation is associated with greater chemosensitivity. When the FLT3/TKD-containing cells are in the minority, however, the overall chemosensitivity is determined by the WT cells. When only a minority of cells contain a mutation, it is reasonable to postulate that the mutation is not present in the leukemic stem cell, which is likely to be responsible for relapse. Notably, therefore, of 9 FLT3/TKD mutant-positive patients for whom mutation status was available at relapse, 3 of 6 patients with a higher level of mutation at presentation relapsed with the same mutation at a similar level. However, 3 patients with a higher level of mutation and all 3 patients with a lower level of mutation lost the mutation at relapse (data not shown), an instability noted in other studies.33 This indicates that although an FLT3/TKD mutation may contribute to the leukemic phenotype, in many cases, it is not an essential requirement for the leukemic clone.
The finding of different clinical outcome for the 2 types of mutation in the same protein is surprising and raises some interesting biologic question regarding the mechanism(s) underlying this difference and the role of TK mutations in human cancers. Both types of FLT3 mutation lead to constitutive activation of the receptor in in vitro studies9,34 and are thought to lead to loss of autoinhibition of the kinase activity. Mutations in the TKD are thought to alter the configuration of the activation loop in a manner similar to that of ligand-induced conformational changes, allowing increased access of ATP and substrates to the kinase, whereas the tandem duplications are thought to disrupt the interaction between the JM domain and the activation loop, which normally stabilizes the kinase in its inactive configuration.35,36 The consequence of these different mechanisms may be quantitatively and/or qualitatively quite variable; for example, they may lead to altered substrate specificities downstream of the kinase.37 Precisely how this may influence outcome is unclear. It is also possible that the difference in clinical outcome observed between the 2 mutant types is not a consequence of differential downstream signaling events but instead reflects the type of mutagenic event giving rise to the FLT3 mutation or the inherent susceptibility of different leukemic stem cells to different types of DNA damage.
These results have important implications for the treatment of patients carrying FLT3 mutations. In some centers, patients with FLT3/ITDs are being considered for allogeneic transplantation in first remission, but in the absence of other poor prognostic factors this might not be appropriate in patients with FLT3/TKDs. Furthermore, several FLT3 inhibitors are now available and, if the different types of FLT3 receptor mutants have different downstream effects, the effects of FLT3 inhibitors might also differ.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Medical Research Council of Great Britain and the Leukaemia Research Fund.
We are grateful to the clinical investigators who entered and managed patients in these 2 trials.
Authorship
Contribution: A.J.M. and R.E.G. performed experimental analysis; R.K.H. performed data analysis; K.W. offered statistical advice and interpretation; D.C.L. and A.K.B. contributed to the design of the study; and A.J.M., D.C.L, and R.E.G. wrote the manuscript with contributions from R.K.H., K.W., and A.K.B.
A complete list of the members of the National Cancer Research Institute Adult Leukaemia Working Party is provided in Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adam J. Mead, Department of Haematology, Royal Free and University College Medical School, 98 Chenies Mews, London WC1E 6HX, United Kingdom; e-mail: adam.mead@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal